Functional Dimorphism Analysis of Sporotrophophyll Leaves and Nest Leaves of Drynaria roosii with Their Connected Rhizomes Based on Multi-Omics Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Detection of Photosynthetic Parameters and Leaves’ Ultrastructure Between NLs and SLs of D. roosii
2.2.1. The Measurement of Photosynthetic Light/CO2 Response Curves of NLs and SLs
2.2.2. Record of Gas Exchange and Fluorescence Parameters of NLs and SLs
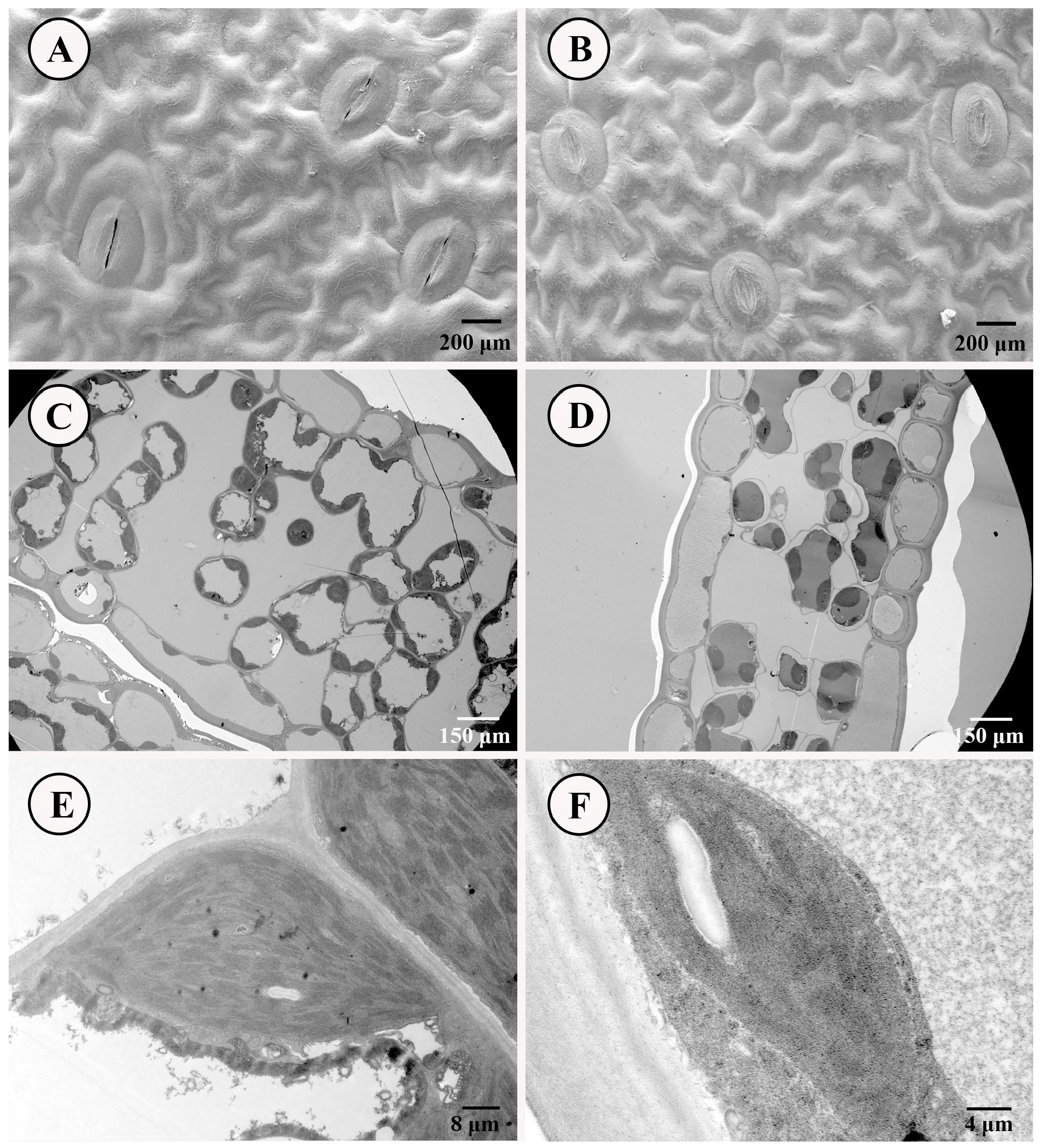
2.2.3. The Scan of Stomata and Ultrastructure of NL and SL
2.3. Transcriptome Measurement of NLs and SLs of D. roosii
2.4. Measurement and Analysis of Widely Targeted Metabolomics Between Connected Rhizomes of NRs and ORs of D. roosii
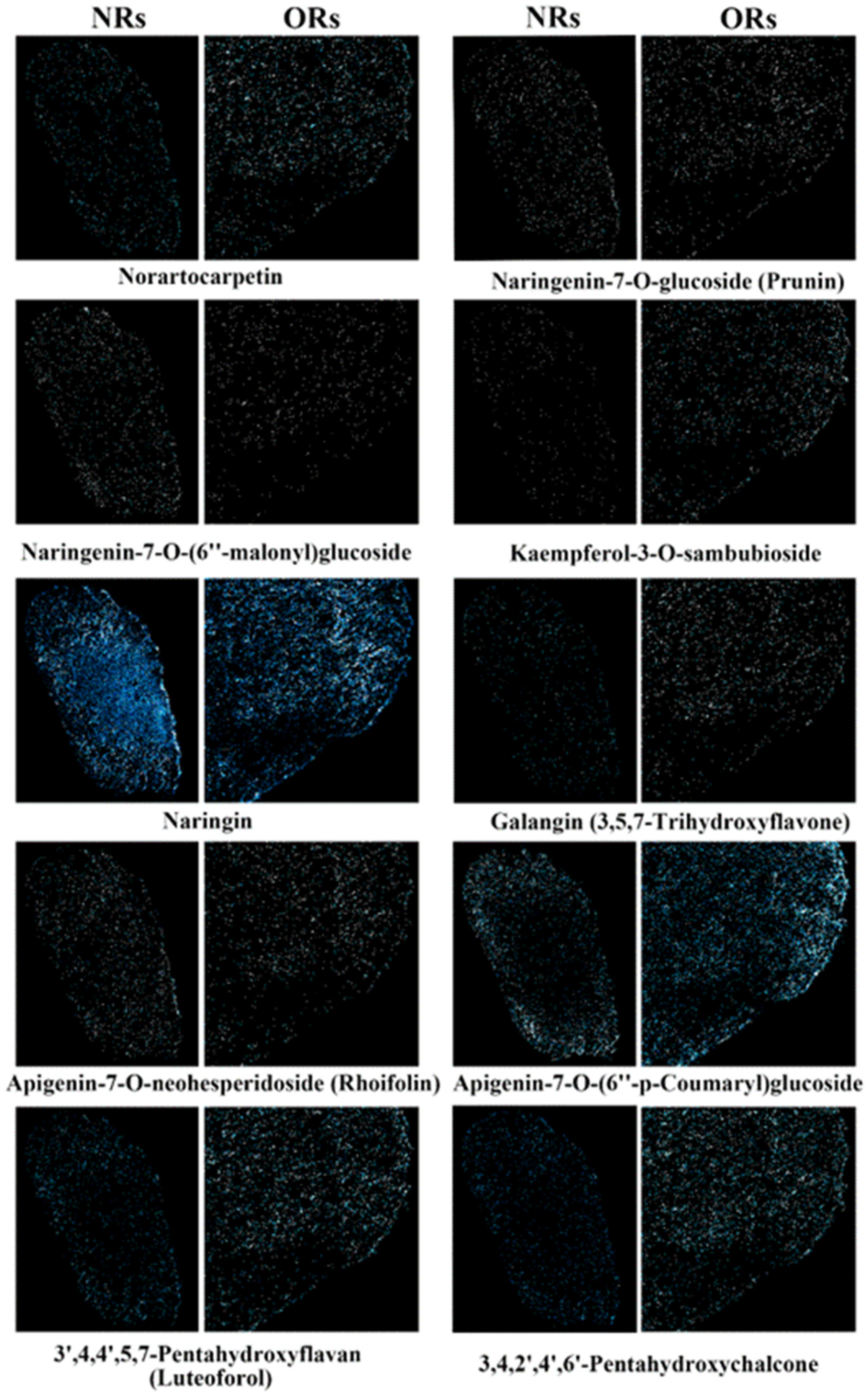
2.5. Measurement and Analysis of Spatial Distribution of Flavonoid Components Between Connected Rhizomes of NRs and ORs of D. roosii
3. Results
3.1. The Morphological Variation in NLs and SLs of D. roosii During the Whole Growth Cycle
3.2. The Comparison of Photosynthetic Parameters and Leaves’ Ultrastructure Between NLs and SLs of D. roosii
3.3. Comparison Results of Differential Expression Gene Networks Between NLs and SLs of D. roosii
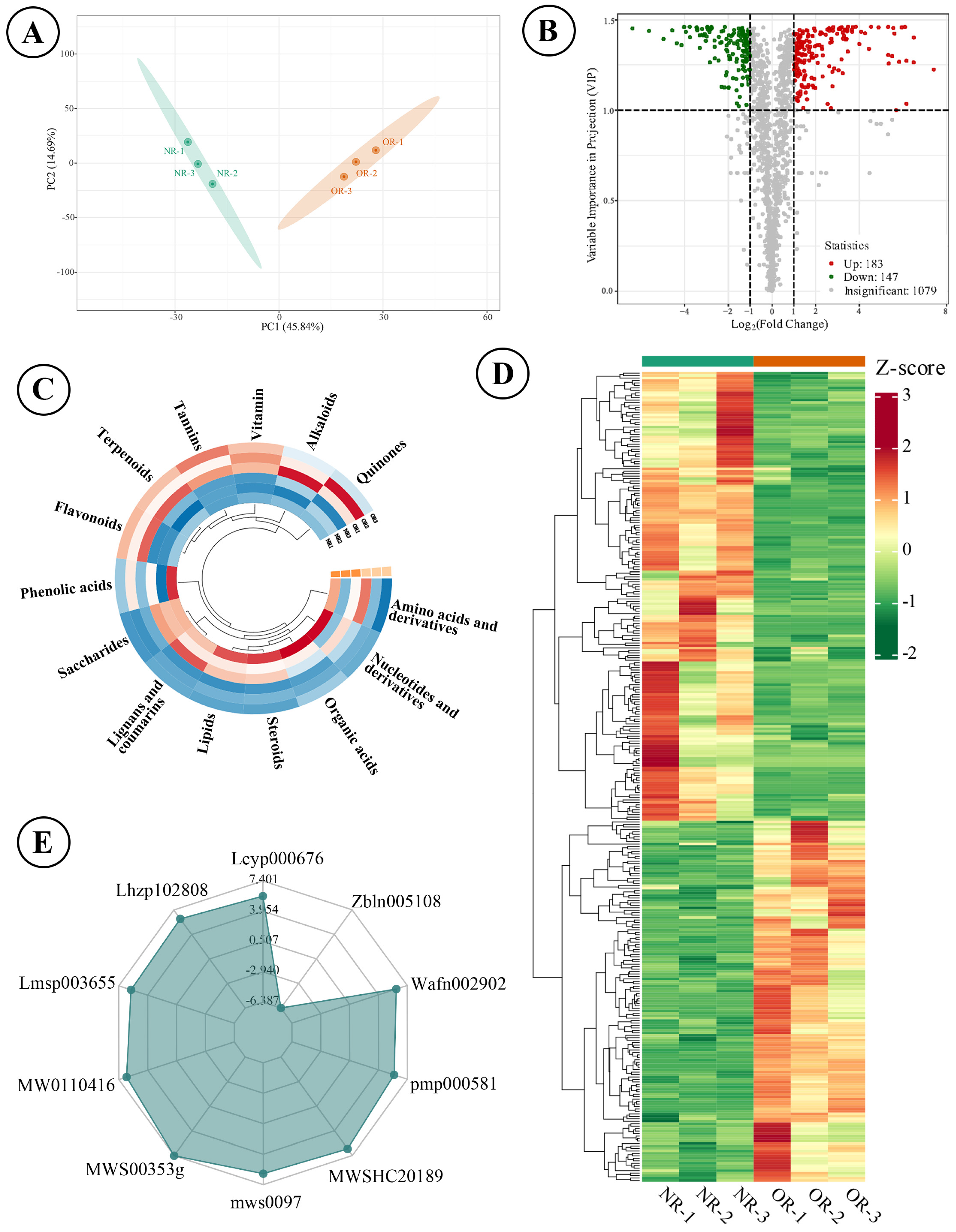
3.4. Metabolite Variation Between Connected Rhizomes of NLs and SLs of D. roosii
4. Discussion
4.1. The Structure Difference in Stomata and Ultrastructure Between SLs and NLs of D. roosii Determined the Variations in Photosynthetic Ability
4.2. The Rational Harvesting of Rhizomes Was Vital for High-Effective Clinical Usage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Huang, W.; Han, X.; Zhao, G.; Zhang, W.; He, W.; Nie, B.; Chen, X.; Zhang, T.; Bai, W.; et al. Diel dynamics of multi-omics in elkhorn fern provide new insights into weak CAM photosynthesis. Plant Commun. 2023, 5, 100594. [Google Scholar] [CrossRef] [PubMed]
- Rut, G.; Krupa, J.; Miszalski, Z.; Rzepka, A.; Ślesak, I. Crassulacean acid metabolism in the epiphytic fern Platycerium bifurcatum. Photosynthetica 2008, 1, 156–160. [Google Scholar]
