Investigating Immune Checkpoint Inhibitor-Induced Pancreatic Injury: When to Discontinue Cancer Therapy
Abstract
1. Immune Checkpoint Inhibitor-Induced Pancreatic Injury
Introduction, Epidemiology, and Risk Factors
2. Pathogenesis of ICI-Induced Pancreatic Injury
3. Clinical Features of ICI-Induced Pancreatic Injury
4. Diagnostic Workup of ICI-Induced Pancreatic Injury
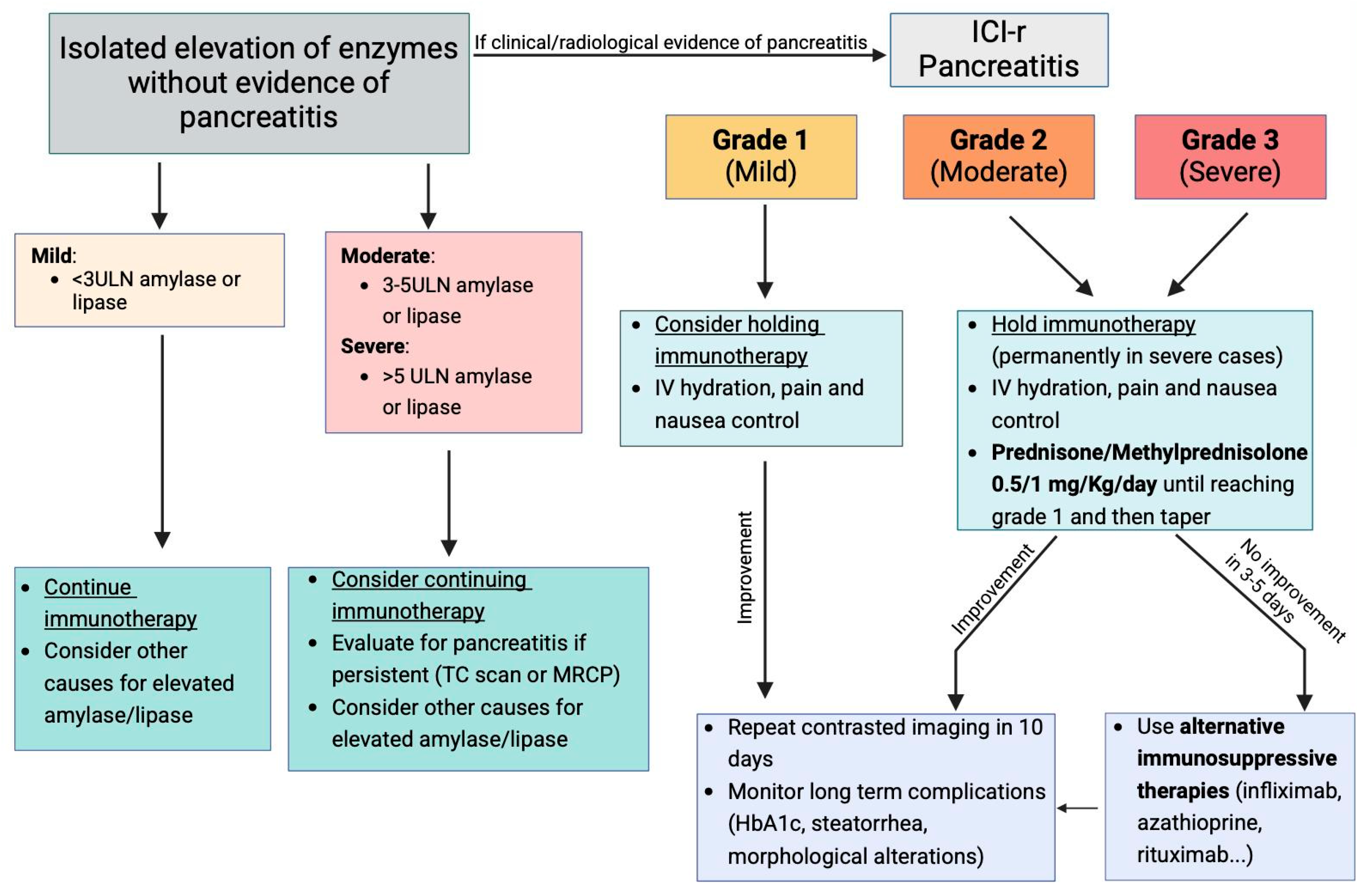
5. Management of ICI-Induced Pancreatic Injury
6. Outcomes
7. Discussion
- -
- Mild Pancreatitis: In these cases, ICIs should be temporarily discontinued, and supportive care, including hydration and control of pain and nausea, should be initiated. If the clinical condition improves with supportive measures, a follow-up radiologic exam should be conducted in 10 days. Long-term complications such as diabetes or steatorrhea should also be evaluated. If the patient continues to improve and no signs of severe complications emerge, the ICIs may potentially be resumed [47].
