Matrix- and Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Methods for Urological Cancer Biomarker Discovery—Metabolomics and Lipidomics Approaches
Abstract
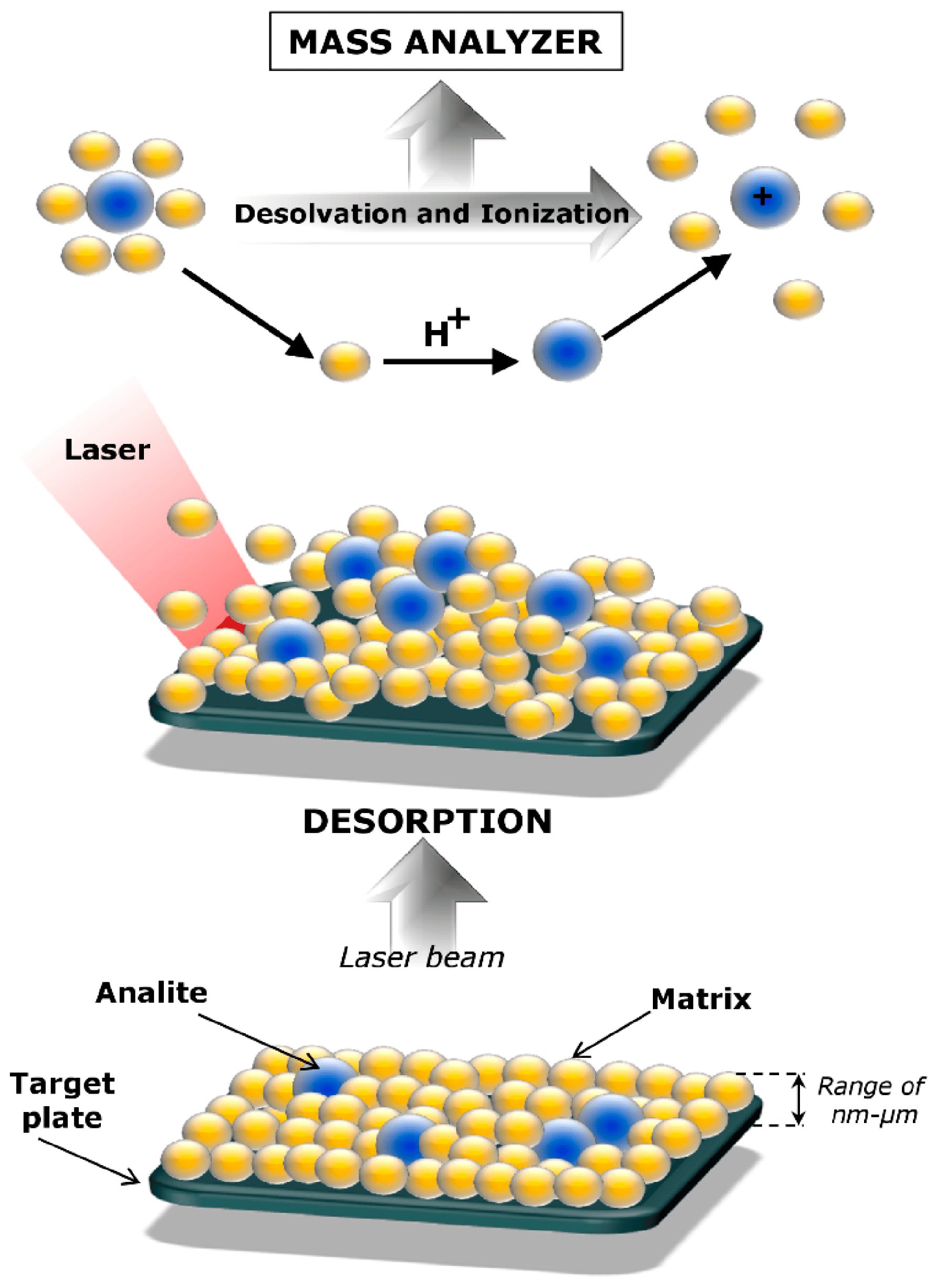
1. Introduction
| Method | MALDI | SALDI | LC-MS | GC-MS | CE-MS | NMR |
|---|---|---|---|---|---|---|
| Sample type | solid | solid | liquid | volatile | liquid | liquid |
| Analysis of LMW compounds | difficult | yes | yes | yes | yes | yes |
| Quantitative analysis | impossible | difficult | yes | yes | yes | yes |
| Typical sample volume | 1 µL | 0.5 µL | 1–20 µL | 0.5–20 µL | 1–50 nL | 0.6 mL |
| LOD (up to) | fmol | amol | amol | fmol | fmol | nmol |
| Sample preparation time | <1 min | <1 min | 10–30 min | 10–60 min | 10–30 min | 1–10 min |
| Analysis time per sample | <1 min | <1 min | 10–30 min | 10–60 min | 3–10 min | 10–300 min |
| References | [27,28] | [29,30] | [31,32] | [33,34] | [35,36] | [37,38,39] |
2. Bladder Cancer
3. Kidney Cancer
| Cancer Type | Sample | Method | Matrix | Main Observation | Ref. |
|---|---|---|---|---|---|
| BC | urine | SALDI-ToF MS | TiO2/MXene | up-regulation of pterin-6-carboxylic acid, phenylacetylglutamine, creatinine, uric acid, gammaglutamylthreonine, canavaninosuccinate, hydroxytyrosol 3′-glucuronide, histidine, down-regulation of leucylproline, tryptophan, and N6-acetyl-L-lysine in BC | [48] |
