Metabolomic Variation in Sugarcane Maturation Under a Temperate Climate
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Preparation
2.3. Sample Pretreatments for NMR Measurements
2.4. NMR Measurements
2.5. Data Analysis
3. Results
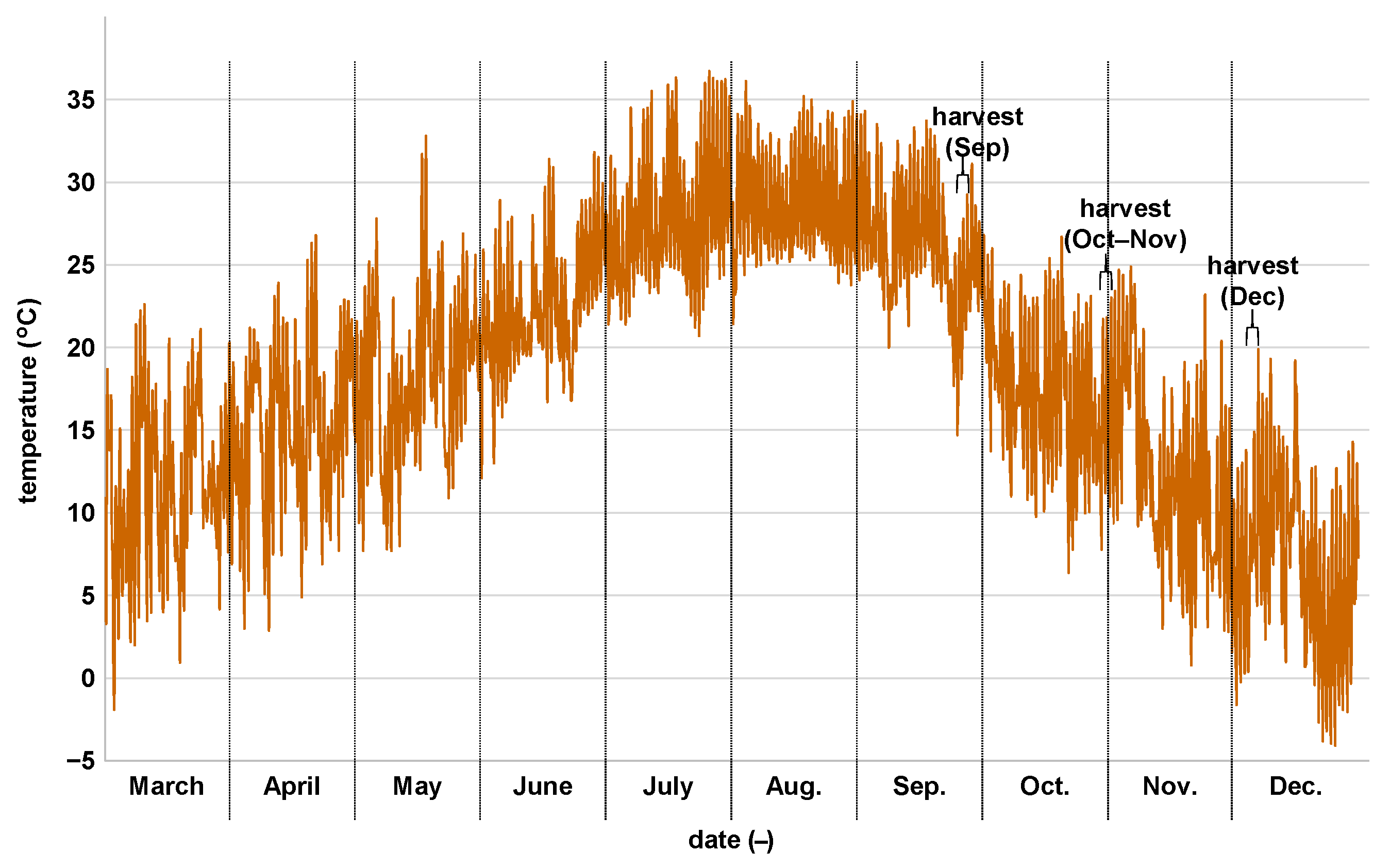
3.1. Growth Characteristics of Sugarcane
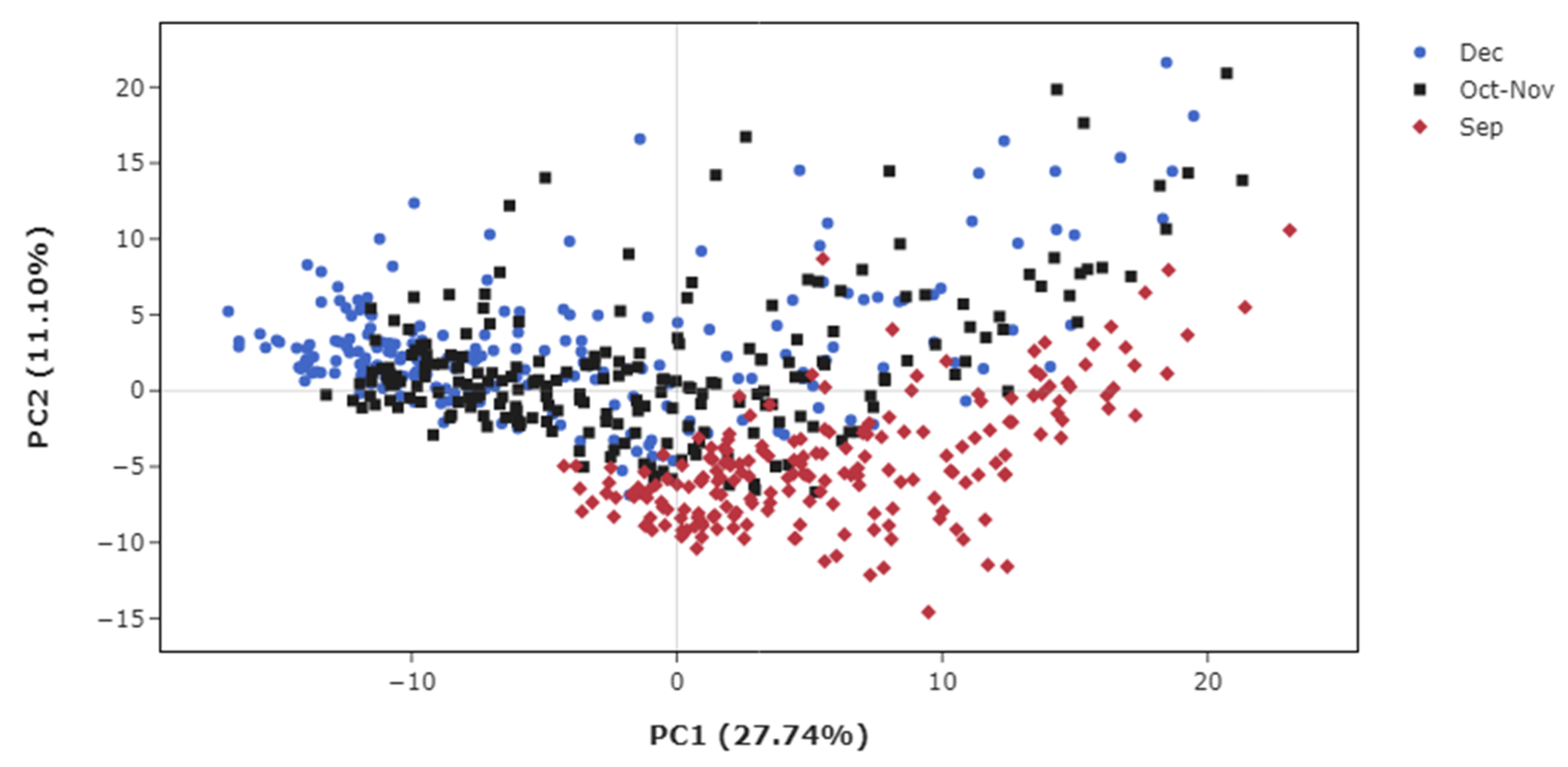
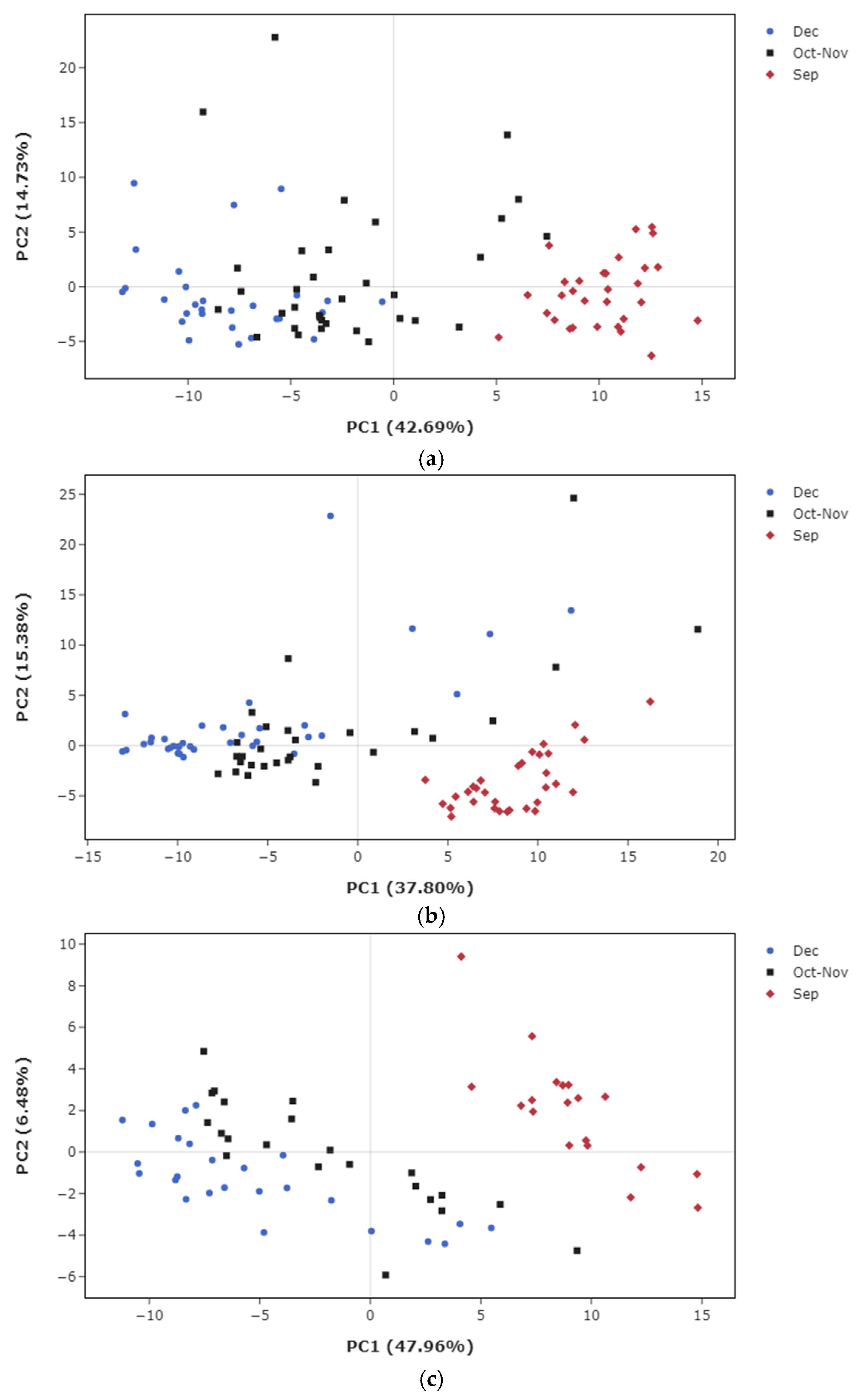
3.2. Metabolic Profiles in Sugarcane Maturation
3.3. Key Metabolites in Sugarcane Maturation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Production Quantities of Sugar Cane. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 23 January 2025).
- Date, Y.; Ishikawa, C.; Umeda, M.; Tarumoto, Y.; Okubo, M.; Tamura, Y.; Ono, H. Sugarcane Metabolome Compositional Stability in Pretreatment Processes for NMR Measurements. Metabolites 2022, 12, 862. [Google Scholar] [CrossRef]
- Ali, S.E.; El Gedaily, R.A.; Mocan, A.; Farag, M.A.; El-Seedi, H.R. Profiling Metabolites and Biological Activities of Sugarcane (Saccharum officinarum Linn.) Juice and its Product Molasses via a Multiplex Metabolomics Approach. Molecules 2019, 24, 934. [Google Scholar] [CrossRef]
- Casarotti, G.G.; Oliveira, B.; Stanisic, D.; Schwab, N.V.; Eberlin, M.N.; Tasic, L.; Lima, C. Enzymatic Browning in Sugarcane: An Insight Into Color Formation in Sugarcane Juice Using Nuclear Magnetic Resonance and ESI-Mass Spectrometry. Food Bioprocess Technol. 2025, 18, 433–448. [Google Scholar] [CrossRef]