- -
- Moderate or Severe Pancreatitis: These conditions warrant the immediate and permanent discontinuation of ICI therapy, particularly in severe pancreatitis, where rechallenge with the same ICI is not recommended [47]. In these cases, patients should receive fluid resuscitation, pain management, and anti-nausea treatment, with glucocorticosteroids initiated at a dose of 0.5–1 mg/kg/day for moderate pancreatitis and 1–2 mg/kg/day for severe pancreatitis, although the response to steroids is not always optimal. If no significant clinical improvement is observed within 3–5 days, additional immunosuppressive treatments may be considered. Thus, alternative immunosuppressive agents, such as TNF-α antagonists, have shown promise in treating steroid-refractory ICI-AP [53]. Infliximab, for instance, has been used successfully in cases of ICI-associated colitis, hepatitis, and myositis, and preliminary evidence suggests it may improve outcomes in ICI-AP as well. In cases of relapse following steroid withdrawal, agents like azathioprine and mycophenolate mofetil, which are commonly used in autoimmune pancreatitis, may be considered as maintenance therapies [54]. This approach is supported by the similarities between ICI-associated pancreatitis and autoimmune pancreatitis, both of which are believed to be T-helper cell-mediated processes.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| (ICIs) | Immune checkpoint inhibitors |
| (ICI-PI) | ICI-induced pancreatic injury |
| (irAEs) | Immune-related adverse events |
| (EPI) | Exocrine pancreatic insufficiency |
| (AIP) | Autoimmune pancreatitis |
| (ICI-AP) | ICI-associated pancreatitis |
| (CTLA-4) | CytotoxicT-lymphocyte antigen 4 |
| (PD-1) | Programmed cell death 1 |
| (PD-L1) | Ligand of programmed cell death 1 |
References
- Yoest, J.M. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: A short review. Immunotargets Ther. 2017, 6, 73–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedoeem, A.; Azoulay-Alfaguter, I.; Strazza, M.; Silverman, G.J.; Mor, A. Programmed death-1 pathway in cancer and autoimmunity. Clin. Immunol. 2014, 153, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Dyck, L.; Mills, K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Valentini, D.; Dodoo, E.; Zumla, A.; Maeurer, M. Anti-PD-1/PD-L1 therapy for infectious diseases: Learning from the cancer paradigm. Int. J. Infect. Dis. 2017, 56, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Jilaveanu, L.B.; Shuch, B.; Zito, C.R.; Parisi, F.; Barr, M.; Kluger, Y.; Chen, L.; Kluger, H.M. PD-L1 Expression in Clear Cell Renal Cell Carcinoma: An Analysis of Nephrectomy and Sites of Metastases. J. Cancer 2014, 5, 166–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Collier, K.A.; Wang, P.; Li, Z.; Monk, P.; Mortazavi, A.; Hu, Z.; Spakowicz, D.; Zheng, L.; Yang, Y. Emerging Immunotherapy Approaches for Advanced Clear Cell Renal Cell Carcinoma. Cells 2023, 13, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.; Chehade, R.; Boldt, R.G.; Raphael, J.; Blanchette, P.; Maleki Vareki, S.; Fernandes, R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors—A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 92, 102134. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, A.; Di Vincenzo, F.; Petito, V.; Giustiniani, M.C.; Gasbarrini, A.; Scaldaferri, F.; Lopetuso, L.R. Focus on Immune Checkpoint Inhibitors-Related Intestinal Inflammation: From Pathogenesis to Therapeutical Approach. Inflamm. Bowel Dis. 2024, 30, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Tang, T.; Lu, Y.; Thirumurthi, S.; Altan, M.; Jazaeri, A.A.; Dadu, R.; Coronel, E.; Wang, Y. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J. Immunother. Cancer 2019, 7, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tirumani, S.H.; Ramaiya, N.H.; Keraliya, A.; Bailey, N.D.; Ott, P.A.; Hodi, F.S.; Nishino, M. Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab. Cancer Immunol. Res. 2015, 3, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- George, J.; Bajaj, D.; Sankaramangalam, K.; Yoo, J.W.; Joshi, N.S.; Gettinger, S.; Price, C.; Farrell, J.J. Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: A systematic review and meta-analysis. Pancreatology 2019, 19, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Naitoh, I.; Naiki-Ito, A.; Kawai, T.; Yoshida, M.; Kato, A.; Kachi, K.; Sahashi, H.; Adachi, A.; Toyohara, T.; et al. Incidence of Pancreatic Injury and Pancreatitis in Patients Treated With Immune Checkpoint Inhibitors. Clin. Transl. Gastroenterol. 2024, 15, e00667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Shi, C.; Liu, X.; Lv, S.; Wang, X.; Li, W. Pancreatic injury following immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 955701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, Q.; Zhang, X.C.; Zhang, C.G.; Hou, Y.L.; Yao, Y.X.; Cao, B.W. Risk of Immune-Related Pancreatitis in Patients with Solid Tumors Treated with Immune Checkpoint Inhibitors: Systematic Assessment with Meta-Analysis. J. Immunol. Res. 2018, 2018, 1027323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, X.; Yao, Z.; Bai, H.; Duan, J.; Wang, Z.; Wang, X.; Zhang, X.; Xu, J.; Fei, K.; Zhang, Z.; et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: A systematic review and meta-analysis. Lancet Oncol. 2021, 22, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Göppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Kohlmann, J.; Wagenknecht, D.; Simon, J.C.; Ziemer, M. Immune-related pancreatitis associated with checkpoint blockade in melanoma. Melanoma Res. 2019, 29, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, B.; Zhang, C.; Xu, T. Immune-related adverse events associated with immune checkpoint inhibitors: An updated comprehensive disproportionality analysis of the FDA adverse event reporting system. Int. Immunopharmacol. 2021, 95, 107498. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1. 2020. J. Natl. Compr. Cancer Netw. 2020, 18, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Sakai, A.; Tsumura, H.; Iemoto, T.; Hirata, Y.; Hori, H.; Ogisu, K.; Kakuyama, S.; Ikegawa, T.; Hirata, T.; et al. Pancreatic injury in patients treated with immune checkpoint inhibitors: A retrospective multicenter study. J. Gastroenterol. 2024, 59, 424–433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, T.; Kudo, M.; Strober, W. Immunopathogenesis of pancreatitis. Mucosal Immunol. 2017, 10, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, O.C.; Lara-Salazar, F.M.; Lara-Salazar, S.; Abdulrahim, A.O.; Chijioke, I.; Singh, J.; Koradia, I.; Gomez, N.M.; Prakash, R.; Gopagoni, R.; et al. Immune Checkpoint Inhibitors in Cancer Treatment and Incidence of Pancreatitis. Cureus 2024, 16, e68043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sullivan, R.J.; Weber, J.S. Immune-related toxicities of checkpoint inhibitors: Mechanisms and mitigation strategies. Nat. Rev. Drug Discov. 2022, 21, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Troiani, T.; Criscuolo, G.; Marone, G.; Ciardiello, F.; Tocchetti, C.G.; Varricchi, G. Holistic Approach to Immune Checkpoint Inhibitor-Related Adverse Events. Front. Immunol. 2022, 13, 804597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, L.C.; Dorak, M.T.; Bettinotti, M.P.; Bingham, C.O.; Shah, A.A. Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology 2019, 58, 476–480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akturk, H.K.; Kahramangil, D.; Sarwal, A.; Hoffecker, L.; Murad, M.H.; Michels, A.W. Immune checkpoint inhibitor-induced Type 1 diabetes: A systematic review and meta-analysis. Diabet. Med. 2019, 36, 1075–1081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Zhang, H.; Zhou, L.; Li, W.; Yang, L.; Li, W.; Li, K.; Liu, X. Immunotherapy-Associated Pancreatic Adverse Events: Current Understanding of Their Mechanism, Diagnosis, and Management. Front. Oncol. 