Taxonomic and Environmental Variation of Metabolite Profiles in Marine Dinoflagellates of the Genus Symbiodinium
Abstract
:1. Introduction
2. Results and Discussion
2.1 Patterns of Metabolite Variability
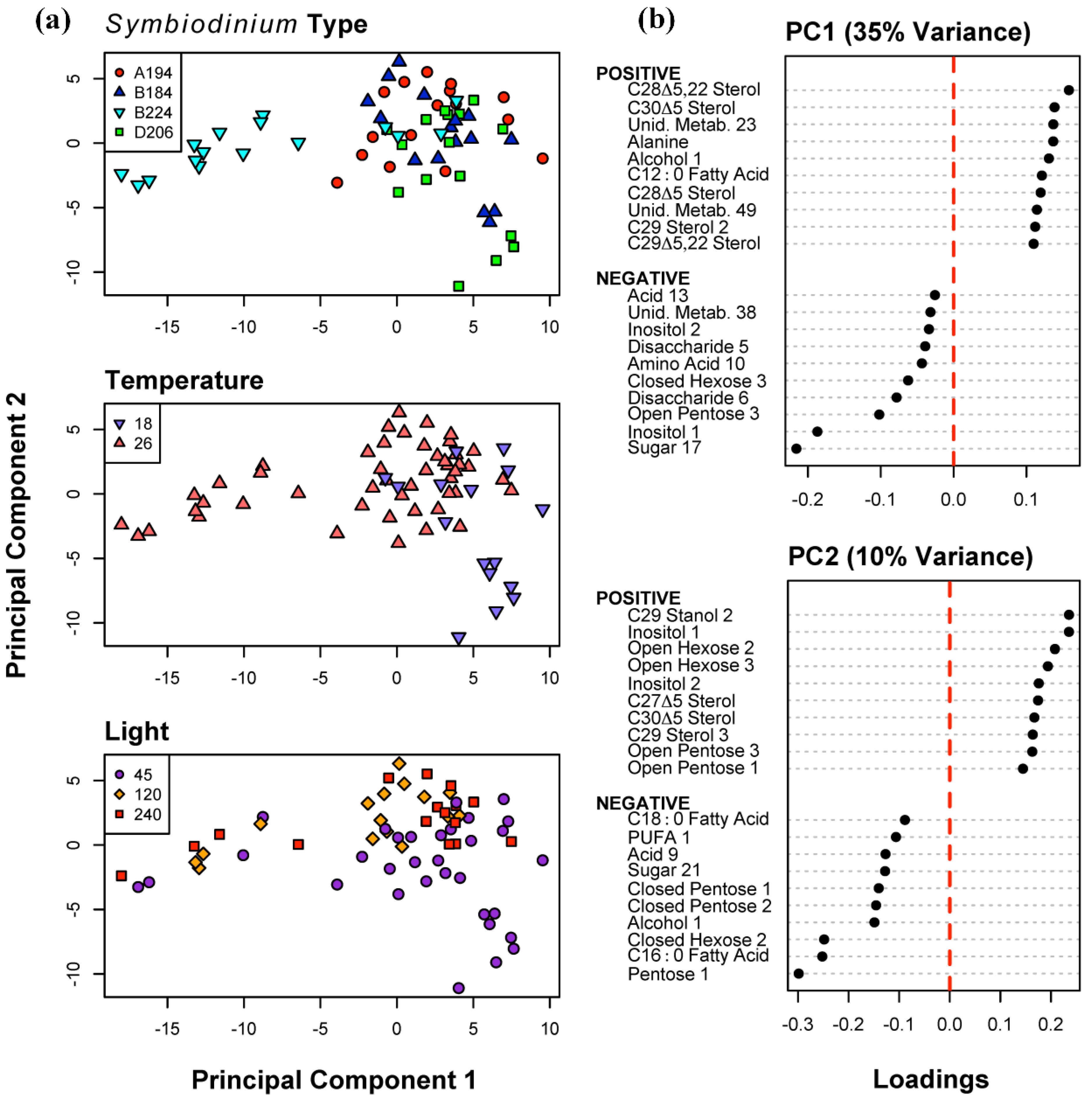
2.2. Classification of Symbiotic Dinoflagellates and Environmental Conditions based on Metabolite Profiles

| (a) | Symbiodinium Type | A194 | B184 | B224 | D206 | Classification Error | Random Error |
| A194 | 14 | 0 | 1 | 1 | 12.5 | 75 | |
| B184 | 0 | 15 | 0 | 1 | 6.3 | 75 | |
| B224 | 0 | 3 | 13 | 0 | 18.8 | 75 | |
| D206 | 0 | 1 | 1 | 14 | 12.5 | 75 | |
| Overall | 12.5 | 75 | |||||
| (b) | Temperature | 18 | 26 | Classification Error | Random Error | ||
| 18 | 10 | 6 | 37.5 | 75 | |||
| 26 | 4 | 44 | 8.3 | 25 | |||
| Overall | 15.6 | 37.5 | |||||
| (c) | Light | 45 | 120 | 240 | Classification Error | Random Error | |
| 45 | 30 | 1 | 1 | 6.3 | 50 | ||
| 120 | 4 | 8 | 4 | 50 | 75 | ||
| 240 | 9 | 5 | 2 | 87.5 | 75 | ||
| Overall | 37.5 | 62.5 |
2.3. Differences among Diagnostic Metabolite Profiles for Symbiotic Dinoflagellates and Environmental Conditions
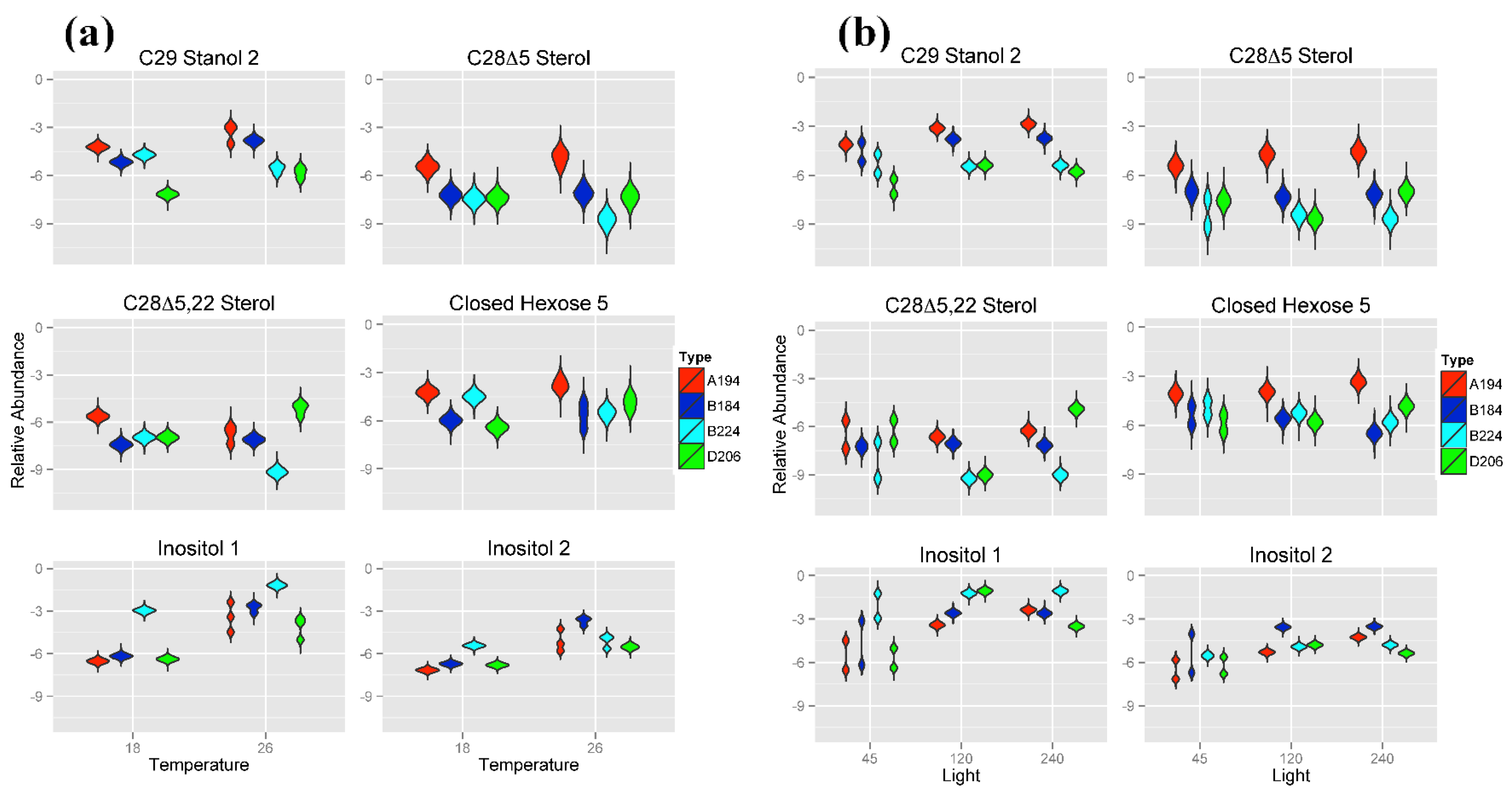
2.3.1. Metabolite Profiles Specific of Different Symbiotic Dinoflagellates
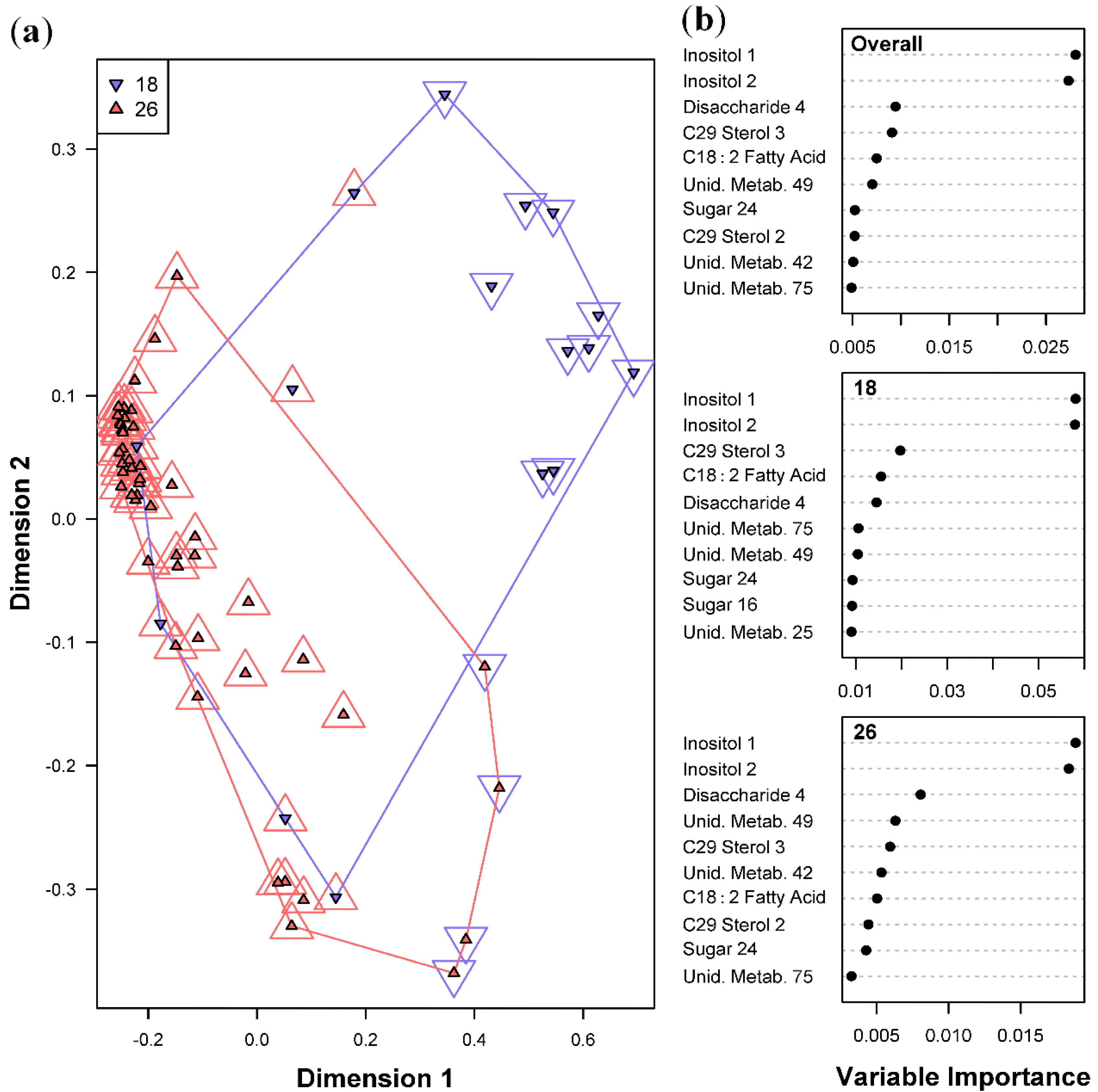
2.3.2. Effect of Temperatures on Metabolite Profiles of Symbiotic Dinoflagellates

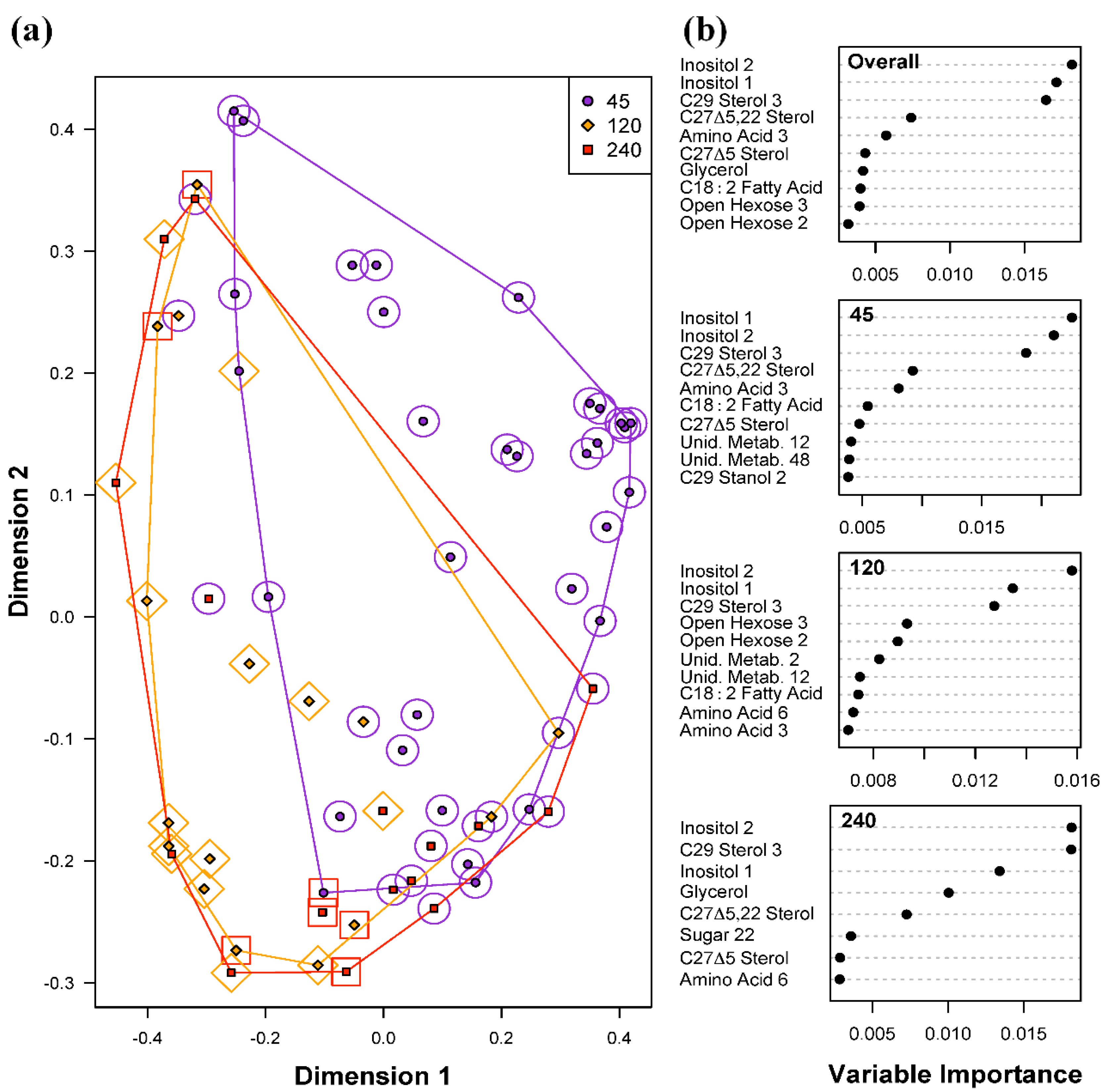
2.3.3. Effect of Variation in Light Intensity on Metabolic Profiles of Symbiotic Dinoflagellates
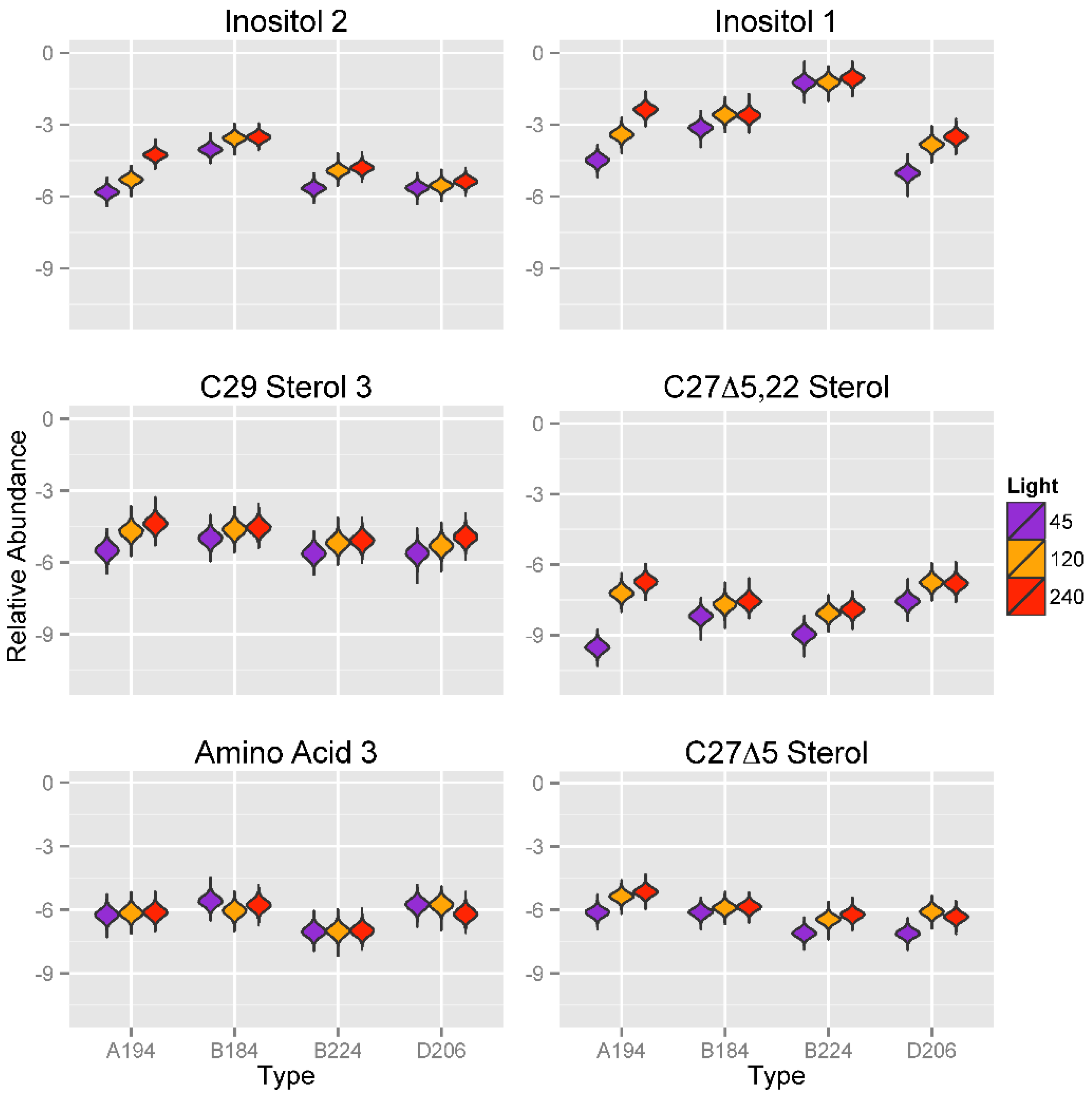
2.4. Physiological and Environmental Correlates of Metabolite Expression in Symbiotic Dinoflagellates
3. Experimental Section
3.1. Experimental Design and Sample Preparation
3.2. Identification of Symbiotic Dinoflagellates
3.3. GC/MS Chromatography and Compound Identification
3.4. Data Formatting
3.5. Random Forests Analysis
3.6. Bayesian Estimation of Means
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Withers, N.W.; Kokke, W.C.M.C.; Fenical, W.; Djerassi, C. Sterol patterns of cultured zooxanthellae isolated from marine-invertebrates—Synthesis of gorgosterol and 23-desmethylgorgosterol by aposymbiotic algae. Proc. Natl. Acad. Sci. Biol. 1982, 79, 3764–3768. [Google Scholar] [CrossRef]
- Muscatine, L. The role of symbiotic algae in carbon and energy flux in reef corals. In Ecosystems of the World: Coral Reefs; Dubinsky, Z., Ed.; Elsevier: Amsterdam, The Netherland, 1990; p. 75. [Google Scholar]
- Stanley, G.D., Jr. Ecology: Photosymbiosis and the evolution of modern coral reefs. Science 2006, 312, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Davy, S.K.; Allemand, D.; Weis, V. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol. Mol. Biol. Rev. 2012, 76, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Tout, J.; Jeffris, T.C.; Webster, N.S.; Stoker, R.; Ralph, P.J.; Seymour, J.R. Variability in microbial community composition and function between different niches within a coral reef. Microb. Ecol. 2014, 67, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- De’ath, G.; Fabricius, K.E.; Sweatman, H.; Poutinen, M. The 27 year decline of coral cover on the great barrier reef and its causes. Proc. Natl. Acad. Sci. USA 2012, 109, 17995–17999. [Google Scholar] [CrossRef] [PubMed]
