Broadly Targeted Metabolomics Analysis of Differential Metabolites Between Bupleurum chinense DC. and Bupleurum scorzonerifolium Willd.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sample Preparation and Extraction
2.3. UPLC Conditions
2.4. ESI-Q TRAP-MS/MS
2.5. Qualitative and Quantitative Determination of Metabolites
2.6. Quality Control Sample Analysis
2.7. Statistical Analysis
3. Results
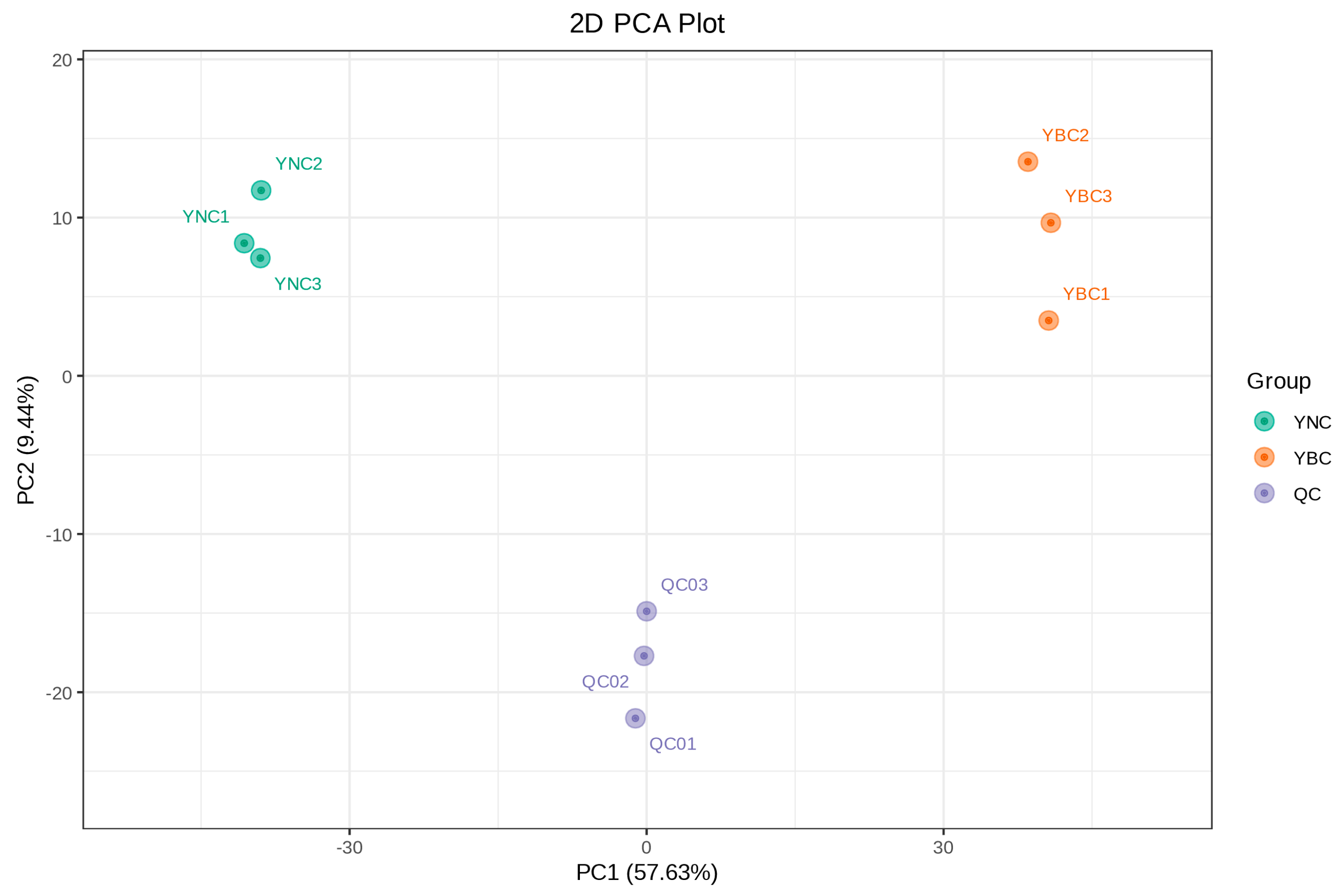
3.1. Quality Control Sample Analysis
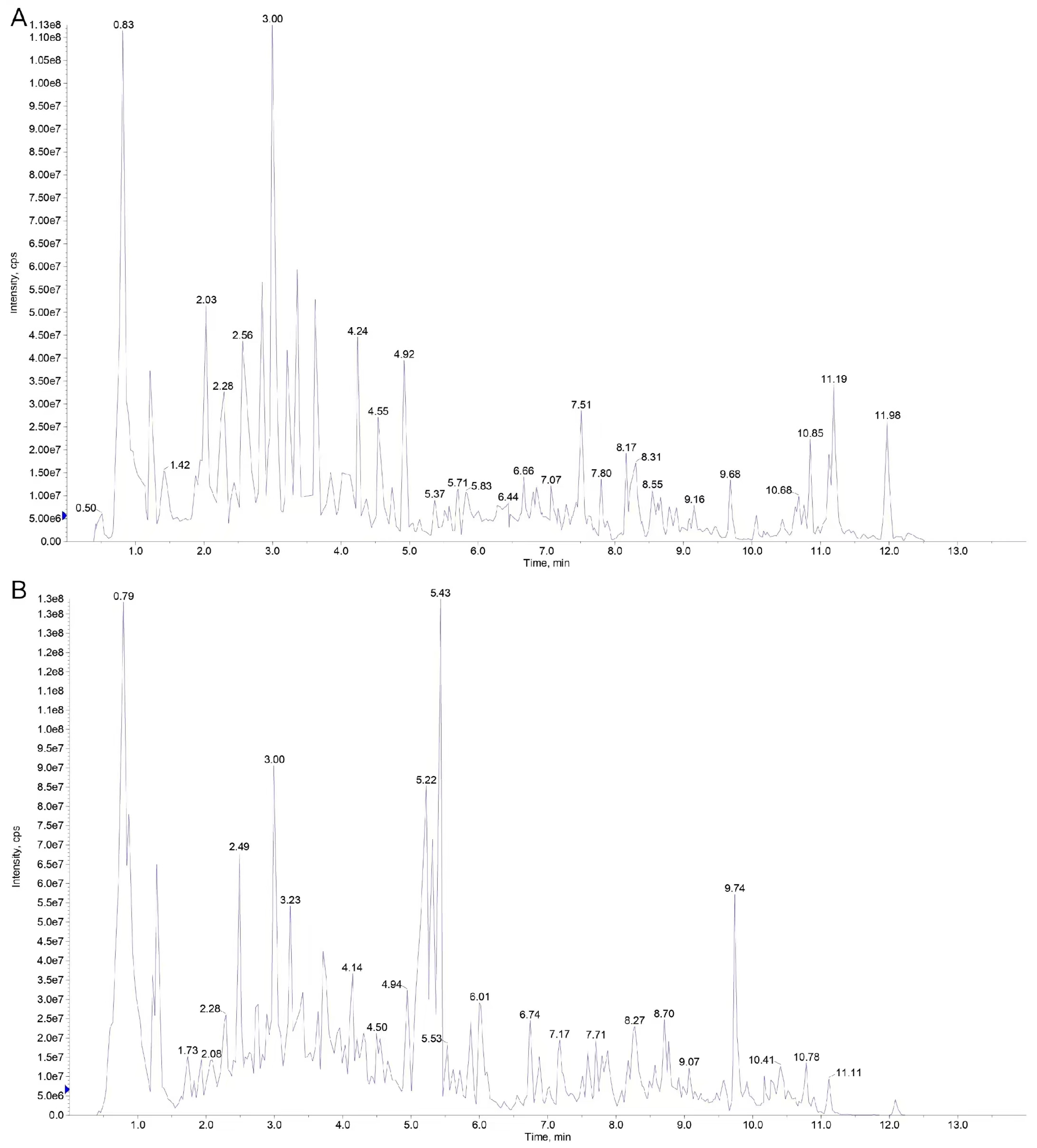
3.2. Metabolite Detection
