Dietary Intervention with Cottonseed and Olive Oil Differentially Affect the Circulating Lipidome and Immunoregulatory Compounds—A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Experimental Design
2.3. Dietary Intervention and Adherence
2.4. Blood Sample Collection
2.5. Fatty Acid Analysis of Oil Samples via Mass Spectrometry
2.6. Untargeted Metabolomic Analysis of Plasma
2.7. Targeted Lipid Mediator (LM) and Total Bulk Lipidomic (TBL) Analyses
2.8. Network Analysis
3. Results
3.1. Participant Characteristics
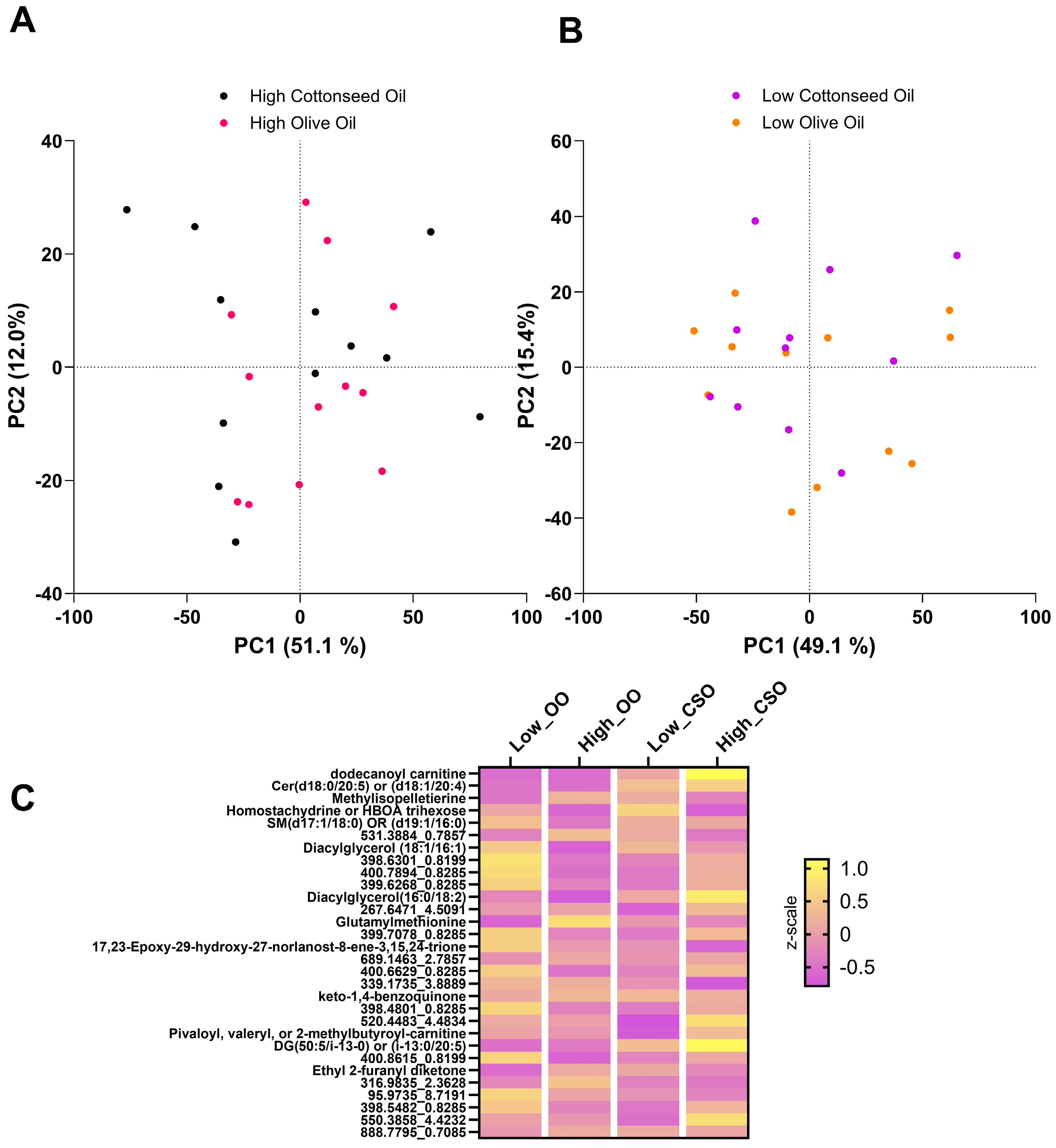
3.2. Impact of CSO and OO on Global Metabolic Status
3.3. Fatty Acid Composition of CSO and OO
3.4. Impact of CSO and OO on Lipidomic Profiles
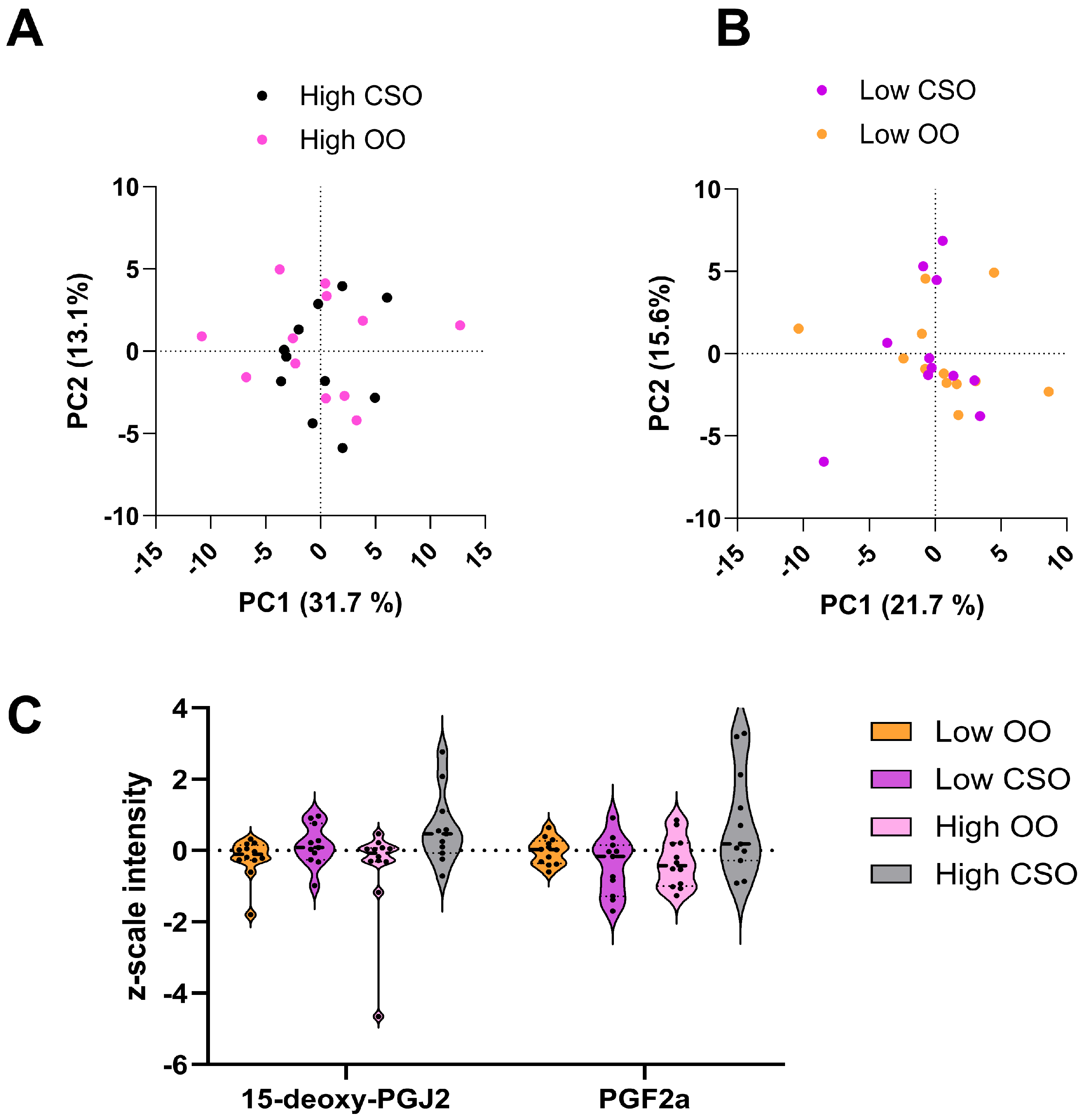
3.5. Immunoregulatory Compounds Affected by Dietary CSO and OO Interventions
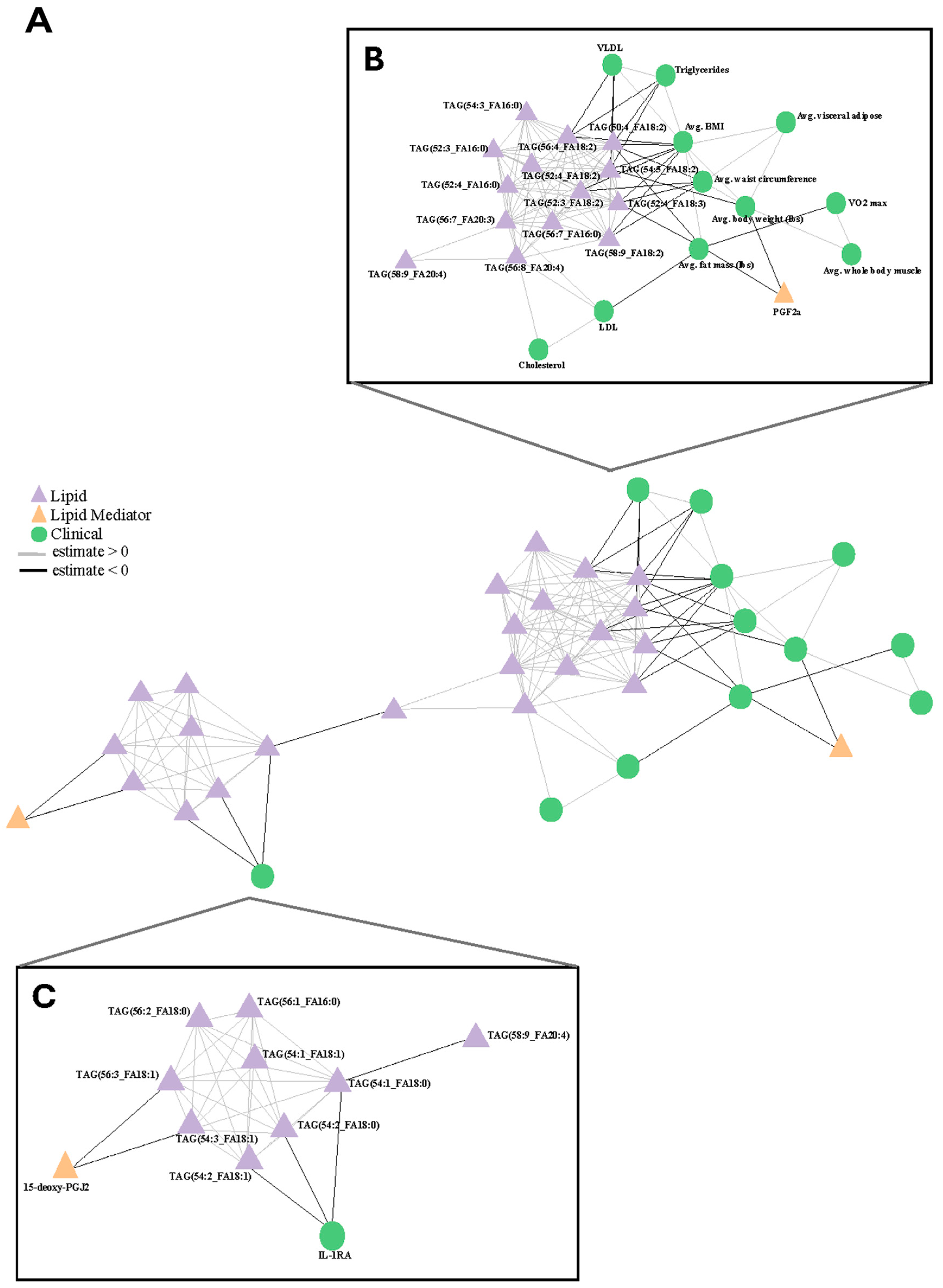
3.6. Correlations Between Oil Consumption, Clinical Measurements, and -Omics Data
4. Discussion
4.1. Metabolomic Profiles of CSO and OO Are Similar
4.2. Decreases in Clinically Relevant Lipids and Acyl Chain Composition Are Observed in CSO Participants
4.3. Immunoregulatory Lipids Are Differentially Affected by CSO and OO
4.4. Bulk Drivers of Dietary Response and Immune Response Are Observed in Response to Interventions
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarate, S.M.; Kirabo, A.; Hinton, A.O.; Santisteban, M.M. Neuroimmunology of Cardiovascular Disease. Curr. Hypertens. Rep. 2024, 26, 339–347. [Google Scholar] [CrossRef]
