Spatially Resolved Plant Metabolomics
Abstract
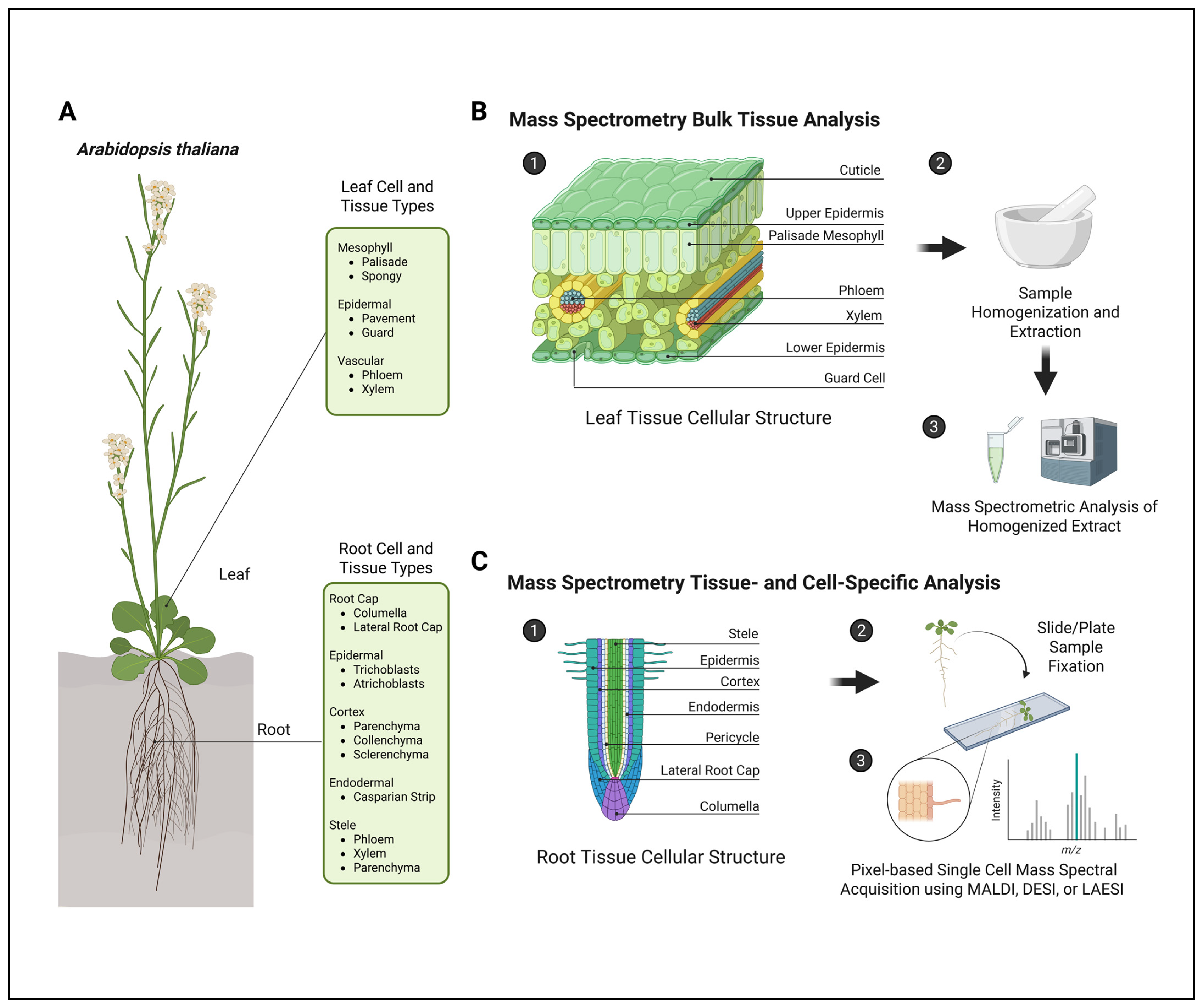
1. Introduction
1.1. Why Do We Need Spatial Metabolomics?
1.2. Bulk Tissue Collections Dilute the Metabolic Phenotype
1.3. Current Perspectives and Potential Outlook
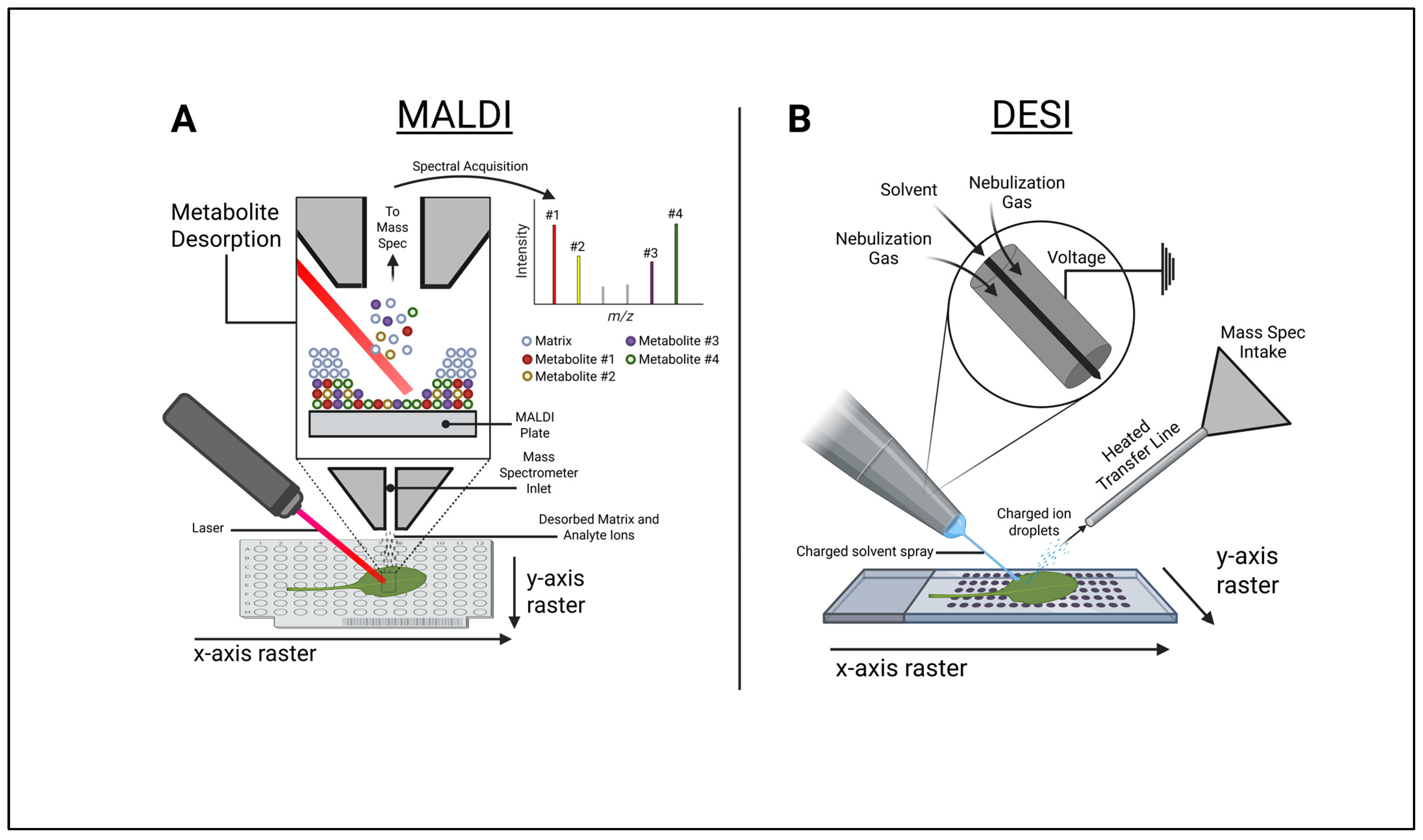
2. Mass Spectral Imaging (MSI) Technologies
2.1. Matrix-Assisted Laser Desorption Ionization–Mass Spectrometry Imaging (MALDI-MSI)
2.2. Desorption Electrospray Ionization (DESI)
2.3. Laser Ablation Electrospray Ionization (LAESI)
2.4. Magnetic Resonance Imaging (MRI)
2.5. Mass Spectral Imaging Software
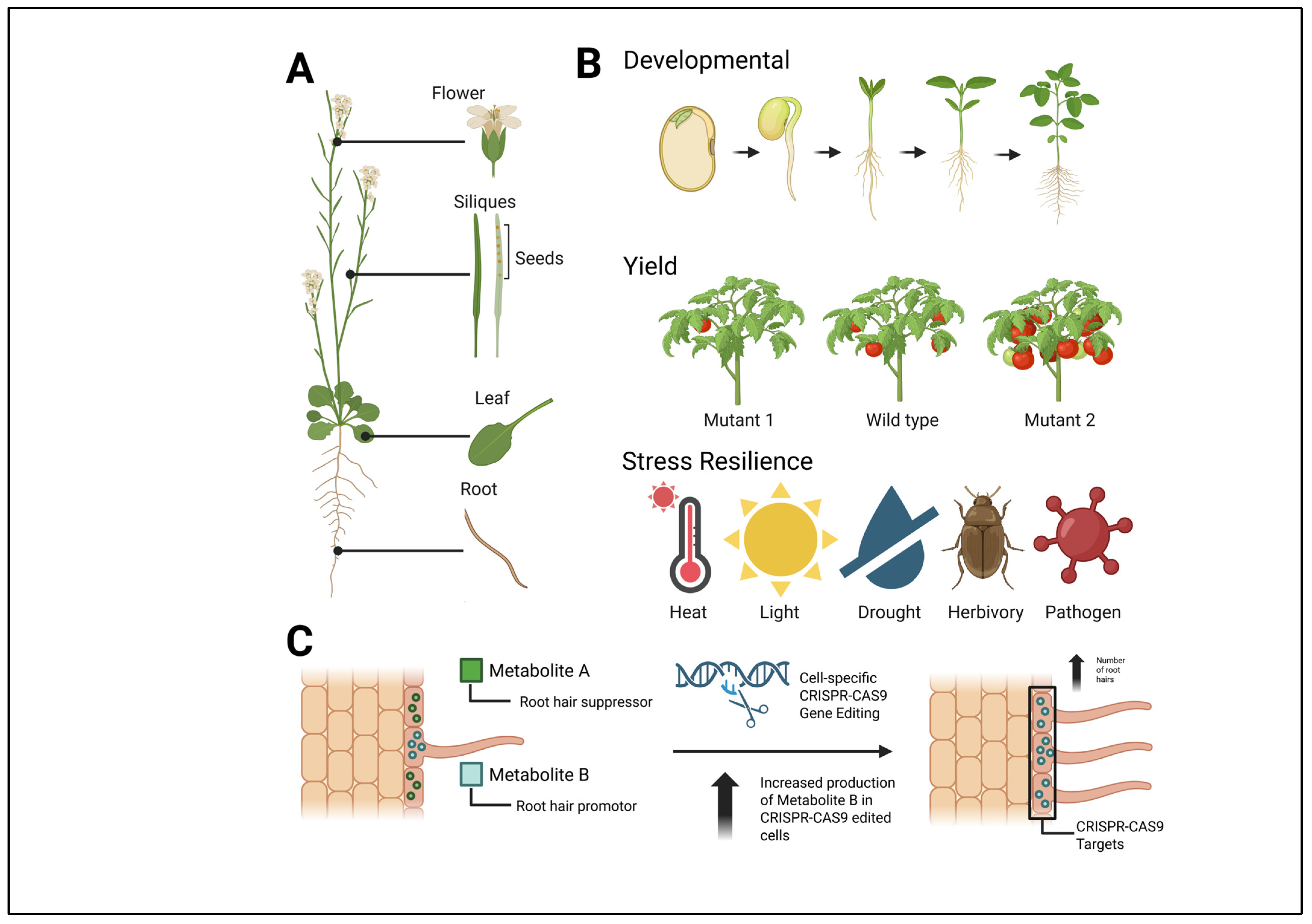
3. Applications of Spatially Resolved Metabolomics in Plant Biochemistry
3.1. Mass Spectral Imaging of Root Metabolites
3.1.1. MSI of Root Metabolites in Abiotic Stress Responses
3.1.2. MSI of Root Metabolites in Abiotic Stress Responses—Nutrient Deficiency and Toxicity
3.1.3. MSI of Root Metabolites in Abiotic Stress Responses—Drought and Salinity
3.1.4. MSI of Root Metabolites in Abiotic Stress Responses—Cold
3.1.5. MSI of Root Metabolites in Metabolic Mapping
3.2. Mass Spectral Imaging of Aerial Tissue
3.2.1. MSI of Aerial Metabolites in Abiotic Stress Response—Drought and Water Deficit
3.2.2. MSI of Aerial Metabolites in Leaf and Fruit Wounding Responses
3.2.3. MSI of Aerial Metabolites in Metabolic Mapping
3.3. Mass Spectral Imaging of Specialized Cell Types
3.3.1. Trichomes
3.3.2. Stomatal Guard Cells
3.3.3. Seed and Seed Coat
3.3.4. Seed and Seed Coat—Drought and Salinity
3.3.5. Seed and Seed Coat—Pathogen Response
3.3.6. Root Border Cells and Nodules
3.3.7. Metabolic Transport and Intracellular Communication
3.4. Integration of Mass Spectral Imaging and Omics Technologies
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic Acid |
| AFADESI-MSI | Air-Flow-Assisted Desorption Electrospray Ionization–Mass Spectrometry |
| Ag | Silver |
| Al | Aluminum |
| AMT | Accurate Mass and Time |
| AP-MALDI | Atmospheric Pressure–Matrix-Assisted Laser Desorption Ionization |
| APPI | Atmospheric Pressure Photoionization |
| AP-SMALDI-MSI | Atmospheric Pressure–Scanning Microprobe Matrix-Assisted Laser Desorption Ionization–Mass Spectrometry Imaging |
| CEST | Chemical Exchange Saturation Transfer |
| CHCA | Cyano-4-HydroxyCinnamic Acid |
| COSY | Homonclear COrrelation SpectroscopY |
| CSI | Chemical Shift Imaging |
| DAN | 1,5-DiAminoNaphthalene |
| DESI | Desorption Electrospray Ionization |
| DHB | 2,5-DihyHroxyBenzoic acid |
| DOSY | Diffusion-Ordered SpectroscopY |
| EMFAFTP | ElectroMagnetic-Field-Assisted Frozen Tissue Planarization |
| ESI | ElectroSpray Ionization |
| Fe3O4 | Iron Oxide |
| FTICR-IMS | Fourier Transform Ion Cyclotron Resonance–Mass Spectrometry |
| GC | Gas Chromatography |
| HMBC | Heteronuclear Multiple Quantum Coherence |
| HRMS/MS | High-Resolution Tandem Mass Spectrometry |
| HSQC | Heteronuclear Single Quantum Coherence |
| HR-MAS | High-Resolution Magic Angle Spinning |
| IAA | Indole-3-Acetic Acid |
| IMS | Ion Mobility Spectrometry |
| LAAPPI | Laser Ablation Atmospheric Pressure Photoionization |
| LAESI | Laser Ablation Electrospray Ionization |
| LC-DAD | Liquid Chromatography–Diode Array Detection |
| JA | Jasmonic Acid |
| MALDI | Matrix-Assisted Laser Desorption Ionization |
| MGs | Monoterpene Glucosides |
| MIAs | Monoterpenoid Indole Alkaloids |
| MMP | Multi-MSI Processer |
| MRI | Magnetic Resonance Imaging |
| MSI | Mass Spectral Imaging |
| NMR | Nuclear Magnetic Resonance |
| OA | Organic Acid |
| P | Phosphorus |
| PCA | Principal Component Analysis |
| PTFE | Porous PolyTetraFluoroEthylene |
| QtoF | Quadropole Time of Flight |
| ROI | Region of Interest |
| ROS | Reactive Oxygen Species |
| SA | Salicylic Acid |
| SIMS | Secondary Ion Mass Spectrometry |
| SSC | Spatial Shrunken Centroids |