2021, 11, 627612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Giacomo, A.M.; Danielli, R.; Guidoboni, M.; Calabrò, L.; Carlucci, D.; Miracco, C.; Volterrani, L.; Mazzei, M.A.; Biagioli, M.; Altomonte, M.; et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: Clinical and immunological evidence from three patient cases. Cancer Immunol. Immunother. 2009, 58, 1297–1306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, F.; Vyas, M.; Gosse, M.; Deshpande, V.; Rosenbaum, M.W. Clinicopathologic Characteristics of Immune Checkpoint Inhibitor-related Pancreatitis. Am. J. Surg. Pathol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Nakano, R.; Shiomi, H.; Fujiwara, A.; Yoshihara, K.; Yoshioka, R.; Kawata, S.; Ota, S.; Yuri, Y.; Takashima, T.; Aizawa, N.; et al. Clinical Characteristics of ICI-Related Pancreatitis and Cholangitis Including Radiographic and Endoscopic Findings. Healthcare 2022, 10, 763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasanna, T.; McNeil, C.M.; Nielsen, T.; Parkin, D. Isolated immune-related pancreatic exocrine insufficiency associated with pembrolizumab therapy. Immunotherapy 2018, 10, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Das, J.P.; Postow, M.A.; Friedman, C.F.; Do, R.K.; Halpenny, D.F. Imaging findings of immune checkpoint inhibitor associated pancreatitis. Eur. J. Radiol. 2020, 131, 109250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, B.; Chen, M.J.; Guo, Q.; Tang, H.; Li, Y.; Jia, X.M.; Xu, Y.; Zhu, L.; Wang, M.Z.; Qian, J.M. Clinical-radiological characteristics and intestinal microbiota in patients with pancreatic immune-related adverse events. Thorac. Cancer 2021, 12, 1814–1823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Capurso, G.; Archibugi, L.; Tessieri, L.; Petrone, M.C.; Laghi, A.; Arcidiacono, P.G. Focal immune-related pancreatitis occurring after treatment with programmed cell death 1 inhibitors: A distinct form of autoimmune pancreatitis? Eur. J. Cancer 2018, 95, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Ono, K. Nivolumab-induced Pancreatitis: An Immune-related Adverse Event. Radiology 2019, 293, 521. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Sakai, A.; Kobayashi, T.; Masuda, A.; Shiomi, H.; Kodama, Y. Nivolumab-related pancreatitis with autoimmune pancreatitis-like imaging features. J. Gastroenterol. Hepatol. 2019, 34, 1274. [Google Scholar] [CrossRef] [PubMed]
- Ofuji, K.; Hiramatsu, K.; Nosaka, T.; Naito, T.; Takahashi, K.; Matsuda, H.; Ohtani, M.; Imamura, Y.; Ishizuka, T.; Nakamoto, Y. Pembrolizumab-induced autoimmune side effects of colon and pancreas in a patient with lung cancer. Clin. J. Gastroenterol. 2021, 14, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Janssens, L.; Takahashi, N.; Majumder, S. Pancreatic Atrophy in Nivolumab-Associated Pancreatitis Mimics Autoimmune Pancreatitis. Pancreas 2021, 50, e28–e29. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Zaid, M.A.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bermas, B.; Braaten, T.; Budde, L.E.; et al. NCCN Guidelines® Insights: Management of Immunotherapy-Related Toxicities, Version 2. 2024. J. Natl. Compr. Cancer Netw. 2024, 22, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126, Erratum in J. Clin. Oncol. 2022, 40, 315. https://doi.org/10.1200/JCO.21.02786. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.J.; Liu, M.; Giobbie-Hurder, A.; Sack, J.S.; LeBoeuf, N.R.; Hodi, F.S.; McNabb-Baltar, J.; Grover, S. Pancreatitis and Hyperlipasemia in the Setting of Immune Checkpoint Inhibitor Therapy. J. Natl. Compr. Cancer Netw. 2023, 21, 831–840.e3. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.S.; Abreo, M.; Ahmed, A.S.; Rao Manikonda, S.P.; Eyada, M.; Issac, A.; Abraham, F.; Jacob, J.S.; Wang, Y.; Yedururi, S.; et al. Immune Checkpoint Inhibitor-Induced Pancreatic Injury: Clinical and Radiological Profile and Response to Steroids. Gastro Hep Adv. 2023, 3, 361–367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruf, T.; Kramer, R.; Forschner, A.; Leiter, U.; Meier, F.; Reinhardt, L.; Dücker, P.; Ertl, C.; Tomsitz, D.; Tietze, J.K.; et al. Second-line therapies for steroid-refractory immune-related adverse events in patients treated with immune checkpoint inhibitors. Eur. J. Cancer 2024, 203, 114028. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Guo, Y.; He, Y.; Wang, C. Clinical characteristics, treatment and outcome of pembrolizumab-induced acute pancreatitis. Investig. New Drugs 2024, 42, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, S.; Ishigami, K.; Yokoyama, Y.; Kazama, T.; Masaki, Y.; Takahashi, M.; Yoshii, S.; Yamano, H.O.; Chiba, H.; Nakase, H. Immune-related colitis and pancreatitis treated with infliximab. Clin. J. Gastroenterol. 2023, 16, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.; van Hee, K.; Blokzijl, H.; van der Heide, F.; Visschedijk, M.C. Immune Checkpoint Inhibitor-related Pancreatitis: A Case Series, Review of the Literature and an Expert Opinion. J. Immunother. 2023, 46, 271–275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santoro, A.; Masini, S.; Cavina, R.; Tronconi, M.C.; De Vincenzo, F. Rituximab in steroid-refractory immune-related pancreatitis: A case report. Front. Oncol. 2023, 13, 1205720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verheijden, R.J.; van Eijs, M.J.M.; May, A.M.; van Wijk, F.; Suijkerbuijk, K.P.M. Immunosuppression for immune-related adverse events during checkpoint inhibition: An intricate balance. NPJ Precis. Oncol. 2023, 7, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horvat, T.Z.; Adel, N.G.; Dang, T.O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef] [PubMed]
- Parvathareddy, V.; Selamet, U.; Sen, A.A.; Mamlouk, O.; Song, J.; Page, V.D.; Abdelrahim, M.; Diab, A.; Abdel-Wahab, N.; Abudayyeh, A. Infliximab for Treatment of Immune Adverse Events and Its Impact on Tumor Response. Cancers 2023, 15, 5181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shirwaikar Thomas, A.; Chari, S.T. Immune Checkpoint Inhibitor-Induced (Type 3) Autoimmune Pancreatitis. Curr. Gastroenterol. Rep. 2023, 25, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Satish, D.; Lin, I.H.; Flory, J.; Gerdes, H.; Postow, M.A.; Faleck, D.M. Exocrine Pancreatic Insufficiency Induced by Immune Checkpoint Inhibitors. Oncologist 2023, 28, 1085–1093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koldenhof, J.J.; Suijkerbuijk, K.P.M. Diarrhoea during checkpoint blockade, not always colitis. Eur. J. Cancer 2017, 87, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.S.; Sarwar, N.; Goldin, R.D.; Dhar, A.; Possamai, L.A. Pembrolizumab-Induced Pancreatic Exocrine Insufficiency Complicated by Severe Hepatic Steatosis. Cureus 2022, 14, e26596. [Google Scholar] [CrossRef]
- Zhang, Z.; Sharma, R.; Hamad, L.; Riebandt, G.; Attwood, K. Incidence of diabetes mellitus in patients treated with immune checkpoint inhibitors (ICI) therapy—A comprehensive cancer center experience. Diabetes Res. Clin. Pract. 2023, 202, 110776. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Riya, I.J.; Piya, I.J.; Muniz, T.P.; Butler, M.O.; Saibil, S.D. Immune Checkpoint Inhibitor-Induced Pancreatic Injury (ICI-PI) in Adult Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 1080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marchand, L.; Disse, E.; Dalle, S.; Reffet, S.; Vouillarmet, J.; Fabien, N.; Thivolet, C.; Cugnet-Anceau, C. The multifaceted nature of diabetes mellitus induced by checkpoint inhibitors. Acta Diabetol. 2019, 56, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Kani, E.R.; Karaviti, E.; Karaviti, D.; Gerontiti, E.; Paschou, I.A.; Saltiki, K.; Stefanaki, K.; Psaltopoulou, T.; Paschou, S.A. Pathophysiology, diagnosis, and management of immune checkpoint inhibitor-induced diabetes mellitus. Endocrine 2025, 87, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Chi Zhang, H.; Peng, Y.; Lu, Y.; Wang, Y. Severe fistulizing pancreatitis in a patient with Merkel cell carcinoma treated with avelumab. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1266–1267. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Day, D.; Nicholls, S.J.; Segelov, E. Immune Checkpoint Inhibitor Therapy in Oncology: Current Uses and Future Directions: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022, 4, 579–597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogers, B.B.; Cuddahy, T.; Zawislak, C. Management of Acute Pancreatitis Associated With Checkpoint Inhibitors. J. Adv. Pract. Oncol. 2020, 11, 49–62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Author, Year, Study Type | Therapy Type | Inhibitor Class | All-Grade PI (%) | Grade ≥ 3 PI (%) | Notes |
|---|---|---|---|---|---|
| George et al. (2019) [16] Systematic review and meta-analysis | Monotherapy | PD-1 | 0.94% (95% CI: 0.48–1.40) | Not specified | Lower PI incidence compared with CTLA-4 inhibitors |
| Su et al. (2018) [20] Systematic review and meta-analysis | Monotherapy | CTLA-4 | 3.98% (95% CI: 2.92–5.05) | Not specified | Higher PI incidence than PD-1 inhibitors |
| Hofmann et al. (2016) [22] Retrospective study | Combination | PD-1 + CTLA-4 | 10.6% (95% CI: 7.89–13.32) | Not specified | Additive increase in PI incidence with combination therapy |
| Zhou et al. (2021) [21] Systematic review and meta-analysis | Monotherapy | PD-1 | 2.0% (95% CI: 1.67–2.39) | 1.8% (95% CI: 1.41–2.29) | Lower severe PI incidence |
| Bagchi et al. (2021) [23] Review | Monotherapy | PD-L1 | 3.01% (95% CI: 1.86–4.87) | 3.1% (95% CI: 1.7–5.64) | Higher PI incidence compared with PD-1 inhibitors |
| Thompson et al. (2020) [26] Guidelines | Monotherapy | CTLA-4 | 2.92% (95% CI: 0.99–8.65) | 2.69% (95% CI: 0.76–9.49) | PI incidence similar to PD-L1 inhibitors |
| Feature | True irAE Pancreatitis | Benign Enzyme Elevation |
|---|---|---|
| Lipase/Amylase Levels | >3× ULN (often >5× ULN) | Mild/moderate elevation, often <3× ULN |
| Abdominal Symptoms | Present (e.g., epigastric pain, nausea, vomiting) | Absent |
| Imaging (CT/MRI/US) | Findings consistent with pancreatitis (edema, swelling) | Normal |
| Timing | Typically, within 4–12 weeks of ICI initiation | Variable; may be incidental |
| Other Laboratory Abnormalities | ↑ CRP, ↑ WBC, possible ↑ transaminases or bilirubin | None or mild inflammatory markers |
| Clinical Course | May require hospitalization and immunosuppression | Self-limiting; often resolves spontaneously |
| Management | Steroids ± ICI interruption; imaging follow-up needed | Monitoring only; no need for steroids or imaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nista, E.C.; De Lucia, S.S.; Archilei, S.; Iaccarino, J.; Piccirilli, G.; Nicoletti, A.; Saviano, A.; Gasbarrini, A.; Ojetti, V. Investigating Immune Checkpoint Inhibitor-Induced Pancreatic Injury: When to Discontinue Cancer Therapy. Metabolites 2025, 15, 385. https://doi.org/10.3390/metabo15060385
Nista EC, De Lucia SS, Archilei S, Iaccarino J, Piccirilli G, Nicoletti A, Saviano A, Gasbarrini A, Ojetti V. Investigating Immune Checkpoint Inhibitor-Induced Pancreatic Injury: When to Discontinue Cancer Therapy. Metabolites. 2025; 15(6):385. https://doi.org/10.3390/metabo15060385
Chicago/Turabian StyleNista, Enrico Celestino, Sara Sofia De Lucia, Sebastiano Archilei, Jacopo Iaccarino, Giulia Piccirilli, Alberto Nicoletti, Angela Saviano, Antonio Gasbarrini, and Veronica Ojetti. 2025. "Investigating Immune Checkpoint Inhibitor-Induced Pancreatic Injury: When to Discontinue Cancer Therapy" Metabolites 15, no. 6: 385. https://doi.org/10.3390/metabo15060385
APA StyleNista, E. C., De Lucia, S. S., Archilei, S., Iaccarino, J., Piccirilli, G., Nicoletti, A., Saviano, A., Gasbarrini, A., & Ojetti, V. (2025). Investigating Immune Checkpoint Inhibitor-Induced Pancreatic Injury: When to Discontinue Cancer Therapy. Metabolites, 15(6), 385. https://doi.org/10.3390/metabo15060385