- Samuel, P.O.; Edo, G.I.; Emakpor, O.L.; Oloni, G.O.; Ezekiel, G.O.; Essaghah, A.E.A.; Agoh, E.; Agbo, J.J. Lifestyle modifications for preventing and managing cardiovascular diseases. Sports Sci. Health 2024, 20, 23–36. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef]
- Parkinson, L.; Keast, R. Oleocanthal, a phenolic derived from virgin olive oil: A review of the beneficial effects on inflammatory disease. Int. J. Mol. Sci. 2014, 15, 12323–12334. [Google Scholar] [CrossRef] [PubMed]
- Kien, C.L.; Bunn, J.Y.; Tompkins, C.L.; Dumas, J.A.; Crain, K.I.; Ebenstein, D.B.; Koves, T.R.; Muoio, D.M. Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood. Am. J. Clin. Nutr. 2013, 97, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, D.; Camacho-Munoz, D.; Certo, M.; Pucino, V.; Nicolaou, A.; Mauro, C. Fatty acids—From energy substrates to key regulators of cell survival, proliferation and effector function. Cell Stress. 2019, 4, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef] [PubMed]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef]
- Corsinovi, L.; Biasi, F.; Poli, G.; Leonarduzzi, G.; Isaia, G. Dietary lipids and their oxidized products in Alzheimer’s disease. Mol. Nutr. Food Res. 2011, 55, S161–S172. [Google Scholar] [CrossRef]
- Delavar, M.A.; Lye, M.S.; Khor, G.L.; Hassan, S.T.; Hanachi, P. Dietary patterns and the metabolic syndrome in middle aged women, Babol, Iran. Asia Pac. J. Clin. Nutr. 2009, 18, 285–292. [Google Scholar]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Ma, C.; Xu, Z.; Lv, H. Low n/6/ n/3 PUFA ratio improves inflammation and myocardial ischemic reperfusion injury. Biochem. Cell Biol. 2019, 97, 621–629. [Google Scholar] [CrossRef]
