The Impact of Gut Microbial Metabolomics on Type 2 Diabetes Development in People Living with HIV
Abstract
1. Introduction
2. Gut Microbiota Alterations in People Living with HIV
3. Metabolomic Alterations in HIV Infection and Type 2 Diabetes Associated with Intestinal Microbiota Dysbiosis
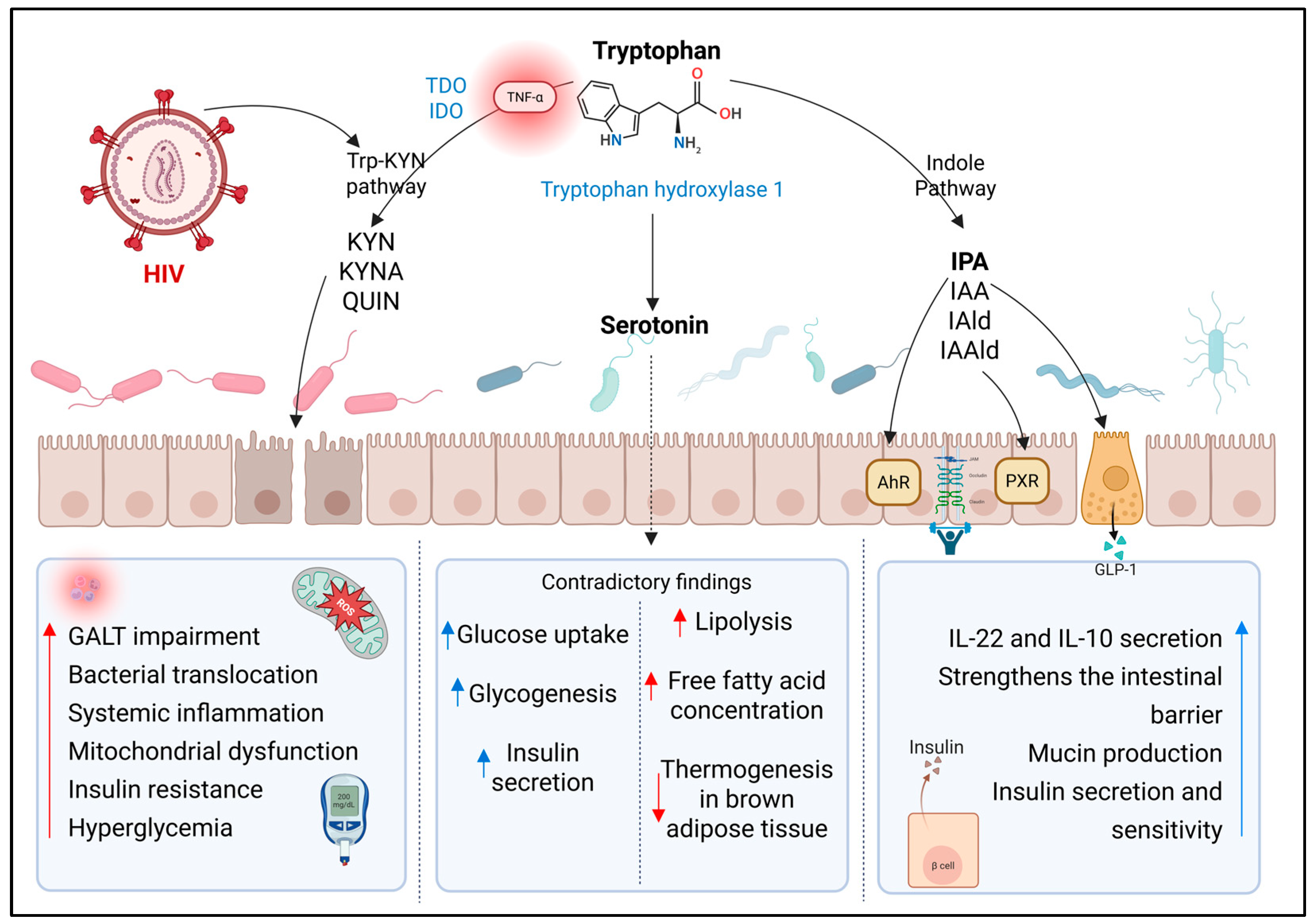
3.1. Metabolites Derived from Tryptophan Catabolism
3.1.1. Kynurenine Pathway (Trp-KYN)
3.1.2. Indole Pathway
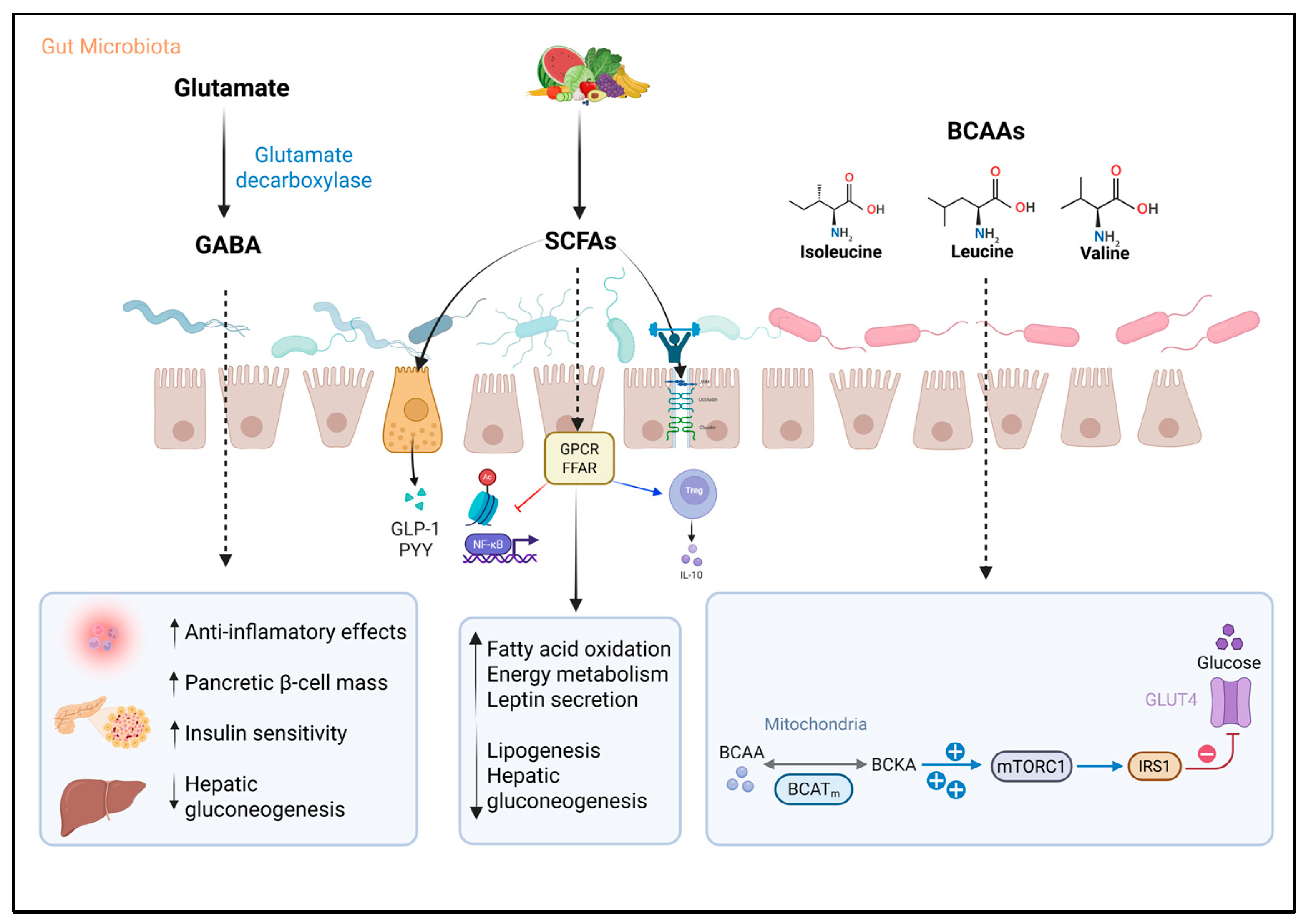
3.2. Serotonin and γ-Aminobutyric Acid
3.3. Short-Chain Fatty Acids
3.4. Branched-Chain Amino Acids
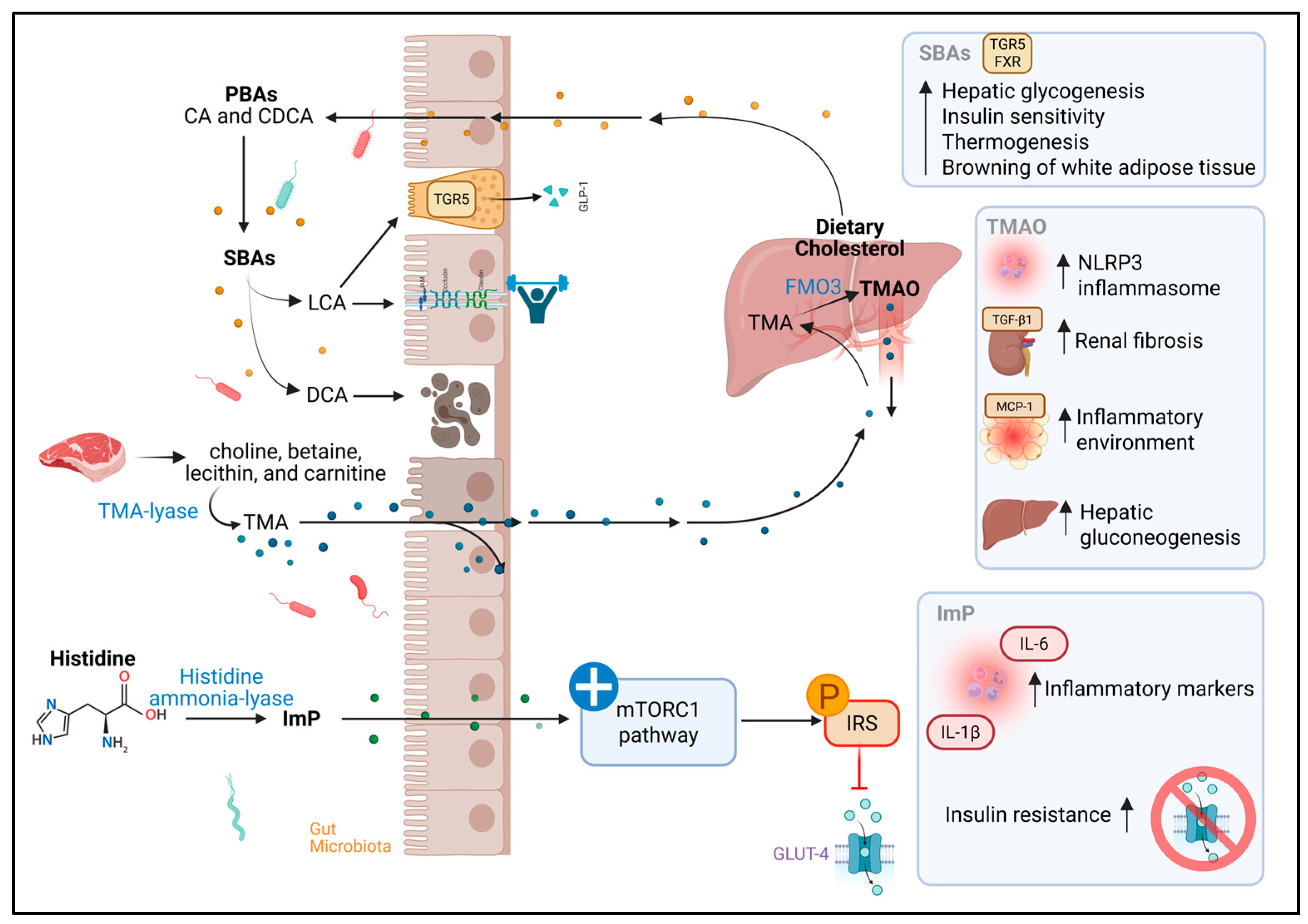
3.5. Bile Acids
3.6. Trimethylamine N-Oxide
3.7. Imidazole Propionate
3.8. Therapeutic and Diagnostic Opportunities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HIV | Human immunodeficiency virus |
| AIDS | Acquired immunodeficiency syndrome |
| PLWHIV | People living with HIV |
| ART | Antiretroviral therapy |
| T2D | Type 2 diabetes |
| GM | Gut microbiota |
| GALT | Gut-associated lymphoid tissue |
| LPS | Lipopolysaccharides |
| Trp-KYN | Kynurenine pathway |
| TDO | Tryptophan 2,3-dioxygenase |
| IDO | Indoleamine 2,3-dioxygenase |
| KYN | Kynurenine |
| KYNA | Kynurenic acid |
| AA | Anthranilic acid |
| 3-HK | 3-hydroxykynurenine |
| QUIN | Quinolinic acid |
| TNF-α | Tumor Necrosis Factor alpha |
| IL-1β | Interleukin 1 beta |
| IR | Insulin resistance |
| IAA | Indole-3-acetic acid |
| IAld | Indole-3-aldehyde |
| IPA | Indole-3-propionic acid |
| IAAld | Indole-3-acetaldehyde |
| AhR | Aryl hydrocarbon receptor |
| PXR | Pregnane X receptor |
| TFF3 | Trefoil factor family 3 |
| RELMβ | Resistin-like molecule beta |
| 5-HT | 5-hydroxytryptamine |