| THAP | 2,4,6-TriHydroxyAcetoPhenone |
| Tims-TOFMS | Trapped Ion Mobility Spectrometry–Time-of-Flight Mass Spectrometry |
| TOCSY | Total Correlation Spectroscopy |
| WO3 | Tungsten Oxide |
| 5GG | 1,2,3,4,6-penta-O-Galloyl-β-D-Glucopyranose |
| P-AA | 9-AminoAcidine |
References
- Oliver, S.G.; Winson, M.K.; Kell, D.B.; Baganz, F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998, 16, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Costa dos Santos, G., Jr.; Renovato-Martins, M.; de Brito, N.M. The remodel of the “central dogma”: A metabolomics interaction perspective. Metabolomics 2021, 17, 48. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Sig Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef]
- Fiehn, O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp. Funct. Genom. 2001, 2, 155–168. [Google Scholar] [CrossRef]
- Hall, R.; Beale, M.; Fiehn, O.; Hardy, N.; Sumner, L.; Bino, R. Plant metabolomics: The missing link in functional genomics strategies. Plant Cell 2002, 14, 1437–1440. [Google Scholar] [CrossRef]
- Garagounis, C.; Delkis, N.; Papadopoulou, K.K. Unraveling the roles of plant specialized metabolites: Using synthetic biology to design molecular biosensors. New Phytol. 2021, 231, 1338–1352. [Google Scholar] [CrossRef]
- Wang, S.; Alseekh, S.; Fernie, A.R.; Luo, J. The Structure and Function of Major Plant Metabolite Modifications. Mol. Plant 2019, 12, 899–919. [Google Scholar] [CrossRef]
- Jorge, T.F.; Rodrigues, J.A.; Caldana, C.; Schmidt, R.; van Dongen, J.T.; Thomas-Oates, J.; António, C. Mass spectrometry-based plant metabolomics: Metabolite responses to abiotic stress. Mass Spec. Rev. 2016, 35, 620–649. [Google Scholar] [CrossRef]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant Metabolomics: An Indispensable System Biology Tool for Plant Science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Mohan, R.; Mishra, S.; Goyal, N.; Shanker, K.; Gupta, N.; Kumar, B. Ultra performance liquid chromatography coupled with principal component and cluster analysis of Swertia chirayita for adulteration check. J. Pharm. Biomed. Anal. 2019, 164, 302–308. [Google Scholar] [CrossRef]
- Caprioli, R.M.; Farmer, T.B.; Gile, J. Molecular imaging of biological samples: Localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 1997, 69, 4751–4760. [Google Scholar] [CrossRef]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.W.; Williams, A.; Uthe, H.; van Dam, N.M.; Mur, L.A.J.; Grant, M.R.; Pétriacq, P. Unravelling Plant Responses to Stress-The Importance of Targeted and Untargeted Metabolomics. Metabolites 2021, 11, 558. [Google Scholar] [CrossRef]
- de Souza, L.P.; Borghi, M.; Fernie, A. Plant Single-Cell Metabolomics—Challenges and Perspectives. Int. J. Mol. Sci. 2020, 21, 8987. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.J.; Chapman, K.D. Imaging plant metabolism in situ. J. Exp. Bot. 2024, 75, 1654–1670. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Z.; Sun, X. Single-cell and spatial multi-omics in the plant sciences: Technical advances, applications, and perspectives. Plant Commun. 2023, 4, 100508. [Google Scholar] [CrossRef]
- Oyarce, P.; Xiao, T.T.; Henkel, C.; Frederiksen, S.F.; Gonzalez-Kise, J.K.; Smet, W.; Wang, J.Y.; Al-Babili, S.; Blilou, I. Microscopy and spatial-metabolomics identify tissue-specific metabolic pathways uncovering salinity and drought tolerance mechanisms in Avicennia marina and Phoenix dactylifera roots. Sci. Rep. 2025, 15, 1076. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Sonawane, P.; Cohen, H.; Polturak, G.; Feldberg, L.; Avivi, S.H.; Rogachev, I.; Aharoni, A. High mass resolution, spatial metabolite mapping enhances the current plant gene and pathway discovery toolbox. New Phytol. 2020, 228, 1986–2002. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, T. Spatial Metabolomics and Imaging Mass Spectrometry in the Age of Artificial Intelligence. Annu. Rev. Biomed. Data Sci. 2020, 3, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, B.; Malitsky, S.; Rogachev, I.; Aharoni, A.; Kaftan, F.; Svatoš, A.; Franceschi, P. Sample Preparation for Mass Spectrometry Imaging of Plant Tissues: A Review. Front. Plant Sci. 2016, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Honeker, L.K.; Hildebrand, G.A.; Fudyma, J.D.; Daber, L.E.; Hoyt, D.; Flowers, S.E.; Gil-Loaiza, J.; Kübert, A.; Bamberger, I.; Anderton, C.R.; et al. Elucidating Drought-Tolerance Mechanisms in Plant Roots through 1H NMR Metabolomics in Parallel with MALDI-MS, and NanoSIMS Imaging Techniques. Environ. Sci. Technol. 2022, 56, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Dilmetz, B.A.; Lee, Y.R.; Condina, M.R.; Briggs, M.; Young, C.; Desire, C.T.; Klingler-Hoffmann, M.; Hoffmann, P. Novel technical developments in mass spectrometry imaging in 2020: A mini review. Anal. Sci. Adv. 2021, 2, 225–237. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, W.; Li, K.; Fernie, A.R.; Yan, S. Advances in mass spectrometry imaging for plant metabolomics—Expanding the analytical toolbox. Plant J. 2024, 119, 2168–2180. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, F.; Wang, J. Spatial Metabolomics and Its Application in Plant Research. Int. J. Mol. Sci. 2025, 26, 3043. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Bachmann, D.; Hillenkamp, F. Influence of the Wavelength in High-Irradiance Ultraviolet Laser Desorption Mass Spectrometry of Organic Molecules. Anal. Chem. 1985, 57, 2935–2939. [Google Scholar] [CrossRef]
- Kompauer, M.; Heiles, S.; Spengler, B. Atmospheric pressure MALDI mass spectrometry imaging of tissues and cells at 1.4-μm lateral resolution. Nat. Methods 2016, 14, 90–96. [Google Scholar] [CrossRef]
- Jones, E.A.; Deininger, S.O.; Hogendoorn, P.C.W.; Deelder, A.M.; McDonnell, L.A. Imaging mass spectrometry statistical analysis. J. Proteom. 2012, 75, 4962–4989. [Google Scholar] [CrossRef] [PubMed]
- Susniak, K.; Krysa, M.; Gieroba, B.; Komaniecka, I.; Sroka-Bartnicka, A. Recent developments of MALDI MSI application in plant tissues analysis. Acta Biochim. Pol. 2020, 67, 277–281. [Google Scholar] [CrossRef]
- Feenstra, A.D.; Alexander, L.E.; Song, Z.; Korte, A.R.; Yandeau-Nelson, M.D.; Nikolau, B.J.; Lee, Y.J. Spatial Mapping and Profiling of Metabolite Distributions during Germination. Plant Physiol. 2017, 174, 2532–2548. [Google Scholar] [CrossRef]
- Becker, L.; Carré, V.; Poutaraud, A.; Merdinoglu, D.; Chaimbault, P. MALDI mass spectrometry imaging for the simultaneous location of resveratrol, pterostilbene and viniferins on grapevine leaves. Molecules 2014, 19, 10587–10600. [Google Scholar] [CrossRef]
- Hansen, R.L.; Lee, Y.J. High-Spatial Resolution Mass Spectrometry Imaging: Toward Single Cell Metabolomics in Plant Tissues. TCR 2018, 18, 65. [Google Scholar] [CrossRef]
- Hu, H.; Qiu, K.; Hao, Q.; He, X.; Qin, L.; Chen, L.; Yang, C.; Dai, X.; Liu, H.; Xu, H.; et al. Electromagnetic Field-Assisted Frozen Tissue Planarization Enhances MALDI-MSI in Plant Spatial Omics. Anal. Chem. 2024, 96, 11809–11822. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Fenn, J.B. Electrospray ion source. Another variation on the free-jet theme. J. Phys. Chem. 1984, 88, 4451–4459. [Google Scholar] [CrossRef]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization for mass spectrometry of large biomolecules. Science 1989, 246, 64–71. [Google Scholar] [CrossRef]
- Morato, N.M.; Cooks, R.G. Desorption Electrospray Ionization Mass Spectrometry: 20 Years. Acc. Chem. Res. 2023, 56, 2526–2536. [Google Scholar] [CrossRef]
- Parrot, D.; Papazian, S.; Foil, D.; Tasdemir, D. Imaging the Unimaginable: Desorption Electrospray Ionization—Imaging Mass Spectrometry (DESI-IMS) in Natural Product Research. Planta Med. 2018, 84, 584–593. [Google Scholar] [CrossRef]
