Metabolomic Analysis of Fission Yeast at the Onset of Nitrogen Starvation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Increase in Cell Size and Number Shortly after N-Starvation
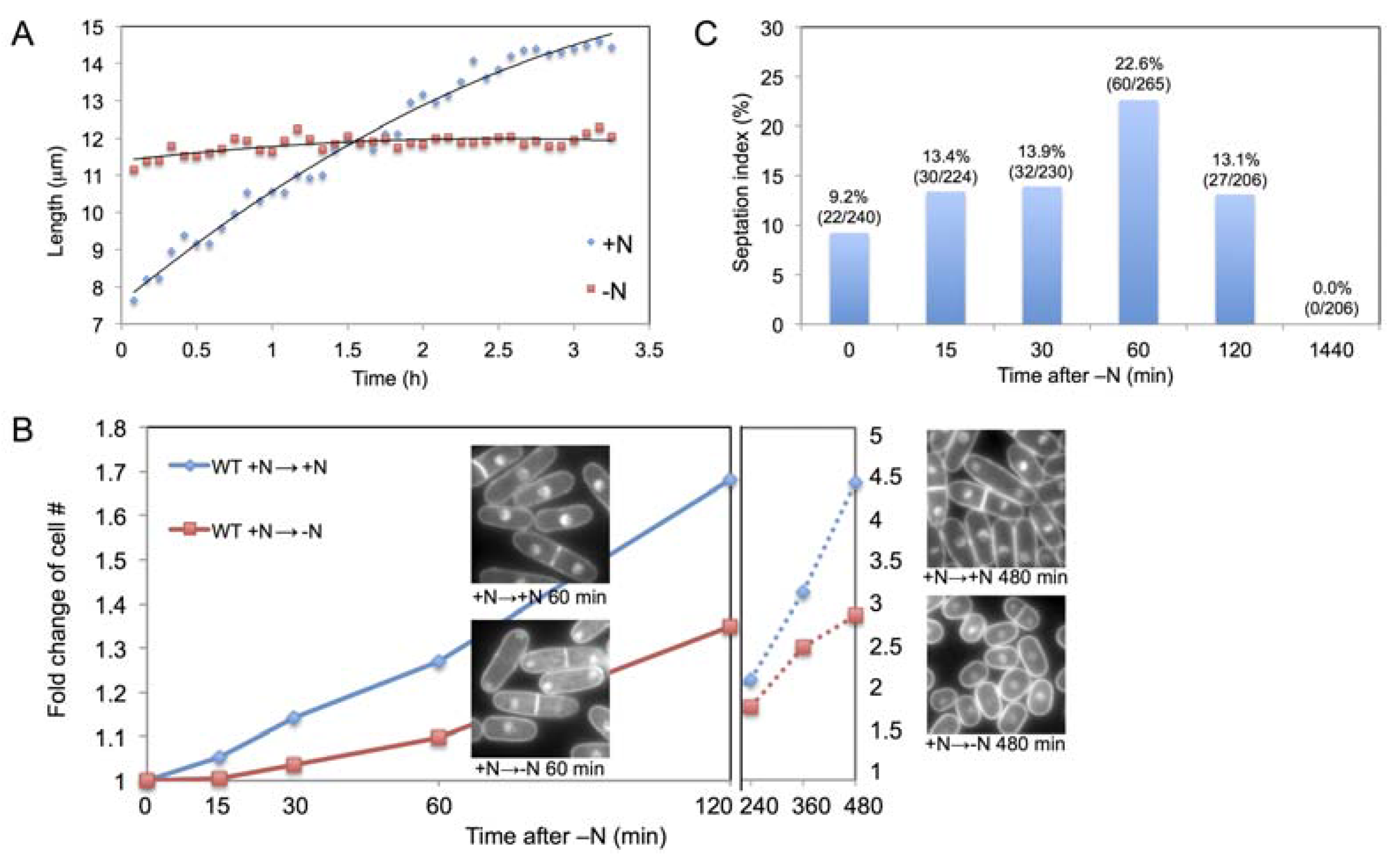
2.2. Time Course Metabolomic Analysis and Data Reproducibility
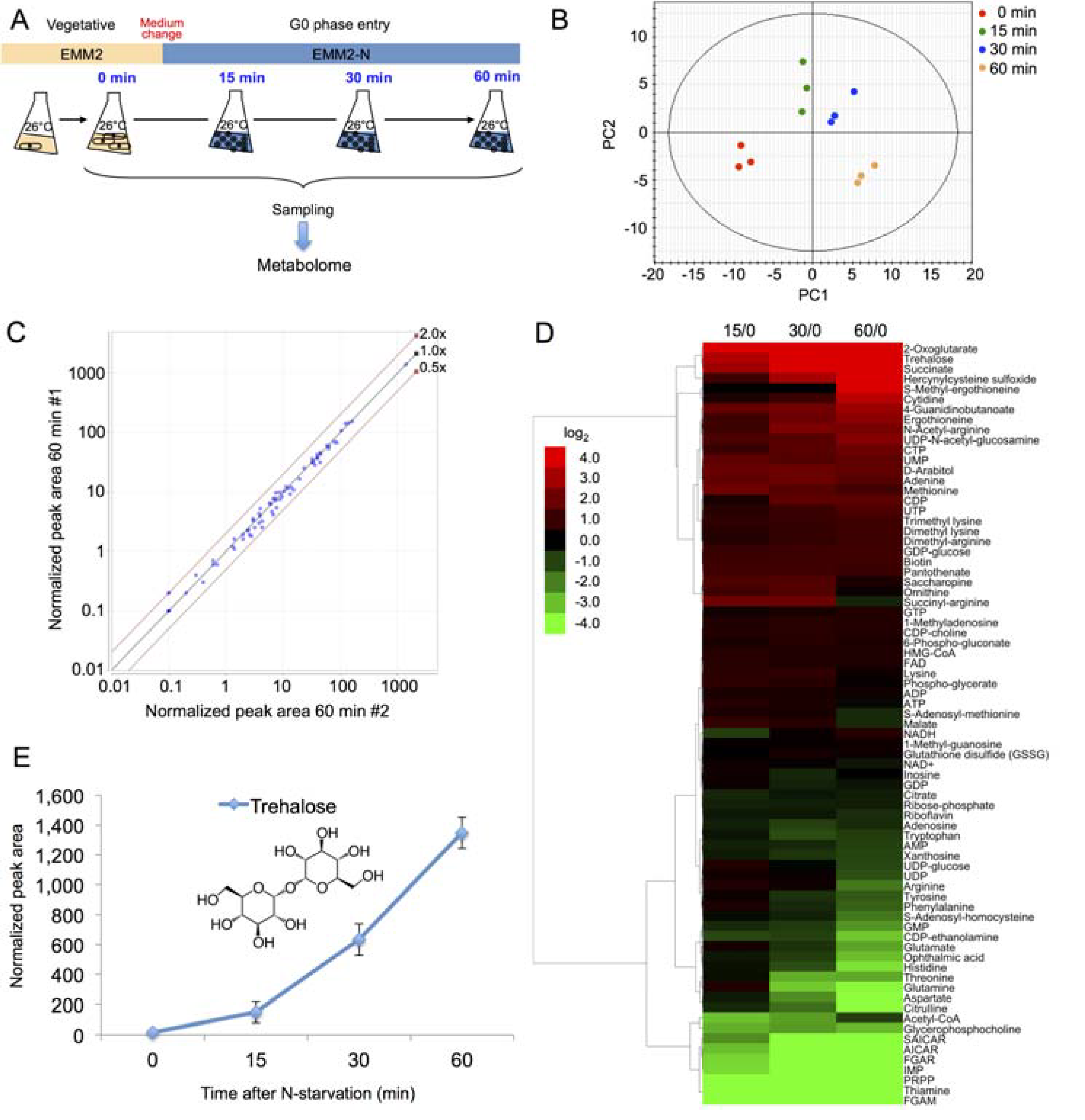
2.3. Partial Remodeling of the Cellular Metabolome after N-Starvation
2.4. Rapid Metabolic Response within 15 min
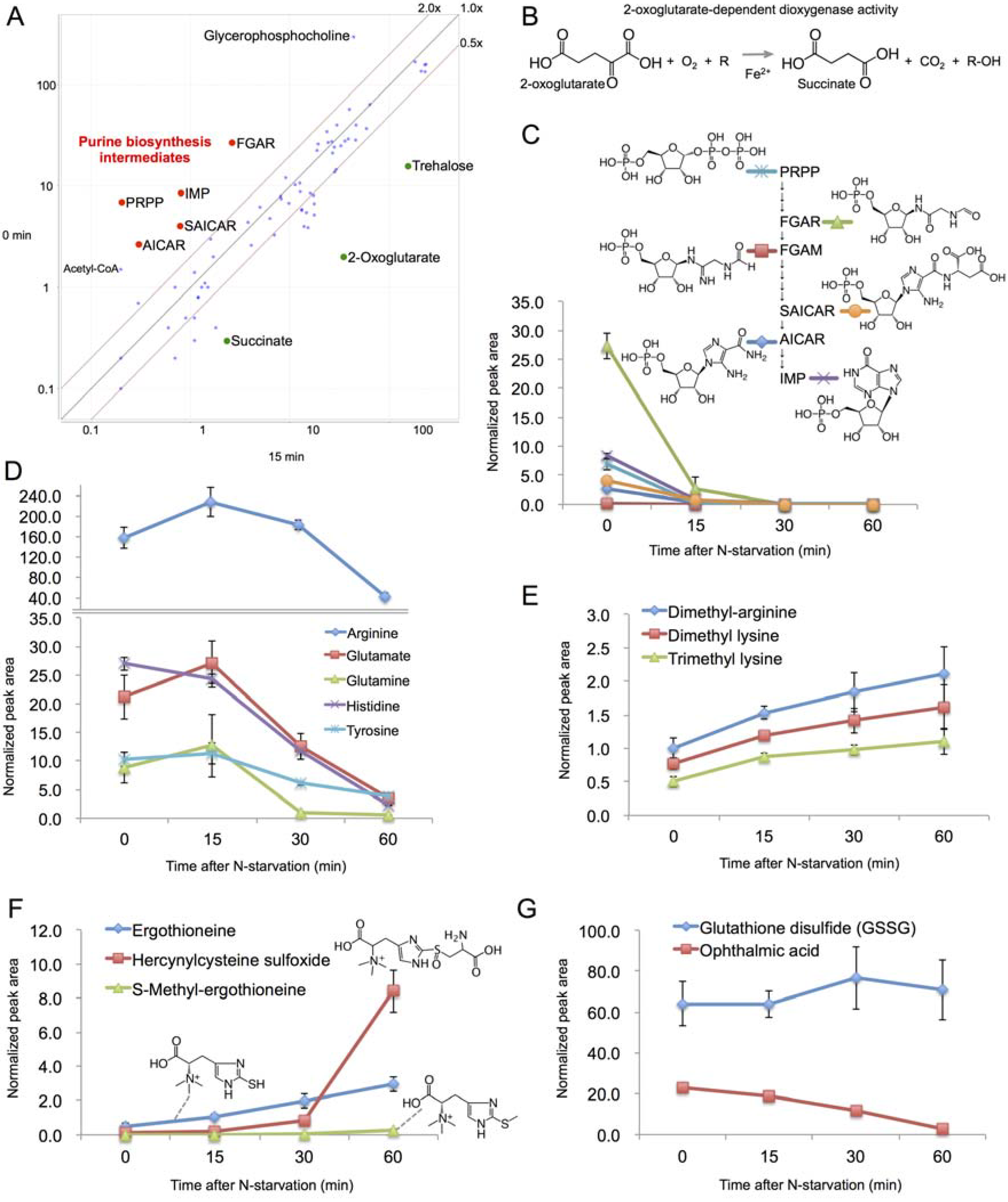
2.5. Changes in Amino Acid Metabolism after 15 min
3. Experimental Section
3.1. Strains and Culture Conditions
3.2. Microscopy
3.3. Metabolome Sample Preparation
3.4. LC-MS Analysis
3.5. LC-MS Data Processing
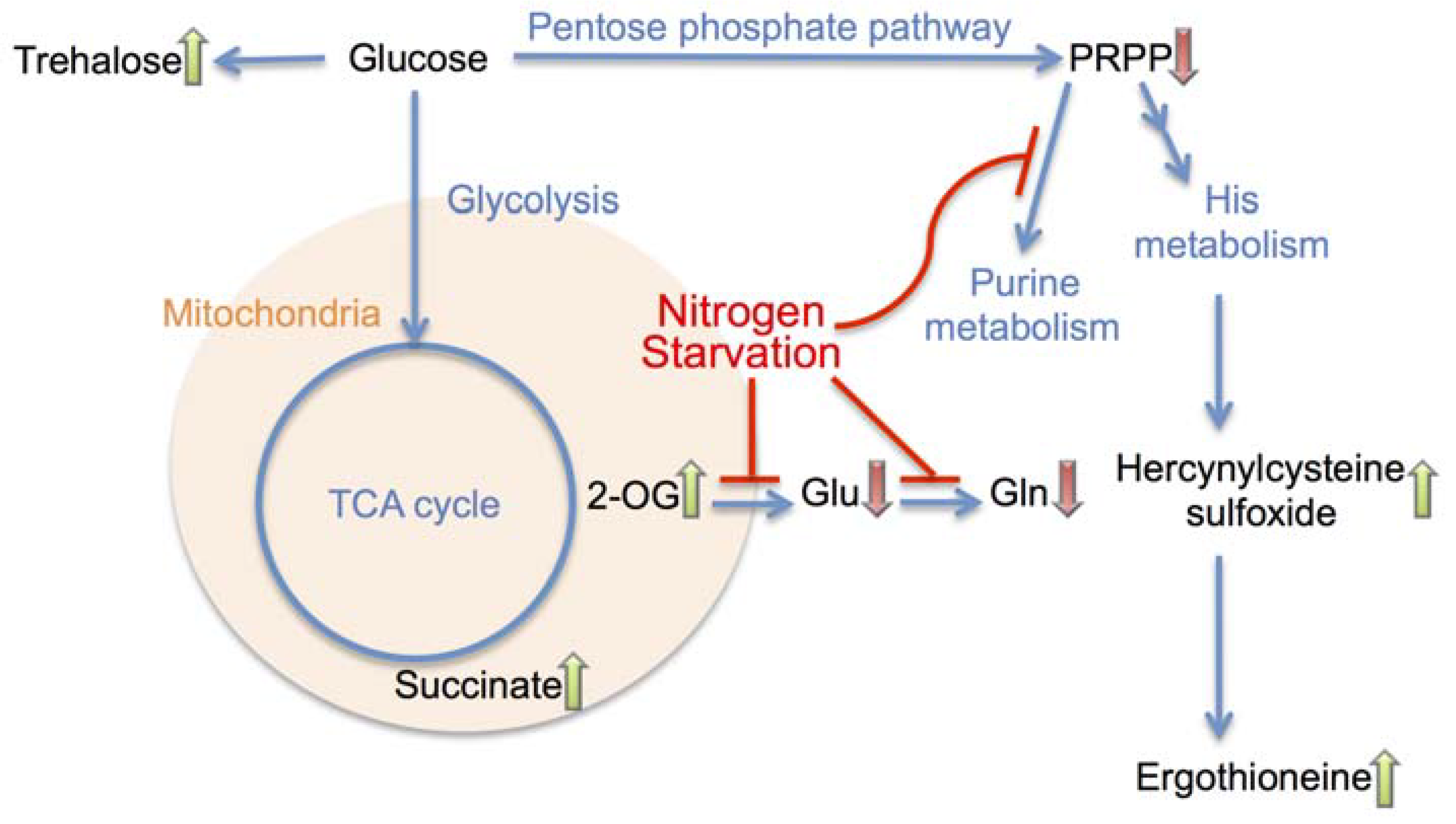
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yanagida, M. Cellular quiescence: Are controlling genes conserved? Trends Cell Biol. 2009, 19, 705–715. [Google Scholar] [CrossRef]
- Shimanuki, M.; Chung, S.Y.; Chikashige, Y.; Kawasaki, Y.; Uehara, L.; Tsutsumi, C.; Hatanaka, M.; Hiraoka, Y.; Nagao, K.; Yanagida, M. Two-step, extensive alterations in the transcriptome from g0 arrest to cell division in schizosaccharomyces pombe. Genes Cells 2007, 12, 677–692. [Google Scholar] [CrossRef]
- Nurse, P.; Bissett, Y. Gene required in g1 for commitment to cell cycle and in g2 for control of mitosis in fission yeast. Nature 1981, 292, 558–560. [Google Scholar] [CrossRef]