- Coffroth, M.A.; Santos, S.R. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist 2005, 156, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Pochon, X.; Montoya-Burgos, J.I.; Stadelmann, B.; Pawlowski, J. Molecular phylogeny, evolutionary rates, and divergence timing of the symbiotic dinoflagellate genus Symbiodinium. Mol. Phylogenetics Evol. 2006, 38, 20–30. [Google Scholar] [CrossRef]
- Pochon, X.; Stat, M.; Takabayashi, M.; Chasqui, L.; Chauka, L.J.; Logan, D.D.K.; Gates, R.D. Comparison of endosymbiotic and free-living Symbiodinium (Dinophyceae) diversity in a Hawaiian reef environment. J. Phycol. 2010, 46, 53–65. [Google Scholar] [CrossRef]
- LaJeunesse, T.C.; Parkinson, J.; Reimer, J.D. A genetics-based description of Symbiodinium minutum sp. nov. and S. Psygmophilum sp. nov. (Dinophyceae), two dinoflagellates symbiotic with Cnidaria. J. Phycol. 2012, 48, 1380–1391. [Google Scholar] [CrossRef]
- Silverstein, R.N.; Correa, A.M.S.; Baker, A.C. Specificity is rarely absolute in coral-algal symbiosis: Implications for coral response to climate change. Proc. R. Soc. B 2012, 7, 2609–2618. [Google Scholar] [CrossRef]
- LaJeunesse, T.C.; Wham, D.C.; Pettay, D.T.; Parksinson, J.E.; Keshavmurthy, S.; Chen, C.A. Ecologically differentiated stress-tolerant endosymbionts in the dinoflagellate genus Symbiodinium (Dinophyceae) Clade D are different species. Phycologia 2014, 53, 305–319. [Google Scholar] [CrossRef]
- Fay, S.A.; Weber, M.X. The occurrence of mixed infections of Symbiodinium (Dinoflagellata) within individual hosts. J. Phycol. 2012, 48, 1306–1316. [Google Scholar] [CrossRef]
- Mellas, R.E.; McIlroy, S.E.; Fitt, W.K.; Coffroth, M.A. Variation in symbiont uptake in the early ontogeny of the upside-down jellyfish, Cassiopea spp. J. Exp. Mar. Biol. Ecol. 2014, 459, 38–44. [Google Scholar] [CrossRef]
- Poland, D.M.; Mansfield, J.M.; Hannes, A.R.; Fairbank Lewis, C.L.; Shearer, T.L.; Connelly, S.J.; Kirk, N.L.; Coffroth, M.A. Variation in Symbiodinium communities in juvenile Briareum asbestinum (Cnidaria: Octocorallia) over temporal and spatial scales. Mar. Ecol. Prog. Ser. 2013, 476, 23–37. [Google Scholar] [CrossRef]
- Kinzie, R.A.; Takayama, M.; Santos, S.R.; Coffroth, M.A. The adaptive bleaching hypothesis: Experimental tests of critical assumptions. Biol. Bull. 2001, 200, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, J.E.; Baums, I.B. The extended phenotypes of marine symbioses: Ecological and evolutionary consequences of intraspecific genetic diversity in coral–algal associations. Front. Microbiol. 2014, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, A.T.; LaJeunesse, T.C.; Trench, R.K. The synthesis of mycosporine-like amino acids (MAAS) by cultured, symbiotic dinoflagellates. J. Exp. Mar. Biol. Ecol. 2000, 249, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.M.; Trapido-Rosenthal, H.; Douglas, A.E. On the functional significance of molecular variation in Symbiodinium, the symbiotic algae of Cnidaria: Photosynthetic response to irradiance. Mar. Ecol. Prog. Ser. 2002, 244, 27–37. [Google Scholar] [CrossRef]