- Sakaigaichi, T.; Terajima, Y.; Matsuoka, M.; Terauchi, T.; Hattori, T.; Ishikawa, S. Evaluation of the Juice Brix of Wild Sugarcanes (Saccharum spontaneum) Indigenous to Japan. Plant Prod. Sci. 2016, 19, 323–329. [Google Scholar] [CrossRef]
- Schmitz, A.; Kennedy, P.L.; Zhang, F.Y. Sugarcane and Sugar Yields in Louisiana (1911–2018): Varietal Development and Mechanization. Crop Sci. 2020, 60, 1303–1312. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Yang, L.T.; Li, Y.R. Physiological and Biochemical Characteristics Related to Cold Resistance in Sugarcane. Sugar Tech 2015, 17, 49–58. [Google Scholar] [CrossRef]
- Hale, A.L.; Todd, J.R.; Gravois, K.A.; Mollov, D.; Malapi-Wight, M.; Momotaz, A.; Laborde, C.; Goenaga, R.; Kimbeng, C.; Solis, A.; et al. Sugarcane Breeding Programs in the USA. Sugar Tech 2022, 24, 97–111. [Google Scholar] [CrossRef]
- Terajima, Y.; Hattori, T.; Shimatani, M.; Sato, M.; Takaragawa, H.; Sakaigaichi, T.; Umeda, M.; Naito, T.; Irei, S. Sugarcane Breeding and Supporting Genetics Research in Japan. Sugar Tech 2022, 24, 134–150. [Google Scholar] [CrossRef]
- Terauchi, T.; Irei, S.; Terajima, Y.; Sakaigaichi, T.; Matsuoka, M.; Sugimoto, A. Sugarcane Breeding of Early Maturing Clone with High Sucrose Content for Earlier Harvest in Japan. JARQ-Jpn. Agric. Res. Q. 2012, 46, 227–235. [Google Scholar] [CrossRef]