| BC | urine | SALDI-ToF MS | FEP@VSiNWs | up-regulation of GABA, serine, proline, cysteine, N-acetylvaline, N-acetylthreonine, valine, allysine, and nicotinic acid and down-regulation of creatinine, taurine, citraconic acid, and lauric acid in BC | [49] |
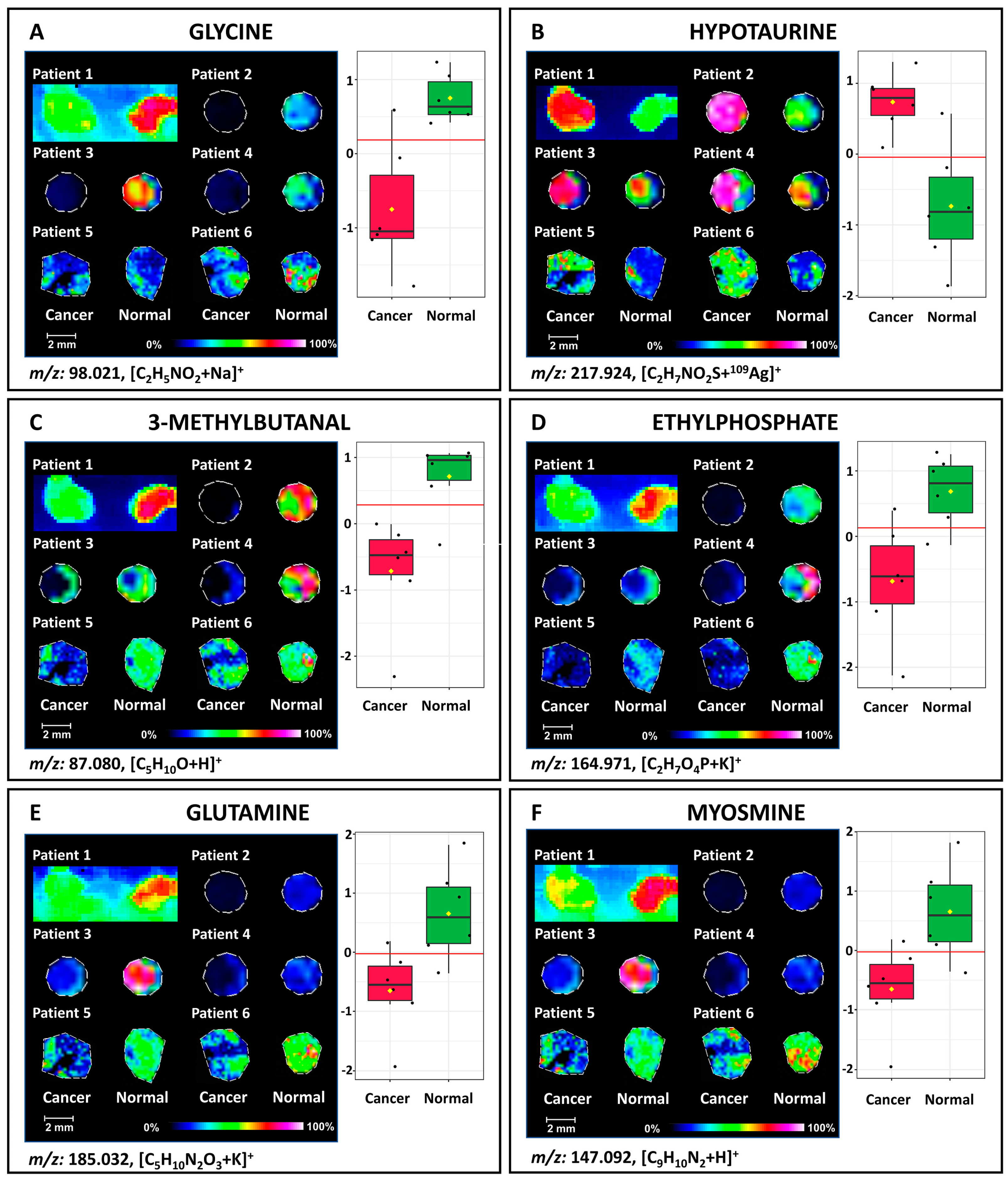
| BC | tissue | SALDI-ToF MSI | 109AgNPs | up-regulation of hypotaurine and down-regulation of glycine, 3-methylbutanal, ethylphosphate, glutamin, myosmine, aminopentanal, proline betaine, and methylguanidine in BC | [52] |
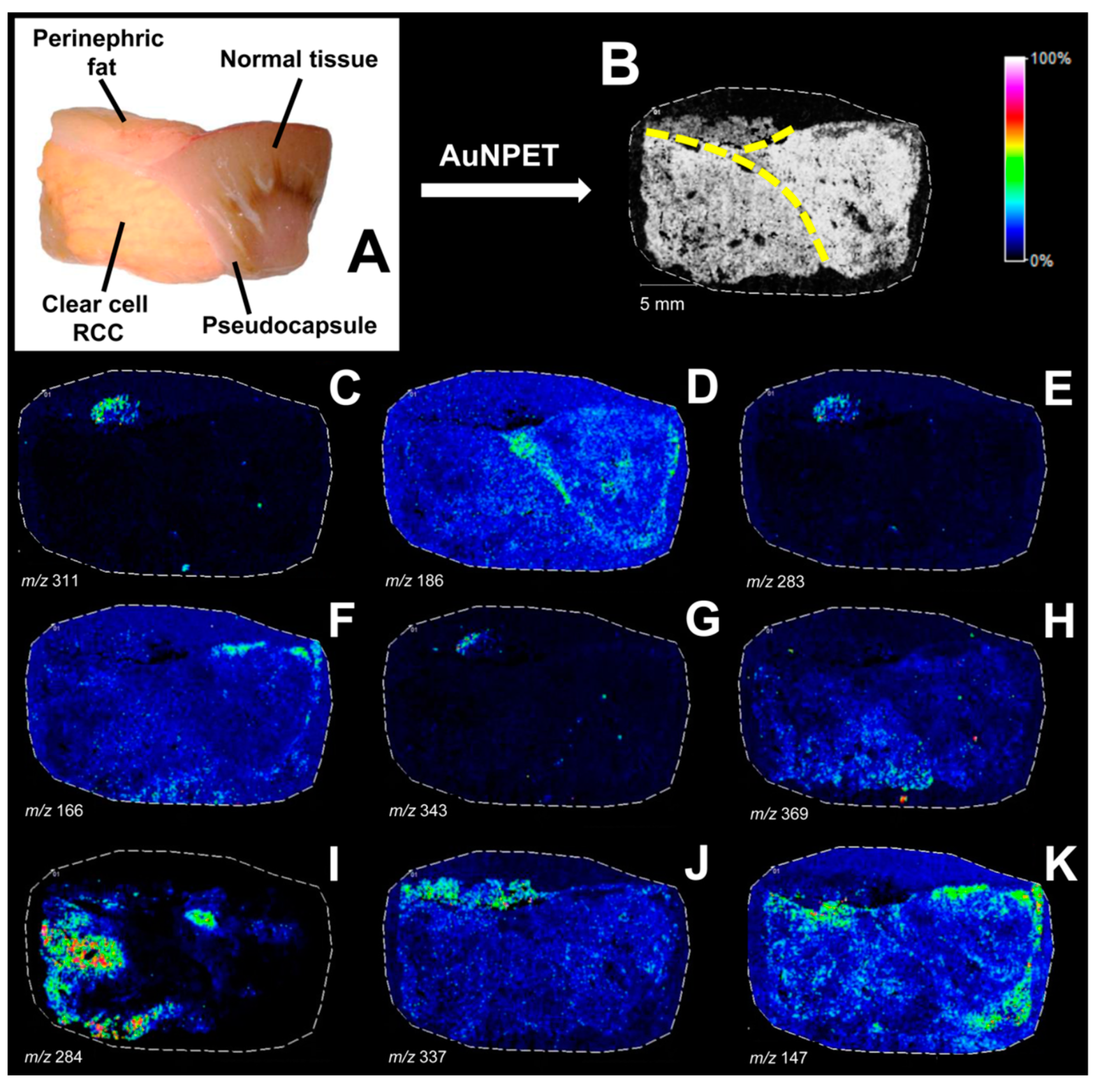
| RCC | tissue | SALDI-ToF MS | 109AgNPs | up-regulation of hydroxyeicosatrienoic acid, octanediol, diethoxypentane, and oxoalanine in RCC | [58] |
| RCC | tissue | SALDI-ToF MSI | 109AgNPs | up-regulation of thymine and inosine and down-regulation of alanine, serine, glutamic acid, methionine, and histidine in RCC | [67] |
| ccRCC | tissue | SALDI-ToF MSI | AgNPs | down-regulation of glucose and phenylacetylglycine and up-regulation of sulfinpyrazone sulfide, riboflavin, and S-adenosyl-L-methionine in ccRCC | [66] |
| kidney cancers | tissue | MALDI-FT-ICR MSI | 9-AA | up-regulation of ribose 5-phosphate in oncocytoma, glucosamine in oncocytoma and chRCC, and 3-dehydrocarnitine in ccRCC and pRCC | [70] |