- Radcliffe, J.D.; Czajka-Narins, D.M.; Imrhan, V. Fatty Acid Composition of Serum, Adipose Tissue, and Liver in Rats Fed Diets Containing Corn Oil or Cottonseed Oil. Plant Foods Hum. Nutr. 2004, 59, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, J.D.; Czajka-Narins, D.M. Lipids and Tocopherols in Serum and Liver of Female Rats Fed Diets Containing Corn Oil or Cottonseed Oil. Plant Foods Hum. Nutr. 2006, 61, 33–36. [Google Scholar] [CrossRef]
- Polley, K.R.; Oswell, N.J.; Pegg, R.B.; Paton, C.M.; Cooper, J.A. A 5-day high-fat diet rich in cottonseed oil improves cholesterol profiles and triglycerides compared to olive oil in healthy men. Nutr. Res. 2018, 60, 43–53. [Google Scholar] [CrossRef]
- Davis, K.E.; Prasad, C.; Imrhan, V. Consumption of a Diet Rich in Cottonseed Oil (CSO) Lowers Total and LDL Cholesterol in Normo-Cholesterolemic Subjects. Nutrients 2012, 4, 602–610. [Google Scholar] [CrossRef]
- Prater, M.C.; Scheurell, A.R.; Paton, C.M.; Cooper, J.A. Blood Lipid Responses to Diets Enriched with Cottonseed Oil Compared With Olive Oil in Adults with High Cholesterol in a Randomized Trial. J. Nutr. 2022, 152, 2060–2071. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Garrison, R.L.; Stamatikos, A.D.; Kang, M.; Cooper, J.A.; Paton, C.M. A High Linoleic Acid Diet does not Induce Inflammation in Mouse Liver or Adipose Tissue. Lipids 2015, 50, 1115–1122. [Google Scholar] [CrossRef]