- Claude, E.; Jones, E.A.; Pringle, S.D. DESI Mass Spectrometry Imaging (MSI). Methods Mol. Biol. 2017, 1618, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Noll, S.E.; Peng, J.T.; Klair, A.; Tripka, A.; Stutzman, N.; Cheng, C.; Zare, R.N.; Dickinson, A.J. Chemical imaging reveals diverse functions of tricarboxylic acid metabolites in root growth and development. Nat. Commun. 2023, 14, 2567. [Google Scholar] [CrossRef]
- Wu, L.; Qi, K.; Liu, C.; Hu, Y.; Xu, M.; Pan, Y. Enhanced Coverage and Sensitivity of Imprint DESI Mass Spectrometry Imaging for Plant Leaf Metabolites by Post-photoionization. Anal. Chem. 2022, 94, 15108–15116. [Google Scholar] [CrossRef]
- Bartels, B.; Svatoš, A. Spatially resolved in vivo plant metabolomics by laser ablation-based mass spectrometry imaging (MSI) techniques: LDI-MSI and LAESI. Front. Plant Sci. 2015, 6, 471. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Smith, B.K.; Márk, L.; Nemes, P.; Nazarian, J.; Vertes, A. Ambient molecular imaging by laser ablation electrospray ionization mass spectrometry with ion mobility separation. Int. J. Mass Spectrom. 2015, 377, 681–689. [Google Scholar] [CrossRef]
- Kulkarni, P.; Wilschut, R.A.; Verhoeven, K.J.F.; van der Putten, W.H.; Garbeva, P. LAESI mass spectrometry imaging as a tool to differentiate the root metabolome of native and range-expanding plant species. Planta 2018, 248, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Liyu, A.; Vertes, A.; Anderton, C.R. Ambient Single-Cell Analysis and Native Tissue Imaging Using Laser-Ablation Electrospray Ionization Mass Spectrometry with Increased Spatial Resolution. J. Am. Soc. Mass Spectrom. 2021, 32, 2490–2494. [Google Scholar] [CrossRef] [PubMed]
- Vaikkinen, A.; Shrestha, B.; Koivisto, J.; Kostiainen, R.; Vertes, A.; Kauppila, T.J. Laser ablation atmospheric pressure photoionization mass spectrometry imaging of phytochemicals from sage leaves. Rapid Commun. Mass Spectrom. 2014, 28, 2490–2496. [Google Scholar] [CrossRef]
- Mayer, S.; Rolletschek, H.; Radchuk, V.; Wagner, S.; Ortleb, S.; Gündel, A.; Dehmer, K.J.; Gutjahr, F.T.; Jakob, P.M.; Borisjuk, L. Metabolic imaging in living plants: A promising field for chemical exchange saturation transfer (CEST) MRI. Sci. Adv. 2024, 10, eadq4424. [Google Scholar] [CrossRef]
- Mahrous, E.A.; Farag, M.A. Two dimensional NMR spectroscopic approaches for exploring plant metabolome: A review. J. Adv. Res. 2015, 6, 3–15. [Google Scholar] [CrossRef]
- Bemis, K.D.; Harry, A.; Eberlin, L.S.; Ferreira, C.; Van de Ven, S.M.; Mallick, P.; Stolowitz, M.L. Cardinal: An R package for statistical analysis of mass spectrometry-based imaging experiments. Bioinformatics 2015, 31, 2418–2420. [Google Scholar] [CrossRef]
- Bemis, K.A.; Föll, M.C.; Guo, D.; Lakkimsetty, S.S.; Vitek, O. Cardinal v.3: A versatile open-source software for mass spectrometry imaging analysis. Nat. Methods 2023, 20, 1883–1886. [Google Scholar] [CrossRef]
- Horn, P.J.; Chapman, K.D. Metabolite Imager: Customized spatial analysis of metabolite distributions in mass spectrometry imaging. Metabolomics 2014, 10, 337–348. [Google Scholar] [CrossRef]
- Bi, S.; Wang, M.; Pu, Q.; Yang, J.; Jiang, N.; Zhao, X.; Qiu, S.; Liu, R.; Xu, R.; Li, X.; et al. Multi-MSIProcessor: Data visualizing and analysis software for spatial metabolomics research. Anal. Chem. 2024, 96, 339–346. [Google Scholar] [CrossRef]
- Tajima, R. Importance of individual root traits to understand crop root system in agronomic and environmental contexts. Breed. Sci. 2021, 71, 13–19. [Google Scholar] [CrossRef]
- Lei, X.; Shen, Y.; Zhao, J.; Huang, J.; Wang, H.; Yu, Y.; Xiao, C. Root Exudates Mediate the Processes of Soil Organic Carbon Input and Efflux. Plants 2023, 12, 630. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 2019. [Google Scholar] [CrossRef]
