Antiviral Action of Tryptanthrin Isolated from Strobilanthes cusia Leaf against Human Coronavirus NL63
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell and Virus
2.2. Preparation of S. cusia Leaf Methanol Extract and Its Related Compounds
2.3. MTT Cytotoxicity Test
2.4. Cytopathic Effect Reduction and Virus Yield Inhibition Assays
2.5. Infectivity Inhibition Assay Using Immunofluorescent Staining
2.6. Analyzing the Inhibitory Effect on the Early and Late Stages Using the Time-of-Addition/RemovalAssay
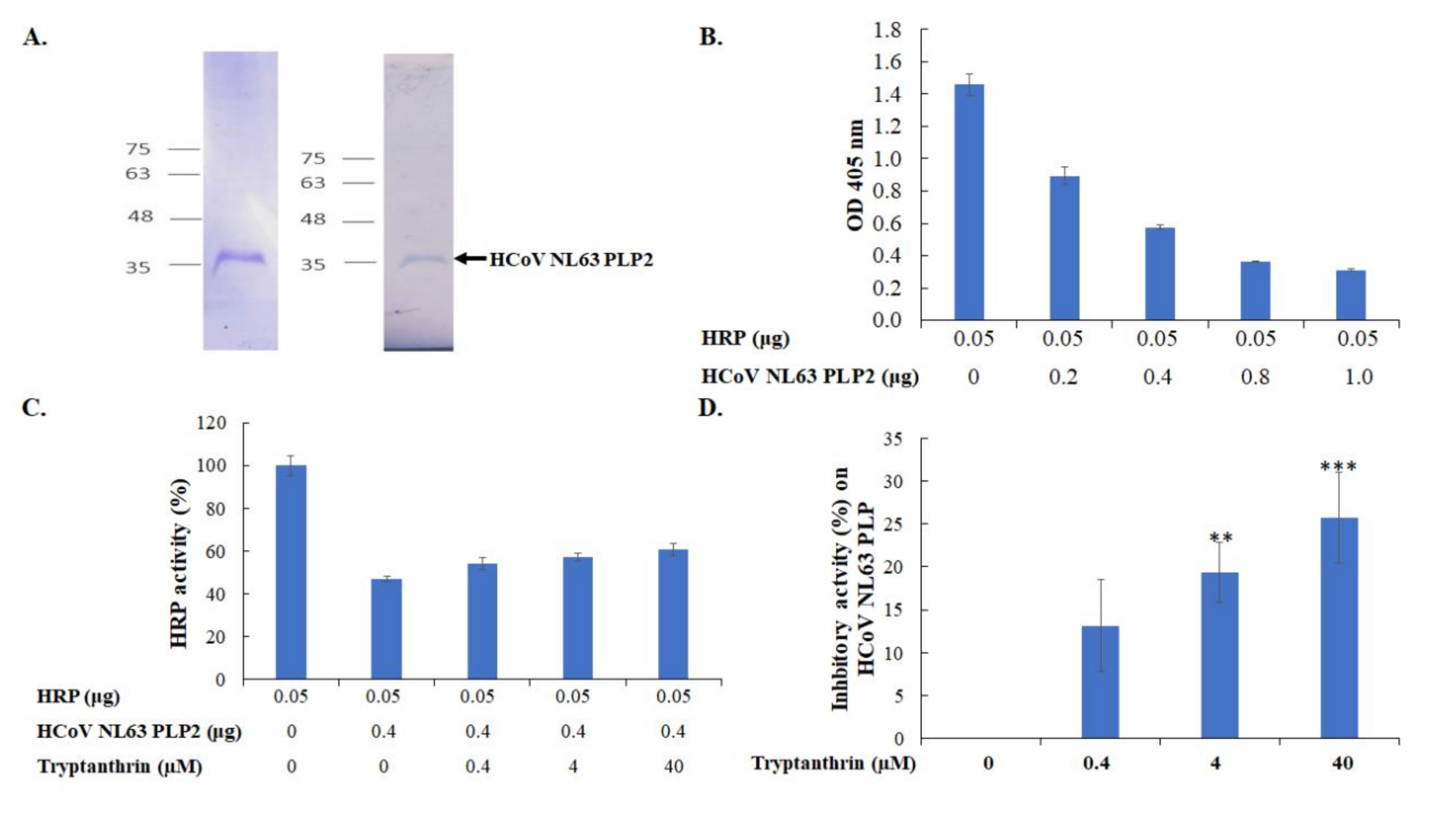
2.7. Construction, Expression, Purification, and In Vitro Trans-Cleavage Activity of Recombinant HCoV-NL63 Papain-Like Protease 2 (PLP2)
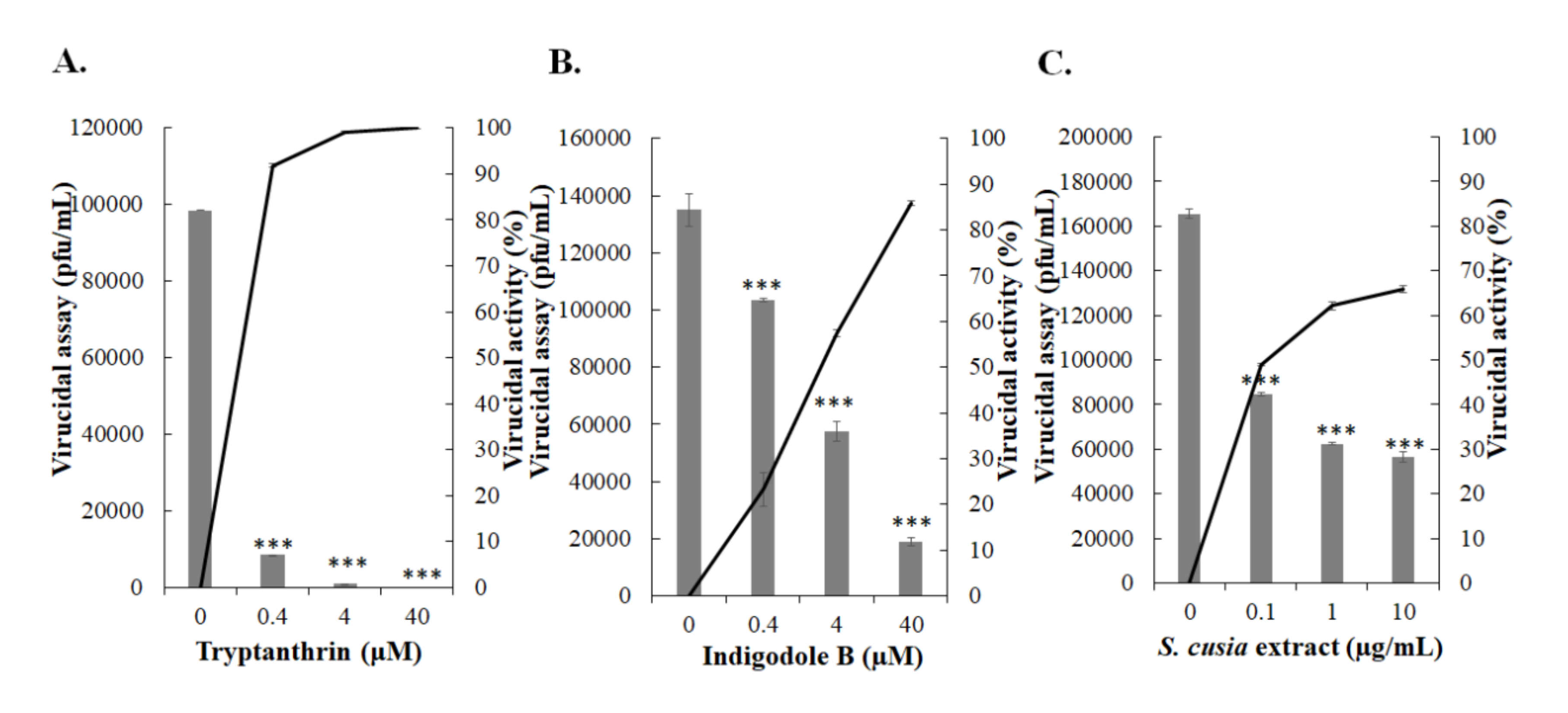
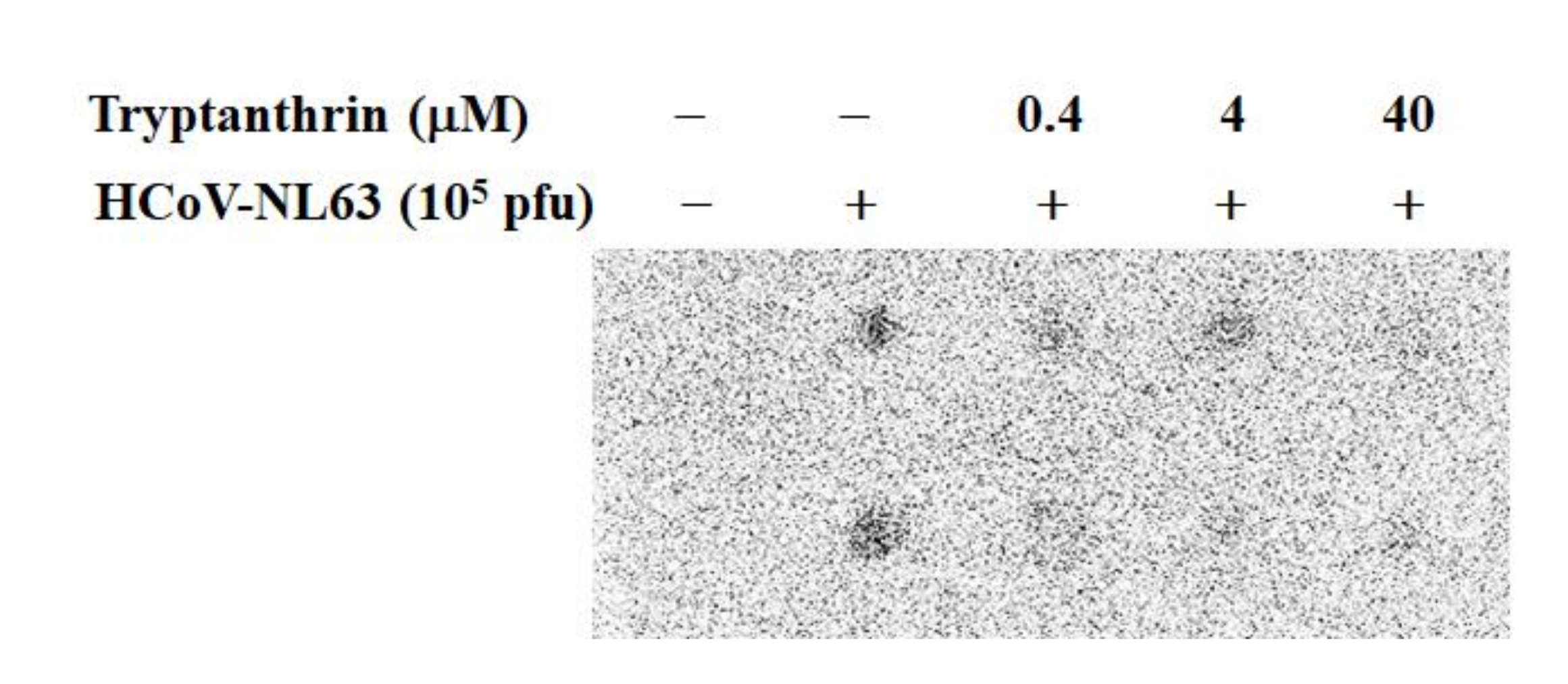
2.8. Assays on Virucidal Activity
2.9. Statistical Analysis
3. Results
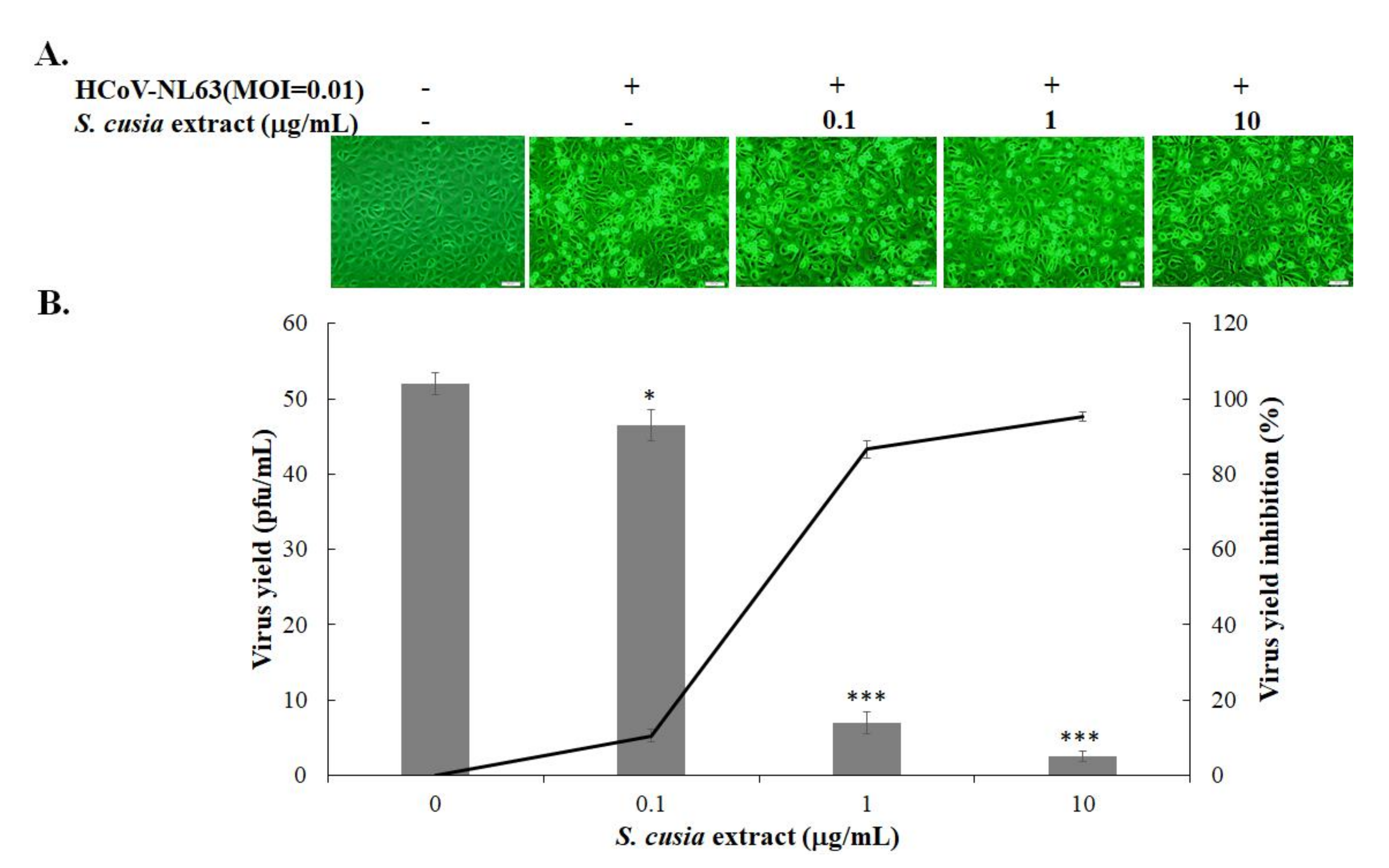
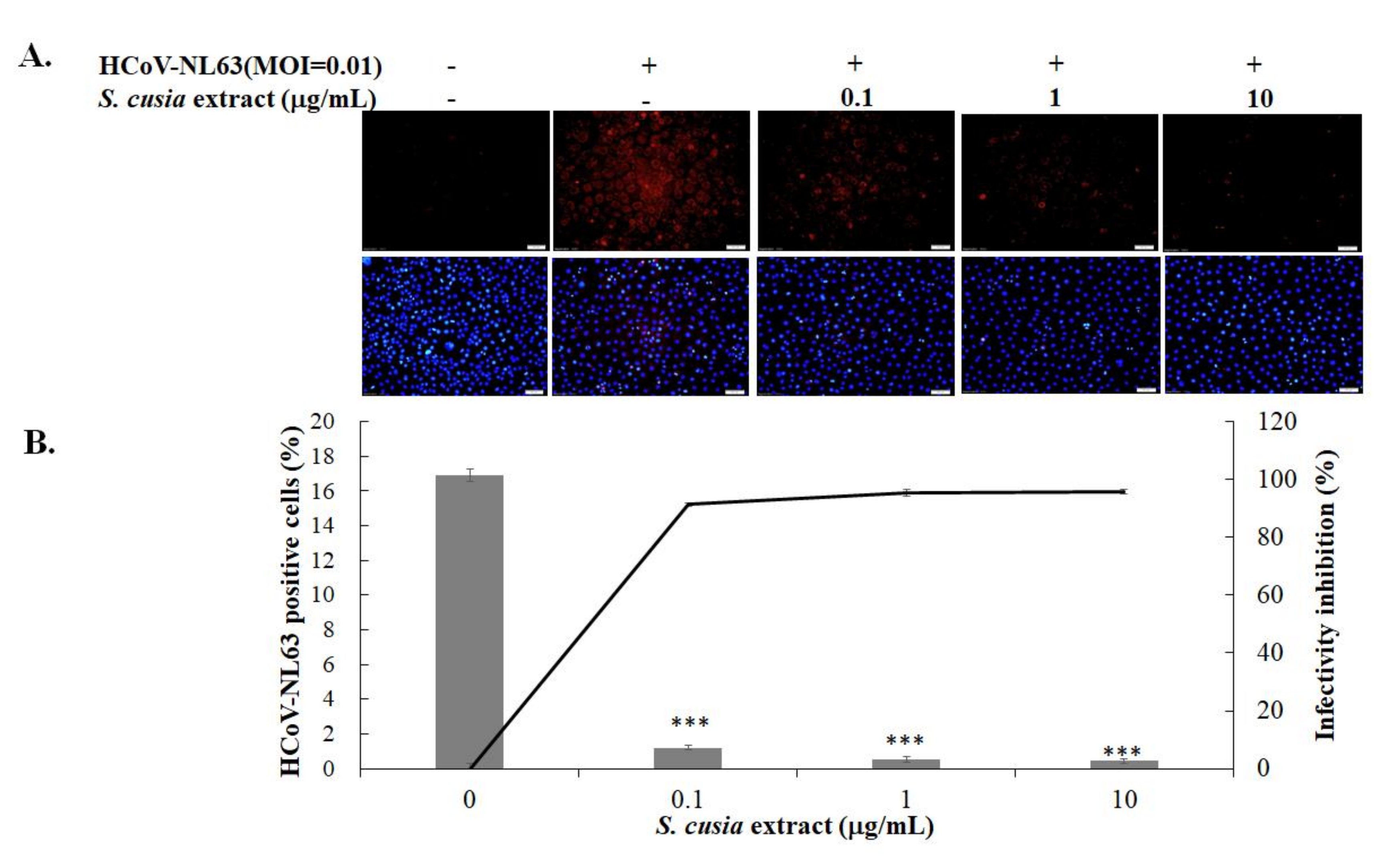
3.1. Antiviral Activity of S. cusia Leaf Methanol Extract against HCoV-NL63
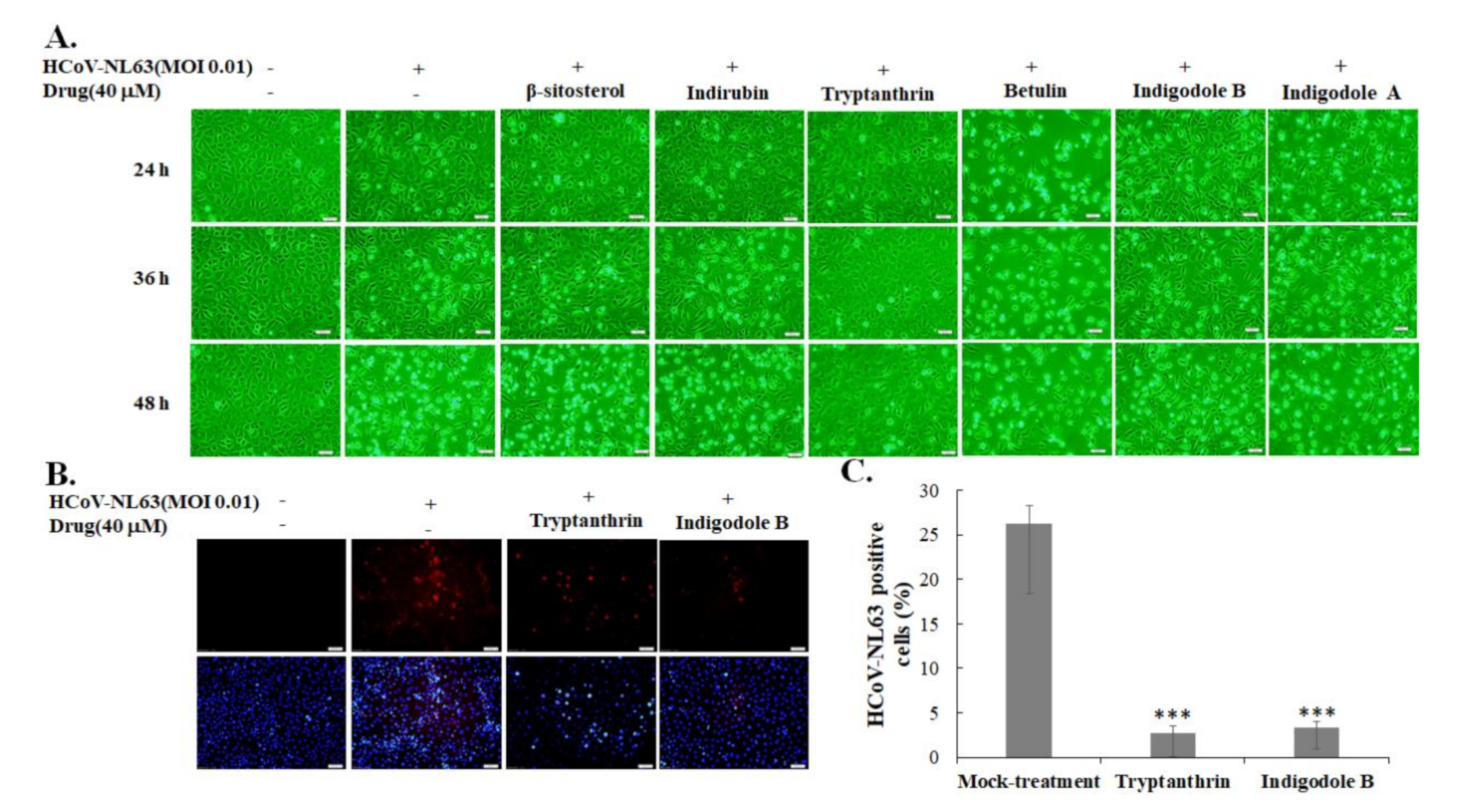
3.2. Action of Active Components from S. cusia Leaf Extract against HCoV-NL63
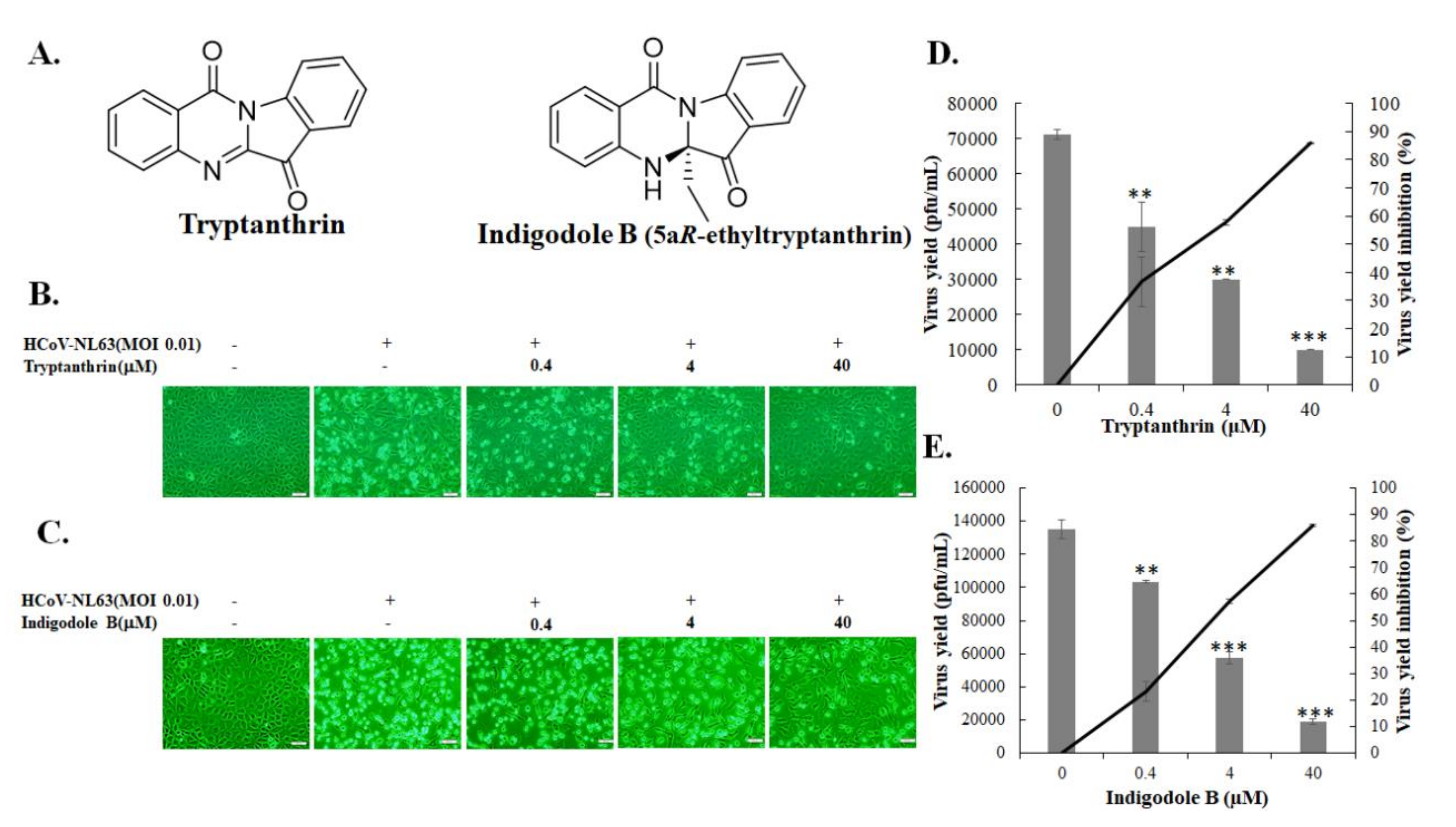
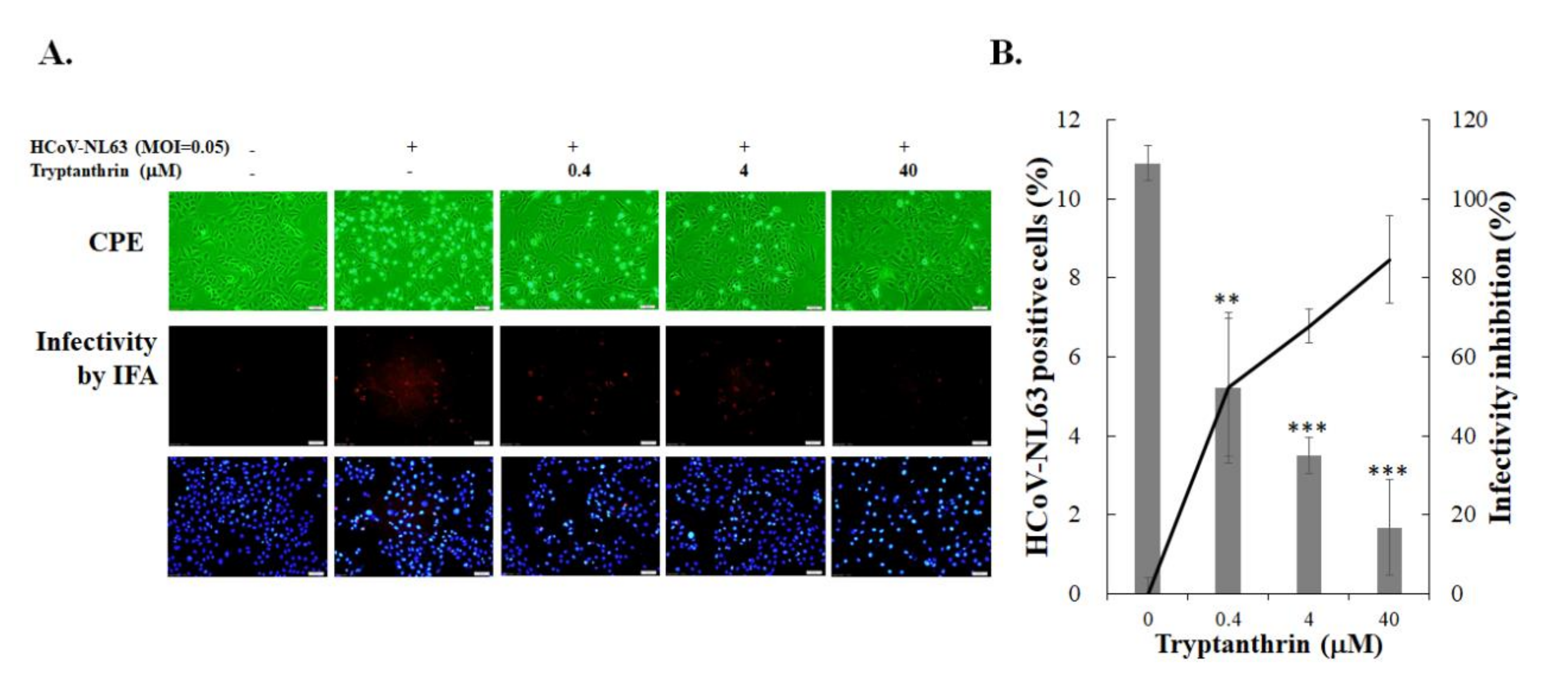
3.3. Evaluation of Anti-HCoV-NL63 Activity of Tryptanthrin in Human Lung Epithelial Cells
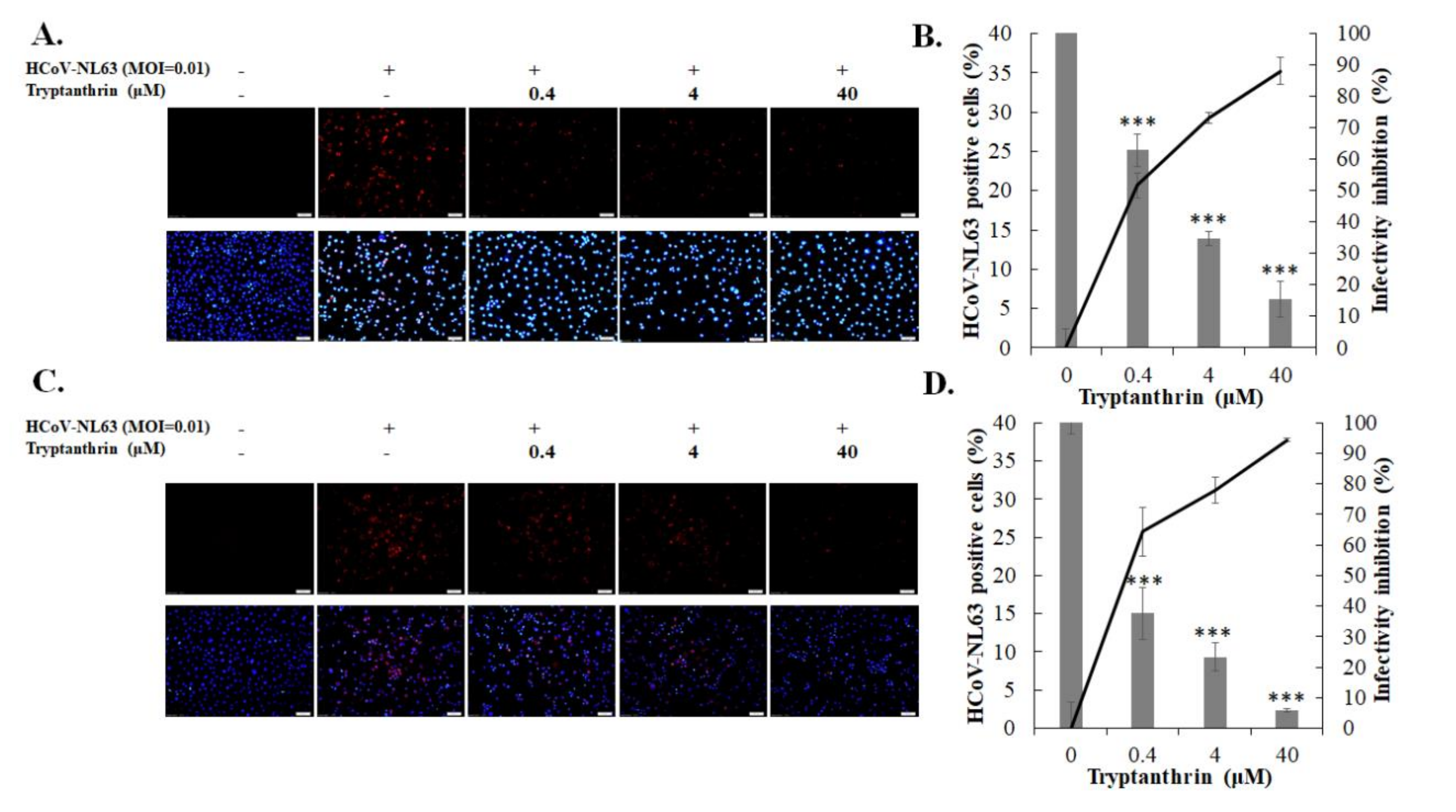
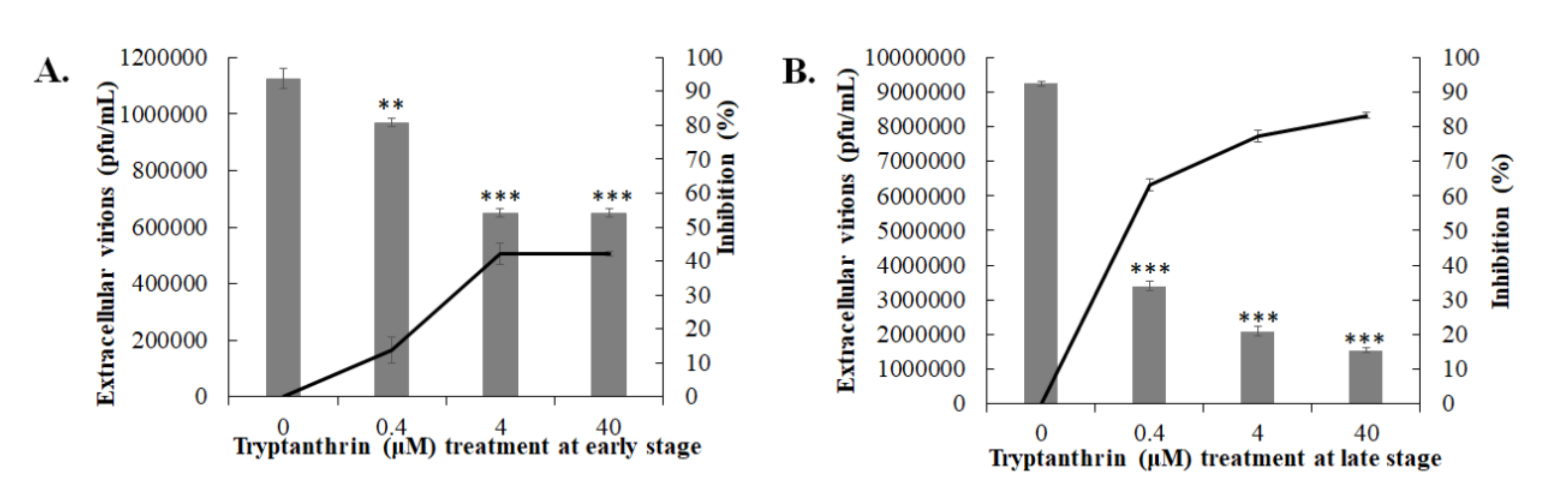
3.4. Antiviral Mechanism Underlying Tryptanthrin Action against HCoV-NL63
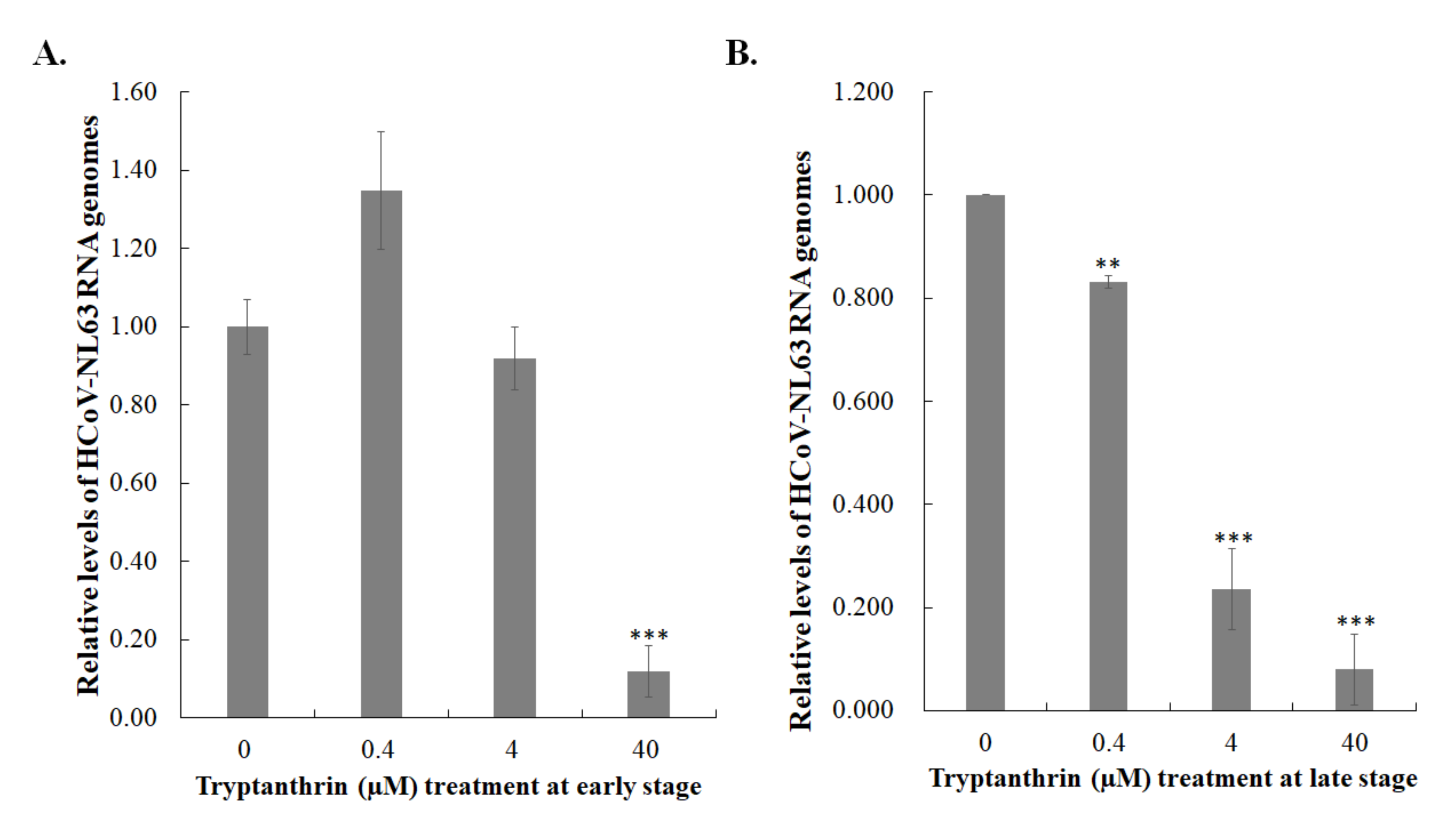
3.5. Tryptanthrin-Mediated Inhibition of HCoV-NL63 RNA Genome Synthesis and Papain-Like Protease 2 Activity
3.6. Virucidal Activity of Tryptanthrin against HCoV-NL63
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, W.; Huang, W.; Ning, S.; Wang, X.; Ye, Q.; Wei, D. De novo characterization of the Baphicacanthus cusia(Nees) Bremek transcriptome and analysis of candidate genes involved in indican biosynthesis and metabolism. PLoS ONE 2018, 13, e0199788. [Google Scholar] [CrossRef]