- Yanagida, M.; Ikai, N.; Shimanuki, M.; Sajiki, K. Nutrient limitations alter cell division control and chromosome segregation through growth-related kinases and phosphatases. Philos. Trans. R. Soc. Lond. Series B Biol. Sci. 2011, 366, 3508–3520. [Google Scholar] [CrossRef]
- Marguerat, S.; Schmidt, A.; Codlin, S.; Chen, W.; Aebersold, R.; Bahler, J. Quantitative analysis of fission yeast transcriptomes and proteomes in proliferating and quiescent cells. Cell 2012, 151, 671–683. [Google Scholar] [CrossRef]
- Costello, G.; Rodgers, L.; Beach, D. Fission yeast enters the stationary phase g0 state from either mitotic g1 or g2. Curr. Genet. 1986, 11, 119–125. [Google Scholar] [CrossRef]
- Su, S.S.; Tanaka, Y.; Samejima, I.; Tanaka, K.; Yanagida, M. A nitrogen starvation-induced dormant g0 state in fission yeast: The establishment from uncommitted g1 state and its delay for return to proliferation. J. Cell Sci. 1996, 109, 1347–1357. [Google Scholar]
- Mata, J.; Bahler, J. Global roles of Ste11p, cell type, and pheromone in the control of gene expression during early sexual differentiation in fission yeast. Proc. Natl. Acad. Sci. USA 2006, 103, 15517–15522. [Google Scholar] [CrossRef]
- Kristell, C.; Westholm, J.O.; Olsson, I.; Ronne, H.; Komorowski, J.; Bjerling, P. Nitrogen depletion in the fission yeast schizosaccharomyces pombe causes nucleosome loss in both promoters and coding regions of activated genes. Genome Res. 2010, 20, 361–371. [Google Scholar] [CrossRef]