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic Stress in Crop Production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef]
- Eckardt, N.A.; Ainsworth, E.A.; Bahuguna, R.N.; Broadley, M.R.; Busch, W.; Carpita, N.C.; Castrillo, G.; Chory, J.; DeHaan, L.R.; Duarte, C.M.; et al. Climate change challenges, plant science solutions. Plant Cell 2023, 35, 24–66. [Google Scholar] [CrossRef]
- Gomez-Zepeda, D.; Frausto, M.; Nájera-González, H.; Herrera-Estrella, L.; Ordaz-Ortiz, J. Mass spectrometry-based quantification and spatial localization of small organic acid exudates in plant roots under phosphorus deficiency and aluminum toxicity. Plant J. 2021, 106, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Witzel, K.; Matros, A. Fructans Are Differentially Distributed in Root Tissues of Asparagus. Cells 2020, 9, 1943. [Google Scholar] [CrossRef]
- Zheng, Z. Carbon and nitrogen nutrient balance signaling in plants. Plant Signal Behav. 2009, 4, 584–591. [Google Scholar] [CrossRef]
- Martín-Cardoso, H.; San Segundo, B. Impact of Nutrient Stress on Plant Disease Resistance. Int. J. Mol. Sci. 2025, 26, 1780. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Bulmwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, M.; Waheed, A.; Wahab, A.; Majeed, M.; Nazim, M.; Liu, Y.; Li, L.; Li, W. Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress 2024, 11, 100319. [Google Scholar] [CrossRef]
- Khan, A.A.; Wang, W.F.; Akbar, R.; Alhoqail, W.A. Mechanistic insights and future perspectives of drought stress management in staple crops. Front. Plant Sci. 2025, 16, 2025. [Google Scholar] [CrossRef]
- Trouvelot, S.; Héloir, M.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef]
- Li, B.; Ge, J.; Liu, W.; Hu, D.; Li, P. Unveiling spatial metabolome of Paeonia suffruticosa and Paeonia lactiflora roots using MALDI MS imaging. New Phytol. 2021, 231, 892–902. [Google Scholar] [CrossRef]
- Li, B.; Bhandari, D.R.; Römpp, A.; Spengler, B. High-resolution MALDI mass spectrometry imaging of gallotannins and monoterpene glucosides in the root of Paeonia lactiflora. Sci. Rep. 2016, 6, 36074. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, N.; Tang, C.; Duan, H.; Wang, Y.; Zhao, G.; Liu, J.; Ye, Y. Differential distribution of characteristic constituents in root, stem and leaf tissues of Salvia miltiorrhiza using MALDI mass spectrometry imaging. Fitoterapia 2020, 146, 104679. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Y.; Wu, C.; Huang, Q. Spatial Distribution and Comparative Analysis of Differential Metabolites in Curcuma longa L. Roots and Rhizomes Using UHPLC-Q-Orbitrap HRMS Combined with DESI-MSI. Phytochem. Anal. 2024, 36, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Kumara, P.M.; Shaanker, R.U.; Pradeep, T. UPLC and ESI-MS analysis of metabolites of Rauvolfia tetraphylla L. and their spatial localization using desorption electrospray ionization (DESI) mass spectrometric imaging. Phytochemistry 2019, 159, 20–29. [Google Scholar] [CrossRef]
- Du, Z.; Zhou, Y.; Guo, S.; Dong, Y.; Xu, Y.; Yu, X. Triterpenoid saponins in tea plants: A spatial and metabolic analysis using UPLC-QTOFMS, molecular networking, and DESI-MSI. Food Chem. 2025, 475, 143323. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Fang, Z.; Zang, Q.; Yang, Y.; Nan, T.; Zhao, Y.; Huang, L. Spatially resolved metabolomics combined with bioactivity analyses to evaluate the pharmacological properties of two Radix Puerariae species. J. Ethnopharmacol. 2023, 313, 116546. [Google Scholar] [CrossRef]
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, X. Reprogramming of Plant Central Metabolism in Response to Abiotic Stresses: A Metabolomics View. Int. J. Mol. Sci. 2022, 23, 5716. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Devireddy, A.R.; Azad, R.K.; Shulaev, V.; Mittler, R. Local and Systemic Metabolic Responses during Light-Induced Rapid Systemic Signaling. Plant Physiol. 2018, 178, 1461–1472. [Google Scholar] [CrossRef]