| HOMA-IR | Homeostatic model assessment of insulin resistance |
| GLP-1 | Glucagon-like peptide-1 |
| GABA | γ-Aminobutyric acid |
| PI3K/AKT | Phosphoinositide 3-kinase/protein kinase B |
| IRS1 | insulin receptor substrate 1 |
| GLUT4 | Glucose transporter type 4 |
| IFN-γ | Interferon gamma |
| SCFAs | Short-chain fatty acids |
| GPCRs | G protein-coupled receptors |
| FFAR | Free fatty acid receptor |
| ERK1/2 | Extracellular signal-regulated kinases 1 and 2 |
| cAMP | Cyclic adenosine monophosphate |
| PYY | Peptide YY |
| AMPK | AMP-activated protein kinase |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPARs | Peroxisome proliferator-activated receptors |
| ATGL | Adipose triglyceride lipase |
| UCPs | Uncoupling proteins |
| PWT2D | People with type 2 diabetes |
| BCAAs | Branched-chain amino acids |
| BCAT2 | Branched-chain aminotransferase 2 |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| BAs | Bile acids |
| CA | Cholic acid |
| CDCA | Chenodeoxycholic acid |
| DCA | Deoxycholic acid |
| LCA | Lithocholic acid |
| TGR5 | Takeda G-protein-coupled receptor 5 |
| ZO-1 | Zonula Occludens-1 |
| TMA | Trimethylamine |
| FMO3 | Flavin-containing monooxygenase 3 enzyme |
| TMAO | Trimethylamine N-oxide |
| PERK | PKR-like Endoplasmic Reticulum Kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| TGF-β1 | Transforming growth factor beta 1 |
| α-SMA | Alpha-smooth muscle actin |
| NLRP3 | NOD-like receptor family pyrin domain-containing 3 |
| ImP | Imidazole propionate |
| MAPK | Mitogen-activated protein kinase |
| S6K1 | Ribosomal protein S6 kinase beta-1 |
References
- UNAIDS. Global HIV & AIDS Statistics. 2023. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 15 July 2025).
