Deciphering the Role of Different Ceramide Synthases in the Human Cardiomyocyte Hypertrophic Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Hypertrophy Induction with CERS2 and CERS5/6 Knockdown (KD)
2.4. Cell Imaging
2.5. Immunoblotting
2.6. Total RNA Isolation
2.7. Library Preparation and mRNA-Sequencing
2.8. Bioinformatics and Pathway Analyses
2.8.1. Gene Expression Analysis
2.8.2. Gene Set Enrichment Analysis (GSEA)
2.8.3. LRT/Cluster Analysis
2.8.4. Pathway Over-Representation Analysis
3. Results
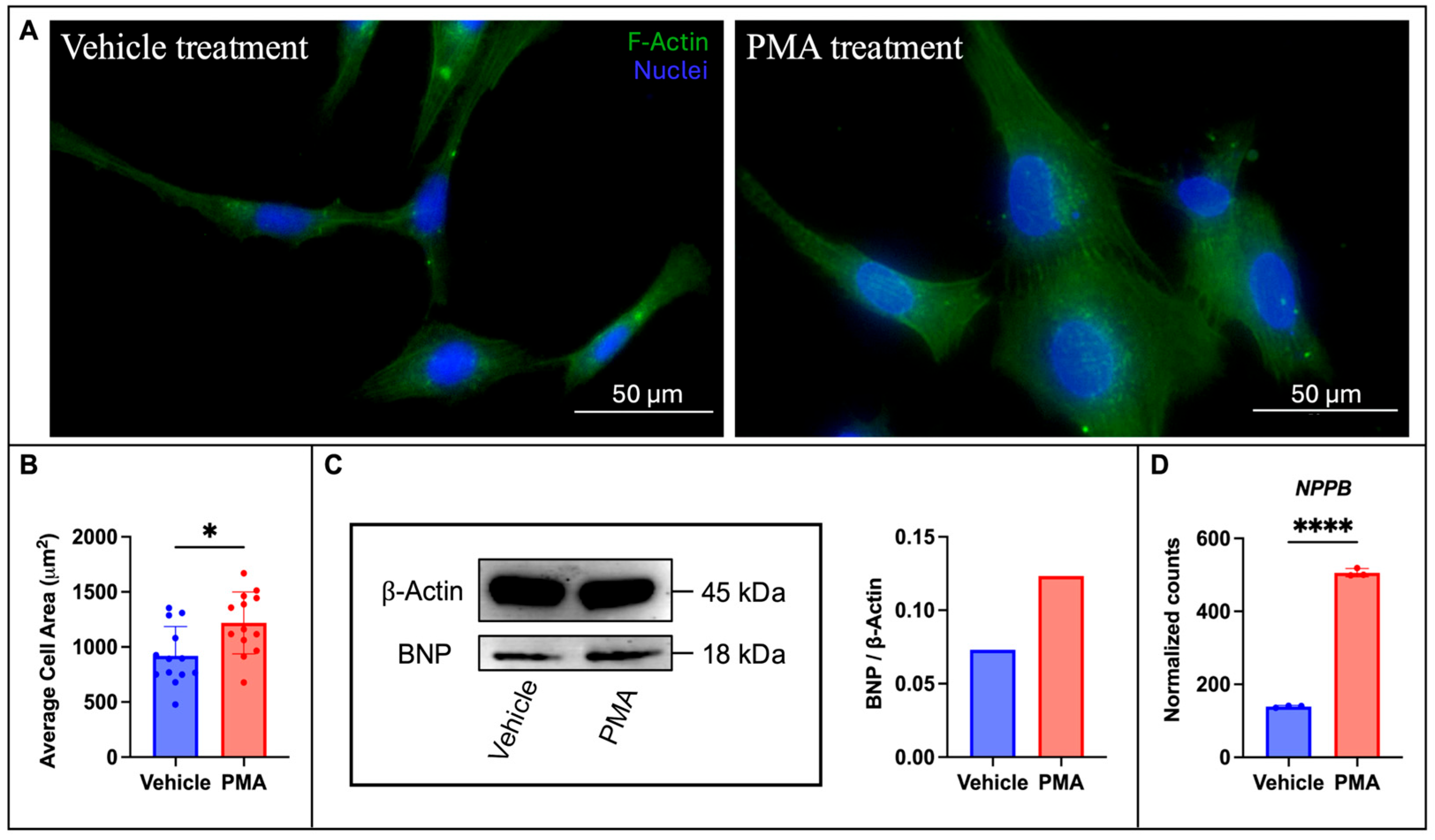
3.1. Establishing a Cell Culture Model of Hypertrophy
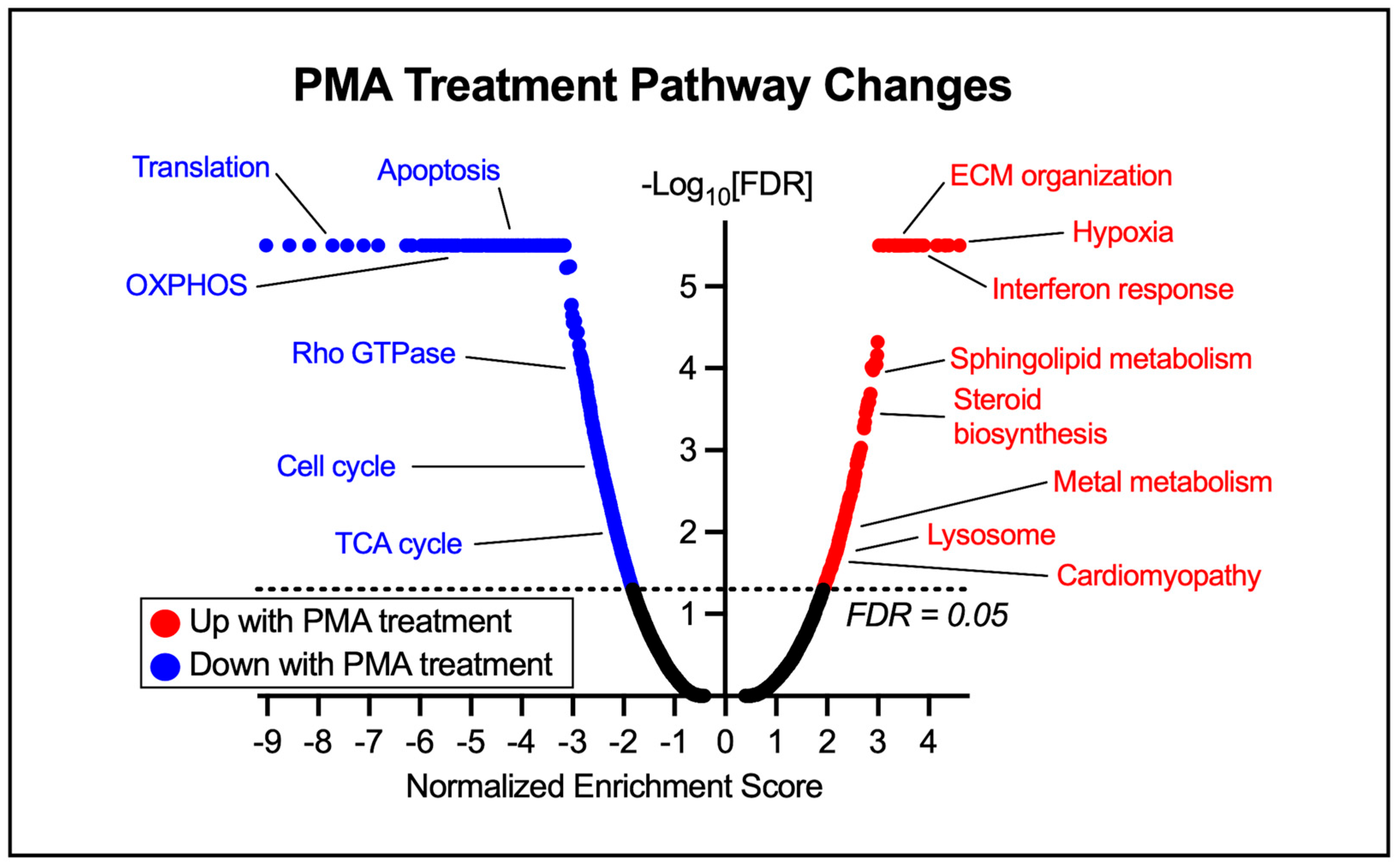
3.2. Leveraging Transcriptomics to Delineate the Role of Ceramides in HF
3.3. Hypertrophy-Specific Responses in Human Cardiomyocytes
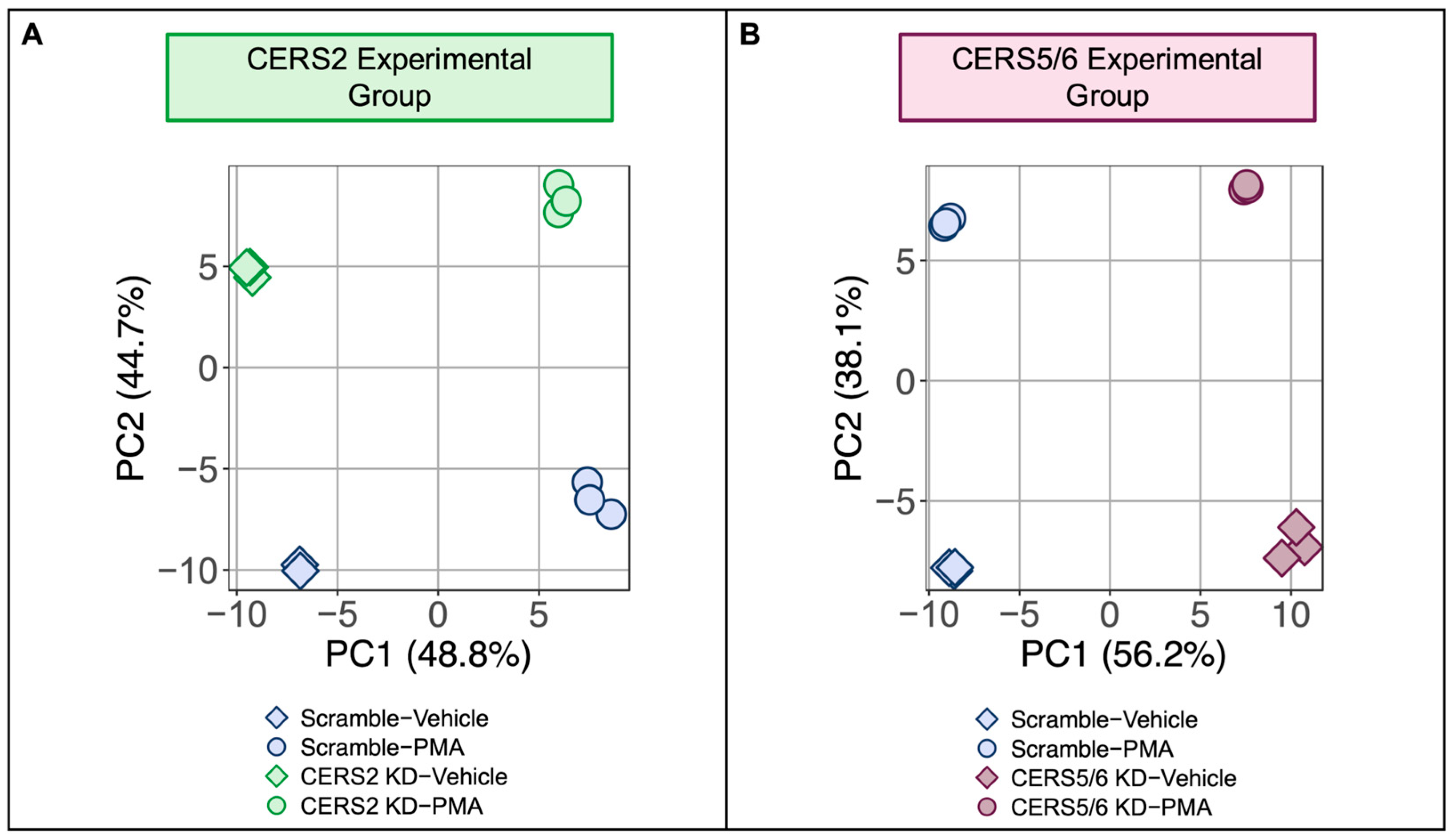
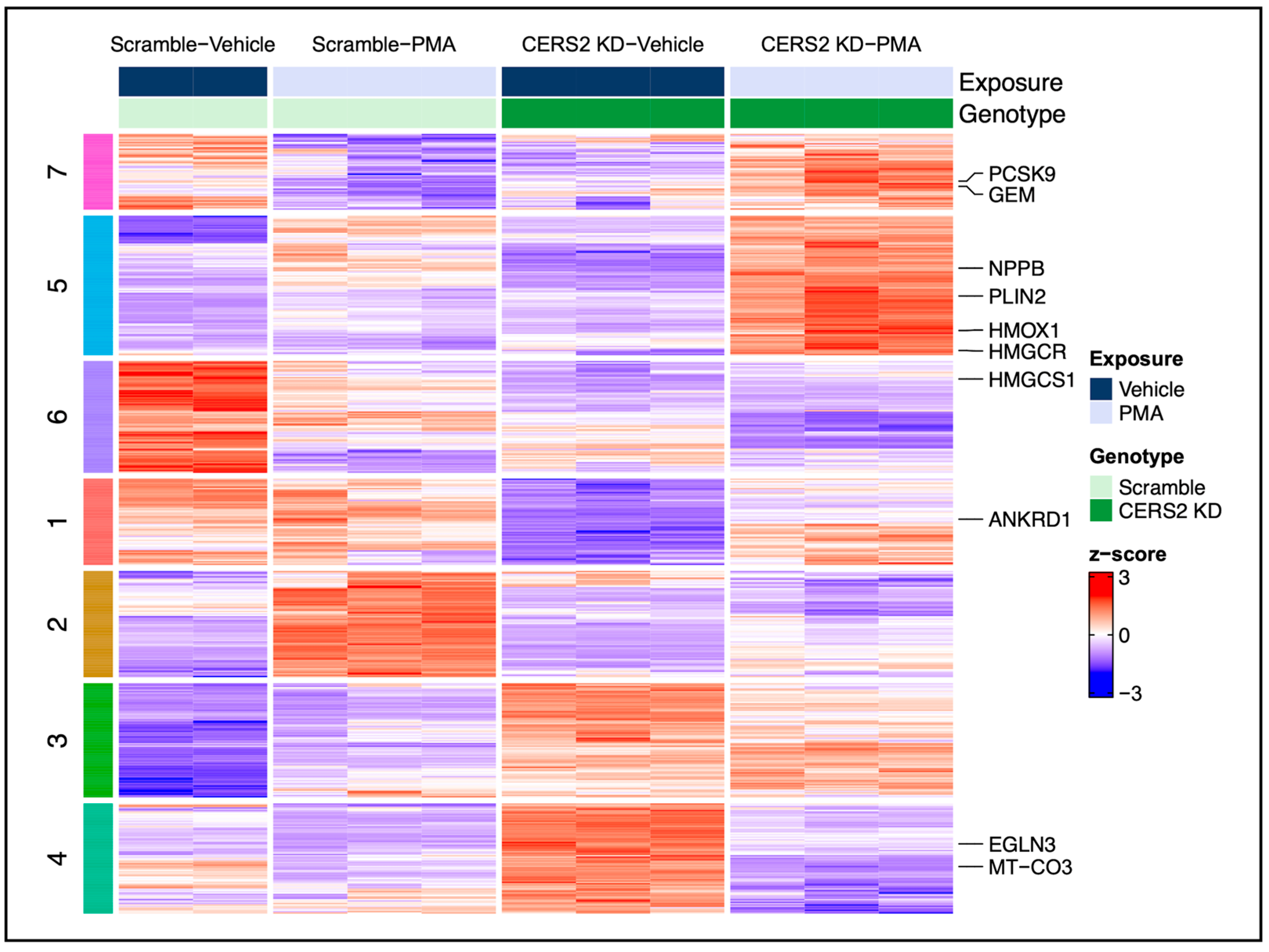
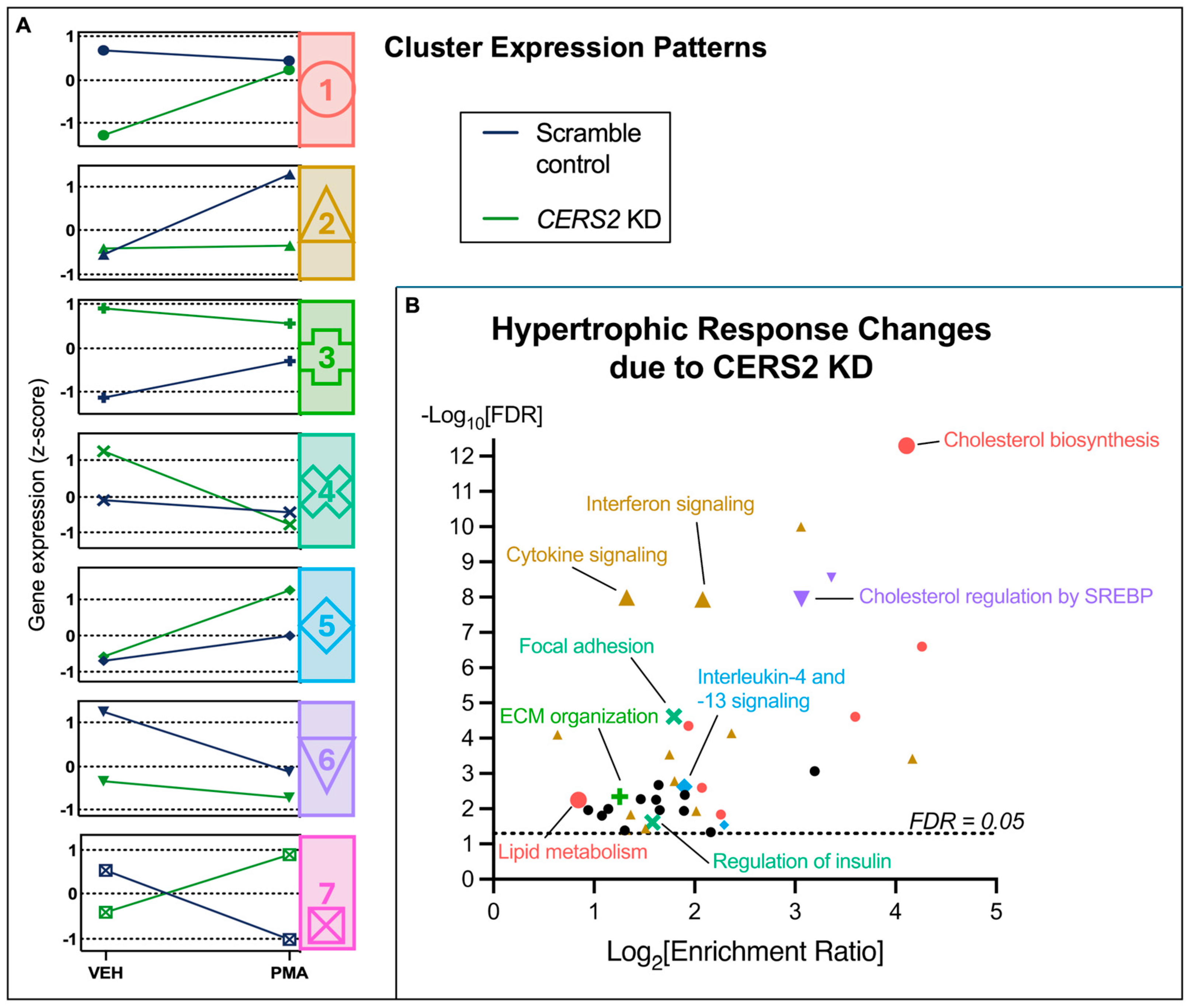
3.4. Hypertrophic Response Alterations with Ceramide Synthase 2 (CERS2) Knockdown
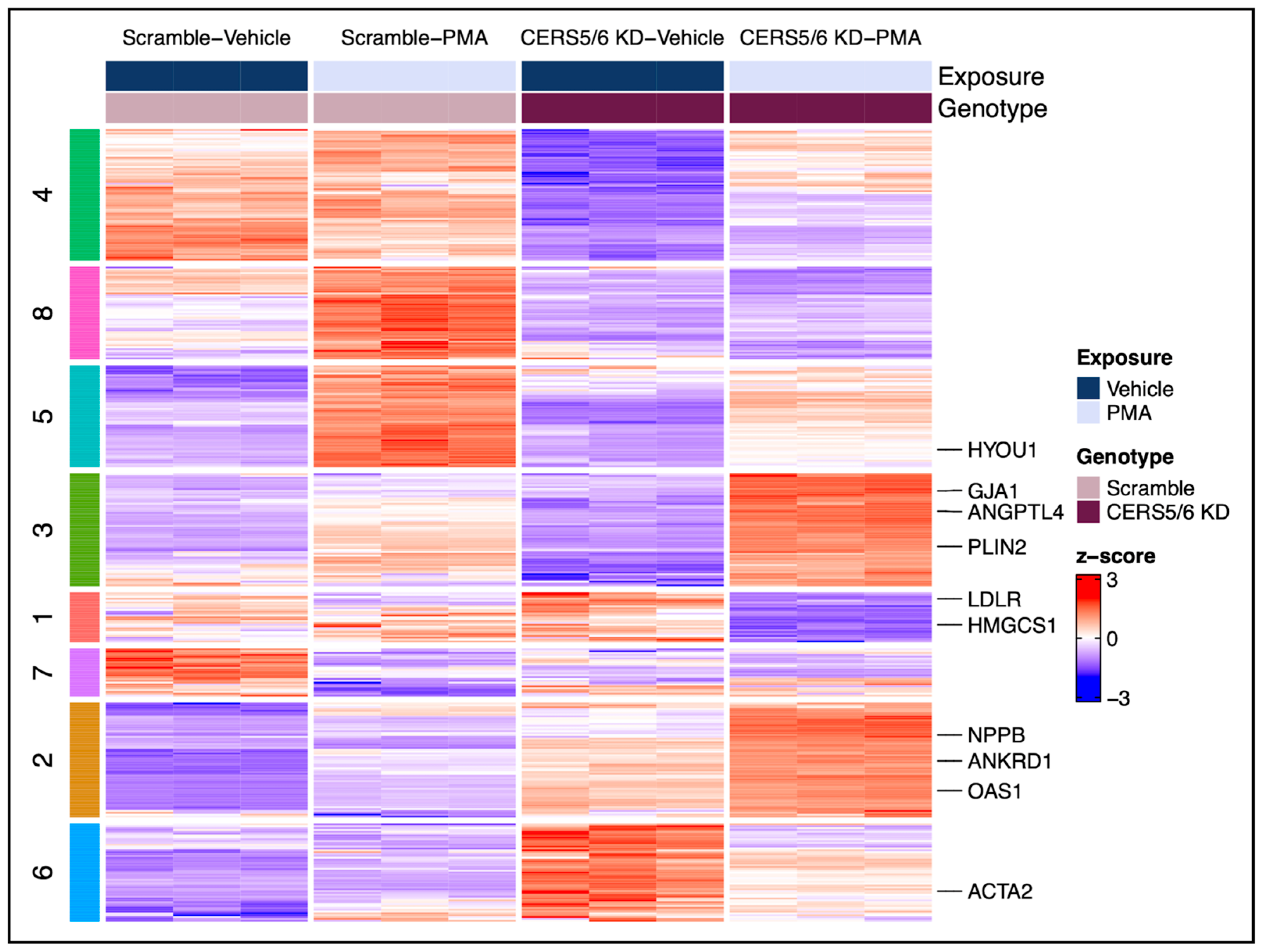
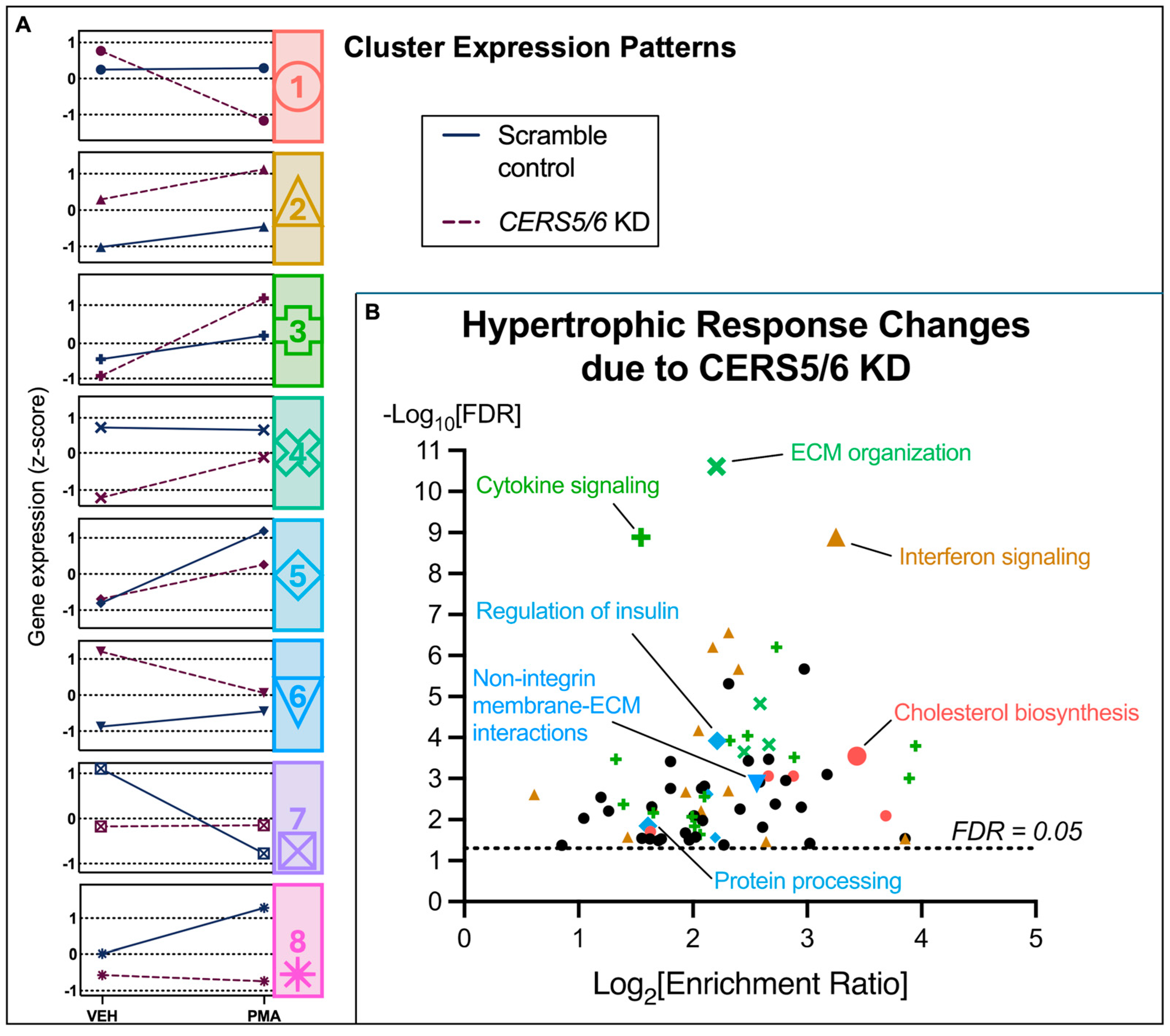
3.5. Hypertrophic Response Alterations with Ceramide Synthase 5 and 6 (CERS5/6) Knockdown
4. Discussion