| RCC | serum | SALDI-ToF MS | 109AgNPs | up-regulation of Phe-Thr-Thr, Glu-Arg-Pro, and His-Ser-Ser-His and down-regulation of Thr-Trp-Cys, Glu-Asp-Phe, and Ala-Cys-Pro-Pro in RCC | [59] |
| RCC | serum | SALDI-ToF MS | AuNPs | down-regulation of dihydrouracil and up-regulation of creatinine, glutamine, tyrosine, 2,3-diaminosalicylic acid, 3-hydroxykynurenine, 2-hydroxylauroylcarnitine, melatonin glucuronide, and palmitoyl glucuronide in RCC | [63] |
| ccRCC | serum | SALDI-ToF MS | AuNPs | up-regulation of melatonin glucuronide in ccRCC samples | [29] |
| RCC | urine | SALDI-ToF MS | 109AgNPs | down-regulation of succinylacetoacetate, Cys-Gly-Ser-His, His-Gly-Ser-Ser, and Met-Thr-His in RCC | [60] |
| RCC | urine | SALDI-ToF MS | AuNPs | up-regulation of heptanol, N-acetylglutamine, and LeuHis and down-regulation of serine, 3-methylene-indolenine, 2-methyl-3-hydroxy-5-formylpyridine-4-carboxylate, phosphodimethylethanolamine, 4-methoxyphenylacetic acid, 3,5-dihydroxyphenylvaleric acid, hydroxyhexanoylglycine, and ValLeu in RCC | [64] |
| PCa | urine | SALDI-ToF MS | AuNPs | down-regulation of peptides, Ile-Ile-Lys-Val and Ala-Arg-His-His, in PCa samples | [30] |
| PCa | serum | SALDI-ToF MS | AuNPs | up-regulation of monodehydroascorbate, Ala-Cys, ascorbate 2-sulfate, homovanillicacidsulfate, 2-oxo-3-hydroxy-4-phosphobutanoate, dITP, and Arg-Leu-Phe-Trp in PCa | [30] |