- Secretaría de Salud. HIV and AIDS Case Surveillance in Mexico. National Registry of HIV and AIDS Cases 2014–2024. 2024. Available online: https://www.gob.mx/censida/documentos/epidemiologia-registro-nacional-de-casos-de-sida (accessed on 15 July 2025).
- Brown, T.T. Antiretroviral Therapy and the Prevalence and Incidence of Diabetes Mellitus in the Multicenter AIDS Cohort Study. Arch. Intern. Med. 2005, 165, 1179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singh, H.; Chakole, S. Exploring the Relation Between Diabetes and HIV: A Narrative Review. Cureus 2023, 15, e43909. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Borrow, P.; Tomaras, G.D.; Goonetilleke, N.; Haynes, B.F. The immune response during acute HIV-1 infection: Clues for vaccine development. Nat. Rev. Immunol. 2010, 10, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, P.; Sahu, A.K.; Mishra, T.; Bhardwaj, V.; Chande, A. From Entry to Egress: Strategic Exploitation of the Cellular Processes by HIV-1. Front. Microbiol. 2020, 11, 559792. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Schacker, T.W.; Ruff, L.E.; Price, D.A.; Taylor, J.H.; Beilman, G.J.; Nguyen, P.L.; Khoruts, A.; Larson, M.; Haase, A.T.; et al. CD4+ T Cell Depletion during all Stages of HIV Disease Occurs Predominantly in the Gastrointestinal Tract. J. Exp. Med. 2004, 200, 749–759. [Google Scholar] [CrossRef]
- Mattapallil, J.J.; Douek, D.C.; Hill, B.; Nishimura, Y.; Martin, M.; Roederer, M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005, 434, 1093–1097. [Google Scholar] [CrossRef]
- Levesque, M.C.; Moody, M.A.; Hwang, K.K.; Marshall, D.J.; Whitesides, J.F.; Amos, J.D.; Gurley, T.C.; Allgood, S.; Haynes, B.B.; Vandergrift, N.A.; et al. Polyclonal B Cell Differentiation and Loss of Gastrointestinal Tract Germinal Centers in the Earliest Stages of HIV-1 Infection. PLoS Med. 2009, 6, e1000107. [Google Scholar] [CrossRef]
- Bonetti, L.; Horkova, V.; Grusdat, M.; Longworth, J.; Guerra, L.; Kurniawan, H.; Franchina, D.G.; Soriano-Baguet, L.; Binsfeld, C.; Verschueren, C.; et al. A Th17 cell-intrinsic glutathione/mitochondrial-IL-22 axis protects against intestinal inflammation. Cell Metab. 2024, 36, 1726–1744.e10. [Google Scholar] [CrossRef]
- Sundaravaradan, V.; Mehta, R.; Harris, D.T.; Zack, J.A.; Ahmad, N. Differential expression and interaction of host factors augment HIV-1 gene expression in neonatal mononuclear cells. Virology 2010, 400, 32–43. [Google Scholar] [CrossRef]
- Christensen-Quick, A.; Lafferty, M.; Sun, L.; Marchionni, L.; DeVico, A.; Garzino-Demo, A. Human Th17 Cells Lack HIV-Inhibitory RNases and Are Highly Permissive to Productive HIV Infection. J. Virol. 2016, 90, 7833–7847. [Google Scholar] [CrossRef]
- Chandrasekar, A.P.; Maynes, M.; Badley, A.D. Dynamic modulation of the non-canonical NF-κB signaling pathway for HIV shock and kill. Front. Cell. Infect. Microbiol. 2024, 14, 1354502. [Google Scholar] [CrossRef]
- Salvador, P.B.U.; Altavas, P.J.d.R.; del Rosario, M.A.S.; Ornos, E.D.B.; Dalmacio, L.M.M. Alterations in the Gut Microbiome Composition of People Living with HIV in the Asia–Pacific Region: A Systematic Review. Clin. Pract. 2024, 14, 846–861. [Google Scholar] [CrossRef] [PubMed]
- Gáspár, Z.; Nagavci, B.; Szabó, B.G.; Lakatos, B. Gut Microbiome Alteration in HIV/AIDS and the Role of Antiretroviral Therapy—A Scoping Review. Microorganisms 2024, 12, 2221. [Google Scholar] [CrossRef]
- Dolo, O.; Coulibaly, F.; Somboro, A.M.; Fofana, D.B.; Togo, J.; Balde, A.; Diallo, D.; Maiga, A.; Diarra, B.; Murphy, R.L.; et al. The impact of HIV antiretroviral therapy on gut microbiota: The need for well-designed longitudinal studies. J. Infect. Dev. Countries. 2024, 18, 1461–1473. [Google Scholar] [CrossRef]
- Pan, Z.; Wu, N.; Jin, C. Intestinal Microbiota Dysbiosis Promotes Mucosal Barrier Damage and Immune Injury in HIV-Infected Patients. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 3080969. [Google Scholar] [CrossRef]
- Krug, S.M.; Grünhagen, C.; Allers, K.; Bojarski, C.; Seybold, J.; Schneider, T.; Schulzke, J.-D.; Epple, H.-J. Macromolecule Translocation across the Intestinal Mucosa of HIV-Infected Patients by Transcytosis and through Apoptotic Leaks. Cells 2023, 12, 1887. [Google Scholar] [CrossRef]
- Tincati, C.; Bono, V.; Cannizzo, E.S.; Tosi, D.; Savi, F.; Falcinella, C.; Casabianca, A.; Orlandi, C.; Luigiano, C.; Augello, M.; et al. Primary HIV infection features colonic damage and neutrophil inflammation yet containment of microbial translocation. AIDS 2024, 38, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Yan, J.; Zhou, X.; Isnard, S.; Harypursat, V.; Cui, H.; Routy, J.-P.; Chen, Y. Relevance of biomarkers indicating gut damage and microbial translocation in people living with HIV. Front. Immunol. 2023, 14, 1173956. [Google Scholar] [CrossRef] [PubMed]
- Ancona, G.; Merlini, E.; Tincati, C.; Barassi, A.; Calcagno, A.; Augello, M.; Bono, V.; Bai, F.; Cannizzo, E.S.; Monforte, A.D.; et al. Long-Term Suppressive cART Is Not Sufficient to Restore Intestinal Permeability and Gut Microbiota Compositional Changes. Front. Immunol. 2021, 12, 639291. [Google Scholar] [CrossRef]
- Tiozzo, E.; Rodriguez, A.; Konefal, J.; Farkas, G.J.; Maher, J.L.; Lewis, J.E. The Relationship between HIV Duration, Insulin Resistance and Diabetes Risk. Int. J. Environ. Res. Public Health 2021, 18, 3926. [Google Scholar] [CrossRef]
- Moon, J.Y.; Zolnik, C.P.; Wang, Z.; Qiu, Y.; Usyk, M.; Wang, T.; Kizer, J.R.; Landay, A.L.; Kurland, I.J.; Anastos, K.; et al. Gut microbiota and plasma metabolites associated with diabetes in women with, or at high risk for, HIV infection. eBioMedicine 2018, 37, 392–400. [Google Scholar] [CrossRef]
- Luo, K.; Peters, B.A.; Moon, J.Y.; Xue, X.; Wang, Z.; Usyk, M.; Hanna, D.B.; Landay, A.L.; Schneider, M.F.; Gustafson, D.; et al. Metabolic and inflammatory perturbation of diabetes associated gut dysbiosis in people living with and without HIV infection. Genome Med. 2024, 16, 59. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Kearns, R. The Kynurenine Pathway in Gut Permeability and Inflammation. Inflammation 2024, 48, 1063–1077. [Google Scholar] [CrossRef]