- Oliwa, J.; Kornas, A.; Skoczowski, A. A low ratio of red/far-red in the light spectrum accelerates senescence in nest leaves of Platycerium bifurcatum. Acta Biol. Cracov. Ser. Bot. 2017, 2, 17–30. [Google Scholar] [CrossRef]
- Vasco, A.; Moran, R.C.; Ambrose, B.A. The evolution, morphology, and development of fern leaves. Front. Plant Sci. 2013, 4, 345. [Google Scholar] [CrossRef]
- Watkins, J.E.; Churchill, A.C.; Holbrook, N.M. A site for sori: Ecophysiology of fertile–sterile leaf dimorphy in ferns. Am. J. Bot. 2016, 5, 845–855. [Google Scholar] [CrossRef]
- Oliwa, J.; Skoczowski, A. Different response of photosynthetic apparatus to high-light stress in sporotrophophyll and nest leaves of Platycerium bifurcatum. Photosynthetica 2019, 1, 147–159. [Google Scholar] [CrossRef]
- Ajuru, M.G.; Joshua, J.A.; Chikere, L.C.; Ibiye, A. Morphological and foliar epidermal studies of fronds of Platycerium bifurcatum and P. superbum in Rivers State, Nigeria. Cell Biol. Dev. 2024, 1, 28–35. [Google Scholar] [CrossRef]
- Chang, N.; Yang, X.; Wang, X.; Chen, C.; Wang, C.; Xu, Y.; Huang, H.; Wang, Y. Epiphytic patterns impacting metabolite diversity of Drynaria roosii rhizomes based on widely targeted metabolomics. Metabolites 2024, 14, 409. [Google Scholar] [CrossRef]
- Chen, S.Q.; Liang, W.; Zhang, X.M.; Li, X.; Zhan, Z.L.; Guo, L.P.; Huang, L.Q.; Zhang, X.M.; Gao, W.Y. Research progress on chemical compositions and pharmacological action of Drynariae Rhizoma. China J. Chin. Mater. Medica 2021, 11, 2737–2745. [Google Scholar]
- Mei, X.; Zhang, T.; Lu, N. Research progress on mechanism of Drynariae Rhizoma in treatment of traumatic fracture. Drugs Clin. 2022, 10, 2386–2389. [Google Scholar]
- Yin, Z.; Tan, W.; Feng, D.; Zhu, C.; Lu, S. The historical research on the herbal medicine of Drynaria fortunei, its preparation and medicinal use evolution. China Pharm. 2019, 12, 1725–1728. [Google Scholar]
- Zhou, Q.; Zeng, X.; Huang, D.; Li, J.; Tang, M.; Li, S. Research progress on the chemical composition and biological activity of Drynariae Rhizoma. World Sci. Technol.-Mod. Tradit. Chin. Med. 2021, 8, 2727–2741. [Google Scholar]