- Itam, M.; Mega, R.; Tadano, S.; Abdelrahman, M.; Matsunaga, S.; Yamasaki, Y.; Akashi, K.; Tsujimoto, H. Metabolic and physiological responses to progressive drought stress in bread wheat. Sci. Rep. 2020, 10, 17189. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.K.; Veličković, D.; Rubio Wilhelmi, M.D.M.; Anderton, C.R.; Stewart, C.N., Jr.; DiFazio, S.; Blumwald, E.; Ahkami, A.H. Spatiotemporal metabolic responses to water deficit stress in distinct leaf cell-types of poplar. Front. Plant Sci. 2024, 15, 1346853. [Google Scholar] [CrossRef]
- Asakura, H.; Taira, S.; Funaki, J.; Yamakawa, T.; Abe, K.; Asakura, T. Mass Spectrometry Imaging Analysis of Metabolic Changes in Green and Red Tomato Fruits Exposed to Drought Stress. Appl. Sci. 2022, 12, 216. [Google Scholar] [CrossRef]
- Lemaire-Chamley, M.; Mounet, F.; Deborde, C.; Maucourt, M.; Jacob, D.; Moing, A. NMR-Based Tissular and Developmental Metabolomics of Tomato Fruit. Metabolites 2019, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Savatin, D.V.; Gramegna, G.; Modesti, V.; Cervone, F. Wounding in the plant tissue: The defense of a dangerous passage. Front. Plant Sci. 2014, 5, 470. [Google Scholar] [CrossRef]
- Zhang, Y.; Turner, J.G. Wound-Induced Endogenous Jasmonates Stunt Plant Growth by Inhibiting Mitosis. PLoS ONE 2008, 3, e3699. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Gao, Y.Q.; Lenzoni, G.; Wolfender, J.L.; Wu, Q. Wound- and mechanostimulated electrical signals control hormone responses. New Phytol. 2020, 227, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.J.; Fichman, Y.; Stacey, G.; Mittler, R. Extracellular ATP plays an important role in systemic wound response activation. Plant Physiol. 2022, 189, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Plant Responses to Herbivory, Wounding, and Infection. Int. J. Mol. Sci. 2022, 23, 7031. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef]
- Zhang, C.; Žukauskaitė, A.; Petřík, I.; Pěnčík, A.; Hönig, M.; Grúz, J.; Široká, J.; Novák, O.; Doležal, K. In situ characterisation of phytohormones from wounded Arabidopsis leaves using desorption electrospray ionisation mass spectrometry imaging. Analyst 2021, 146, 2653–2663. [Google Scholar] [CrossRef]
- Veličković, D.; Chu, R.K.; Henkel, C.; Nyhuis, A.; Tao, N.; Kyle, J.E.; Adkins, J.N.; Anderton, C.R.; Paurus, V.; Bloodsworth, K.; et al. Preserved and variable spatial-chemical changes of lipids across tomato leaves in response to central vein wounding reveals potential origin of linolenic acid in signal transduction cascade. PEI 2021, 2, 28–35. [Google Scholar] [CrossRef]
- Dai, W.; Hu, Z.; Xie, D.; Tan, J.; Lin, Z. A novel spatial-resolution targeted metabolomics method in a single leaf of the tea plant (Camellia sinensis). Food Chem. 2020, 311, 126007. [Google Scholar] [CrossRef]
- Nakamura, J.; Morikawa-Ichinose, T.; Fujimura, Y.; Hayakawa, E.; Takahashi, K.; Ishii, T.; Miura, D.; Wariishi, H. Spatially resolved metabolic distribution for unraveling the physiological change and responses in tomato fruit using matrix-assisted laser desorption/ionization-mass spectrometry imaging (MALDI-MSI). Anal. Bioanal. Chem. 2017, 409, 1697–1706. [Google Scholar] [CrossRef]