- Kozieł, K.; Urbanska, E.M. Kynurenine Pathway in Diabetes Mellitus—Novel Pharmacological Target? Cells 2023, 12, 460. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, Q. Gut Microbial Metabolites Associated with HIV Infection. Future Virol. 2019, 14, 335–347. [Google Scholar] [CrossRef]
- Trøseid, M.; Nielsen, S.D.; Vujkovic-Cvijin, I. Gut microbiome and cardiometabolic comorbidities in people living with HIV. Microbiome 2024, 12, 106. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Dunham, R.M.; Iwai, S.; Maher, M.C.; Albright, R.G.; Broadhurst, M.J.; Hernandez, R.D.; Lederman, M.M.; Huang, Y.; Somsouk, M.; et al. Dysbiosis of the Gut Microbiota Is Associated with HIV Disease Progression and Tryptophan Catabolism. Sci. Transl. Med. 2013, 5, 193ra91. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res. Rev. 2022, 75, 101573. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Li, J.; Yu, B.; Moon, J.-Y.; Chai, J.C.; Merino, J.; Hu, J.; Ruiz-Canela, M.; Rebholz, C.; Wang, Z.; et al. Host and gut microbial tryptophan metabolism and type 2 diabetes: An integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut 2022, 71, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Papandreou, C.; Ruiz-Canela, M.; Guasch-Ferre, M.; Clish, C.B.; Dennis, C.; Liang, L.; Corella, D.; Fitó, M.; Razquin, C.; et al. Association of Tryptophan Metabolites with Incident Type 2 Diabetes in the PREDIMED Trial: A Case–Cohort Study. Clin. Chem. 2018, 64, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Hoel, H.; Hove-Skovsgaard, M.; Hov, J.R.; Gaardbo, J.C.; Holm, K.; Kummen, M.; Rudi, K.; Nwosu, F.; Valeur, J.; Gelpi, M.; et al. Impact of HIV and Type 2 diabetes on Gut Microbiota Diversity, Tryptophan Catabolism and Endothelial Dysfunction. Sci. Rep. 2018, 8, 6725. [Google Scholar] [CrossRef]
- Biernacki, T.; Sandi, D.; Bencsik, K.; Vécsei, L. Kynurenines in the Pathogenesis of Multiple Sclerosis: Therapeutic Perspectives. Cells 2020, 9, 1564. [Google Scholar] [CrossRef]
- Wu, J.; Yang, K.; Fan, H.; Wei, M.; Xiong, Q. Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1114424. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Luo, K.; Wang, Z.; Peters, B.A.; Hanna, D.B.; Wang, T.; Sollecito, C.C.; Grassi, E.; Wiek, F.; Peter, L.S.; Usyk, M.; et al. Tryptophan metabolism, gut microbiota, and carotid artery plaque in women with and without HIV infection. AIDS 2024, 38, 223–233. [Google Scholar] [CrossRef]
- Boyd, A.; Boccara, F.; Meynard, J.L.; Ichou, F.; Bastard, J.-P.; Fellahi, S.; Samri, A.; Sauce, D.; Haddour, N.; Autran, B.; et al. Serum tryptophan-derived quinolinate and indole-3-acetate are associated with carotid intima-media thickness and its evolution in HIV-infected treated adults. Open Forum Infect Dis. 2019, 6, ofz516. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Li, S.; Zhang, Q.; Cai, Y.; Li, P.; Li, H.; Shen, B.; Liao, Q.; Hong, Y.; et al. Indoleacrylic acid produced by Parabacteroides distasonis alleviates type 2 diabetes via activation of AhR to repair intestinal barrier. BMC Biol. 2023, 21, 90. [Google Scholar] [CrossRef]
- Wang, M.; Guo, J.; Hart, A.L.; Li, J.V. Indole-3-Aldehyde Reduces Inflammatory Responses and Restores Intestinal Epithelial Barrier Function Partially via Aryl Hydrocarbon Receptor (AhR) in Experimental Colitis Models. J. Inflamm. Res. 2023, 16, 5845–5864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Guo, X.; Li, X.; Zhang, R.; Wang, M.; Zhu, W.; Yu, K. The gut microbial metabolite indole-3-aldehyde alleviates impaired intestinal development by promoting intestinal stem cell expansion in weaned piglets. J. Anim. Sci. Biotechnol. 2024, 15, 150. [Google Scholar] [CrossRef]
- de Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Tuomainen, M.; Lindström, J.; Lehtonen, M.; Auriola, S.; Pihlajamäki, J.; Peltonen, M.; Tuomilehto, J.; Uusitupa, M.; de Mello, V.D.; Hanhineva, K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr. Diabetes 2018, 8, 35. [Google Scholar] [CrossRef]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic Bacterial Metabolites Regulate Gastrointestinal Barrier Function via the Xenobiotic Sensor PXR and Toll-like Receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021, 69, 1487–1495. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.-X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef]
- Dong, T.S.; Mayer, E.A.; Osadchiy, V.; Chang, C.; Katzka, W.; Lagishetty, V.; Gonzalez, K.; Kalani, A.; Stains, J.; Jacobs, J.P.; et al. A Distinct Brain-Gut-Microbiome Profile Exists for Females with Obesity and Food Addiction. Obesity 2020, 28, 1477–1486. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Zhang, F.; Zhong, S.; Sun, Y.; Huo, J.; Zhu, J.; Wu, C.; Manichanh, C. The Gut Microbiota-Produced Indole-3-Propionic Acid Confers the Antihyperlipidemic Effect of Mulberry-Derived 1-Deoxynojirimycin. mSystems 2020, 5, e00313-20. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch. Physiol. Biochem. 2018, 124, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, B.; Hu, Y.; Zhao, Y. New Insights Into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases. Front. Pharmacol. 2021, 12, 769501. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Aldrich, C.C.; Sherman, D.H. Molecular Analysis of the Rebeccamycin L-Amino Acid Oxidase from Lechevalieria aerocolonigenes ATCC 39243. J. Bacteriol. 2005, 187, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, Q.; Yi, H.; Kuang, T.; Tang, Y.; Fan, G. Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Biomed. Pharmacother. 2022, 149, 112839. [Google Scholar] [CrossRef]
- Averina, O.V.; Zorkina, Y.A.; Yunes, R.A.; Kovtun, A.S.; Ushakova, V.M.; Morozova, A.Y.; Kostyuk, G.P.; Danilenko, V.N.; Chekhonin, V.P. Bacterial Metabolites of Human Gut Microbiota Correlating with Depression. Int. J. Mol. Sci. 2020, 21, 9234. [Google Scholar] [CrossRef] [PubMed]
- Özoğul, F. Production of biogenic amines by Morganella morganii, Klebsiella pneumoniae and Hafnia alvei using a rapid HPLC method. Eur. Food Res. Technol. 2004, 219, 465–469. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Hu, A.; Zaongo, S.D.; Harypursat, V.; Wang, X.; Ouyang, J.; Chen, Y. HIV-associated neurocognitive disorder: Key implications of the microbiota-gut-brain axis. Front. Microbiol. 2024, 15, 1428239. [Google Scholar] [CrossRef]