- Dong, Y.; Toume, K.; Kimijima, S.; Zhang, H.; Zhu, S.; He, Y.; Cai, S.; Maruyama, T.; Komatsu, K. Metabolite profiling of Drynariae Rhizoma using 1H NMR and HPLC coupled with multivariate statistical analysis. J. Nat. Med. 2023, 4, 839–857. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Mo, Y.; Sun, Q.; Xu, W.; Guo, W.; Lu, X.; Tan, L. Comparative study on the quality of Drynariae Rhizoma from two different habitats. Guizhou Sci. 2020, 1, 25–30. [Google Scholar]
- Wu, Y.; Ma, H.; Ma, S.; Li, W.; Tan, L. Physiological, proteomic and metabolomic analysis provide insights into Ca2+ tolerance in Drynaria roosii leaves. Plant Stress 2023, 7, 100132. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Ma, S.; Ma, H.; Tan, L. Physiological and differential protein expression analyses of the calcium stress response in the Drynaria roosii rhizome. Heliyon 2024, 19, e38260. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Ma, H.; Li, W.; Tan, L. Analysis of differentially expressed metabolites in Drynaria roosii rhizome in response to calcium stress. Guihaia 2024, 3, 531–540. [Google Scholar]
- Qiu, D.; Luo, Y.; Li, C.; Du, C.; Yuan, X. Comparative analysis of broad-targeted metabolomics between greenhouse cultivated and wild collected Drynaria fortune. Guangdong Agric. Sci. 2024, 5, 30–43. [Google Scholar]
- Li, J.; Li, D.; Du, X.; Li, H.; Wang, D.; Xing, Q.; Yao, R.; Sun, M.; Shi, L. Modular organization analysis of specific naringin/neoeriocitrin related gene expression induced by UVC irradiation in Drynaria roosii. Environ. Exp. Bot. 2018, 156, 298–315. [Google Scholar] [CrossRef]
- Sun, M.; Li, J.; Li, D.; Huang, F.; Wang, D.; Li, H.; Xing, Q.; Zhu, H.; Shi, L. Full-Length transcriptome sequencing and modular organization analysis of the naringin/neoeriocitrin-related gene expression pattern in Drynaria roosii. Plant Cell Physiol. 2018, 7, 1398–1414. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 7, 644–652. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.; Dillies, M. SARTools: A DESeq2- and EdgeR-based R pipeline for comprehensive differential analysis of RNA-seq data. PLoS ONE 2016, 6, e0157022. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, H.; Yang, T.; Gao, D.; Li, X. Primary investigation of phenotypic plasticity in Fritillaria cirrhosa based on metabolome and transcriptome analyses. Cells 2022, 11, 3844. [Google Scholar] [CrossRef]