- Sajiki, K.; Hatanaka, M.; Nakamura, T.; Takeda, K.; Shimanuki, M.; Yoshida, T.; Hanyu, Y.; Hayashi, T.; Nakaseko, Y.; Yanagida, M. Genetic control of cellular quiescence in S. pombe. J. Cell Sci. 2009, 122, 1418–1429. [Google Scholar] [CrossRef]
- Alfredsson-Timmins, J.; Kristell, C.; Henningson, F.; Lyckman, S.; Bjerling, P. Reorganization of chromatin is an early response to nitrogen starvation in schizosaccharomyces pombe. Chromosoma 2009, 118, 99–112. [Google Scholar] [CrossRef]
- De Hoon, M.J.; Imoto, S.; Nolan, J.; Miyano, S. Open source clustering software. Bioinformatics 2004, 20, 1453–1454. [Google Scholar] [CrossRef]
- Saldanha, A.J. Java treeview—extensible visualization of microarray data. Bioinformatics 2004, 20, 3246–3248. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinforma 2010, 11. [Google Scholar] [CrossRef]
- Klosinska, M.M.; Crutchfield, C.A.; Bradley, P.H.; Rabinowitz, J.D.; Broach, J.R. Yeast cells can access distinct quiescent states. Genes Dev. 2011, 25, 336–349. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Chen, D.; Toone, W.M.; Mata, J.; Lyne, R.; Burns, G.; Kivinen, K.; Brazma, A.; Jones, N.; Bahler, J. Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell 2003, 14, 214–229. [Google Scholar] [CrossRef]