- Al-Zoairy, R.; Pedrini, M.T.; Khan, M.I.; Engl, J.; Tschoner, A.; Ebenbichler, C.; Gstraunthaler, G.; Salzmann, K.; Bakry, R.; Niederwanger, A. Serotonin improves glucose metabolism by Serotonylation of the small GTPase Rab4 in L6 skeletal muscle cells. Diabetol. Metab. Syndr. 2017, 9, 1. [Google Scholar] [CrossRef]
- Derkach, K.V.; Bondareva, V.M.; Chistyakova, O.V.; Berstein, L.M.; Shpakov, A.O. The Effect of Long-Term Intranasal Serotonin Treatment on Metabolic Parameters and Hormonal Signaling in Rats with High-Fat Diet/Low-Dose Streptozotocin-Induced Type 2 Diabetes. Int. J. Endocrinol. 2015, 2015, 245459. [Google Scholar] [CrossRef]
- Paulmann, N.; Grohmann, M.; Voigt, J.P.; Bert, B.; Vowinckel, J.; Bader, M.; Skelin, M.; Jevšek, M.; Fink, H.; Rupnik, M.; et al. Intracellular Serotonin Modulates Insulin Secretion from Pancreatic β-Cells by Protein Serotonylation. PLoS Biol. 2009, 7, e1000229. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayyar, A.; Hammad, M.M.; Williams, M.R.; Al-Onaizi, M.; Abubaker, J.; Alzaid, F. Neurotransmitters in Type 2 Diabetes and the Control of Systemic and Central Energy Balance. Metabolites 2023, 13, 384. [Google Scholar] [CrossRef]
- Cai, Y.; Li, X.; Zhou, H.; Zhou, J. The serotonergic system dysfunction in diabetes mellitus. Front. Cell. Neurosci. 2022, 16, 899069. [Google Scholar] [CrossRef]
- Hara, K.; Hirowatari, Y.; Shimura, Y.; Takahashi, H. Serotonin levels in platelet-poor plasma and whole blood in people with type 2 diabetes with chronic kidney disease. Diabetes Res. Clin. Pract. 2011, 94, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Oh, C.; Kim, H. Serotonin in the regulation of systemic energy metabolism. J. Diabetes Investig. 2022, 13, 1639–1645. [Google Scholar] [CrossRef]
- Watanabe, H.; Saito, R.; Nakano, T.; Takahashi, H.; Takahashi, Y.; Sumiyoshi, K.; Sato, K.; Chen, X.; Okada, N.; Iwasaki, S.; et al. Effect of Peripheral 5-HT on Glucose and Lipid Metabolism in Wether Sheep. PLoS ONE 2014, 9, e88058. [Google Scholar] [CrossRef] [PubMed]
- Shaibe, E.; Metzer, E.; Halpern, Y.S. Metabolic pathway for the utilization of L-arginine, L-ornithine, agmatine, and putrescine as nitrogen sources in Escherichia coli K-12. J. Bacteriol. 1985, 163, 933–937. [Google Scholar] [CrossRef]
- Smith, D.K.; Kassam, T.; Singh, B.; Elliott, J.F. Escherichia coli has two homologous glutamate decarboxylase genes that map to distinct loci. J. Bacteriol. 1992, 174, 5820–5826. [Google Scholar] [CrossRef] [PubMed]
- Otaru, N.; Ye, K.; Mujezinovic, D.; Berchtold, L.; Constancias, F.; Cornejo, F.A.; Krzystek, A.; de Wouters, T.; Braegger, C.; Lacroix, C.; et al. GABA Production by Human Intestinal Bacteroides spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance. Front. Microbiol. 2021, 12, 656895. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of Gamma-Aminobutyric Acid from Lactic Acid Bacteria: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef]
- Cotter, P.D.; Gahan, C.G.M.; Hill, C. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol. Microbiol. 2001, 40, 465–475. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2017, 29, e12904. [Google Scholar] [CrossRef]
- Rodriguez, M.T.; McLaurin, K.A.; Shtutman, M.; Kubinak, J.L.; Mactutus, C.F.; Booze, R.M. Therapeutically targeting the consequences of HIV-1-associated gastrointestinal dysbiosis: Implications for neurocognitive and affective alterations. Pharmacol. Biochem. Behav. 2023, 229, 173592. [Google Scholar] [CrossRef]
- Sarnobat, D.; Charlotte Moffett, R.; Flatt, P.R.; Irwin, N.; Tarasov, A.I. GABA and insulin but not nicotinamide augment α- to β-cell transdifferentiation in insulin-deficient diabetic mice. Biochem. Pharmacol. 2022, 199, 115019. [Google Scholar] [CrossRef]
- Barakat, H.; Aljutaily, T. Role of γ-Aminobutyric Acid (GABA) as an Inhibitory Neurotransmitter in Diabetes Management: Mechanisms and Therapeutic Implications. Biomolecules 2025, 15, 399. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, Y.; Yang, F.; Deng, H.; Wang, Y.; Yuan, L. Angiotensin-converting enzyme 2 improves hepatic insulin resistance by regulating GABAergic signaling in the liver. J. Biol. Chem. 2022, 298, 102603. [Google Scholar] [CrossRef]
- van Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin signalling and GLUT4 trafficking in insulin resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef]
- Groeger, M.; Matsuo, K.; Heidary Arash, E.; Pereira, A.; Le Guillou, D.; Pino, C.; Telles-Silva, K.A.; Maher, J.J.; Hsiao, E.C.; Willenbring, H. Modeling and therapeutic targeting of inflammation-induced hepatic insulin resistance using human iPSC-derived hepatocytes and macrophages. Nat. Commun. 2023, 14, 3902. [Google Scholar] [CrossRef]
- Freeland, K.R.; Wolever, T.M.S. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-α. Br. J. Nutr. 2010, 103, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.P.; Zhang, Y.N.; Li, Y.; Gu, L.T.; Sun, H.H.; Liu, M.D.; Zhou, H.L.; Wang, Y.S.; Xu, Z.X.; et al. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Liu, T.; Song, X.; An, Y.; Wu, X.; Zhang, W.; Li, J.; Sun, Y.; Jin, G.; Liu, X.; Guo, Z.; et al. Lactobacillus rhamnosus GG Colonization in Early Life Ameliorates Inflammaging of Offspring by Activating SIRT1/AMPK/PGC-1α Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 3328505. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell. Mol. Life Sci. 2010, 67, 3407–3423. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Münte, E.; Hartmann, P. The Role of Short-Chain Fatty Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease and Other Metabolic Diseases. Biomolecules 2025, 15, 469. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, J.; Yuan, Y.; Chen, J.; Cheng, S.; Wang, H.; Xu, Y. Sodium butyrate mitigates type 2 diabetes by inhibiting PERK-CHOP pathway of endoplasmic reticulum stress. Environ. Toxicol. Pharmacol. 