- Zhu, M.; Li, J.; Ye, L.; Di Lei Liu, H.; Liu, Y.; Sen, L. Study actuality of medicinal pteridophyes literature via visualized bibliometrics. Mod. Tradit. Chin. Med. Mater. Medica-World Sci. Technol. 2024, 8, 2154–2167. [Google Scholar]
- Taylor, A.; Zotz, G.; Weigelt, P.; Cai, L.; Karger, D.N.; König, C.; Kreft, H. Vascular epiphytes contribute disproportionately to global centres of plant diversity. Glob. Ecol. Biogeogr. 2022, 1, 62–74. [Google Scholar] [CrossRef]
- Yang, Y.J.; Bi, M.H.; Nie, Z.F.; Jiang, H.; Liu, X.D.; Fang, X.W.; Brodribb, T.J. Evolution of stomatal closure to optimize water-use efficiency in response to dehydration in ferns and seed plants. N. Phytol. 2021, 5, 2001–2010. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Xu, F.; Li, X. Resistance evaluation of different phenotypes of farmland Ginseng Radix et Rhizoma germplasm under high light stress. Chin. J. Exp. Tradit. Med. Formulae 2021, 20, 121–129. [Google Scholar]
- Chen, Z.; Liu, Z.; Han, S.; Jiang, H.; Xu, S.; Zhao, H.; Ren, S. Using the diurnal variation characteristics of effective quantum yield of PSII photochemistry for drought stress detection in maize. Ecol. Indic. 2022, 138, 108842. [Google Scholar] [CrossRef]
- Helm, L.T.; Shi, H.; Lerdau, M.T.; Yang, X. Solar-induced chlorophyll fluorescence and short-term photosynthetic response to drought. Ecol. Appl. 2020, 5, e02101. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Zhang, A. Comparison of chlorophyll fluorescence parameters among different Rosa rugosa varieties. J. Anhui Agric. Sci. 2022, 1, 50–54. [Google Scholar]
- Feng, J.; Shen, X.; Liu, S.; Lei, P.; Han, L.; Gao, P. Effects of drought stress on photosynthetic characteristics of two medicinal plants. Mol. Plant Breed. 2023, 21, 5211–5230. [Google Scholar]