- Pluskal, T.; Nakamura, T.; Villar-Briones, A.; Yanagida, M. Metabolic profiling of the fission yeast S. pombe: Quantification of compounds under different temperatures and genetic perturbation. Mol. Biosyst. 2010, 6, 182–198. [Google Scholar] [CrossRef]
- Pluskal, T.; Hayashi, T.; Saitoh, S.; Fujisawa, A.; Yanagida, M. Specific biomarkers for stochastic division patterns and starvation-induced quiescence under limited glucose levels in fission yeast. FEBS J. 2011, 278, 1299–1315. [Google Scholar] [CrossRef]
- Perysinakis, A.; Kinghorn, J.R.; Drainas, C. Glutamine synthetase/glutamate synthase ammonium-assimilating pathway in schizosaccharomyces pombe. Curr. Microbiol. 1995, 30, 367–372. [Google Scholar] [CrossRef]
- Clifton, I.J.; McDonough, M.A.; Ehrismann, D.; Kershaw, N.J.; Granatino, N.; Schofield, C.J. Structural studies on 2-oxoglutarate oxygenases and related double-stranded beta-helix fold proteins. J. Inorg. Biochem. 2006, 100, 644–669. [Google Scholar] [CrossRef]
- Zhang, Y.; Morar, M.; Ealick, S.E. Structural biology of the purine biosynthetic pathway. Cell. Mol. Life Sci. 2008, 65, 3699–3724. [Google Scholar] [CrossRef]
- Yang, P.; Du, H.; Hoffman, C.S.; Marcus, S. The phospholipase b homolog plb1 is a mediator of osmotic stress response and of nutrient-dependent repression of sexual differentiation in the fission yeast Schizosaccharomyces pombe. Mol. Genet. Genomics 2003, 269, 116–125. [Google Scholar]
- Cheah, I.K.; Halliwell, B. Ergothioneine, antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2012, 1822, 784–793. [Google Scholar]