2018, 64, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Castellanos, J.F.; Serrano-Villar, S.; Latorre, A.; Artacho, A.; Ferrús, M.L.; Madrid, N.; Vallejo, A.; Sainz, T.; Martínez-Botas, J.; Ferrando-Martínez, S.; et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 2015, 8, 760–772. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, Y.; Lin, P.; Tang, Y.-W.; Yang, L.; Shen, Y.; Zhang, R.; Liu, L.; Cheng, J.; Shao, J.; et al. Fecal bacterial microbiome diversity in chronic HIV-infected patients in China. Emerg. Microbes Infect. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Dinh, D.M.; Volpe, G.E.; Duffalo, C.; Bhalchandra, S.; Tai, A.K.; Kane, A.V.; Wanke, C.A.; Ward, H.D. Intestinal Microbiota, Microbial Translocation, and Systemic Inflammation in Chronic HIV Infection. J. Infect. Dis. 2015, 211, 19–27. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Vázquez-Castellanos, J.F.; Vallejo, A.; Latorre, A.; Sainz, T.; Ferrando-Martínez, S.; Rojo, D.; Martínez-Botas, J.; del Romero, J.; Madrid, N.; et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol. 2017, 10, 1279–1293. [Google Scholar] [CrossRef]
- Dillon, S.M.; Kibbie, J.; Lee, E.J.; Guo, K.; Santiago, M.L.; Austin, G.L.; Gianella, S.; Landay, A.L.; Donovan, A.M.; Frank, D.N.; et al. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS 2017, 31, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Kantor, B.; Ma, H.; Webster-Cyriaque, J.; Monahan, P.E.; Kafri, T. Epigenetic activation of unintegrated HIV-1 genomes by gut-associated short chain fatty acids and its implications for HIV infection. Proc. Natl. Acad. Sci. USA 2009, 106, 18786–18791. [Google Scholar] [CrossRef] [PubMed]
- Baltazar-Díaz, T.A.; Andrade-Villanueva, J.F.; Sánchez-Álvarez, P.; Amador-Lara, F.; Holguín-Aguirre, T.; Sánchez-Reyes, K.; Álvarez-Zavala, M.; López-Roa, R.I.; Bueno-Topete, M.R.; González-Hernández, L.A. A Two-Faced Gut Microbiome: Butyrogenic and Proinflammatory Bacteria Predominate in the Intestinal Milieu of People Living with HIV from Western Mexico. Int. J. Mol. Sci. 2024, 25, 4830. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? eBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Zhai, L.; Wu, J.; Lam, Y.Y.; Kwan, H.Y.; Bian, Z.X.; Wong, H.L.X. Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 12846. [Google Scholar] [CrossRef]
- Cummings, N.E.; Williams, E.M.; Kasza, I.; Konon, E.N.; Schaid, M.D.; Schmidt, B.A.; Poudel, C.; Sherman, D.S.; Yu, D.; Apelo, S.I.A.; et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 2018, 596, 623–645. [Google Scholar] [CrossRef]
- Ramzan, I.; Ardavani, A.; Vanweert, F.; Mellett, A.; Atherton, P.J.; Idris, I. The Association between Circulating Branched Chain Amino Acids and the Temporal Risk of Developing Type 2 Diabetes Mellitus: A Systematic Review & Meta-Analysis. Nutrients 2022, 14, 4411. [Google Scholar] [CrossRef]
- Moghei, M.; Tavajohi-Fini, P.; Beatty, B.; Adegoke, O.A.J. Ketoisocaproic acid, a metabolite of leucine, suppresses insulin-stimulated glucose transport in skeletal muscle cells in a BCAT2-dependent manner. Am. J. Physiol.-Cell Physiol. 2016, 311, C518–C527. [Google Scholar] [CrossRef]
- Giesbertz, P.; Daniel, H. Branched-chain amino acids as biomarkers in diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag Complex Targets mTORC1 to the Lysosomal Surface and Is Necessary for Its Activation by Amino Acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Li, K.; Wei, J.; Lin, Y.; Liu, Y. The contradictory role of branched-chain amino acids in lifespan and insulin resistance. Front. Nutr. 2023, 10, 1189982. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.R.; Rawls, J.F. Essential Amino Acid Metabolites as Chemical Mediators of Host-Microbe Interaction in the Gut. Annu. Rev. Microbiol. 2023, 77, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yue, C.; Zhang, H.; Chen, H.; Liu, Y.; Li, J. Deoxycholic Acid and Lithocholic Acid Alleviate Liver Injury and Inflammation in Mice with Klebsiella pneumoniae-Induced Liver Abscess and Bacteremia. J. Inflamm. Res. 2021, 14, 777–789. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, L.; Hu, X.; Wang, X.; Xu, F.; Chen, B.; Liang, X.; Xia, J.; Wang, P.; Aibara, D.; et al. Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J. Clin. Investig. 2021, 131, e142865. [Google Scholar] [CrossRef]
- Hou, Y.; Zhai, X.; Wang, X.; Wu, Y.; Wang, H.; Qin, Y.; Han, J.; Meng, Y. Research progress on the relationship between bile acid metabolism and type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2023, 15, 235. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Li, C.; Xie, Z.; Dai, L. Amelioration of Colitis by a Gut Bacterial Consortium Producing Anti-Inflammatory Secondary Bile Acids. Microbiol. Spectr. 2023, 11, e0333022. [Google Scholar] [CrossRef]
- Taylor, B.C.; Weldon, K.C.; Ellis, R.J.; Franklin, D.; Groth, T.; Gentry, E.C.; Tripathi, A.; McDonald, D.; Humphrey, G.; Bryant, M.; et al. Depression in Individuals Coinfected with HIV and HCV Is Associated with Systematic Differences in the Gut Microbiome and Metabolome. mSystems 2020, 5, e00465-20. [Google Scholar] [CrossRef]
- Duboc, H.; Rajca, S.; Rainteau, D.; Benarous, D.; Maubert, M.-A.; Quervain, E.; Thomas, G.; Barbu, V.; Humbert, L.; Despras, G.; et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut 2013, 62, 531–539. [Google Scholar] [CrossRef]
- Chong, S.; Lin, M.; Chong, D.; Jensen, S.; Lau, N.S. A systematic review on gut microbiota in type 2 diabetes mellitus. Front. Endocrinol. 2025, 15, 1486793. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, S.; Dai, H.; Xuan, L.; Deng, C.; Wang, T.; Zhao, Z.; Li, M.; Lu, J.; Xu, Y.; et al. Serum total bile acids associate with risk of incident type 2 diabetes and longitudinal changes in glucose-related metabolic traits. J. Diabetes 2020, 12, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine-N-Oxide. mBio 2015, 6, e02481-14. [Google Scholar] [CrossRef]