| PCa | interstitial fluid | SALDI-ToF MS | AuNPs | down-regulation of maleylpyruvate, 3.2′,3′-cyclic uridine monophosphate, and Arg-Asp-Gln-His in PCa | [30] |
| PCa | tissue | MALDI-ToF MSI | NEDC | down-regulation of aspartate and citrate in PCa | [72] |
4. Prostate Cancer
| Cancer Type | Sample | Method | Matrix | Main Observation | Ref. |
|---|---|---|---|---|---|
| BC | tissue | SALDI-ToF MSI | 109AgNPs | down-regulation of PI(22:0/0:0) in BC | [52] |
| RCC | tissue | MALDI-Orbitrap MS | 9-AA | up-regulation of SulfoHexCer, SulfoHex2Cer, and SulfoHex2Cer (OH) in RCC | [28] |
| ccRCC | tissue | MALDI-FT-ICR MSI | 2,5-DHB | up-regulation of PC 30:3 and down-regulation of PC 26:0 and PC 30:2 in ccRCC samples | [68] |
| ccRCC | tissue | SALDI-ToF MSI | AuNPs | up-regulation of DG(18:1/20:0) and octadecanamide in ccRCC | [65] |
| ccRCC | tissue | SALDI-ToF MSI | AgNPs | up-regulation of octadecanamide, arachidonic acid, eicosenoic acid, and N-(2-hydroxypentadecanoyl)-4,8- sphingadienine in ccRCC | [66] |
| RCC | serum | SALDI-ToF MS | 109AgNPs | up-regulation of [FA(20:4)] eicosatetraenoyl amine and MG(0:0/16:0/0:0) in RCC | [59] |
| RCC | serum | SALDI-ToF MS | AuNPs | up-regulation of TG(52:4) and PC(42:0) in RCC | [63] |
| ccRCC | serum | SALDI-ToF MS | AuNPs | up-regulation of 2-hydroxylauroylcarnitine in ccRCC samples | [29] |