- Chen, H.; Shao, J.; Zhang, H.; Jiang, M.; Huang, L.; Zhang, Z.; Yang, D.; He, M.; Ronaghi, M.; Luo, X.; et al. Sequencing and Analysis of Strobilanthes cusia (Nees) Kuntze Chloroplast Genome Revealed the Rare Simultaneous Contraction and Expansion of the Inverted Repeat Region in Angiosperm. Front. Plant. Sci. 2018, 9, 324. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, Y.; Hao, X.J.; Yang, F.M.; Sun, Q.Y.; Morris-Natschke, S.L.; Lee, K.H.; Wang, Y.H.; Long, C.L. Indole alkaloid glycosides from the aerial parts of Strobilanthes cusia. J. Nat. Prod. 2014, 77, 2590–2604. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ikeda, T.; Kaku, M.; Zhu, X.H.; Okawa, M.; Yokomizo, K.; Uyeda, M.; Nohara, T. A new lignan glycoside and phenylethanoid glycosides from Strobilanthes cusia BREMEK. Chem. Pharm. Bull. (Tokyo) 2004, 52, 1242–1245. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, Z.; Feng, Q.; Liang, X.; Li, J.; Zanin, M.; Jiang, Z.; Zhong, N. ; Aurantiamide acetate from baphicacanthus cusia root exhibits anti-inflammatory and anti-viral effects via inhibition of the NF-kappaB signaling pathway in Influenza A virus-infected cells. J. Ethnopharmacol. 2017, 199, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wang, W.; Li, X.N.; Zhang, Y.; Wang, L.P.; Yuan, C.M.; Huang, L.J.; Hao, X.J. A novel isocoumarin with anti-influenza virus activity from Strobilanthes cusia. Fitoterapia 2015, 107, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.L.; Kao, K.C.; Tsai, H.Y.; Chueh, F.Y.; Chang, Y.S. ; Evaluation of antinociceptive, anti-inflammatory and antipyretic effects of Strobilanthes cusia leaf extract in male mice and rats. Am. J. Chin. Med. 2003, 31, 61–69. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Liu, B.; Guo, Z.; Huang, W.; Wu, X.; Li, S.; Zhou, J.; Lei, Q.; Long, C. Ethnobotany of dye plants in Dong communities of China. J. Ethnobiol. Ethnomed. 2014, 10, 23. [Google Scholar] [CrossRef]