- Wu, J.H.Y.; Lemaitre, R.N.; King, I.B.; Song, X.; Psaty, B.M.; Siscovick, D.S.; Mozaffarian, D. Circulating Omega-6 Polyunsaturated Fatty Acids and Total and Cause-Specific Mortality. Circulation 2014, 130, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Bishop-Bailey, D. Lipid mediators in immune regulation and resolution. Br. J. Pharmacol. 2019, 176, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Bhupathiraju, S.N.; Hu, F.B. Use of Metabolomics in Improving Assessment of Dietary Intake. Clin. Chem. 2018, 64, 82–98. [Google Scholar] [CrossRef]
- Gika, H.; Virgiliou, C.; Theodoridis, G.; Plumb, R.S.; Wilson, I.D. Untargeted LC/MS-based metabolic phenotyping (metabonomics/metabolomics): The state of the art. J. Chromatogr. B 2019, 1117, 136–147. [Google Scholar] [CrossRef]
- Belhaj, M.R.; Lawler, N.G.; Hoffman, N.J. Metabolomics and Lipidomics: Expanding the Molecular Landscape of Exercise Biology. Metabolites 2021, 11, 151. [Google Scholar] [CrossRef]
- Carbone, F.; Bruzzaniti, S.; Fusco, C.; Colamatteo, A.; Micillo, T.; De Candia, P.; Bonacina, F.; Norata, G.D.; Matarese, G. Metabolomics, Lipidomics, and Immunometabolism. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 319–328. [Google Scholar] [CrossRef]
- Jones, D.P.; Park, Y.; Ziegler, T.R. Nutritional Metabolomics: Progress in Addressing Complexity in Diet and Health. Annu. Rev. Nutr. 2012, 32, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S. ACSM’s New Preparticipation Health Screening Recommendations from ACSM’s Guidelines for Exercise Testing and Prescription, Ninth Edition. Curr. Sports Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Jamnik, V.K.; Bredin, S.S.D.; Gledhill, N. The Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and Electronic Physical Activity Readiness Medical Examination (ePARmed-X+). Health Fit. J. Can. 2011, 4, 3–17. [Google Scholar] [CrossRef]