- Terajima, Y.; Sugimoto, A.; Matsuoka, M.; Ujihara, K.; Sakaigaichi, T.; Fukuhara, S.; Maeda, H.; Katsuta, Y.; Oka, M.; Shimoda, S. New Sugarcane Cultivar” NiTn18” with Excellent Ratooning Ability in Mulch-free Cultivation. Bull. NARO Agric. Res. Kyushu Okinawa Reg. 2010, 54, 23–41. [Google Scholar]
- Hale, A.L.; Viator, R.P.; Eggleston, G.; Hodnett, G.; Stelly, D.M.; Boykin, D.; Miller, D.K. Estimating Broad Sense Heritability and Investigating the Mechanism of Genetic Transmission of Cold Tolerance Using Mannitol as a Measure of Post-freeze Juice Degradation in Sugarcane and Energycane (spp.). J. Agric. Food Chem. 2016, 64, 1657–1663. [Google Scholar] [CrossRef]
- Hale, A.L.; Viator, R.P.; Veremis, J.C. Identification of Freeze Tolerant Accessions Through a Pot-based Study for Use in Sugarcane Germplasm Enhancement for Adaptation to Temperate Climates. Biomass Bioenergy 2014, 61, 53–57. [Google Scholar] [CrossRef]
- Ando, S.; Sugiura, M.; Yamada, T.; Katsuta, M.; Ishikawa, S.; Terajima, Y.; Sugimoto, A.; Matsuoka, M. Overwintering Ability and Dry Matter Production of Sugarcane Hybrids and Relatives in the Kanto Region of Japan. JARQ-Jpn. Agric. Res. Q. 2011, 45, 259–267. [Google Scholar] [CrossRef]
- Uchimiya, M.; Reis, A.F.D.; Lago, B.C.; Kimbeng, C. Early Detection Cold Markers to Expedite the Sugarcane (spp. Hybrids) Breeding. ACS Agric. Sci. Technol. 2023, 3, 1117–1124. [Google Scholar] [CrossRef]