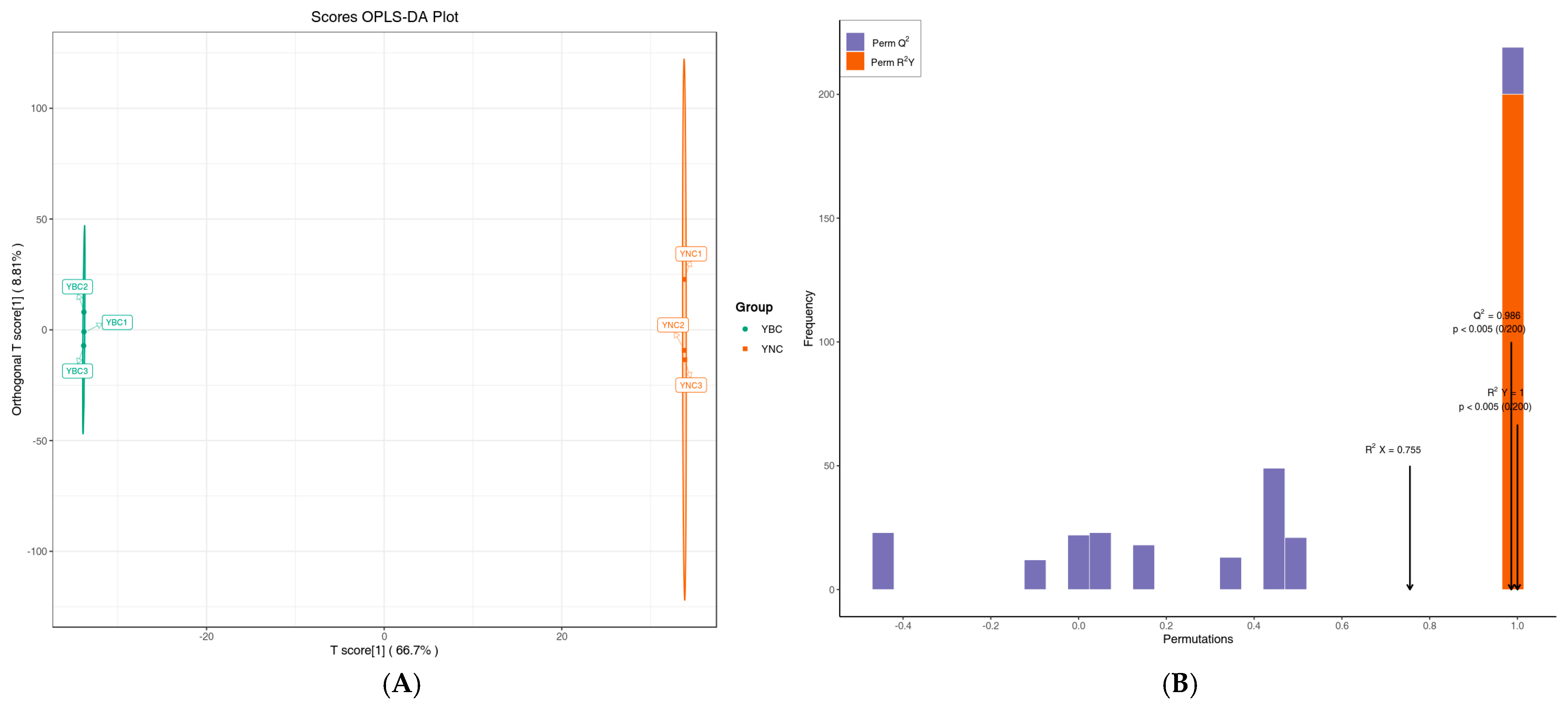
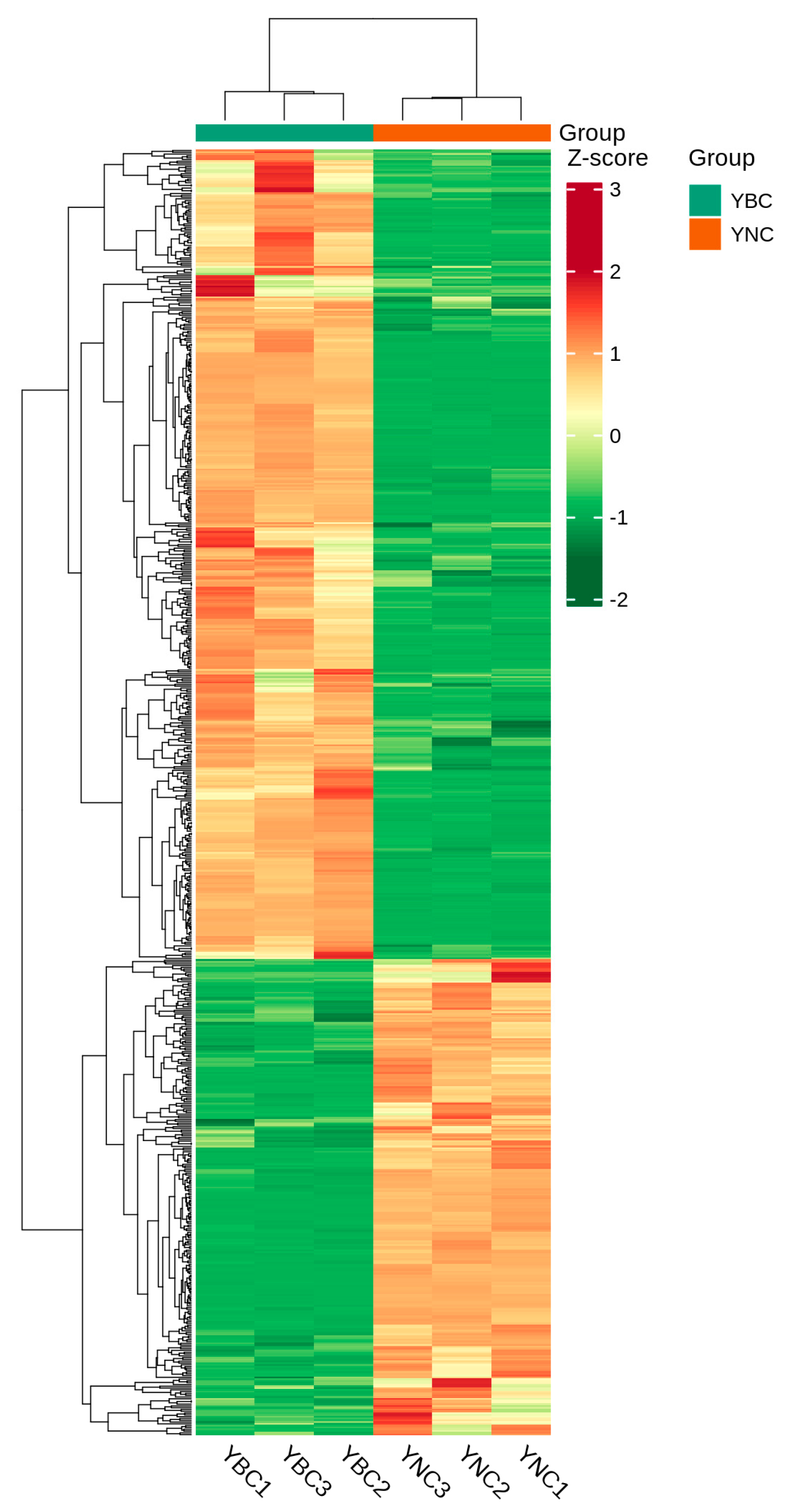
3.3. Analysis of Metabolomics Difference Between YBC and YNC by Multivariate Analysis
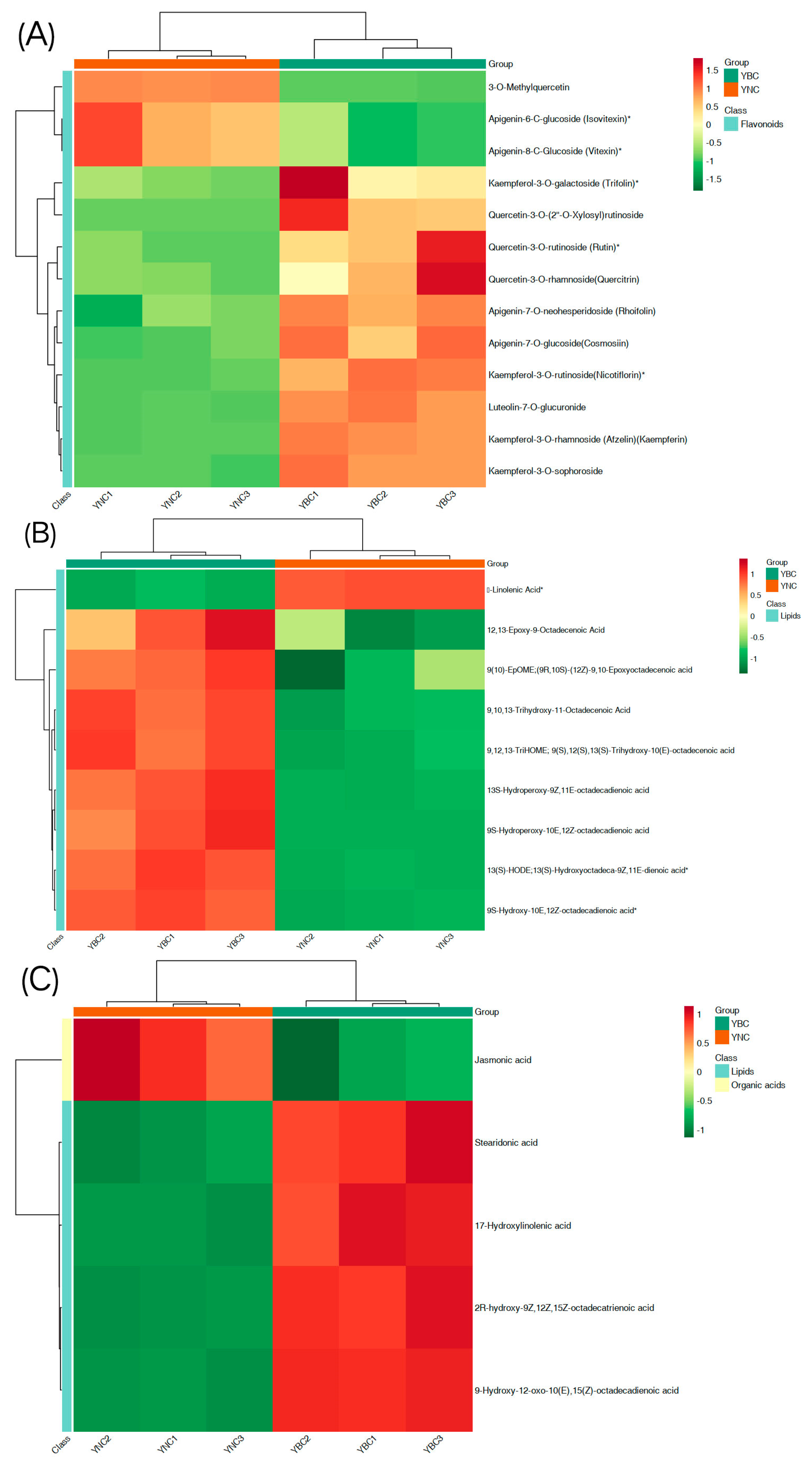
3.4. Different Metabolite Analyses
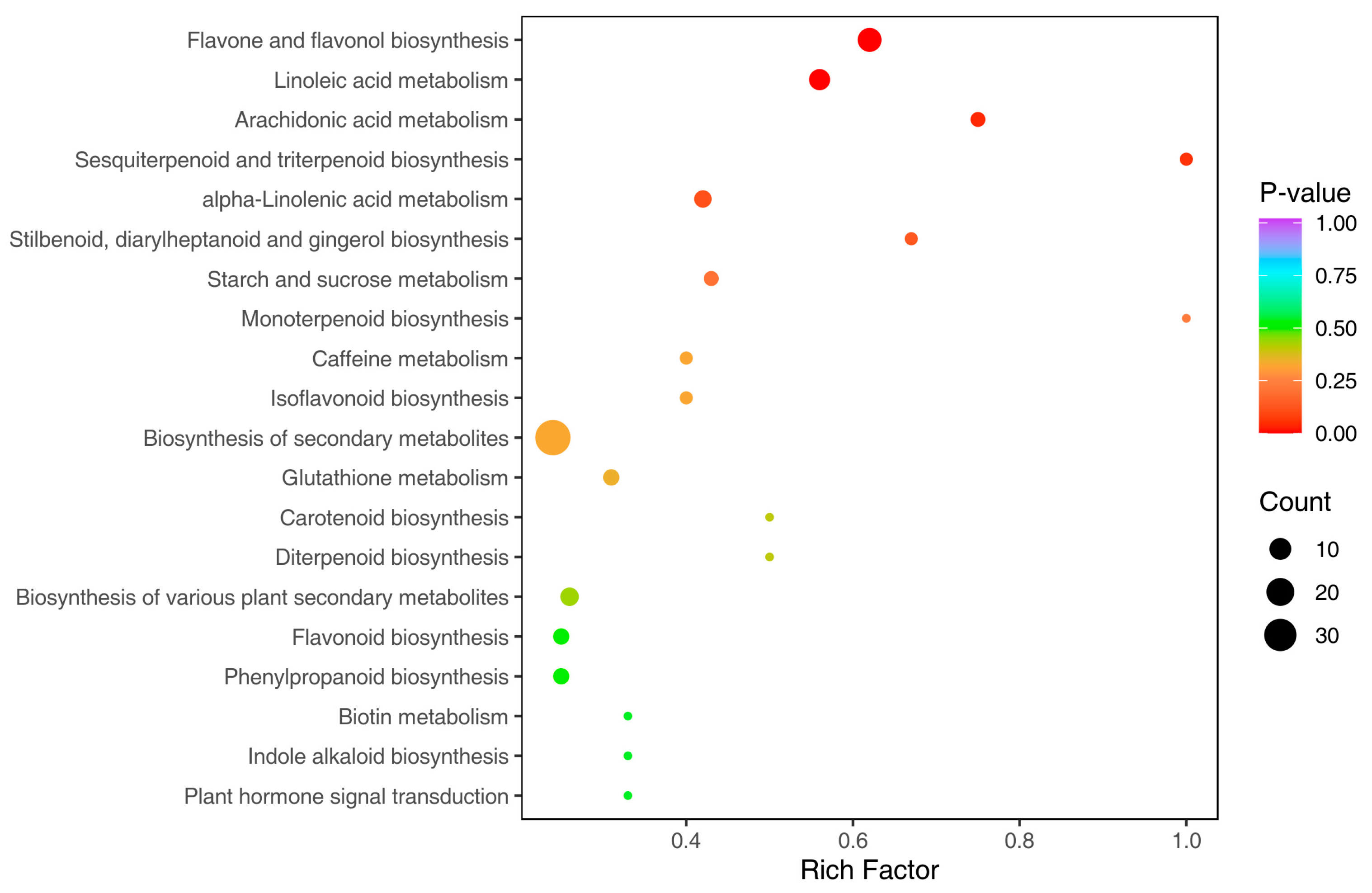
3.5. Kyoto Encyclopedia of Genes and Genomes Annotation and Enrichment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pharmacopoeia of the People’s Republic of China: Part I, 2020 ed.; China Medical Science Press: Beijing, China, 2015; pp. 156–157.