4.1. PMA Treatment Leads to a Cardiac Hypertrophy Phenotype
4.2. Hypertrophy Response Changes Due to CERS KD
4.2.1. The Effect of CERS2 KD on the Hypertrophic Response
4.2.2. The Effect of CERS5/6 KD on the Hypertrophic Response
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CVD | cardiovascular disease |
| LDL | low density lipoprotein |
| CerS | ceramide synthase |
| HF | heart failure |
| SM | sphingomyelin |
| LC | long-chain |
| VLC | very long-chain |
| HCM | human ventricular cardiomyocytes |
| KD | knockdown |
| PMA | phorbol 12-myristate 13-acetate |
| GSEA | gene set enrichment analysis |
| FDR | false discovery rate |
| LRT | likelihood ratio test |
| BNP | B-type natriuretic peptide |
| NPPB | gene name for BNP |
| PCA | principal component analysis |
| DEGs | differentially expressed genes |
| ECM | extracellular matrix |
| LDLR | low density lipoprotein receptor |
| ACTA2 | actin alpha 2 |
| PLIN2 | perilipin 2 |
| ANKRD1 | ankyrin repeat domain 1 |
| PKC | protein kinase C |
| OXPHOS | oxidative phosphorylation |
| TCA | tricarboxylic acid cycle |
References