- Wang, Y.; Li, P. Effect of cultivation years on saponins in Paris Polyphylla var. yunnanensis using ultra-high liquid chromatography–tandem mass spectrometry and Fourier transform infrared spectroscopy. Plant Growth Regul. 2018, 2, 373–381. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Zuo, Z.; Wang, Y. Comprehensive quality assessment of Dendrubium officinale using ATR-FTIR spectroscopy combined with random forest and support vector machine regression. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Yuba-Kubo, A.; Sugiura, Y.; Zaima, N.; Hayasaka, T.; Goto-Inoue, N.; Wakui, M.; Suematsu, M.; Takeshita, K.; Ogawa, K.; et al. Visualization of volatile substances in different organelles with an atmospheric-pressure mass microscope. Anal. Chem. 2009, 21, 9153–9157. [Google Scholar] [CrossRef]
- Du, Y.; Peng, S.; Chen, H.; Li, J.; Huang, F.; Chen, W.; Wang, J.; Fang, X.; Liu, L.; Wei, L.; et al. Unveiling the spatiotemporal landscape of Ganoderma lingzhi: Insights into ganoderic acid distribution and biosynthesis. Engineering, 2025; in press. [Google Scholar] [CrossRef]
- Huang, L.; Nie, L.; Dai, Z.; Dong, J.; Jia, X.; Yang, X.; Yao, L.; Ma, S. The application of mass spectrometry imaging in traditional Chinese medicine: A review. Chin. Med. 2022, 1, 35. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, Y.; Liu, Y.; He, H.; Han, M.; Li, Y.; Zeng, M.; Wang, X. Recent advances in matrix-assisted laser desorption/ionisation mass spectrometry imaging (MALDI-MSI) for in situ analysis of endogenous molecules in plants. Phytochem. Anal. 2018, 4, 351–364. [Google Scholar] [CrossRef]
- Ge, J.; Hu, D.; Zhang, Y.; Li, P.; Li, B. Analytical capabilities of matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) and its potential applications in medicinal plants. Acta Pharm. Sin. 2019, 7, 1179–1189. [Google Scholar]
- Li, B.; Ge, J.; Liu, W.; Hu, D.; Li, P. Unveiling spatial metabolome of Paeonia suffruticosa and Paeonia lactiflora roots using MALDI MS imaging. N. Phytol. 2021, 2, 892–902. [Google Scholar] [CrossRef]
- Wang, S.; Bai, H.; Cai, Z.; Gao, D.; Jiang, Y.; Liu, J.; Liu, H. MALDI imaging for the localization of saponins in root tissues and rapid differentiation of three Panax herbs. Electrophoresis 2016, 13, 1956–1966. [Google Scholar] [CrossRef] [PubMed]