- USDA. Dietary Guidelines for Americans, 2020–2025; USDA: Washington, DC, USA, 2020. [Google Scholar]
- Welhaven, H.D.; Vahidi, G.; Walk, S.T.; Bothner, B.; Martin, S.A.; Heveran, C.M.; June, R.K. The Cortical Bone Metabolome of C57BL/6J Mice Is Sexually Dimorphic. JBMR Plus 2022, 6, e10654. [Google Scholar] [CrossRef]
- Schwarz, B.; Roberts, L.M.; Bohrnsen, E.; Jessop, F.; Wehrly, T.D.; Shaia, C.; Bosio, C.M. Contribution of Lipid Mediators in Divergent Outcomes following Acute Bacterial and Viral Lung Infections in the Obese Host. J. Immunol. 2022, 209, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, B.; Sharma, L.; Roberts, L.; Peng, X.; Bermejo, S.; Leighton, I.; Casanovas-Massana, A.; Minasyan, M.; Farhadian, S.; Ko, A.I.; et al. Cutting Edge: Severe SARS-CoV-2 Infection in Humans Is Defined by a Shift in the Serum Lipidome, Resulting in Dysregulation of Eicosanoid Immune Mediators. J. Immunol. 2021, 206, 329–334. [Google Scholar] [CrossRef]
- Toubiana, D.; Sade, N.; Liu, L.; Rubio Wilhelmi, M.d.M.; Brotman, Y.; Luzarowska, U.; Vogel, J.P.; Blumwald, E. Correlation-based network analysis combined with machine learning techniques highlight the role of the GABA shunt in Brachypodium sylvaticum freezing tolerance. Sci. Rep. 2020, 10, 4489. [Google Scholar] [CrossRef]
- Csardi, G.N.T. The Igraph Software Package for Complex Network Research. 2006. Available online: https://igraph.org (accessed on 1 May 2025).