- Lee, C.L.; Wang, C.M.; Hu, H.C.; Yen, H.R.; Song, Y.C.; Yu, S.J.; Chen, C.J.; Li, W.C.; Wu, Y.C. Indole alkaloids indigodoles A–C from aerial parts of Strobilanthes cusia in the traditional Chinese medicine Qing Dai have anti-IL-17 properties. Phytochemistry 2019, 162, 39–46. [Google Scholar] [CrossRef]
- Liau, B.C.; Jong, T.T.; Lee, M.R.; Chen, S.S. LC-APCI-MS method for detection and analysis of tryptanthrin, indigo, and indirubin in daqingye and banlangen. J. Pharm. Biomed. Anal. 2007, 43, 346–351. [Google Scholar] [CrossRef]
- Chang, S.J.; Chang, Y.C.; Lu, K.Z.; Tsou, Y.Y.; Lin, C.W. Antiviral Activity of Isatis indigotica Extract and Its Derived Indirubin against Japanese Encephalitis Virus. Evid. Based. Complement. Alternat. Med. 2012, 2012, 925830. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tu, Y.; Xia, Y.; Cheng, P.; Sun, W.; Shi, Y.; Guo, L.; He, H.; Xiong, C.; Chen, S.; et al. Identification and Verification of Indirubin-Containing Medicinal Plants. Evid. Based. Complement. Alternat. Med. 2015, 2015, 484670. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mak, N.K.; Leung, C.Y.; Wei, X.Y.; Shen, X.L.; Wong, R.N.; Leung, K.N.; Fung, M.C. Inhibition of RANTES expression by indirubin in influenza virus-infected human bronchial epithelial cells. Biochem. Pharmacol. 2004, 67, 167–74. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Tsai, F.J.; Tsai, C.H.; Lai, C.C.; Wan, L.; Ho, T.Y.; Hsieh, C.C.; Chao, P.D. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 2005, 68, 36–42. [Google Scholar] [CrossRef]