- Sun, P.; Li, Y.; Wei, S.; Zhao, T.; Wang, Y.; Song, C.; Xue, L.; Wang, F.; Xiao, L.; Wu, J. Pharmacological effects and chemical constituents of bupleurum. Mini Rev. Med. Chem. 2019, 19, 34–55. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, L.; Hou, A.; Zhang, J.; Wang, S.; Man, W.; Zheng, S.; Yu, H.; Wang, X.; Yang, B.; et al. Botany, traditional uses, phytochemistry, analytical methods, processing, pharmacology and pharmacokinetics of Bupleuri Radix: A systematic review. Biomed. Pharmacother. 2020, 131, 110679. [Google Scholar] [CrossRef]
- Li, L.; Chen, L.; Hu, X.; Chen, C.; Zheng, X. Research Progress on Chemical Components and Pharmacological Effects of Radix Bupleuri. Acta Chin. Med. Pharmacol. 2023, 51, 109–112. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, X.; Tong, L.; Wang, H.; Qin, X. Comparison of chemical constituents of Bupleurum marginatum var. stenophyllum and Bupleurum chinense DC. using UHPLC–Q-TOF–MS based on a metabonomics approach. Biomed. Chromatogr. 2021, 35, e5133. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, X.; Lin, M.; Shen, Y.; Yan, S.; Zhang, W. Characterization and identification of saikosaponins in crude extracts from three Bupleurum species using LC-ESI-MS. J. Sep. Sci. 2008, 31, 3190–3201. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, X. Research Progress on Chemical Composition and Quality Control Methods of Bupleurum chinense. Mod. Chin. Med. 2021, 23, 940–949. [Google Scholar]
- Yuan, B.; Yang, R.; Ma, Y.; Zhou, S.; Zhang, X.; Liu, Y. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm. Biol. 2017, 55, 620. [Google Scholar] [CrossRef]
- Chen, S.; Wang, K.; Wang, H.; Gao, Y.; Nie, K.; Jiang, X. The therapeutic effects of saikosaponins on depression through the modulation of neuroplasticity: From molecular mechanisms to potential clinical applications. Pharmacol. Res. 2024, 201, 107090. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Gao, X.; Qin, X. To investigate the difference, clinical application and toxicity of Bupleurum. J. Chin. Med. Mater. 2019, 42, 2200–2205. [Google Scholar]
- Zhang, G. Survey and Identification of Cultivated Bupleurum spp. Germplasm Resources in China. Ph.D. Thesis, Peking Union Medical College, Beijing, China, 2020. [Google Scholar]
- Wang, W.; Du, X.; Hou, B.; Zhu, H.; Yu, D. Correlations between appearance traits and internal quality of Bupleurum scorzonerifolium roots based on theory of “quality evaluation through morphological identification”. China J. Chin. Mater. Medica 2023, 48, 1815–1823. [Google Scholar]
- Liang, Z.; Liu, L.; Chao, Z. Investigation of Medicinal Bupleurum Resources and Current situation of Chaihu Production. Lishizhen Med. Mater. Medica Res. 2012, 23, 2011–2013. [Google Scholar]
- Sun, R.; Wang, L.; Ren, H.; Lv, L.; Yin, J. Experimental Research of Influences on Acute Toxicity and Saponins Substances Caused by Different Original of Bupleurum chinense. Chin. J. Pharmacovigil. 2009, 6, 705–708. [Google Scholar]
- Zhang, S.; Xing, J.; Li, Z.; Qin, X. Metabolic Essential Oil of Different Radix Bupleuri by GC-MS. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 84–87. [Google Scholar]
- Guo, X.Q.; Tian, J.S.; Shi, B.Y.; Xing, J.; Jia, J.P.; Zhang, L.Z. 1H-NMR metabonomics on antidepression of Xiaoyao Powder composed of Bupleurum scorzonerifolium or Bupleurum chinense. Chin. Tradit. Herb. Drugs 2012, 43, 2209–2216. [Google Scholar]