- Bello, M.H.; Barrera-Perez, V.; Morin, D.; Epstein, L. The neurospora crassa mutant ncdeltaegt-1 identifies an ergothioneine biosynthetic gene and demonstrates that ergothioneine enhances conidial survival and protects against peroxide toxicity during conidial germination. Fungal Genet. Biol. 2012, 49, 160–172. [Google Scholar] [CrossRef]
- Soga, T.; Baran, R.; Suematsu, M.; Ueno, Y.; Ikeda, S.; Sakurakawa, T.; Kakazu, Y.; Ishikawa, T.; Robert, M.; Nishioka, T.; et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 2006, 281, 16768–16776. [Google Scholar] [CrossRef]
- Gutz, H.; Heslet, H.; Leupold, U.; Loprieno, N. Schizosaccharomyces pombe. In Handbook of Genetics; Plenum Press: New York, NY, USA, 1974. [Google Scholar]
- Mitchison, J.M. Physiological and cytological methods for Schizosaccharomyces pombe. Method Cell Physiol. 1970, 4, 131–165. [Google Scholar]
- Meley, D.; Bauvy, C.; Houben-Weerts, J.H.; Dubbelhuis, P.F.; Helmond, M.T.; Codogno, P.; Meijer, A.J. Amp-activated protein kinase and the regulation of autophagic proteolysis. J. Biol. Chem. 2006, 281, 34870–34879. [Google Scholar]
- Samari, H.R.; Seglen, P.O. Inhibition of hepatocytic autophagy by adenosine, aminoimidazole-4-carboxamide riboside, and n6-mercaptopurine riboside. Evidence for involvement of amp-activated protein kinase. J. Biol. Chem. 1998, 273, 23758–23763. [Google Scholar] [CrossRef]