- Iglesias-Prieto, R.; Trench, R.K. Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density. Mar. Ecol. Prog. Ser. 1994, 113, 163–175. [Google Scholar] [CrossRef]
- Warner, M.E.; Fitt, W.K.; Schmidt, G.W. In Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc. Natl. Acad. Sci. USA 1999, 96, 8007–8012. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.M.; Goodson, M.S.; Visram, S.; Trapido-Rosenthal, H.; Wiedenmann, J.; Douglas, A.E. Molecular diversity of symbiotic algae at the latitudinal margins of their distribution: Dinoflagellates of the genus Symbiodinium in corals and sea anemones. Mar. Ecol. Prog. Ser. 2002, 244, 17–26. [Google Scholar] [CrossRef]
- Saxby, T.; Dennison, W.C.; Hoegh-Guldberg, O. Photosynthetic responses of the coral Montipora digitata to cold temperature stress. Mar. Ecol. Prog. Ser. 2003, 248, 85–97. [Google Scholar] [CrossRef]
- Tchernov, D.; Gorbunov, M.Y.; de Vargas, C.; Yadav, S.N.; Milligan, A.J.; Haggblom, M.; Falkowski, P.G. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl. Acad. Sci. USA 2004, 101, 13531–13535. [Google Scholar] [CrossRef] [PubMed]
- Goulet, T.L.; Cook, C.B.; Goulet, D. Effect of short-term exposure to elevated temperatures and light levels on photosynthesis of different host-symbiont combinations in the Aiptasia pallidal-Symbiodinium symbiosis. Limnol. Oceanogr. 2005, 50, 1490–1498. [Google Scholar] [CrossRef]
- Thornhill, D.J.; Kemp, D.W.; Bruns, B.U.; Fitt, W.K.; Schmidt, G.W. Correspondence between cold tolerance and temperate biogeography in a western Atlantic Symbiodinium (Dinophyta) lineage. J. Phycol. 2008, 44, 1126–1135. [Google Scholar] [CrossRef]
- Iglesias-Prieto, R.; Beltran, V.H.; LaJeunesse, T.C.; Reyes-Bonilla, H.; Thome, P.E. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern pacific. Proc. Natl. Acad. Sci. B 2004, 271, 1757–1763. [Google Scholar]
- Karako-Lampert, S.; Katcoff, D.J.; Achituv, Y.; Dubinsky, Z.; Stambler, N. Do clades of symbiotic dinoflagellates in scleractinan corals of the Gulf of Eilat (Red Sea) differ from those of other coral reefs? J. Exp. Mar. Biol. Ecol. 2004, 311, 301–314. [Google Scholar] [CrossRef]
- Van Oppen, M.J.H.; Palstra, F.P.; Piquet, A.M.T.; Miller, D.J. Patterns of coral-dinoflagellate associations in Acropora: Significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proc. R. Soc. B 2001, 268, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Rowan, R. Coral bleaching—thermal adaptation in reef coral symbionts. Nature 2004, 430. [Google Scholar] [CrossRef]