- Oellgaard, J.; Winther, S.A.; Hansen, T.S.; Rossing, P.; von Scholten, B.J. Trimethylamine N-oxide (TMAO) as a New Potential Therapeutic Target for Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23, 3699–3712. [Google Scholar] [CrossRef]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z.; et al. Gut microbe–generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes. Rev. 2019, 20, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Kalagi, N.A.; Thota, R.N.; Stojanovski, E.; Alburikan, K.A.; Garg, M.L. Association between Plasma Trimethylamine N-Oxide Levels and Type 2 Diabetes: A Case Control Study. Nutrients 2022, 14, 2093. [Google Scholar] [CrossRef]
- Dehghan, P.; Farhangi, M.A.; Nikniaz, L.; Nikniaz, Z.; Asghari-Jafarabadi, M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: An exploratory systematic review and dose-response meta- analysis. Obes. Rev. 2020, 21, e12993. [Google Scholar] [CrossRef]
- Fu, B.C.; Hullar, M.A.; Randolph, T.W.; Franke, A.A.; Monroe, K.R.; Cheng, I.; Wilkens, L.R.; Shepherd, J.A.; Madeleine, M.M.; Le Marchand, L.; et al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am. J. Clin. Nutr. 2020, 111, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019, 30, 1141–1151.e5. [Google Scholar] [CrossRef]
- Fang, Q.; Zheng, B.; Liu, N.; Liu, J.; Liu, W.; Huang, X.; Zeng, X.; Chen, L.; Li, Z.; Ouyang, D. Trimethylamine N-Oxide Exacerbates Renal Inflammation and Fibrosis in Rats with Diabetic Kidney Disease. Front. Physiol. 2021, 12, 682482. [Google Scholar] [CrossRef]
- Velasquez, M.; Ramezani, A.; Manal, A.; Raj, D. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Haissman, J.M.; Haugaard, A.K.; Ostrowski, S.R.; Berge, R.K.; Hov, J.R.; Trøseid, M.; Nielsen, S.D. Microbiota-dependent metabolite and cardiovascular disease marker trimethylamine-N-oxide (TMAO) is associated with monocyte activation but not platelet function in untreated HIV infection. BMC Infect. Dis. 2017, 17, 445. [Google Scholar] [CrossRef]
- Miller, P.E.; Haberlen, S.A.; Brown, T.T.; Margolick, J.B.; DiDonato, J.A.; Hazen, S.L.; Witt, M.D.; Kingsley, L.A.D.; Palella, F.J.J.; Budoff, M.; et al. Brief Report. JAIDS J. Acquir. Immune Defic. Syndr. 2016, 72, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, S.; Fitch, K.V.; Lo, J.; Kadar, H.; Knight, R.; Wong, K.; Abbara, S.; Gauguier, D.; Capeau, J.; Boccara, F.; et al. Plaque burden in HIV-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS 2015, 29, 443–452. [Google Scholar] [CrossRef]
- Li, S.Y.; Chen, S.; Lu, X.T.; Fang, A.-P.; Chen, Y.-M.; Huang, R.-Z.; Lin, X.-L.; Huang, Z.-H.; Ma, J.-F.; Huang, B.-X.; et al. Serum trimethylamine-N-oxide is associated with incident type 2 diabetes in middle-aged and older adults: A prospective cohort study. J. Transl. Med. 2022, 20, 374. [Google Scholar] [CrossRef]
- Kaminskas, E.; Kimhi, Y.; Magasanik, B. Urocanase and N-Formimino-l-glutamate Formiminohydrolase of Bacillus subtilis, Two Enzymes of the Histidine Degradation Pathway. J. Biol. Chem. 1970, 245, 3536–3544. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961.e17. [Google Scholar] [CrossRef]
- Molinaro, A.; Bel Lassen, P.; Henricsson, M.; Wu, H.; Adriouch, S.; Belda, E.; Chakaroun, R.; Nielsen, T.; Bergh, P.-O.; Rouault, C.; et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat. Commun. 2020, 11, 5881. [Google Scholar] [CrossRef]
- Trøseid, M.; Molinaro, A.; Gelpi, M.; Vestad, B.; Kofoed, K.F.; Fuchs, A.; Køber, L.; Holm, K.; Benfield, T.; Ueland, P.M.; et al. Gut Microbiota Alterations and Circulating Imidazole Propionate Levels Are Associated with Obstructive Coronary Artery Disease in People with HIV. J. Infect. Dis. 2024, 229, 898–907. [Google Scholar] [CrossRef]
- Wang, Z.; Peters, B.A.; Bryant, M.; Hanna, D.B.; Schwartz, T.; Wang, T.; Sollecito, C.C.; Usyk, M.; Grassi, E.; Wiek, F.; et al. Gut microbiota, circulating inflammatory markers and metabolites, and carotid artery atherosclerosis in HIV infection. Microbiome 2023, 11, 119. [Google Scholar] [CrossRef]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef]
- Garber, A.J. Incretin Effects on β-Cell Function, Replication, and Mass. Diabetes Care 2011, 34 (Suppl. S2), S258–S263. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Xin, F.Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.-L.; Cui, A.; Liu, Z.; et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Marques, A.M.; Sarandy, M.M.; Novaes, R.D.; Gonçalves, R.V.; Freitas, M.B. Preclinical relevance of probiotics in type 2 diabetes: A systematic review. Int. J. Exp. Pathol. 2020, 101, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, E.; Liu, C.; Wicks, H.; Yildiz, S.; Razack, F.; Ying, Z.; Kooijman, S.; Koonen, D.P.; Heijink, M.; et al. Dietary butyrate ameliorates metabolic health associated with selective proliferation of gut Lachnospiraceae bacterium 28-4. JCI Insight 2023, 8, e166655. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Sun, D.; Li, X.; DiDonato, J.A.; A Bray, G.; Sacks, F.M.; Qi, L. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: The POUNDS Lost trial. Gut 2019, 68, 263–270. [Google Scholar] [CrossRef] [PubMed]
| Metabolites | Main Microbial Sources | Key Metabolic Pathways | Relevance in HIV | Relevance in T2D |
|---|---|---|---|---|
| Kynurenine pathway-derived metabolites | Pseudomonas, Xanthomonas, Burkholderia, Stenotrophomonas, and Shewanella, and members of the genus Bacillus | Multiple pathways promoting oxidative stress, chronic immune activation, and inflammation | Associated with disease progression, CD4+ T cell depletion, and systemic immune dysfunction. An imbalance between Th17 and Treg cells exacerbates immunosuppression and intestinal epithelial damage | Linked to increased IR through chronic low-grade inflammation, oxidative stress, and decreased tryptophan availability for the synthesis of protective metabolites |
| Indole Pathway-derived metabolites | Roseburia, Eubacterium, Lachnospira, Coprobacter Peptostreptococcus russellii, Lactobacillus spp., Clostridium sporogenes, and Clostridium paraputrificum | Acts on AhR and PXR receptors, modulating immune functions, intestinal barrier integrity, and cytokine production | Reduced production is associated with decreased IL-12, impaired intestinal epithelial barrier integrity, and dysbiosis | Protective metabolite associated with lower fasting glucose, enhanced insulin secretion, and improved insulin sensitivity. Levels are reduced in T2D |