- Kim, S.; Lim, S.-W.; Choi, J. Drug discovery inspired by bioactive small molecules from nature. Anim. Cell Syst. 2022, 26, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Hu, S.; Li, T.; Zhang, J.; Wang, B.; Liu, C. Metabolomics Analysis Reveals the Differences Between Bupleurum chinense DC. and Bupleurum scorzonerifolium Willd. Front Plant Sci. 2022, 13, 933849. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, H.; Liu, S.; Li, Y.; Sui, C.; Hou, D.; Wei, J. Differential Expression of Genes Involved in Saikosaponin Biosynthesis Between Bupleurum chinense DC. and Bupleurum scorzonerifolium Willd. Front. Genet. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, C.; Wu, Z.; Jin, Y.; Cao, D.; Wu, S. Overview on Application of Metabonomics in Study on Processing Mechanism of Traditional Chinese Materia Medica. Chin. Arch. Tradit. Chin. Med. 2018, 36, 275–279. [Google Scholar]
- Chen, L. Study on Quality Evaluation of Bupleurum chinensis. Ph.D. Thesis, Harbin University of Commerce, Harbin, China, 2024. [Google Scholar]
- Zhou, X.; Yang, G.; Zhou, L.; Shao, A.; Wei, Y.; Chi, X.; Li, X. Study on Characteristics and Microscopic identification of Fruits of Bupleurum chinense and Bupleurum scorzonerifolium. Mod. Chin. Med. 2018, 20, 1251–1254. [Google Scholar]
- Zou, S.; Wu, J.; Shahid, M.; He, Y.; Lin, S.; Liu, Z.; Yang, X. Identification of key taste components in loquat using widely targeted metabolomics. Food Chem. 2020, 323, 126822. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Liu, H.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.; Clowers, B.; Moore, R.; Zink, E. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Kettanehwold, N.; Trygg, J. Multi and Megavariate Data Analysis: Part I: Basic Principles and Applications; Umetrics Inc.: Umeaa, Sweden, 2006. [Google Scholar]
- Wang, Y.; Yuan, C.; Qian, J.; Wang, Y.; Liu, Y.; Liu, Y.; Nan, T.; Kang, L.; Zhan, Z.; Guo, L.; et al. Reviews and Recommendations in Comparative Studies on Quality of Wild and Cultivated Chinese Crude Drugs. Chin. J. Exp. Tradit. Med. Formulae 2024, 30, 1–20. [Google Scholar]
- Thévenot, E.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Zhu, L.; Liang, Z.; Yi, T.; Ma, Y.; Zhao, Z.; Guo, B.; Zhang, J.; Chen, H. Comparison of chemical profiles between the root and aerial parts from three Bupleurum species based on a UHPLC-QTOF-MS metabolomics approach. BMC Complement. Altern. Med. 2017, 17, 305. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.; Chen, X.; Liu, G.; Zhang, Z.; Tan, Q.; Hu, Y.; Fan, Y.; Liu, Y.; Zhu, T.; et al. Chromosome-Level Genome Assembly of Bupleurum chinense DC Provides Insights into the Saikosaponin Biosynthesis. Front. Genet. 2022, 13, 878431. [Google Scholar] [CrossRef]
- Li, P.; Song, J.; Guan, H.; Zhang, Y.; Zhou, G. Analysis and Countermeasures on Use of Endangered Wild Fauna and Floran Resources in Traditional Chinese Medicine. Food Drug 2021, 23, 168–172. [Google Scholar]
- Zhang, C.; Sun, J.; Wang, Y.; Wang, H.; Guo, X.; Yan, B.; Sun, Z.; Wang, S.; Guo, L. Research progress in microevolutionary process of excellent traits and quality of Dao-di herbs. China J. Chin. Mater. Medica 2023, 48, 6021–6029. [Google Scholar]
- Wang, C.; Zhao, H.; Yan, Y.; Shi, J.; Chen, S.; Tan, M.; Chen, J.; Liu, Z.; Chen, C.; Zou, L.; et al. Thoughts of omics research on quality formation in Dao-di herbs. China J. Chin. Mater. Medica 2018, 43, 2407–2412. [Google Scholar]