- Van Oppen, M.J.H.; Baker, A.C.; Coffroth, M.A.; Willis, B.L. Bleaching resistance and the role of algal endosymbionts. In Coral bleaching; Oppen, M.J.H., Lough, J.M., Eds.; Springer Berlin Heidelberg: Heidelberg, Germany, 2009; Volume 205, pp. 83–102. [Google Scholar]
- Trench, R.K. The physiology and biochemistry of zooxanthellae symbiotic with marine coelenterates. I. The assimilation of photosynthetic products of zooxanthellae by two marine coelenterates. Proc. R. Soc. B 1971, 177, 225–235. [Google Scholar] [CrossRef]
- Trench, R.K. The physiology and biochemistry of zooxanthellae symbiotic with marine coelenterates. II. Liberation of fixed 14C by zooxanthellae in vitro. Proc. R. Soc. B 1971, 177, 237–250. [Google Scholar] [CrossRef]
- Trench, R.K. The physiology and biochemistry of zooxanthellae symbiotic with marine coelenterates. III. The effect of homogenates of the host tissues on the excretion of the photosynthetic products in vitro by zooxanthellae from two marine coelenterates. Proc. R. Soc. B 1971, 177, 251–264. [Google Scholar] [CrossRef]
- Whitehead, L.F.; Douglas, A.E. Metabolite comparisons and the identity of nutrients translocated from symbiotic algae to an animal host. J. Exp. Biol. 2003, 206, 3149–3157. [Google Scholar] [CrossRef] [PubMed]
- Buddemeier, R.W.; Fautin, D.G. Coral bleaching as an adaptive mechanism—A testable hypothesis. BioScience 1993, 43, 320–326. [Google Scholar] [CrossRef]
- Rowan, R.; Knowlton, N. Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc. Natl. Acad. Sci. USA 1995, 92, 2850–2853. [Google Scholar] [CrossRef] [PubMed]
- Rowan, R.; Knowlton, N.; Baker, A.; Jara, J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 1997, 388, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.C. Flexibility and specificity in coral-algal symbiosis: Diversity, ecology, and biogeography of symbiodinium. Ann. Rev. Ecol. Evol. Syst. 2003, 34, 661–689. [Google Scholar] [CrossRef]
- Michal, G.; Schomburg, D. Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology; Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Fernie, A.R.; Aharoni, A.; Willmitzer, L.; Stitt, M.; Tohge, T.; Kopka, J.; Carroll, A.J.; Saito, K.; Fraser, P.D.; DeLuca, V. Recommendations for reporting metabolite data. Plant Cell 2011, 23, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Hrydziuszko, O.; Viant, M.R. Missing values in mass spectrometry based metabolomics: An undervalued step in the data processing pipeline. Metabolomics 2012, 8, S161–S174. [Google Scholar] [CrossRef]
- Chaston, J.; Douglas, A.E. Making the most of “omics” for symbiosis research. Biol. Bull. 2012, 223, 21–29. [Google Scholar] [PubMed]
- Meyer, E.; Weis, V.M. Study of cnidarian-algal symbiosis in the “omics” age. Biol. Bull. 2012, 223, 44–65. [Google Scholar] [PubMed]
- Viant, M.R. Metabolomics of aquatic organisms: The new “omics” on the block. Mar. Ecol. Progr. Ser. 2007, 332, 301–306. [Google Scholar] [CrossRef]
- Barofsky, A.; Vidoudez, C.; Pohnert, G. Metabolic profiling reveals growth stage variability in diatom exudates. Limnol. Oceanogr. 2009, 7, 382–390. [Google Scholar] [CrossRef]