- Ishikawa, C.; Date, Y.; Umeda, M.; Tarumoto, Y.; Okubo, M.; Morimitsu, Y.; Tamura, Y.; Nishiba, Y.; Ono, H. A Data-Driven Approach to Sugarcane Breeding Programs with Agronomic Characteristics and Amino Acid Constituent Profiling. Metabolites 2024, 14, 243. [Google Scholar] [CrossRef]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. 2019, 58, 968–994. [Google Scholar] [CrossRef]
- Ministry of Agriculture Forestry and Fisheries of Japan. Plant Variety Protection Database. 2019. Available online: https://www.hinshu2.maff.go.jp/vips/cmm/apCMM112.aspx?TOUROKU_NO=28825&LANGUAGE=English (accessed on 7 February 2025).
- Ministry of Agriculture Forestry and Fisheries of Japan. Plant Variety Protection Database. 2013. Available online: https://www.hinshu2.maff.go.jp/vips/cmm/apCMM112.aspx?TOUROKU_NO=26950&LANGUAGE=English (accessed on 7 February 2025).
- Ministry of Agriculture Forestry and Fisheries of Japan. Plant Variety Protection Database. 2011. Available online: https://www.hinshu2.maff.go.jp/vips/cmm/apCMM112.aspx?TOUROKU_NO=24575&LANGUAGE=English (accessed on 7 February 2025).
- Ministry of Agriculture Forestry and Fisheries of Japan. Plant Variety Protection Database. 2006. Available online: https://www.hinshu2.maff.go.jp/vips/cmm/apCMM112.aspx?TOUROKU_NO=16150&LANGUAGE=English (accessed on 7 February 2025).
- Ministry of Agriculture Forestry and Fisheries of Japan. Plant Variety Protection Database. 2009. Available online: https://www.hinshu2.maff.go.jp/vips/cmm/apCMM112.aspx?TOUROKU_NO=19542&LANGUAGE=English (accessed on 7 February 2025).