- Yang, A.; Qi, M.; Wang, X.; Wang, S.; Sun, L.; Qi, D.; Zhu, L.; Duan, Y.; Gao, X.; Ali Rajput, S.; et al. Refined cottonseed oil as a replacement for soybean oil in broiler diet. Food Sci. Nutr. 2019, 7, 1027–1034. [Google Scholar] [CrossRef]
- Zhang, Z.; Tong, Z.; Shao, Y.; Su, G.; Li, K.; Li, C. Comparing and Evaluating the Oil Composition of Olive Oil of 85 Olive Varieties in the Liangshan Region, China. Agronomy 2024, 14, 304. [Google Scholar] [CrossRef]
- Vanni, S.; Riccardi, L.; Palermo, G.; De Vivo, M. Structure and Dynamics of the Acyl Chains in the Membrane Trafficking and Enzymatic Processing of Lipids. Acc. Chem. Res. 2019, 52, 3087–3096. [Google Scholar] [CrossRef]
- Barneda, D.; Janardan, V.; Niewczas, I.; Collins, D.M.; Cosulich, S.; Clark, J.; Stephens, L.R.; Hawkins, P.T. Acyl chain selection couples the consumption and synthesis of phosphoinositides. EMBO J. 2022, 41, e110038. [Google Scholar] [CrossRef]
- Basu, S. Bioactive Eicosanoids: Role of Prostaglandin F2α and F2-Isoprostanes in Inflammation and Oxidative Stress Related Pathology. Mol. Cells 2010, 30, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Pillinger, M.H. 15d-PGJ2: The anti-inflammatory prostaglandin? Clin. Immunol. 2005, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Nassar, H.; Espinosa, C.; Stelzer, I.A.; Feyaerts, D.; Berson, E.; Bidoki, N.H.; Chang, A.L.; Saarunya, G.; Culos, A.; et al. Large-scale correlation network construction for unraveling the coordination of complex biological systems. Nat. Comput. Sci. 2023, 3, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Biadgo, B.; Abebe, S.M.; Baynes, H.W.; Yesuf, M.; Alemu, A.; Abebe, M. Correlation between Serum Lipid Profile with Anthropometric and Clinical Variables in Patients with Type 2 Diabetes Mellitus. Ethiop. J. Health Sci. 2017, 27, 215–226. [Google Scholar] [CrossRef]
- Ikezaki, H.; Lim, E.; Cupples, L.A.; Liu, C.T.; Asztalos, B.F.; Schaefer, E.J. Small Dense Low-Density Lipoprotein Cholesterol Is the Most Atherogenic Lipoprotein Parameter in the Prospective Framingham Offspring Study. J. Am. Heart Assoc. 2021, 10, e019140. [Google Scholar] [CrossRef]
- Catherine Prater, M.; Polley, K.R.; Cooper, J.A. Improvements in markers of inflammation and coagulation potential following a 5-day high-fat diet rich in cottonseed oil vs. Olive oil in healthy males. Cytokine 2024, 175, 156494. [Google Scholar] [CrossRef]
- Prater, M.C.; Scheurell, A.R.; Paton, C.M.; Cooper, J.A. Metabolic responses to 8 weeks of consuming cottonseed oil versus olive oil in adults with dyslipidaemia: A randomised trial. J. Hum. Nutr. Diet. 2023, 36, 1079–1089. [Google Scholar] [CrossRef]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef]
- Akerele, O.A.; Cheema, S.K. Fatty acyl composition of lysophosphatidylcholine is important in atherosclerosis. Med. Hypotheses 2015, 85, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Kabarowski, J.H. G2A and LPC: Regulatory functions in immunity. Prostaglandins Other Lipid Mediat. 2009, 89, 73–81. [Google Scholar] [CrossRef]
- Schwenke, D.C. Antioxidants and atherogenesis. J. Nutr. Biochem. 1998, 9, 424–445. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sakurai, T.; Chen, Z.; Inoue, N.; Chiba, H.; Hui, S.-P. Lysophosphatidylethanolamine Affects Lipid Accumulation and Metabolism in a Human Liver-Derived Cell Line. Nutrients 2022, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Kolar, M.J.; McGregor, G.H.; Nelson, A.T.; Wallace, M.; Metallo, C.M. Quantifying acyl-chain diversity in isobaric compound lipids containing monomethyl branched-chain fatty acids. J. Lipid Res. 2024, 65, 100677. [Google Scholar] [CrossRef]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef]