| Parameters | SLs | NLs | p Value |
|---|---|---|---|
| Transpiration rate (E, mol·m−2·s−1) | 0.0003 ± 0.0002 | 0.0001 ± 0.0001 | 0.190 |
| Net photosynthetic rate (Pn, µmol·m−2 s−1) | 1.0039 ± 0.2687 | 0.3063 ± 0.1068 ** | 0.001 |
| Intercellular CO2 concentration (Ci, µmol mol−1) | 262.4504 ± 62.4487 | 325.115 ± 50.7267 | 0.135 |
| Stomatal conductance to water vapor (GSW, mol·m−2·s−1) | 0.0165 ± 0.0109 | 0.0108 ± 0.0065 | 0.379 |
| Stomatal conductance to boundary layer water vapor (GBW, m−2·s−1) | 3.0215 ± 0.005 | 3.0508 ± 0.0014 ** | 0.000 |
| Total conductance to water vapor (GTW, mol·m−2·s−1) | 0.0164 ± 0.0108 | 0.0108 ± 0.0065 | 0.379 |
| Total conductance to CO2 (GTC, mol m−2·s−1) | 0.0103 ± 0.0068 | 0.0067 ± 0.0041 | 0.379 |
| Vapor pressure deficit at leaf surface (VPD, kPa) | 1.6554 ± 0.0132 | 1.2694 ± 0.0229 ** | 0.000 |
| Steady state fluorescence under light (Fs) | 256.2163 ± 24.5645 | 336.4477 ± 15.4555 ** | 0.000 |
| Maximum fluorescence under light (Fm′) | 913.6633 ± 114.7845 | 988.939 ± 58.948 | 0.267 |
| Actual quantum yield of photosystem II photochemistry (PhiPS2) | 0.7175 ± 0.0279 | 0.6591 ± 0.021 ** | 0.008 |
| Electron transport rate (ETR, µmol m−2 s−1) | 11.1807 ± 0.4356 | 8.345 ± 0.2614 | 0.000 |
| Quantum efficiency of CO2 assimilation (PhiCO2, µmol·µmol−1) | 0.0442 ± 0.0086 | −0.2962 ± 0.004 ** | 0.000 |
| The difference between maximum fluorescence and minimum fluorescence under light (Fv′) | 0.7865 ± 0.0179 | 1.0009 ± 0.0001 ** | 0.000 |
| Minimum initial fluorescence (Fo) | 191.3968 ± 40.6225 | 277.0105 ± 22.5607 ** | 0.006 |
| Maximum fluorescence after dark adaption (Fm) | 1025.38 ± 236.7404 | 1203.0767 ± 54.1949 | 0.236 |
| Maximum photochemical efficiency of PSII reaction centers when fully open under dark adaptation (Fv/Fm) | 0.8105 ± 0.0272 | 0.77 ± 0.0086 | 0.031 |
| Net photosynthetic rate under dark adaptation (Adark, µmol·m−2·s−1) | −0.5237 ± 0.1861 | −0.7954 ± 0.2911 | 0.074 |
| Curve Type | Fitting Parameters | SLs | NLs |
|---|---|---|---|