- Ministry of Agriculture Forestry and Fisheries of Japan. Plant Variety Protection Database. 1990. Available online: https://www.hinshu2.maff.go.jp/vips/cmm/apCMM112.aspx?TOUROKU_NO=2990&LANGUAGE=English (accessed on 7 February 2025).
- Ministry of Agriculture Forestry and Fisheries of Japan. Plant Variety Protection Database. 2004. Available online: https://www.hinshu2.maff.go.jp/vips/cmm/apCMM112.aspx?TOUROKU_NO=14531&LANGUAGE=English (accessed on 7 February 2025).
- Ministry of Agriculture Forestry and Fisheries of Japan. Plant Variety Protection Database. 2019. Available online: https://www.hinshu2.maff.go.jp/vips/cmm/apCMM112.aspx?TOUROKU_NO=28318&LANGUAGE=English (accessed on 7 February 2025).
- Lewis, I.A.; Schommer, S.C.; Markley, J.L. rNMR: Open Source Software for Identifying and Quantifying Metabolites in NMR Spectra. Magn. Reson. Chem. 2009, 47 (Suppl. S1), S123–S126. [Google Scholar] [CrossRef]
- Ito, K.; Date, Y.; Kawamura, T.; Oshiro, M.; Eguchi, H.; Ono, H. Development of Remote NMR Analytical System Coupled with NARO Linked Database and AI Supercomputer “Shiho”. J. NARO Res. Dev. 2023, 2023, 3–22. [Google Scholar]
- Helmus, J.J.; Jaroniec, C.P. Nmrglue: An Open Source Python Package for the Analysis of Multidimensional NMR Data. J. Biomol. NMR 2013, 55, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J. Python Data Analysis with pandas. In Python Recipes Handbook: A Problem-Solution Approach; Apress: Berkeley, CA, USA, 2016; pp. 37–48. [Google Scholar]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array Programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. icoshift: A Versatile Tool for the Rapid Alignment of 1D NMR Spectra. J. Magn. Reson. 2010, 202, 190–202. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Novikov, A.V. PyClustering: Data Mining Library. J. Open Source Softw. 2019, 4, 1230. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Lefort, G.; Liaubet, L.; Canlet, C.; Tardivel, P.; Père, M.-C.; Quesnel, H.; Paris, A.; Iannuccelli, N.; Vialaneix, N.; Servien, R. ASICS: An R Package for a Whole Analysis Workflow of 1D 1H NMR spectra. Bioinformatics 2019, 35, 4356–4363. [Google Scholar] [CrossRef] [PubMed]
- Tardivel, P.J.; Canlet, C.; Lefort, G.; Tremblay-Franco, M.; Debrauwer, L.; Concordet, D.; Servien, R. ASICS: An Automatic Method for Identification and Quantification of Metabolites in Complex 1D 1H NMR Spectra. Metabolomics 2017, 13, 109. [Google Scholar] [CrossRef]
- Chen, L.; Weng, Z.Q.; Goh, L.Y.; Garland, M. An Efficient Algorithm for Automatic Phase Correction of NMR Spectra based on Entropy Minimization. J. Magn. Reson. 2002, 158, 164–168. [Google Scholar] [CrossRef]
- Wang, K.C.; Wang, S.Y.; Kuo, C.H.; Tseng, Y.F.J. Distribution-Based Classification Method for Baseline Correction of Metabolomic 1D Proton Nuclear Magnetic Resonance Spectra. Anal. Chem. 2013, 85, 1231–1239. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures.: Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Ono, H. Production and Processing of Brown Sugar. J. Cook. Sci. Jpn. 2020, 53, 61–64. [Google Scholar]