- Paul, C.; Barofsky, A.; Vidoudez, C.; Pohnert, G. Diatom exudates influence metabolism and cell growth of co-cultured diatom species. Mar. Ecol. Progr. Ser. 2009, 389, 61–70. [Google Scholar] [CrossRef]
- Wilson, N.G.; Maschek, J.A.; Baker, B.J. A species flock driven by predation? Secondary metabolites support diversification of slugs in Antarctica. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Lannig, G.; Eilers, S.; Portner, H.O.; Sokolova, I.M.; Bock, C. Impact of ocean acidification on energy metabolism of oyster, Crassostrea gigas—Changes in metabolic pathways and thermal response. Mar. Drugs 2010, 8, 2318–2339. [Google Scholar] [CrossRef] [PubMed]
- Solanky, K.S.; Burton, I.W.; MacKinnon, S.L.; Walter, J.A.; Dacanay, A. Metabolic changes in Atlantic salmon exposed to Aeromonas salmonicida detected by H-1-Nuclear Magnetic Resonance spectroscopy of plasma. Dis. Aquat. Org. 2005, 65, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Viant, M.R.; Rosenblum, E.S.; Tjeerdema, R.S. NMR-based metabolomics: A powerful approach for characterizing the effects of environmental stressors on organism health. Environ. Sci. Technol. 2003, 37, 4982–4989. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, E.S.; Viant, M.R.; Braid, B.M.; Moore, J.D.; Friedman, C.S.; Tjeerdema, R.S. Characterizing the metabolic actions of natural stresses in the California red abalone, Haliotis rufescens using H-1 NMR metabolomics. Metabolomics 2005, 1, 199–209. [Google Scholar] [CrossRef]
- Coelho, S.M.; Simon, N.; Ahmed, S.; Cock, J.M.; Partensky, F. Ecological and evolutionary genomics of marine photosynthetic organisms. Mol. Ecol. 2013, 22, 867–907. [Google Scholar] [CrossRef] [PubMed]
- Robison, J.D.; Warner, M.E. Differential impacts of photoacclimation and thermal stress on the photobiology of four different phylotypes of Symbiodinium (Pyrrhophyta). J. Phycol. 2006, 42, 568–579. [Google Scholar] [CrossRef]
- Suggett, D.J.; Warner, M.E. Photosynthesis and production of hydrogen peroxide by Symbiodinium (Pyrrhophyta) phylotypes with different thermal tolerances. J. Phycol. 2008, 44, 948–956. [Google Scholar] [CrossRef]
- Hennige, S.; Suggett, D.; Warner, M.; McDougall, K.; Smith, D. Photobiology of Symbiodinium revisited: Bio-physical and bio-optical signatures. Coral Reefs 2009, 28, 179–195. [Google Scholar] [CrossRef]
- Diaz-Almeyda, E.; Thome, P.E.; el Hafidi, M.; Iglesias-Prieto, R. Differential stability of photosynthetic membranes and fatty acid composition at elevated temperature in Symbiodinium. Coral Reefs 2011, 30, 217–225. [Google Scholar] [CrossRef]
- Sorek, M.; Levy, O. The effect of temperature compensation on the circadian rhythmicity of photosynthesis in Symbiodinium, coral-symbiotic alga. Science 2012. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Berk, R.A. An introduction to ensemble methods for data analysis. Sociol. Methods Res. 2006, 34, 263–295. [Google Scholar] [CrossRef]