- Li, S.W.; Lin, C.W. Human coronaviruses: Clinical features and phylogenetic analysis. Biomedicine (Taipei) 2013, 3, 43–50. [Google Scholar] [CrossRef]
- Milewska, A.; Nowak, P.; Owczarek, K.; Szczepanski, A.; Zarebski, M.; Hoang, A.; Berniak, K.; Wojarski, J.; Zeglen, S.; Baster, Z.; et al. Entry of Human Coronavirus NL63 into the Cell. J. Virol. 2018, 92, e01933–17. [Google Scholar] [CrossRef]
- van der Hoek, L.; Pyrc, K.; Berkhout, B. Human coronavirus NL63, a new respiratory virus. FEMS Microbiol. Rev. 2006, 30, 760–73. [Google Scholar] [CrossRef]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, in press. [Google Scholar] [CrossRef]
- Huang, S.H.; Su, M.C.; Tien, N.; Huang, C.J.; Lan, Y.C.; Lin, C.S.; Chen, C.H.; Lin, C.W. Epidemiology of human coronavirus NL63 infection among hospitalized patients with pneumonia in Taiwan. J. Microbiol. Immunol. Infect. 2017, 50, 763–70. [Google Scholar] [CrossRef]
- Su, J.H.; Diao, R.G.; Lv, S.G.; Mou, X.D.; Li, K. Modes of Antiviral Action of Chemical Portions and Constituents from Woad Root Extract against Influenza Virus A FM1. Evid. Based Complement. Alternat. Med. 2016, 2016, 2537294. [Google Scholar] [CrossRef]
- Hand, J.; Rose, E.B.; Salinas, A.; Lu, X.; Sakthivel, S.K.; Schneider, E.; Watson, J.T. Severe Respiratory Illness Outbreak Associated with Human Coronavirus NL63 in a Long-Term Care Facility. Emerg. Infect. Dis. 2018, 24, 1964–66. [Google Scholar] [CrossRef] [PubMed]
- Beau De Rochars, V.M.; Lednicky, J.; White, S.; Loeb, J.; Elbadry, M.A.; Telisma, T.; Chavannes, S.; Anilis, M.G.; Cella, E.; Ciccozzi, M.; et al. Isolation of Coronavirus NL63 from Blood from Children in Rural Haiti: Phylogenetic Similarities with Recent Isolates from Malaysia. Am. J. Trop. Med. Hyg. 2017, 96, 144–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lednicky, J.A.; Waltzek, T.B.; McGeehan, E.; Loeb, J.C.; Hamilton, S.B.; Luetke, M.C. Isolation