- Hattori, T.; Tanaka, M.; Sakaigaichi, T.; Tarumoto, Y.; Ishikawa, S.; Terauchi, T. Relationship Between Stalk Fiber Content of Sugarcane and Juice Extraction Efficiency. In Proceedings of the 237th Meeting of CSSJ, Chiba, Japan, 29–30 March 2014; The Crop Science Society of Japan: Tokyo, Japan, 2014; pp. 430–431. [Google Scholar]
- Gravois, K.A.; Milligan, S.B. Genetic-Relationships between Fiber and Sugarcane Yield Components. Crop Sci. 1992, 32, 62–67. [Google Scholar] [CrossRef]
- Hattori, T.; Terajima, Y.; Sakaigaichi, T.; Terauchi, T.; Tarumoto, Y.; Adachi, K.; Hayano, M.; Tanaka, M.; Ishikawa, S.; Umeda, M.; et al. High Ratoon Yield Sugarcane Cultivar “Harunoogi” Developed for Kumage Region by Using an Interspecific Hybrid between a Commercial Cultivar and Saccharum spontaneum L. J. NARO Res. Dev. 2019, 2019, 21–44. [Google Scholar]
- Glasziou, K.T.; Gayler, K.R. Storage of Sugars in Stalks of Sugar-Cane. Bot. Rev. 1972, 38, 471–490. [Google Scholar] [CrossRef]
- Batta, S.K.; Singh, R. Sucrose Metabolism in Sugarcane Grown under Varying Climatic Conditions—Synthesis and Storage of Sucrose in Relation to the Activities of Sucrose Synthase, Sucrose Phosphate Synthase and Invertase. Phytochemistry 1986, 25, 2431–2437. [Google Scholar] [CrossRef]
- Terauchi, T. [Sugarcane cultivar Kurokaido developed for Honsyu such as Kyusyu and Shikoku regions—Toward 6th industrialization focusing on brown sugar products] Kyusyu Shikoku tou no hondomuke satoukibi hinsyu kurokaido -kokuto seihin wo chushin to shita 6ji sangyouka ni mukete. Tokusanshubyo 2011, 12, 75. (In Japanese) [Google Scholar]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric Acid (GABA): A Comprehensive Review of Dietary Sources, Enrichment Technologies, Processing Effects, Health Benefits, and its Applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 8852–8874. [Google Scholar] [CrossRef]
- Sun, Y.; Mehmood, A.; Battino, M.; Xiao, J.; Chen, X. Enrichment of Gamma-Aminobutyric Acid in Foods: From Conventional Methods to Innovative Technologies. Food Res. Int. 2022, 162, 111801. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma Aminobutyric Acid (GABA) and Plant Responses to Stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]

| Module | Description/Parameter Options |
|---|---|
| (1) Data import | |
| Loading NMR data | Bruker fid data, Bruker spectra pdata, CSV data |
| Loading group data | CSV data |
| Loading objective variables | CSV data |
| (2) Spectral processing | |
| Zero filling (FID only) | Input a value (points) |
| Line broadening (FID only) | Input a value (Hz) |
| Phase correction (FID only) | Minimization around maximum peak, automated phase correction based on minimization of entropy [38] |
| Baseline correction | Distribution-based classification method [39] |
| Reference calibration | Select a reference peak range (ppm) |
| Field trimming | Select a range (ppm) |
| Binning/bucketing | Input binning/bucketing size (ppm) |
| Solvent peak removal | Select a range of solvent peaks (ppm) |