- Institute for Health Metrics and Evaluations (IHME). GBD Results; IHME, University of Washington: Seattle, WA, USA, 2024; Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 15 May 2024).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, R.N.; Jensen, P.N.; Hoofnagle, A.; McKnight, B.; Fretts, A.M.; King, I.B.; Siscovick, D.S.; Psaty, B.M.; Heckbert, S.R.; Mozaffarian, D.; et al. Plasma Ceramides and Sphingomyelins in Relation to Heart Failure Risk: The Cardiovascular Health Study. Circ. Heart Fail. 2019, 12, e005708. [Google Scholar] [CrossRef]
- Summers, S.A. Could Ceramides Become the New Cholesterol? Cell Metab. 2018, 27, 276–280. [Google Scholar] [CrossRef]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Augusto, S.N.; Suresh, A.; Tang, W.H.W. Ceramides as Biomarkers of Cardiovascular Diseases and Heart Failure. Curr. Heart Fail. Rep. 2025, 22, 2. [Google Scholar] [CrossRef]
- Grösch, S.; Schiffmann, S.; Geisslinger, G. Chain length-specific properties of ceramides. Prog. Lipid Res. 2012, 51, 50–62. [Google Scholar] [CrossRef]
- Pettus, B.J.; Chalfant, C.E.; Hannun, Y.A. Ceramide in apoptosis: An overview and current perspectives. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2002, 1585, 114–125. [Google Scholar] [CrossRef]