and genetic characterization of human coronavirus NL63 in primary human renal proximal tubular epithelial cells obtained from a commercial supplier, and confirmation of its replication in two different types of human primary kidney cells. Virol. J. 2013, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Alex, V.V.; Nisthul, A.A.; Bava, S.V.; Sundaram, S.; Retnakumari, A.P.; Chittalakkottu, S.; Anto, R.J. Pre-clinical evidences for the efficacy of tryptanthrin as a potent suppressor of skin cancer. Cell Prolif. 2020, 53, e12710. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Kim, H.M.; Jeong, H.J. Tryptanthrin reduces mast cell proliferation promoted by TSLP through modulation of MDM2 and p53. Biomed. Pharmacother. 2016, 79, 71–87. [Google Scholar] [CrossRef]
- Honda, G.; Tabata, M. Isolation of antifungal principle tryptanthrin, from Strobilanthes cusia O. Kuntze. Planta Med. 1979, 36, 85–90. [Google Scholar] [CrossRef]
- Honda, G.; Tosirisuk, V.; Tabata, M. Isolation of an antidermatophytic, tryptanthrin, from indigo plants, Polygonum tinctorium and Isatis tinctoria. Planta Med. 1980, 38, 275–6. [Google Scholar] [CrossRef]
- Plitzko, I.; Mohn, T.; Sedlacek, N.; Hamburger, M. Composition of Indigo naturalis. Planta Med. 2009, 75, 860–863. [Google Scholar] [CrossRef]
- Kataoka, M.; Hirata, K.; Kunikata, T.; Ushio, S.; Iwaki, K.; Ohashi, K.; Ikeda, M.; Kurimoto, M. Antibacterial action of tryptanthrin and kaempferol, isolated from the indigo plant (Polygonum tinctorium Lour.), against Helicobacter pylori-infected Mongolian gerbils. J. Gastroenterol. 2001, 36, 5–9. [Google Scholar] [CrossRef]
- Ishihara, T.; Kohno, K.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Tryptanthrin inhibits nitric oxide and prostaglandin E(2) synthesis by murine macrophages. Eur. J. Pharmacol. 2000, 407, 197–204. [Google Scholar] [CrossRef]