| Normalization | Reference peak, mean, probabilistic quotient normalization [40] |
| Peak alignment | icoshift algorithm |
| (3) Multivariate analysis | |
| Hierarchical cluster analysis | Method: ward, complete, average, etc. Metric: Euclidean, Bray–Curtis, cosine, etc. |
| Nonhierarchical cluster analysis | K-means, g-means, x-means |
| PCA | - |
| Discriminant analysis | Partial least squares, orthogonal partial least squares |
| Correlation heatmap | Pearson, Spearman, and Kendall correlations |
| (4) Machine learning | |
| Random forest | Classification, regression |
| Support vector machine | Classification, regression |
| (5) Peak annotation | |
| ASICS | - |
| Stalk Weight (g) | Stalk Length (cm) | Stalk Diameter (mm) | Internode Count | Stalk Count | |
|---|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | Total | |
| Harunoogi | 506.4 ± 205.4 | 176.6 ± 45.2 | 16.9 ± 2.3 | 10.8 ± 2.8 | 92 |
| Sep | 489.9 ± 170.9 | 170.9 ± 34.4 | 17.4 ± 1.9 | 10.6 ± 2.3 | 30 |
| Oct–Nov | 503.3 ± 224.8 | 170.0 ± 52.5 | 17.0 ± 2.2 | 10.3 ± 3.1 | 34 |
| Dec | 527.8 ± 212.6 | 190.8 ± 42.6 | 16.3 ± 2.7 | 11.6 ± 2.7 | 28 |
| KTn03-54 | 575.7 ± 280.3 | 137.7 ± 57.8 | 21.4 ± 2.0 | 9.5 ± 4.1 | 43 |
| Sep | 646.1 ± 235.5 | 150.3 ± 44.5 | 22.1 ± 1.9 | 10.4 ± 3.2 | 13 |
| Oct–Nov | 534.9 ± 284.1 | 125.5 ± 58.9 | 21.5 ± 1.9 | 8.5 ± 4.2 | 16 |
| Dec | 557.0 ± 301.3 | 140.1 ± 64.3 | 20.6 ± 2.0 | 9.9 ± 4.6 | 14 |
| Kurokaido | 449.4 ± 182.2 | 153.3 ± 42.7 | 17.2 ± 1.9 | 8.1 ± 2.2 | 95 |
| Sep | 431.2 ± 147.9 | 148.1 ± 30.1 | 17.1 ± 1.8 | 8.0 ± 1.6 | 32 |
| Oct–Nov | 454.7 ± 191.5 | 148.2 ± 47.6 | 17.6 ± 1.6 | 7.8 ± 2.4 | 28 |
| Dec | 461.8 ± 200.6 | 162.2 ± 46.7 | 16.9 ± 2.1 | 8.5 ± 2.4 | 35 |
| NCo310 | 398.3 ± 205.8 | 155.8 ± 60.8 | 16.1 ± 2.1 | 9.2 ± 3.5 | 83 |
| Sep | 365.9 ± 151.0 | 149.2 ± 45.3 | 16.0 ± 2.1 | 8.8 ± 2.6 | 26 |
| Oct–Nov | 416.4 ± 223.5 | 162.2 ± 67.1 | 16.0 ± 1.8 | 9.5 ± 3.8 | 29 |
| Dec | 409.8 ± 226.3 | 155.4 ± 65.6 | 16.4 ± 2.3 | 9.4 ± 3.8 | 28 |
| Ni22 | 639.5 ± 220.4 | 200.6 ± 48.6 | 18.3 ± 1.6 | 10.6 ± 2.7 | 69 |
| Sep | 576.1 ± 208.4 | 180.2 ± 37.1 | 18.5 ± 1.7 | 9.4 ± 2.3 | 20 |
| Oct–Nov | 673.8 ± 201.5 | 204.8 ± 45.7 | 18.4 ± 1.6 | 10.8 ± 2.3 | 27 |
| Dec | 655.0 ± 240.3 | 213.9 ± 55.0 | 18.0 ± 1.5 | 11.5 ± 3.1 | 22 |
| Ni27 | 897.7 ± 274.6 | 203.1 ± 37.7 | 21.9 ± 2.2 | 11.6 ± 2.5 | 66 |
| Sep | 855.2 ± 254.4 | 186.7 ± 41.4 | 22.8 ± 1.7 | 10.6 ± 2.7 | 19 |
| Oct–Nov | 872.3 ± 313.0 | 200.1 ± 38.5 | 21.6 ± 2.5 | 11.4 ± 2.4 | 22 |
| Dec | 952.4 ± 242.3 | 218.3 ± 26.3 | 21.6 ± 1.9 | 12.5 ± 2.0 | 25 |
| NiF8 | 691.8 ± 356.3 | 166.2 ± 63.3 | 20.9 ± 3.3 | 9.3 ± 3.5 | 56 |
| Sep | 623.3 ± 370.9 | 147.6 ± 63.6 | 21.0 ± 4.5 | 8.5 ± 3.5 | 17 |
| Oct–Nov | 721.8 ± 339.8 | 171.5 ± 58.0 | 21.0 ± 2.3 | 9.3 ± 3.3 | 20 |
| Dec | 721.5 ± 351.5 | 177.4 ± 64.7 | 20.6 ± 2.9 | 10.2 ± 3.4 | 19 |
| NiTn18 | 450.2 ± 266.4 | 150.4 ± 75.4 | 17.5 ± 2.0 | 9.7 ± 4.9 | 88 |
| Sep | 472.6 ± 229.2 | 155.8 ± 56.1 | 17.9 ± 1.7 | 9.8 ± 3.7 | 28 |
| Oct–Nov | 443.7 ± 297.9 | 144.9 ± 83.0 | 17.4 ± 2.1 | 9.5 ± 5.5 | 31 |