- Kohda, T.A.; Tanaka, K.; Konomi, M.; Sato, M.; Osumi, M.; Yamamoto, M. Fission yeast autophagy induced by nitrogen starvation generates a nitrogen source that drives adaptation processes. Genes Cells 2007, 12, 155–170. [Google Scholar] [CrossRef]
- Boer, V.M.; Crutchfield, C.A.; Bradley, P.H.; Botstein, D.; Rabinowitz, J.D. Growth-limiting intracellular metabolites in yeast growing under diverse nutrient limitations. Mol. Biol. Cell 2010, 21, 198–211. [Google Scholar] [CrossRef]
Supplementary Material
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sajiki, K.; Pluskal, T.; Shimanuki, M.; Yanagida, M. Metabolomic Analysis of Fission Yeast at the Onset of Nitrogen Starvation. Metabolites 2013, 3, 1118-1129. https://doi.org/10.3390/metabo3041118
Sajiki K, Pluskal T, Shimanuki M, Yanagida M. Metabolomic Analysis of Fission Yeast at the Onset of Nitrogen Starvation. Metabolites. 2013; 3(4):1118-1129. https://doi.org/10.3390/metabo3041118
Chicago/Turabian StyleSajiki, Kenichi, Tomáš Pluskal, Mizuki Shimanuki, and Mitsuhiro Yanagida. 2013. "Metabolomic Analysis of Fission Yeast at the Onset of Nitrogen Starvation" Metabolites 3, no. 4: 1118-1129. https://doi.org/10.3390/metabo3041118
APA StyleSajiki, K., Pluskal, T., Shimanuki, M., & Yanagida, M. (2013). Metabolomic Analysis of Fission Yeast at the Onset of Nitrogen Starvation. Metabolites, 3(4), 1118-1129. https://doi.org/10.3390/metabo3041118