- Recio, M.C.; Cerdá-Nicolás, M.; Potterat, O.; Hamburger, M.; Ríos, J.L. Anti-inflammatory and antiallergic activity in vivo of lipophilic Isatis tinctoria extracts and tryptanthrin. Planta Med. 2006, 72, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, T.; Hino, K.; Koya-Miyata, S.; Yamamoto, Y.; Takeuchi, M.; Nishizaki, Y.; Micallef, M.J.; Ushio, S.; Iwaki, K.; Ikeda, M.; et al. Cell differentiation and apoptosis of monocytic and promyelocytic leukemia cells (U-937 and HL-60) by tryptanthrin, an active ingredient of Polygonum tinctorium Lour. Pathol. Int. 2001, 51, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Shi, X.; Zhang, H.; Wang, S.; Sun, J.; Hua, W.; Miao, Q.; Zhao, Y.; Zhang, C. Proliferation-attenuating and apoptosis-inducing effects of tryptanthrin on human chronic myeloid leukemia K562 cell line in vitro. Int. J. Mol. Sci. 2011, 12, 3831–3845. [Google Scholar] [CrossRef]
- Yu, S.T.; Chen, T.M.; Chern, J.W.; Tseng, S.Y.; Chen, Y.H. Downregulation of GSTpi expression by tryptanthrin contributing to sensitization of doxorubicin-resistant MCF-7 cells through C-Jun NH2-terminal kinase-mediated apoptosis. Anticancer Drugs 2009, 20, 382–388. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Ma, G.; Yan, J.; Wang, H.; Yang, Q. Transport characteristics of tryptanthrin and its inhibitory effect on P-gp and MRP2 in Caco-2 cells. J. Pharm. Pharm. Sci. 2011, 14, 325–35. [Google Scholar] [CrossRef]
- Chang, H.N.; Yeh, Y.C.; Chueh, H.Y.; Pang, J.S. The anti-angiogenic effect of tryptanthrin is mediated by the inhibition of apelin promoter activity and shortened mRNA half-life in human vascular endothelial cells. Phytomedicine 2019, 58, 152879. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, D.C.; Baek, H.Y.; Lee, K.D.