- Goodman, S.N. Toward evidence-based medical statistics. 1: The p value fallacy. Ann. Intern. Med. 1999, 130, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Gliner, J.A.; Leech, N.L.; Morgan, G.A. Problems with null hypothesis testing (NHST): What do the textbooks say? J. Exp. Educ. 2002, 71, 83–92. [Google Scholar] [CrossRef]
- Kruschke, J.K. Bayesian estimation supersedes the t test. J. Exp. Psychol. 2013, 142, 573–603. [Google Scholar] [CrossRef]
- Thornhill, D.J.; Xiang, Y.; Pettay, D.T.; Zhong, M.; Santos, S.R. Population genetic data of a model symbiotic cnidarian system reveal remarkable symbiotic and vectored introductions across ocean basins. Mol. Ecol. 2013, 22, 4499–4515. [Google Scholar] [CrossRef] [PubMed]
- LaJeunesse, T.C. “Species” radiations of symbiotic dinoflagellates in the atlantic and indo-pacific since the miocene-pliocene transition. Mol. Biol. Evol. 2005, 22, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Prada, C.; McIlroy, S.E.; Beltran, D.M.; Viant, D.J.; Ford, S.A.; Hellberg, M.E.; Coffroth, M.A. Cryptic diversity hides host and habitat specialization in a gorgonian-algal symbiosis. Mol. Ecol. 2014, 23, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Hannah, M.A.; Caldana, C.; Steinhauser, D.; Balbo, I.; Fernie, A.R.; Willmitzer, L. Combined transcript and metabolite profiling of Arabidopsis grown under widely variant growth conditions facilitates the identification of novel metabolite-mediated regulation of gene expression. Plant Physiol. 2010, 152, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Tonk, L.; Sampayo, E.M.; Weeks, S.; Magno-Canto, M.; Hoegh-Guldberg, O. Host-specific interactions with environmental factors shape the distribution of Symbiodinium across the Great Barrier Reef. PLoS One 2013. [Google Scholar] [CrossRef]
- Peng, S.E.; Chen, C.-S.; Song, Y.-F.; Huang, H.-T.; Jiang, P.-L.; Chen, W.-N.U.; Fang, L.S.; Lee, Y-C. Assessment of metabolic modulation in free-living versus endosymbiotic Symbiodinium using synchrotron radiation-based infrared mirospectroscopy. Biol. Lett. 2011, 8, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Teece, M.A.; Estes, B.; Gelsleichter, E.; Lirman, D. Heterotrophic and autotrophic assimilation of fatty acids by two scleractinian corals, Montastraea faveolata and Porites astreoides. Limnol. Oceanogr. 2011, 56, 1285–1296. [Google Scholar] [CrossRef]
- Chen, W.-N.U.; Kang, H.-J.; Weis, V.M.; Mayfield, A.B.; Jiang, P.-L.; Fang, L.-S.; Chen, C.-S. Diel rhythmicity of lipid-body formation in a coral-Symbiodinium endosymbiosis. Coral Reefs 2012, 31, 521–534. [Google Scholar] [CrossRef]
- Yancey, P.H.; Heppenstall, M.; Ly, S.; Andrell, R.M.; Gates, R.D.; Carter, V.L.; Hagedorn, M. Betaines and dimethylsulfoniopropionate as major osmolytes in Cnidaria with endosymbiotic dinoflagellates. Physiol. Biochem. Zool. 2009, 83, 163–173. [Google Scholar]
- Gordon, B.R.; Leggat, W. Symbiodinium-invertebrate symbioses and the role of metabolomics. Mar. Drugs 2010, 8, 2546–2568. [Google Scholar] [CrossRef] [PubMed]