- Zhao, M.; Xiao, L.; Ke, G.; Zhao, G.; Chen, Q.; Shen, L.; Parsa, D.; Chen, M.; Hu, Y.; Zhang, J.; et al. Comprehensive comparison on the anti-inflammation and GC-MS-based metabolomics discrimination between Bupleuri chinense DC. and B. scorzonerifolium Willd. Front. Pharmacol. 2022, 13, 1005011. [Google Scholar] [CrossRef]
- Moghadam, A.; Taghizadeh, M.; Haghi, R.; Tahmasebi, A.; Niazi, A.; Ebrahimie, E. Exploring novel insights: Methyl jasmonate treatment reveals novel lncRNA-mediated regulation of secondary metabolite biosynthesis pathways in Echinacea purpurea. Food Biosci. 2023, 52, 103457. [Google Scholar] [CrossRef]

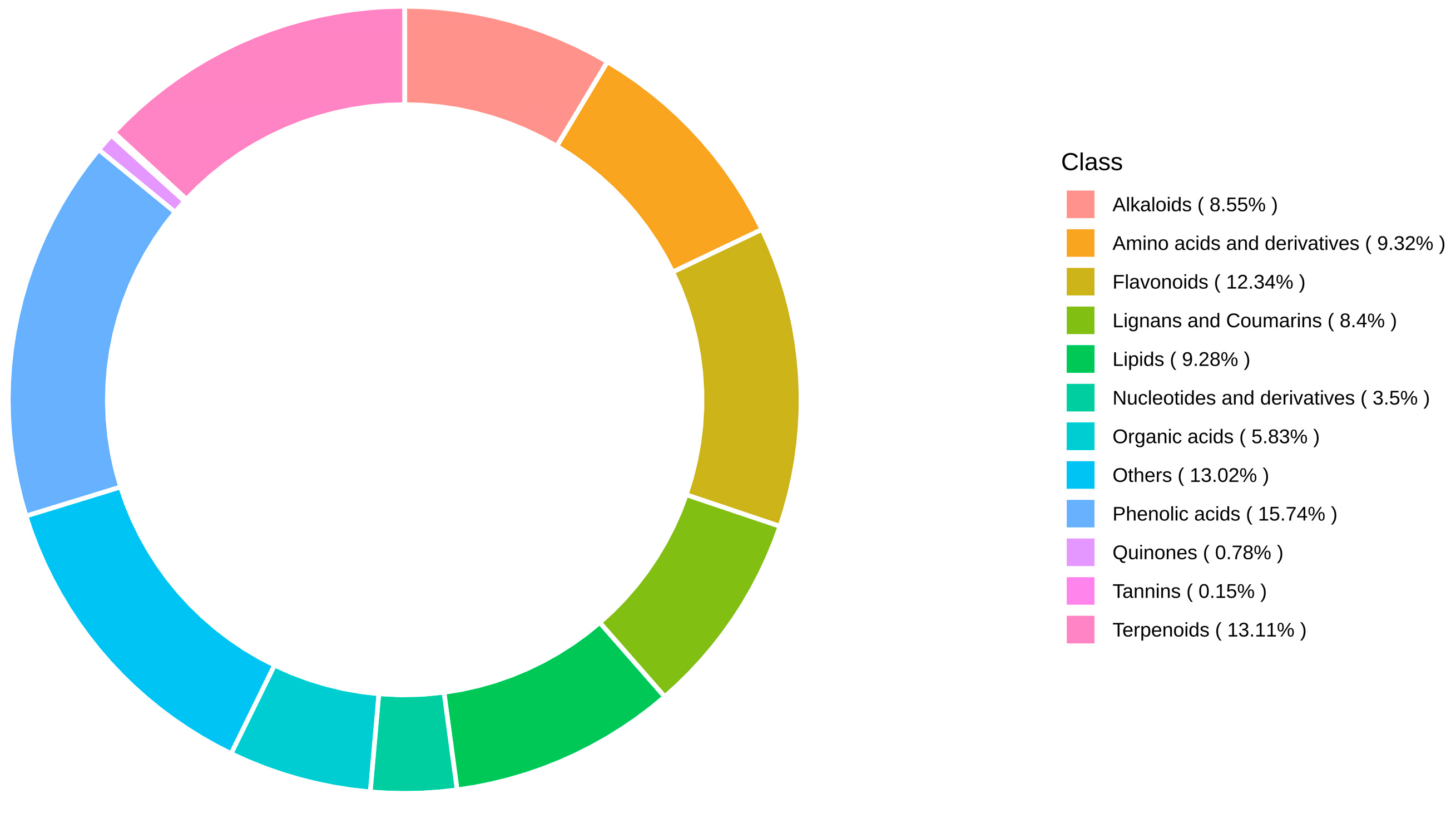
| Total | Common | Difference | YBC | YNC | |
|---|---|---|---|---|---|
| Phenolic acids | 324 | 319 | 133 | 2(p-Coumaroylcaffeoyltartaric acid, 2,4,6-Trihydroxybenzoic acid) | 3(Acropyrone, Leonoside F, 3,5-Dicaffeoylquinic acid) |
| Dx rTerpenoids | 270 | 256 | 117 | 6(Shanzhiside methyl eter,3-Hydroxy-11-oxoolean-12-en-30-oic acid (18α-Glycyrrhetinic acid), 27,28-Dicarboxyl ursolicaid, Clerodendrin I, Achyranthoside D, Armatoside) | 8(Ceanothenicaid, Semiaquilegin A, 13-Methyl-27-norolean-14-en-3-ol (Taraxerol), Nootkatol, Icariside B1, Lupane-20(29)-en-3-on- 28-oic acid, lada-8(17), 12-diene-15,16-dial, [6-[acetyloxy-[1-(furan-3-yl)-5-hydroxy-8a-methyl-3,6-dioxo-7,8-dihydro-1H-isochromen-5-yl]methyl]-3-(2-methoxy-2-oxoethyl)-2,2,4-trimethyl-5-oxocyclohexyl]2-methylbutanoate) |
| Flavonoids | 254 | 247 | 138 | 5(Quercetin-5-O-glucuronide, Dihydrocharcone-4′-O-glucoside, Genistein-8-C-glucoside-O-apiosyl, Luteolin-7-O-(6″-sinapoyl)glucoside, Quercetin-3-O-(2″-O-Xylosyl rutinoside) | 2(Diosmetin-8-C(2″-Orhamnosyl) glucosde, Luteolin-3′-O-glucoside) |
| Amino acids and derivatives | 192 | 192 | 38 | ||
| Lipids | 191 | 191 | 41 | ||
| Alkaloids | 176 | 173 | 42 | 3(Ethy11,2-dihydro-1-methyl-2-oxoquinoline-4-carboxylate, Caffeoylcholine-3-O-glucoside, Caffeoylcholine-4-O-glucoside) | |
| Lignans and Coumarins | 173 | 172 | 81 | 1(5′-Methoxylariciresino1) | |
| Organic acids | 120 | 118 | 28 | 1(Pyrrole-2-carboxylic acid) | 1(Dihydrojasmonic acid) |
| Nucleotides and derivatives | 72 | 72 | 10 | ||
| Quinones | 16 | 16 | 8 | ||
| Tannins | 3 | 3 | |||
| Others | 268 | 267 | 82 | 1(Senkyunolide I) | |
| Total | 2059 | 2026 | 718 | 19 | 14 |
| No. | Compounds | Class I | Class II | Formula | log2FC | Type |
|---|---|---|---|---|---|---|
| 1 | 3,5-dihydroxy-4-{[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-[(sulfooxy)methyl]oxan-2-yl]oxy}benzoic acid | Phenolic acids | Phenolic acids | C13H16O13S | 12.45 | up |
| 2 | 3,5-dihydroxy-4-[(2S,3R,4S,5S,6R)-3,4,5-trih-droxy-6-(sulfooxymethyl)oxan-2-yl]oxybenzoic acid | Phenolic acids | Phenolic acids | C13H16O13S | 12.89 | up |
| 3 | 5-O-Feruloylquinic acid | Phenolic acids | Phenolic acids | C17H20O9 | 7.21 | up |
| 4 | Kaempferol-3-O-rhamnoside (Afzelin)(Kaempferin) | Flavonoids | Flavonoids | C21H20O10 | 8.32 | up |
| 5 | 8-Methoxykaempferol-7-O-rhamnoside | Flavonoids | Flavonoids | C22H22O11 | 7.17 | up |
| 6 | Kaempferol-7-O-rhamnoside | Flavonoids | Flavonoids | C21H20O10 | 8.48 | up |
| 7 | Hispidulin-7-O-(6″-O-p-Coumaroyl)Glucoside | Flavonoids | Flavonoids | C31H28O13 | 7.02 | up |
| 8 | Quercetin-3,7-Di-O-rhamnoside | Flavonoids | Flavonoids | C27H30O15 | 6.82 | up |
| 9 | 5,7-Dimethoxycoumarin (Limettin)(Citropten) | Lignans and Coumarins | Coumarins | C11H10O4 | 7.98 | up |