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory effects of tryptanthrin from Polygonum tinctorium Lour. in lipopolysaccharide-stimulated BV2 microglial cells. Arch. Pharm. Res. 2018, 41, 419–30. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.H.; Jung, J.Y.; Ko, H.L.; Kim, J.K.; Park, S.M.; Jung, D.H.; Park, C.A.; Kim, Y.W.; Ku, S.K.; Cho, I.J.; et al. Tryptanthrin prevents oxidative stress-mediated apoptosis through AMP-activated protein kinase-dependent p38 mitogen-activated protein kinase activation. Arch. Pharm. Res. 2017, 40, 1071–86. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Zheng, Z.; Zhao, S.; Zhao, J.; Lin, Q.; Li, C.; Zhu, Q.; Zhong, N. Antiviral activity of Isatis indigotica root-derived clemastanin B against human and avian influenza A and B viruses in vitro. Int. J. Mol. Med. 2013, 31, 867–73. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, J.; Xie, L. Structure Analysis of Effective Chemical Compounds against Dengue Viruses Isolated from Isatis tinctoria. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 3217473. [Google Scholar] [CrossRef] [PubMed]
- Hsuan, S.L.; Chang, S.C.; Wang, S.Y.; Liao, T.L.; Jong, T.T.; Chien, M.S.; Lee, W.C.; Chen, S.S.; Liao, J.W. The cytotoxicity to leukemia cells and antiviral effects of Isatis indigotica extracts on pseudorabies virus. J. Ethnopharmacol. 2009, 123, 61–7. [Google Scholar] [CrossRef] [PubMed]
| Tests | S. cusia Extract (μg/mL)a | Indigodole B (μM)a | Tryptanthrin (μM)a |
|---|---|---|---|
| LCC-MK2 cells at 37 °C | |||
| Cytotoxicity (CC50) | > 100 | > 4 00 | > 400 |
| Virus yield inhibition using plaque assay (IC50) | 0.64 ± 0.43 | 2.60 ± 0.11 | 1.52 ± 0.13 |
| Virucidal activity using plaque assay | 0.12 ± 0.03 | 2.09 ± 0.89 | 0.06 ± 0.04 |