- Suescún-Bolívar, L.P.; Iglesias-Prieto, R.; Thome, P.E. Induction of glycerol synthesis and release in cultured Symbiodinium. PLoS One 2012. [Google Scholar] [CrossRef]
- Treignier, C.; Grover, R.; Ferrier-Pagès, C.; Tolosa, I. Effect of light and feeding on the fatty acid and sterol composition of zooxanthellae and host tissue isolated from the scleractinian coral Turbinaria reniformis. Limnol. Oceanogr. 2008, 53, 2702–2710. [Google Scholar] [CrossRef]
- Kuhl, M.; Cohen, Y.; Dalsgaard, T.; Jorgensen, B.B.; Revsbech, N.P. Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, ph and light. Mar. Ecol. Progr. Ser. 1995, 117, 159–172. [Google Scholar] [CrossRef]
- Ulstrup, K.E.; van Oppen, M.J.H. Geographic and habitat partitioning of genetically distinct zooxanthellae (Symbiodinium) in Acropora corals on the Great Barrier Reef. Mol. Ecol. 2003, 12, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.F.; Lai, M.; Ulstrup, K.E.; Saunders, S.M.; Flematti, G.R.; Radford, B.; van Oppen, M.J.H. Symbiodinium genotypic and environmental controls on lipids in reef building corals. PLoS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.W.; Hernandez-Pech, X.; Iglesias-Prieto, R.; Fitt, W.K.; Schmidt, G.W. Community dynamics and physiology of Symbiodinium spp. Before, during, and after a coral bleaching event. Limnol. Oceanogr. 2014, 59, 788–797. [Google Scholar] [CrossRef]
- Roth, M.S.; Goericke, R.; Deheyn, D.D. Cold induces acute stress but heat is ultimately more deleterious for the reef-building coral Acropora yongei. Sci. Rep. 2012. [Google Scholar] [CrossRef]
- Cooper, T.F.; Ulstrup, K.E.; Dandan, S.S.; Heyward, A.J.; Kuhl, M.; Muirhead, A.; O’Leary, R.A.; Ziersen, B.E.F.; van Oppen, M.J.H. Niche specialization of reef-building corals in the mesophotic zone: Metabolic trade-offs between divergent Symbiodinium types. Proc. R. Soc. B 2011, 278, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Sougata, R.; Letourneau, L.; Morse, D. Cold-induced cysts of the photosynthetic dinoflagellate Lingulodinium polyedrum have an arrested circadian bioluminescence rhythm and lower levels of protein phosphorylation. Plant Physiol. 2014, 164, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Tsim, S.-T.; Wong, J.T.Y.; Wong, Y.H. Calcium ion dependency and the role of inositol phosphates in melatonininduced encystment of dinoflagellates. J. Cell Sci. 1997, 110, 1387–1393. [Google Scholar] [PubMed]
- Wen, R.; Sui, Z.; Bao, Z.; Zhou, W.; Wang, C. Isolation and characterization of calmodulin gene of Alexandrium catenella (Dinoflagellate) and its performance in cell growth and heat stress. J. Ocean Univ. China 2014, 13, 290–296. [Google Scholar] [CrossRef]
- Sogin, E.M.; Anderson, P.; Williams, P.; Chen, C.-S.; Gates, R.D. Application of 1H-NMR metabolomic profiling for reef-building corals. PLoS One 2014. [Google Scholar] [CrossRef]
- Freudenthal, H. Symbiodinium gen. nov. and Symbiodinium microadriaticum sp. npv., a zooxanthella: Taxonomy, life cycle and morphology. J. Protozool. 1962, 9, 45–52. [Google Scholar] [CrossRef]