- Gulbins, E.; Li, P.L. Physiological and pathophysiological aspects of ceramide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R11–R26. [Google Scholar] [CrossRef] [PubMed]
- Summers, S. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 2006, 45, 42–72. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Many Ceramides. J. Biol. Chem. 2011, 286, 27855–27862. [Google Scholar] [CrossRef]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008, 20, 1010–1018. [Google Scholar] [CrossRef]
- Hammerschmidt, P.; Brüning, J.C. Contribution of specific ceramides to obesity-associated metabolic diseases. Cell. Mol. Life Sci. 2022, 79, 395. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.D.; Spassieva, S.; Jenkins, R.W.; Kitatani, K.; Bielawski, J.; Hannun, Y.A.; Obeid, L.M. Selective knockdown of ceramide synthases reveals complex interregulation of sphingolipid metabolism. J. Lipid Res. 2011, 52, 68–77. [Google Scholar] [CrossRef]
- Wiley, A.M.; Krueger, M.A.; Becker, J.O.; Karasu, M.; Sotoodehnia, N.; Umans, J.G.; Hoofnagle, A.N.; Gharib, S.A.; Totah, R.A.; Lemaitre, R.N. Selective Knockdown of Ceramide Synthases Reveals Opposite Roles of Different Ceramide Species in Cardiac Homeostasis. Metabolites 2025, 15, 584. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef]