| Infectivity inhibition with the compound in 0.01 N HCl (pH 2.0) for 15 min using IFAb (IC50) | 0.67 ± 0.03 | ||
| Infectivity inhibition with the compound in 0.01 N HCl (pH 2.0) for 60 min using IFA (IC50) | 1.58 ± 0.15 | ||
| Infectivity inhibition at the early stage using IFA (IC50) | 0.32 ± 0.05 | ||
| Infectivity inhibition at the late stage using IFA (IC50) | 0.06 ± 0.03 | ||
| Production inhibition of extracellular virions at the early stage using plaque assay (IC50) | 6.99 ± 2.18 | ||
| Production inhibition of extracellular virions at the late stage using plaque assay (IC50) | 0.05 ± 0.03 | ||
| Calu-3 cells at 32 °C | |||
| Cytotoxicity (CC50) | 72.5 ± 0.77 | 173.2 ± 1.3 | |
| Infectivity inhibition using IFA (IC50) | 0.30 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-C.; Lee, C.-L.; Yen, H.-R.; Chang, Y.-S.; Lin, Y.-P.; Huang, S.-H.; Lin, C.-W. Antiviral Action of Tryptanthrin Isolated from Strobilanthes cusia Leaf against Human Coronavirus NL63. Biomolecules 2020, 10, 366. https://doi.org/10.3390/biom10030366
Tsai Y-C, Lee C-L, Yen H-R, Chang Y-S, Lin Y-P, Huang S-H, Lin C-W. Antiviral Action of Tryptanthrin Isolated from Strobilanthes cusia Leaf against Human Coronavirus NL63. Biomolecules. 2020; 10(3):366. https://doi.org/10.3390/biom10030366
Chicago/Turabian StyleTsai, Yu-Chi, Chia-Lin Lee, Hung-Rong Yen, Young-Sheng Chang, Yu-Ping Lin, Su-Hua Huang, and Cheng-Wen Lin. 2020. "Antiviral Action of Tryptanthrin Isolated from Strobilanthes cusia Leaf against Human Coronavirus NL63" Biomolecules 10, no. 3: 366. https://doi.org/10.3390/biom10030366
APA StyleTsai, Y.-C., Lee, C.-L., Yen, H.-R., Chang, Y.-S., Lin, Y.-P., Huang, S.-H., & Lin, C.-W. (2020). Antiviral Action of Tryptanthrin Isolated from Strobilanthes cusia Leaf against Human Coronavirus NL63. Biomolecules, 10(3), 366. https://doi.org/10.3390/biom10030366