- Eichelmann, F.; Prada, M.; Sellem, L.; Jackson, K.G.; Salas Salvadó, J.; Razquin Burillo, C.; Estruch, R.; Friedén, M.; Rosqvist, F.; Risérus, U.; et al. Lipidome changes due to improved dietary fat quality inform cardiometabolic risk reduction and precision nutrition. Nat. Med. 2024, 30, 2867–2877. [Google Scholar] [CrossRef]
- Meuronen, T.; Lankinen, M.A.; Fauland, A.; Shimizu, B.-i.; de Mello, V.D.; Laaksonen, D.E.; Wheelock, C.E.; Erkkilä, A.T.; Schwab, U.S. Intake of Camelina Sativa Oil and Fatty Fish Alter the Plasma Lipid Mediator Profile in Subjects with Impaired Glucose Metabolism—A Randomized Controlled Trial. Prostaglandins Leukot. Essent. Fat. Acids 2020, 159, 102143. [Google Scholar] [CrossRef]
- Irún, P.; Carrera-Lasfuentes, P.; Sánchez-Luengo, M.; Belio, Ú.; Domper-Arnal, M.J.; Higuera, G.A.; Hawkins, M.; de la Rosa, X.; Lanas, A. Pharmacokinetics and Changes in Lipid Mediator Profiling after Consumption of Specialized Pro-Resolving Lipid-Mediator-Enriched Marine Oil in Healthy Subjects. Int. J. Mol. Sci. 2023, 24, 6143. [Google Scholar] [CrossRef]
- Dasilva, G.; Lois, S.; Méndez, L.; Miralles-Pérez, B.; Romeu, M.; Ramos-Romero, S.; Torres, J.L.; Medina, I. Fish Oil Improves Pathway-Oriented Profiling of Lipid Mediators for Maintaining Metabolic Homeostasis in Adipose Tissue of Prediabetic Rats. Front. Immunol. 2021, 12, 608875. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; De Jesus, D.F.; Ju, C.W.; Wei, J.B.; Hu, J.; DiStefano-Forti, A.; Tsuji, T.; Cero, C.; Männistö, V.; Manninen, S.M.; et al. m(6)A mRNA methylation in brown fat regulates systemic insulin sensitivity via an inter-organ prostaglandin signaling axis independent of UCP1. Cell Metab. 2024, 36, 2207–2227.V2209. [Google Scholar] [CrossRef]
- Riaposova, L.; Kim, S.H.; Hanyaloglu, A.C.; Sykes, L.; MacIntyre, D.A.; Bennett, P.R.; Terzidou, V. Prostaglandin F2α requires activation of calcium-dependent signalling to trigger inflammation in human myometrium. Front. Endocrinol. 2023, 14, 1150125. [Google Scholar] [CrossRef] [PubMed]
- Janczura, M.; Gielicz, A.; Kotula-Horowitz, K.; Iwaniec, T.; Stanisz, A.; Rosa, R.; Dropinski, J.; Domagala, T. Plasma but not urine 8-ISO-PGF2ALFA is a predictor of insulin resistance in the middle-aged subjects—An occupational, cohort-based study. Atherosclerosis 2018, 275, e63. [Google Scholar] [CrossRef]
- Akour, A.; Farha, R.A.; Alefishat, E.; Kasabri, V.; Bulatova, N. Insulin resistance and levels of cardiovascular biomarkers in night-shift workers. Atherosclerosis 2020, 315, e117–e118. [Google Scholar] [CrossRef]
- Kim, W.; Jang, J.-H.; Zhong, X.; Seo, H.; Surh, Y.-J. 15-Deoxy-Δ12,14-Prostaglandin J2 Promotes Resolution of Experimentally Induced Colitis. Front. Immunol. 2021, 12, 615803. [Google Scholar] [CrossRef]
- Chiang, K.-M.; Chen, J.-F.; Yang, C.-A.; Xiu, L.; Yang, H.-C.; Shyur, L.-F.; Pan, W.-H. Identification of Serum Oxylipins Associated with the Development of Coronary Artery Disease: A Nested Case-Control Study. Metabolites 2022, 12, 495. [Google Scholar] [CrossRef]
- Katsumata, Y.; Shinmura, K.; Sugiura, Y.; Tohyama, S.; Matsuhashi, T.; Ito, H.; Yan, X.; Ito, K.; Yuasa, S.; Ieda, M.; et al. Endogenous Prostaglandin D2 and Its Metabolites Protect the Heart Against Ischemia–Reperfusion Injury by Activating Nrf2. Hypertension 2014, 63, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Armour, C.R.; Hu, C.; Mei, M.; Tian, C.; Sharpton, T.J.; Jiang, Y. Microbiome Multi-Omics Network Analysis: Statistical Considerations, Limitations, and Opportunities. Front. Genet. 2019, 10, 995. [Google Scholar] [CrossRef]
- Borén, J.; Taskinen, M.-R.; Björnson, E.; Packard, C.J. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat. Rev. Cardiol. 2022, 19, 577–592. [Google Scholar] [CrossRef]