| 10 | 6,7-Dimethoxy-4-methylcoumarin | Lignans and Coumarins | Coumarins | C12H12O4 | 9.81 | up |
| 11 | 3-Methyl-6-methoxy-8-hydroxy-3,4-dihydroisocoumarin | Lignans and Coumarins | Coumarins | C11H12O4 | 8.21 | up |
| 12 | Eugenin | Others | Others | C11H10O4 | 8.67 | up |
| 13 | leptorumol | Others | Others | C11H10O4 | 8.23 | up |
| 14 | 30-O-Aangeloylhamaudol | Others | Chromone | C20H22O6 | 8.40 | up |
| 15 | Vanillin acetate | Phenolic acids | Phenolic acids | C10H10O4 | −7.62 | down |
| 16 | 6-Methylaloe emodin | Quinones | Anthraquinone | C16H12O5 | −6.28 | down |
| 17 | Hydroxystemofoline | Alkaloids | Pyrrole alkaloids | C22H29NO6 | −7.52 | down |
| 18 | Cinnamoyltyramin | Alkaloids | Phenolamine | C17H17NO2 | −6.35 | down |
| 19 | Ajugamacrin C* | Terpenoids | Diterpenoids | C34H50O11 | −6.60 | down |
| 20 | Ajugamacrin D* | Terpenoids | Diterpenoids | C34H50O11 | −7.17 | down |
| KEGG Pathway | Ko_ID | Number of Metabolites | p-Value |
|---|---|---|---|
| Flavone and flavanol biosynthesis | ko00944 | 13 | 0.0001 |
| Linoleic acid metabolism | ko00591 | 9 | 0.0033 |
| Arachidonic acid metabolism | ko00590 | 3 | 0.0388 |
| Sesquiterpenoid and triterpenoid biosynthesis | ko00909 | 2 | 0.0518 |
| alpha-Linolenic acid metabolism | ko00592 | 5 | 0.1133 |
| Stilbene, diarylheptanoid and gingerol biosynthesis | ko00945 | 2 | 0.1321 |
| Starch and sucrose metabolism | ko00500 | 3 | 0.1988 |
| Monoterpenoid biosynthesis | ko00902 | 1 | 0.2285 |
| Caffeine metabolism | ko00232 | 2 | 0.3217 |
| Isoflavonoid biosynthesis | ko00943 | 2 | 0.3217 |
| Biosynthesis of secondary metabolites | ko01110 | 38 | 0.3249 |
| Glutathione metabolism | ko00480 | 4 | 0.3429 |
| Carotenoid biosynthesis | ko00906 | 1 | 0.4052 |
| Diterpenoid biosynthesis | ko00904 | 1 | 0.4052 |
| Biosynthesis of various plant secondary metabolites | ko00999 | 6 | 0.4341 |
| Flavonoid biosynthesis | ko00941 | 4 | 0.5166 |
| Phenylpropanoid biosynthesis | ko00940 | 4 | 0.5166 |
| Biotin metabolism | ko00780 | 1 | 0.5418 |
| Indole alkaloid biosynthesis | ko00901 | 1 | 0.5418 |
| Plant hormone signal transduction | ko04075 | 1 | 0.5418 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, Q.; Zhu, T.; Liu, G.; Chen, W.; Chen, Y.; Bu, X.; Zhang, Z.; Zhang, Y. Broadly Targeted Metabolomics Analysis of Differential Metabolites Between Bupleurum chinense DC. and Bupleurum scorzonerifolium Willd. Metabolites 2025, 15, 119. https://doi.org/10.3390/metabo15020119
Li M, Zhang Q, Zhu T, Liu G, Chen W, Chen Y, Bu X, Zhang Z, Zhang Y. Broadly Targeted Metabolomics Analysis of Differential Metabolites Between Bupleurum chinense DC. and Bupleurum scorzonerifolium Willd. Metabolites. 2025; 15(2):119. https://doi.org/10.3390/metabo15020119
Chicago/Turabian StyleLi, Min, Quanfang Zhang, Tongshan Zhu, Guoxia Liu, Wenxiao Chen, Yanli Chen, Xun Bu, Zhifeng Zhang, and Yongqing Zhang. 2025. "Broadly Targeted Metabolomics Analysis of Differential Metabolites Between Bupleurum chinense DC. and Bupleurum scorzonerifolium Willd." Metabolites 15, no. 2: 119. https://doi.org/10.3390/metabo15020119
APA StyleLi, M., Zhang, Q., Zhu, T., Liu, G., Chen, W., Chen, Y., Bu, X., Zhang, Z., & Zhang, Y. (2025). Broadly Targeted Metabolomics Analysis of Differential Metabolites Between Bupleurum chinense DC. and Bupleurum scorzonerifolium Willd. Metabolites, 15(2), 119. https://doi.org/10.3390/metabo15020119