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef] [PubMed]
- Ho, Q.W.C.; Zheng, X.; Ali, Y. Ceramide Acyl Chain Length and Its Relevance to Intracellular Lipid Regulation. Int. J. Mol. Sci. 2022, 23, 9697. [Google Scholar] [CrossRef] [PubMed]
- Zietzer, A.; Düsing, P.; Reese, L.; Nickenig, G.; Jansen, F. Ceramide Metabolism in Cardiovascular Disease: A Network with High Therapeutic Potential. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1220–1228. [Google Scholar] [CrossRef]
- Nicholson, R.J.; Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hopkins, P.N.; Hunt, S.C.; Playdon, M.C.; Holland, W.L.; Summers, S. Characterizing a Common CERS2 Polymorphism in a Mouse Model of Metabolic Disease and in Subjects from the Utah CAD Study. J. Clin. Endocrinol. Metab. 2021, 106, e3098–e3109. [Google Scholar] [CrossRef]
- De La Monte, S.M. Triangulated Mal-Signaling in Alzheimer’s Disease: Roles of Neurotoxic Ceramides, ER Stress, and Insulin Resistance Reviewed. J. Alzheimer’s Dis. 2012, 30, S231–S249. [Google Scholar] [CrossRef]
- Vega, R.B.; Harrison, B.C.; Meadows, E.; Roberts, C.R.; Papst, P.J.; Olson, E.N.; McKinsey, T.A. Protein Kinases C and D Mediate Agonist-Dependent Cardiac Hypertrophy through Nuclear Export of Histone Deacetylase 5. Mol. Cell. Biol. 2004, 24, 8374–8385. [Google Scholar] [CrossRef]
- Miao, L.N.; Pan, D.; Shi, J.; Du, J.P.; Chen, P.F.; Gao, J.; Yu, Y.; Shi, D.Z.; Guo, M. Role and Mechanism of PKC-δ for Cardiovascular Disease: Current Status and Perspective. Front. Cardiovasc. Med. 2022, 9, 816369. [Google Scholar] [CrossRef]
- Russo, S.B.; Baicu, C.F.; Van Laer, A.; Geng, T.; Kasiganesan, H.; Zile, M.R.; Cowart, L.A. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Investig. 2012, 122, 3919–3930. [Google Scholar] [CrossRef]
- Dai, Z.; Cheng, J.; Liu, B.; Yi, D.; Feng, A.; Wang, T.; An, L.; Gao, C.; Wang, Y.; Zhu, M.M.; et al. Loss of Endothelial Hypoxia Inducible Factor-Prolyl Hydroxylase 2 Induces Cardiac Hypertrophy and Fibrosis. J. Am. Heart Assoc. 2021, 10, e022077. [Google Scholar] [CrossRef] [PubMed]
- Kehat, I.; Molkentin, J.D. Molecular Pathways Underlying Cardiac Remodeling During Pathophysiological Stimulation. Circulation 2010, 122, 2727–2735. [Google Scholar] [CrossRef]