| Photosynthetic light response curve | Dark respiration rate | 0.5284 | 0.7224 |
| Maximum net photosynthetic rate (Pnmax, µmol·m−2 s−1) | 2.5738 | 0.4315 | |
| Light saturation point (LSP, µmol·m−2·s−1) | 467.7510 | 206.8240 | |
| Light compensation point (LCP, µmol·m−2·s−1) | 11.5740 | 16.8850 | |
| Apparent quantum yield (AQY) | 0.0117 | 0.0042 | |
| Fitting residuals | 1.7085 | 0.1802 | |
| Determination coefficient | 0.8364 | 0.8682 | |
| Photosynthetic CO2 response curve | Dark respiration rate | 4.0670 | 1.3667 |
| Maximum net photosynthetic rate (Pnmax, µmol·m−2 s−1) | 3.4172 | −1.1921 | |
| Saturation intercellular CO2 concentration (SIC, µmol mol−1) | 1125.4500 | 343.0470 | |
| CO2 compensation point (CCP, µmol mol−1) | 250.5410 | 460.9870 | |
| Fitting residuals | 8.8957 | 0.6672 | |
| Determination coefficient | 0.7960 | 0.9110 | |
| Dark respiration rate | 4.0670 | 1.3667 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Ren, Y.; Han, Y.; Wang, X.; Li, H.; Zeng, Y.; Li, X.; Wang, Y. Functional Dimorphism Analysis of Sporotrophophyll Leaves and Nest Leaves of Drynaria roosii with Their Connected Rhizomes Based on Multi-Omics Analysis. Metabolites 2025, 15, 805. https://doi.org/10.3390/metabo15120805
Cao Y, Ren Y, Han Y, Wang X, Li H, Zeng Y, Li X, Wang Y. Functional Dimorphism Analysis of Sporotrophophyll Leaves and Nest Leaves of Drynaria roosii with Their Connected Rhizomes Based on Multi-Omics Analysis. Metabolites. 2025; 15(12):805. https://doi.org/10.3390/metabo15120805
Chicago/Turabian StyleCao, Ye, Yan Ren, Yanlei Han, Xiaoqing Wang, Hui Li, Yong Zeng, Xiwen Li, and Ye Wang. 2025. "Functional Dimorphism Analysis of Sporotrophophyll Leaves and Nest Leaves of Drynaria roosii with Their Connected Rhizomes Based on Multi-Omics Analysis" Metabolites 15, no. 12: 805. https://doi.org/10.3390/metabo15120805
APA StyleCao, Y., Ren, Y., Han, Y., Wang, X., Li, H., Zeng, Y., Li, X., & Wang, Y. (2025). Functional Dimorphism Analysis of Sporotrophophyll Leaves and Nest Leaves of Drynaria roosii with Their Connected Rhizomes Based on Multi-Omics Analysis. Metabolites, 15(12), 805. https://doi.org/10.3390/metabo15120805