- Han, F.Y.; Conboy-Schmidt, L.; Rybachuk, G.; Volk, H.A.; Zanghi, B.; Pan, Y.; Borges, K. Dietary medium chain triglycerides for management of epilepsy: New data from human, dog, and rodent studies. Epilepsia 2021, 62, 1790–1806. [Google Scholar] [CrossRef]
- Dimache, A.M.; Șalaru, D.L.; Sascău, R.; Stătescu, C. The Role of High Triglycerides Level in Predicting Cognitive Impairment: A Review of Current Evidence. Nutrients 2021, 13, 2118. [Google Scholar] [CrossRef]
- Berezhnoy, G.; Bissinger, R.; Liu, A.; Cannet, C.; Schäfer, H.; Kienzle, K.; Bitzer, M.; Häberle, H.; Göpel, S.; Trautwein, C.; et al. Maintained imbalance of triglycerides, apolipoproteins, energy metabolites and cytokines in long-term COVID-19 syndrome patients. Front. Immunol. 2023, 14, 1144224. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P.; Malyak, M.; Guthridge, C.J.; Gabay, C. Interleukin-1 receptor antagonist: Role in biology. Annu. Rev. Immunol. 1998, 16, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor. Rev. 2002, 13, 323–340. [Google Scholar] [CrossRef]
- McGillicuddy, F.C.; Harford, K.A.; Reynolds, C.M.; Oliver, E.; Claessens, M.; Mills, K.H.G.; Roche, H.M. Lack of Interleukin-1 Receptor I (IL-1RI) Protects Mice From High-Fat Diet–Induced Adipose Tissue Inflammation Coincident With Improved Glucose Homeostasis. Diabetes 2011, 60, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Luotola, K. IL-1 Receptor Antagonist (IL-1Ra) Levels and Management of Metabolic Disorders. Nutrients 2022, 14, 3422. [Google Scholar] [CrossRef] [PubMed]
- Salomaa, V.; Havulinna, A.; Saarela, O.; Zeller, T.; Jousilahti, P.; Jula, A.; Muenzel, T.; Aromaa, A.; Evans, A.; Kuulasmaa, K.; et al. Thirty-One Novel Biomarkers as Predictors for Clinically Incident Diabetes. PLoS ONE 2010, 5, e10100. [Google Scholar] [CrossRef] [PubMed]
- Paton, C.M.; Vaughan, R.A.; Selen Alpergin, E.S.; Assadi-Porter, F.; Dowd, M.K. Dihydrosterculic acid from cottonseed oil suppresses desaturase activity and improves liver metabolomic profiles of high-fat-fed mice. Nutr. Res. 2017, 45, 52–62. [Google Scholar] [CrossRef]

| Participants | Age (years) | BMI (kg/m2) | WC (cm) | VAT (L) | Glucose (mmol/L) | Triglycerides (mmol/L) |
|---|---|---|---|---|---|---|
| All (n = 47) | 31.49 ± 11.83 | 23.13 ± 1.98 | 77.40 ± 7.67 | 0.71 ± 0.57 | 5.3 ± 0.4 | 0.93 ± 0.33 |
| Male (n = 24) | 31.25 ± 12.25 | 23.58 ± 1.91 | 80.55 ± 6.86 | 0.95 ± 0.58 | 5.4 ± 0.4 | 1.01 ± 0.4 |
| Female (n = 23) | 31.74 ± 11.38 | 22.66 ± 1.94 | 74.12 ± 7.07 | 0.46 ± 0.44 | 5.2 ± 0.3 | 0.84 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, G.; Bhattarai, P.; Sather, B.; Bailey, M.L.; Chamberlin, M.; Miles, M.; Bothner, B. Dietary Intervention with Cottonseed and Olive Oil Differentially Affect the Circulating Lipidome and Immunoregulatory Compounds—A Randomized Clinical Trial. Metabolites 2025, 15, 599. https://doi.org/10.3390/metabo15090599
Cooper G, Bhattarai P, Sather B, Bailey ML, Chamberlin M, Miles M, Bothner B. Dietary Intervention with Cottonseed and Olive Oil Differentially Affect the Circulating Lipidome and Immunoregulatory Compounds—A Randomized Clinical Trial. Metabolites. 2025; 15(9):599. https://doi.org/10.3390/metabo15090599
Chicago/Turabian StyleCooper, Gwendolyn, Prabina Bhattarai, Brett Sather, Marguerite L. Bailey, Morgan Chamberlin, Mary Miles, and Brian Bothner. 2025. "Dietary Intervention with Cottonseed and Olive Oil Differentially Affect the Circulating Lipidome and Immunoregulatory Compounds—A Randomized Clinical Trial" Metabolites 15, no. 9: 599. https://doi.org/10.3390/metabo15090599
APA StyleCooper, G., Bhattarai, P., Sather, B., Bailey, M. L., Chamberlin, M., Miles, M., & Bothner, B. (2025). Dietary Intervention with Cottonseed and Olive Oil Differentially Affect the Circulating Lipidome and Immunoregulatory Compounds—A Randomized Clinical Trial. Metabolites, 15(9), 599. https://doi.org/10.3390/metabo15090599