- Korte, A.R.; Yandeau-Nelson, M.D.; Nikolau, B.J.; Lee, Y.J. Subcellular-level resolution MALDI-MS imaging of maize leaf metabolites by MALDI-linear ion trap-Orbitrap mass spectrometer. Anal. Bioanal. Chem. 2015, 407, 2301–2309. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Hashimoto, K.; Mori, T.; Toyooka, K.; Sudo, H.; Saito, K. Spatial metabolomics using imaging mass spectrometry to identify the localization of asparaptine A in Asparagus officinalis. Plant Biotechnol. 2021, 38, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, Y.; Linghu, C.; Xiao, J.; Gu, R. Metabolic profiling, in-situ spatial distribution, and biosynthetic pathway of functional metabolites in Dendrobium nobile stem revealed by combining UPLC-QTOF-MS with MALDI-TOF-MSI. Front. Plant Sci. 2023, 13, 1125872. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Wang, R.Z.; Sun, Z.L.; Su, Y.; Xiao, L.T. A mass spectrometry imaging approach on spatiotemporal distribution of multiple alkaloids in Gelsemium elegans. Front. Plant Sci. 2022, 13, 1051756. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Meng, P.; Tan, G.; Lv, L. Analysis and review of trichomes in plants. BMC Plant Biol. 2021, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Perazza, D.; Herzog, M.; Hülskamp, M.; Brown, S.; Dorne, A.M.; Bonneville, J.M. Trichome cell growth in Arabidopsis thaliana can be derepressed by mutations in at least five genes. Genetics 1999, 152, 461–476. [Google Scholar] [CrossRef]
- Hieta, J.; Sipari, N.; Räikkönen, H.; Keinänen, M.; Kostiainen, R. Mass Spectrometry Imaging of Arabidopsis thaliana Leaves at the Single-Cell Level by Infrared Laser Ablation Atmospheric Pressure Photoionization (LAAPPI). J. Am. Soc. Mass Spectrom. 2021, 32, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, D.H.; Kopischke, M.; Robatzek, S. Gate control: Guard cell regulation by microbial stress. New Phytol. 2014, 203, 1049–1063. [Google Scholar] [CrossRef]
- Dong, H.; Bai, L.; Zhang, Y.; Zhang, G.; Mao, Y.; Min, L.; Xiang, F.; Qian, D.; Zhu, X.; Song, C.P. Modulation of Guard Cell Turgor and Drought Tolerance by a Peroxisomal Acetate–Malate Shunt. Mol. Plant 2018, 11, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- El-Maarouf-Bouteau, H. The Seed and the Metabolism Regulation. Biology 2022, 11, 168. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2015, 67, 567–591. [Google Scholar] [CrossRef]
- Enomoto, H.; Miyamoto, K. Unique localization of jasmonic acid-related compounds in developing Phaseolus vulgaris L. (common bean) seeds revealed through desorption electrospray ionization-mass spectrometry imaging. Phytochemistry 2021, 188, 112812. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; Hanumantha-Rao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef]
- Irik, H.A.; Bikmaz, G. Effect of different salinity on seed germination, growth parameters and biochemical contents of pumpkin (Cucurbita pepo L.) seeds cultivars. Sci. Rep. 2024, 14, 6929. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Li, K.; Cai, Z. Spatially Resolved Metabolomics and Lipidomics Reveal Salinity and Drought-Tolerant Mechanisms of Cottonseeds. J. Agric. Food Chem. 2021, 69, 8028–8037. [Google Scholar] [CrossRef]
- Righetti, L.; Gottwald, S.; Tortorella, S.; Spengler, B.; Bhandari, D.R. Mass Spectrometry Imaging Disclosed Spatial Distribution of Defense-Related Metabolites in Triticum spp. Metabolites 2022, 12, 48. [Google Scholar] [CrossRef]
- Hawes, M.; Allen, C.; Turgeon, B.G.; Curlango-Rivera, G.; Minh Tran, T.; Huskey, D.A.; Xiong, Z. Root Border Cells and Their Role in Plant Defense. Annu. Rev. Phytopathol. 2016, 54, 143–161. [Google Scholar] [CrossRef]
- Watson, B.S.; Bedair, M.F.; Urbanczyk-Wochniak, E.; Huhman, D.V.; Yang, D.S.; Allen, S.N.; Li, W.; Tang, Y.; Sumner, L.W. Integrated metabolomics and transcriptomics reveal enhanced specialized metabolism in Medicago truncatula root border cells. Plant Physiol. 2015, 167, 1699–1716. [Google Scholar] [CrossRef] [PubMed]
- Huisman, R.; Geurts, R. A Roadmap toward Engineered Nitrogen-Fixing Nodule Symbiosis. Plant Commun. 2019, 1, 100019. [Google Scholar] [CrossRef] [PubMed]