- LaJeunesse, T.C. Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: In search of a “species” level marker. J. Phycol. 2001, 37, 866–880. [Google Scholar] [CrossRef]
- Santos, S.R.; Taylor, D.J.; Kinzie, R.A.; Hidaka, M.; Sakai, K.; Coffroth, M.A. Molecular phylogeny of symbiotic dinoflagellates inferred from partial chloroplast large subunit (23S)-rDNA sequences. Mol. Phylogenetics Evol. 2002, 23, 97–111. [Google Scholar] [CrossRef]
- Santos, S.R.; Taylor, D.J.; Coffroth, M.A. Genetic comparisons of freshly isolated versus cultured symbiotic dinoflagellates: Implications for extrapolating to the intact symbiosis. J. Phycol. 2001, 37, 900–912. [Google Scholar] [CrossRef]
- Guillard, R.; Ryther, J. Studies on marine planktonic diatoms. I. Cyclotella nana husted and Detonula confervacea cleve. Can. J. Microbiol. 1963, 8, 229–239. [Google Scholar]
- Coffroth, M.A.; Lasker, H.R.; Diamond, M.E.; Bruenn, J.A.; Bermingham, E. DNA fingerprints of a gorgonian coral—A method for detecting clonal structure in a vegetative species. Mar. Biol. 1992, 114, 317–325. [Google Scholar] [CrossRef]
- Santos, S.R.; Gutierrez-Rodriguez, C.; Coffroth, M.A. Phylogenetic identification of symbiotic dinoflagellates via length heteroplasmy in domain V of chloroplast large subunit (cp23S)-ribosomal DNA sequences. Mar. Biotechnol. 2003, 5, 130–140. [Google Scholar] [PubMed]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.F.; Southam, A.D.; Hines, A.; Viant, M.R. High-throughput tissue extraction protocol for NMR- and MS-based metabolomics. Anal. Biochem. 2008, 372, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Templ, M.; Alfons, A.; Filzmoser, P. Exploring incomplete data using visualization tools. J. Adv. Data Anal. Classif. 2012, 6, 29–47. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomforest. R News 2002, 2, 18–22. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klueter, A.; Crandall, J.B.; Archer, F.I.; Teece, M.A.; Coffroth, M.A. Taxonomic and Environmental Variation of Metabolite Profiles in Marine Dinoflagellates of the Genus Symbiodinium. Metabolites 2015, 5, 74-99. https://doi.org/10.3390/metabo5010074
Klueter A, Crandall JB, Archer FI, Teece MA, Coffroth MA. Taxonomic and Environmental Variation of Metabolite Profiles in Marine Dinoflagellates of the Genus Symbiodinium. Metabolites. 2015; 5(1):74-99. https://doi.org/10.3390/metabo5010074
Chicago/Turabian StyleKlueter, Anke, Jesse B. Crandall, Frederick I. Archer, Mark A. Teece, and Mary Alice Coffroth. 2015. "Taxonomic and Environmental Variation of Metabolite Profiles in Marine Dinoflagellates of the Genus Symbiodinium" Metabolites 5, no. 1: 74-99. https://doi.org/10.3390/metabo5010074
APA StyleKlueter, A., Crandall, J. B., Archer, F. I., Teece, M. A., & Coffroth, M. A. (2015). Taxonomic and Environmental Variation of Metabolite Profiles in Marine Dinoflagellates of the Genus Symbiodinium. Metabolites, 5(1), 74-99. https://doi.org/10.3390/metabo5010074