- Ahuja, P.; Sdek, P.; MacLellan, W.R. Cardiac Myocyte Cell Cycle Control in Development, Disease, and Regeneration. Physiol. Rev. 2007, 87, 521–544. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Merrill, A.H. Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling, and disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; Sullards, M.C.; Allegood, J.; Wang, E.; Schmelz, E.M.; Hartl, M.; Humpf, H.-U.; Liotta, D.; Peng, Q.; Merrill, A.H. Fumonisins and fumonisin analogs as inhibitors of ceramide synthase and inducers of apoptosis. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2002, 1585, 188–192. [Google Scholar] [CrossRef]
- Wang, S.; Jin, Z.; Wu, B.; Morris, A.J.; Deng, P. Role of dietary and nutritional interventions in ceramide-associated diseases. J. Lipid Res. 2025, 66, 100726. [Google Scholar] [CrossRef]
- Walker, M.E.; Xanthakis, V.; Peterson, L.R.; Duncan, M.S.; Lee, J.; Ma, J.; Bigornia, S.; Moore, L.L.; Quatromoni, P.; Vasan, R.S.; et al. Dietary Patterns, Ceramide Ratios, and Risk of All-Cause and Cause-Specific Mortality: The Framingham Offspring Study. J. Nutr. 2020, 150, 2994–3004. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiley, A.M.; Krueger, M.A.; Sotoodehnia, N.; Umans, J.G.; Hoofnagle, A.N.; Lemaitre, R.N.; Totah, R.A.; Gharib, S.A. Deciphering the Role of Different Ceramide Synthases in the Human Cardiomyocyte Hypertrophic Response. Metabolites 2025, 15, 635. https://doi.org/10.3390/metabo15090635
Wiley AM, Krueger MA, Sotoodehnia N, Umans JG, Hoofnagle AN, Lemaitre RN, Totah RA, Gharib SA. Deciphering the Role of Different Ceramide Synthases in the Human Cardiomyocyte Hypertrophic Response. Metabolites. 2025; 15(9):635. https://doi.org/10.3390/metabo15090635
Chicago/Turabian StyleWiley, Alexandra M., Melissa A. Krueger, Nona Sotoodehnia, Jason G. Umans, Andrew N. Hoofnagle, Rozenn N. Lemaitre, Rheem A. Totah, and Sina A. Gharib. 2025. "Deciphering the Role of Different Ceramide Synthases in the Human Cardiomyocyte Hypertrophic Response" Metabolites 15, no. 9: 635. https://doi.org/10.3390/metabo15090635
APA StyleWiley, A. M., Krueger, M. A., Sotoodehnia, N., Umans, J. G., Hoofnagle, A. N., Lemaitre, R. N., Totah, R. A., & Gharib, S. A. (2025). Deciphering the Role of Different Ceramide Synthases in the Human Cardiomyocyte Hypertrophic Response. Metabolites, 15(9), 635. https://doi.org/10.3390/metabo15090635