- Agtuca, B.; Stopka, S.; Evans, S.; Samarah, L.; Liu, Y.; Xu, D.; Stacey, M.; Koppenaal, D.; Paša-Tolić, L.; Anderton, C.; et al. Metabolomic profiling of wild-type and mutant soybean root nodules using laser-ablation electrospray ionization mass spectrometry reveals altered metabolism. Plant J. 2020, 103, 1937–1958. [Google Scholar] [CrossRef] [PubMed]
- Gani, U.; Vishwakarma, R.A.; Misra, P. Membrane transporters: The key drivers of transport of secondary metabolites in plants. Plant Cell Rep. 2021, 40, 1–18. [Google Scholar] [CrossRef]
- Griffiths, C.A.; Paul, M.J.; Foyer, C.H. Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. Biochim. Biophys. Acta 2016, 1857, 1715–1725. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Long-distance transport in non-vascular plants. Plant Cell Environ. 2003, 26, 73–85. [Google Scholar] [CrossRef]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid responses to abiotic stress: Priming the landscape for the signal transduction network. Trend Plant Sci 2019, 24, 25–37. [Google Scholar] [CrossRef]
- Fichman, Y.; Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020, 102, 887–896. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dang, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal 2009, 2, ra45. [Google Scholar] [CrossRef]
- Christmann, A.; Grill, E.; Huang, J. Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 2013, 16, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Miller, G.; Salazar, C.; Mondal, H.A.; Shulaev, E.; Cortes, D.F.; Shuman, J.L.; Luo, X.; Shah, J.; Schlauch, K.; et al. Temporal–spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 2013, 25, 3553–3569. [Google Scholar] [CrossRef] [PubMed]
- Brechenmacher, L.; Nguyen, T.H.N.; Hixson, K.; Libault, M.; Aldrich, J.; Pasa-Tolic, L.; Stacey, G. Identification of Soybean Proteins from a Single Cell Type: The Root Hair. Proteomics 2012, 12, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Yu, X.; Liu, Z.; Yuan, J.; Qin, A.; Wang, Y.; Chen, Y.; Qin, W.; Liu, Y.; Liu, X.; et al. Spatiotemporal transcriptome and metabolome landscapes of cotton somatic embryos. Nat. Commun. 2025, 16, 859. [Google Scholar] [CrossRef]
- Jozwiak, A.; Panda, S.; Akiyama, R.; Yoneda, A.; Umemoto, N.; Saito, K.; Yasumoto, S.; Muranaka, T.; Gharat, S.A.; Kazachkova, Y.; et al. A cellulose synthase-like protein governs the biosynthesis of Solanum alkaloids. Science 2024, 386, eadq5721. [Google Scholar] [CrossRef] [PubMed]
- Pascual, L.S.; Segarra-Medina, C.; Gómez-Cadenas, A.; López-Climent, M.F.; Vives-Peris, V.; Zandalinas, S.I. Climate change-associated multifactorial stress combination: A present challenge for our ecosystems. J. Plant Physiol. 2022, 276, 153764. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myers, R.J., Jr.; Tretter, Z.M.; Daffron, A.G.; Fritschi, E.X.; Santos, W.T.; Foster, M.L.; Klotz, M.; Stafford, K.M.; Kasch, C.; Taylor, T.J.; et al. Spatially Resolved Plant Metabolomics. Metabolites 2025, 15, 539. https://doi.org/10.3390/metabo15080539
Myers RJ Jr., Tretter ZM, Daffron AG, Fritschi EX, Santos WT, Foster ML, Klotz M, Stafford KM, Kasch C, Taylor TJ, et al. Spatially Resolved Plant Metabolomics. Metabolites. 2025; 15(8):539. https://doi.org/10.3390/metabo15080539
Chicago/Turabian StyleMyers, Ronald J., Jr., Zachary M. Tretter, Abigail G. Daffron, Eric X. Fritschi, William Thives Santos, Maiya L. Foster, Matthew Klotz, Kristin M. Stafford, Christina Kasch, Thomas J. Taylor, and et al. 2025. "Spatially Resolved Plant Metabolomics" Metabolites 15, no. 8: 539. https://doi.org/10.3390/metabo15080539
APA StyleMyers, R. J., Jr., Tretter, Z. M., Daffron, A. G., Fritschi, E. X., Santos, W. T., Foster, M. L., Klotz, M., Stafford, K. M., Kasch, C., Taylor, T. J., Tellefson, L. C., Hartman, T., Hackler, D., Stephen, P., & Sumner, L. W. (2025). Spatially Resolved Plant Metabolomics. Metabolites, 15(8), 539. https://doi.org/10.3390/metabo15080539