| Serotonin | Streptococcus, Escherichia, Enterococcus, Hafnia alvei, Klebsiella pneumoniae, Lactobacillus plantarum, Morganella morganii, Akkermansia, Alistipes, and Roseburia | - Serotonylation of specific GTPases enhances GLUT4 translocation, increases insulin secretion, and inhibits glucagon release | An imbalance of GM significantly affects serotonin production | Results are contradictory |
| - Can induce hyperglycemia through adrenaline release and alter glycogen synthesis | ||||
| GABA | Escherichia coli, Listeria monocytogenes, Bifidobacterium spp., Bacteroides spp., and lactic acid bacteria like Lactobacillus, Lactococcus, and Streptococcus | Acts on specific type A receptors, modulating glucose-induced insulin secretion. Activates the PI3K/AKT pathway, enhancing IRS1 and GLUT4 expression | Exhibits anti-inflammatory effects. HIV is associated with reduced abundance of GABA-producing intestinal bacteria | Increase insulin sensitivity and secretion. Reduces hepatic glucose production and lipid accumulation |
| SCFAs | Faecalibacterium, Roseburia, Coprococcus, and Eubacterium | Activates GPCRs, inhibits HDAC2, stimulates PPAR activity for fatty acid oxidation, and modulates key metabolic regulators (ATGL, UCPs) | Significant decrease in SCFA-producing bacteria, which may compromise intestinal barrier function and contribute to microbial translocation and chronic immune activation characteristic of the infection | Enhances intestinal barrier and hormone secretion (GLP-1, PYY, leptin), improves insulin sensitivity, glucose uptake, and lipid metabolism, and preserves β-cell mass |
| BCAAs | Prevotella copri, Bacteroides vulgatus, and Clostridium sporogenes | Incomplete catabolism of BCAAs leads to the accumulation of intermediate metabolites such as α-ketoisocaproic acid. BCAAs can continuously activate the mTORC1 cellular pathway, which negatively affects the function of IRS1 | Strong and relevant association with intestinal dysbiosis and IR, suggesting a significant impact on metabolic pathophysiology and chronic inflammation in the context of HIV | Elevated BCAAs are associated with IR, impaired glucose uptake, and lipotoxicity. Accumulation of catabolic intermediates disrupts insulin signaling. Gut bacteria modulate circulating BCAA levels, influencing T2D risk |
| Bile Acids (Primary and Secondary) | Lactobacillus, Bifidobacterium, Staphylococcus, Clostridium perfringens and members of the Lachnospiraceae y Ruminococcaceae | FXR/TGR5 activation: improves glucose metabolism, insulin sensitivity, appetite regulation, and thermogenesis; DCA cytotoxic at high levels; LCA protective | Elevated concentrations of primary and secondary BAs in PLWHIV with chronic hepatitis C virus coinfection and a history of depression, related to significant alterations in intestinal microbiota composition. Much remains to be investigated | Trend toward higher total BA concentrations. Many results are contradictory |
| TMAO | Anaerococcus hydrogenalis, Clostridium asparagiforme, Clostridium hathewayi, Clostridium sporogenes, Escherichia fergusonii, Proteus penneri, Providencia rettgeri and Salmonella enterica | Activates PERK pathway; promotes hyperglycemia and IR; increases adipose inflammation (↑ MCP-1, ↓ IL-10); stimulates hepatic gluconeogenesis; implicated in renal fibrosis and NLRP3 inflammasome activation | Many studies have reported higher TMAO levels, especially associated with ART, but this has not been consistent in other research | TMAO has been widely studied for its potential role in the development and progression of disease. Elevated levels of this metabolite have been associated with IR, impaired glucose tolerance, and increased systemic inflammation |
| ImP | Clostridium baumannii, Clostridium parasymbiotics, Ruminococcus gnavus, and Veillonella | Activates MAPK p38γ, which triggers phosphorylation of the p62 protein, abnormally activating the mTORC1 pathway and inducing defective phosphorylation of IRS, promoting its degradation and thus favoring the development of IR | In PLWHIV, ImP has gained attention as a potential indicator of dysbiosis and cardiovascular risk. Several studies report consistent findings linking this metabolite to intestinal dysbiosis in HIV | Positive correlations have been reported between ImP levels, systemic inflammatory markers, and IR in people with T2D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Rodríguez, Y.L.; Anaya-Ambriz, E.J.; Méndez-Ríos, P.C.; Andrade-Villanueva, J.F.; González-Hernández, L.A.; Holguín-Aguirre, T.E.; Martínez-Ayala, P.; Ruiz-Herrera, V.V.; Alvarez-Zavala, M.; Sánchez-Reyes, K. The Impact of Gut Microbial Metabolomics on Type 2 Diabetes Development in People Living with HIV. Metabolites 2025, 15, 627. https://doi.org/10.3390/metabo15090627
Díaz-Rodríguez YL, Anaya-Ambriz EJ, Méndez-Ríos PC, Andrade-Villanueva JF, González-Hernández LA, Holguín-Aguirre TE, Martínez-Ayala P, Ruiz-Herrera VV, Alvarez-Zavala M, Sánchez-Reyes K. The Impact of Gut Microbial Metabolomics on Type 2 Diabetes Development in People Living with HIV. Metabolites. 2025; 15(9):627. https://doi.org/10.3390/metabo15090627
Chicago/Turabian StyleDíaz-Rodríguez, Yusnier Lázaro, Elsa Janneth Anaya-Ambriz, Paula Catalina Méndez-Ríos, Jaime F. Andrade-Villanueva, Luz A. González-Hernández, Tania Elisa Holguín-Aguirre, Pedro Martínez-Ayala, Vida V. Ruiz-Herrera, Monserrat Alvarez-Zavala, and Karina Sánchez-Reyes. 2025. "The Impact of Gut Microbial Metabolomics on Type 2 Diabetes Development in People Living with HIV" Metabolites 15, no. 9: 627. https://doi.org/10.3390/metabo15090627
APA StyleDíaz-Rodríguez, Y. L., Anaya-Ambriz, E. J., Méndez-Ríos, P. C., Andrade-Villanueva, J. F., González-Hernández, L. A., Holguín-Aguirre, T. E., Martínez-Ayala, P., Ruiz-Herrera, V. V., Alvarez-Zavala, M., & Sánchez-Reyes, K. (2025). The Impact of Gut Microbial Metabolomics on Type 2 Diabetes Development in People Living with HIV. Metabolites, 15(9), 627. https://doi.org/10.3390/metabo15090627