| Dec | 435.4 ± 262.6 | 150.9 ± 82.4 | 17.2 ± 2.1 | 9.8 ± 5.2 | 29 |
| RK03-3010 | 450.7 ± 250.2 | 123.0 ± 59.7 | 20.5 ± 1.6 | 7.4 ± 3.3 | 36 |
| Sep | 361.7 ± 188.6 | 105.8 ± 48.7 | 19.9 ± 2.0 | 6.4 ± 2.6 | 13 |
| Oct–Nov | 421.4 ± 243.6 | 115.2 ± 58.8 | 20.5 ± 1.1 | 7.0 ± 3.2 | 13 |
| Dec | 604.4 ± 258.8 | 155.5 ± 61.2 | 21.4 ± 1.2 | 9.3 ± 3.3 | 10 |
| Juice Extraction Efficiency (%) | Brix (%) | pH | |
|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | |
| Harunoogi | 51.1 ± 2.7 | 13.3 ± 2.2 | 5.20 ± 0.09 |
| Sep | 51.6 ± 2.2 | 10.8 ± 0.9 | 5.11 ± 0.04 |
| Oct–Nov | 50.8 ± 3.0 | 13.6 ± 1.4 | 5.21 ± 0.05 |
| Dec | 51.0 ± 2.7 | 15.5 ± 0.9 | 5.30 ± 0.04 |
| KTn03-54 | 58.7 ± 2.7 | 11.6 ± 2.0 | 5.22 ± 0.10 |
| Sep | 58.4 ± 1.5 | 9.9 ± 1.2 | 5.11 ± 0.05 |
| Oct–Nov | 58.8 ± 3.1 | 12.0 ± 1.5 | 5.22 ± 0.08 |
| Dec | 58.8 ± 3.2 | 12.6 ± 2.1 | 5.31 ± 0.06 |
| Kurokaido | 55.4 ± 2.7 | 15.7 ± 2.8 | 5.15 ± 0.08 |
| Sep | 54.9 ± 2.3 | 13.2 ± 0.8 | 5.15 ± 0.09 |
| Oct–Nov | 55.2 ± 2.5 | 16.2 ± 2.7 | 5.13 ± 0.06 |
| Dec | 55.9 ± 3.1 | 17.6 ± 2.3 | 5.17 ± 0.07 |
| NCo310 | 55.9 ± 3.3 | 10.2 ± 2.8 | 5.11 ± 0.13 |
| Sep | 55.0 ± 3.5 | 8.9 ± 1.8 | 5.00 ± 0.06 |
| Oct–Nov | 55.5 ± 3.0 | 10.1 ± 3.0 | 5.15 ± 0.12 |
| Dec | 57.3 ± 2.9 | 11.4 ± 2.8 | 5.19 ± 0.10 |
| Ni22 | 55.2 ± 2.2 | 13.2 ± 2.1 | 5.22 ± 0.09 |
| Sep | 55.4 ± 2.0 | 10.7 ± 0.7 | 5.16 ± 0.06 |
| Oct–Nov | 54.8 ± 2.7 | 13.7 ± 0.8 | 5.23 ± 0.08 |
| Dec | 55.4 ± 1.8 | 15.0 ± 1.8 | 5.28 ± 0.07 |
| Ni27 | 57.5 ± 2.1 | 13.6 ± 2.4 | 5.23 ± 0.10 |
| Sep | 57.7 ± 1.5 | 10.8 ± 1.4 | 5.13 ± 0.06 |
| Oct–Nov | 56.5 ± 2.3 | 14.5 ± 1.8 | 5.22 ± 0.09 |
| Dec | 58.2 ± 1.8 | 14.8 ± 1.6 | 5.32 ± 0.05 |
| NiF8 | 55.6 ± 2.6 | 11.4 ± 2.4 | 5.13 ± 0.11 |
| Sep | 54.1 ± 2.8 | 8.8 ± 1.2 | 5.01 ± 0.03 |
| Oct–Nov | 56.6 ± 2.0 | 12.3 ± 1.1 | 5.14 ± 0.08 |
| Dec | 56.1 ± 2.3 | 12.9 ± 2.2 | 5.25 ± 0.04 |
| NiTn18 | 56.7 ± 2.5 | 10.6 ± 2.6 | 5.19 ± 0.11 |
| Sep | 56.4 ± 1.7 | 8.7 ± 1.9 | 5.11 ± 0.06 |
| Oct–Nov | 56.6 ± 3.2 | 11.5 ± 2.1 | 5.19 ± 0.13 |
| Dec | 57.1 ± 2.3 | 11.6 ± 2.5 | 5.25 ± 0.07 |
| RK03-3010 | 52.3 ± 2.5 | 9.4 ± 2.9 | 5.20 ± 0.12 |
| Sep | 50.9 ± 2.6 | 7.6 ± 1.3 | 5.08 ± 0.05 |
| Oct–Nov | 52.7 ± 2.4 | 9.8 ± 2.7 | 5.23 ± 0.10 |
| Dec | 53.5 ± 1.6 | 11.4 ± 3.1 | 5.31 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Date, Y.; Ishikawa, C.; Ono, H. Metabolomic Variation in Sugarcane Maturation Under a Temperate Climate. Metabolites 2025, 15, 558. https://doi.org/10.3390/metabo15080558
Date Y, Ishikawa C, Ono H. Metabolomic Variation in Sugarcane Maturation Under a Temperate Climate. Metabolites. 2025; 15(8):558. https://doi.org/10.3390/metabo15080558
Chicago/Turabian StyleDate, Yasuhiro, Chiaki Ishikawa, and Hiroshi Ono. 2025. "Metabolomic Variation in Sugarcane Maturation Under a Temperate Climate" Metabolites 15, no. 8: 558. https://doi.org/10.3390/metabo15080558
APA StyleDate, Y., Ishikawa, C., & Ono, H. (2025). Metabolomic Variation in Sugarcane Maturation Under a Temperate Climate. Metabolites, 15(8), 558. https://doi.org/10.3390/metabo15080558