| RCC | urine | SALDI-ToF MS | AuNPs | up-regulation of oleamide, 9,12,13-trihydroxyoctadecenoic acid, stearidonyl carnitine, and squalene in RCC | [64] |
| ccRCC | urine | SALDI-ToF MS | AuNPs | up-regulation of 9,12,13-trihydroxyoctadecenoic acid and 3-hydroxydecanoyl carnitine in ccRCC samples | [29] |
| PCa | urine | SALDI-ToF MS | AuNPs | up-regulation of TG(12:0/20:1) in PCa | [30] |
| PCa | serum | SALDI-ToF MS | AuNPs | down-regulation of nonanoylcarnitine, palmitoyl glucuronide, squalene, calcitriol, and (9Z, 12Z, 15Z)-octadecatrienoic acid in PCa | [30] |
| PCa | interstitial fluid | SALDI-ToF MS | AuNPs | down-regulation of pregnanediol in PCa | [30] |
| PCa | tissue | MALDI-ToF MS | CHCA | down-regulation of PC(18:0/22:5) in PCa samples | [76] |
| PCa | tissue/urine | MALDI-ToF MS | 9-AA | ratio of PC(34:2) + PC(34:1) to LPC(16:0) is higher in PCa | [77] |
| PCa | tissue | MALDI-QToF MS | 9-AA | up-regulation of PI(18:0/18:1), PI(18:0/20:3), and PI(18:0/20:2) in PCa | [78] |
| PCa | tissue | MALDI-ToF MSI | CHCA | up-regulation of PE(42:6) and down-regulation of PI(36:4) in malignant PCa tissues | [80] |
5. Future Directions
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ilyin, S.E.; Belkowski, S.M.; Plata-Salamán, C.R. Biomarker Discovery and Validation: Technologies and Integrative Approaches. Trends Biotechnol. 2004, 22, 411–416. [Google Scholar] [CrossRef]
- Romero Otero, J.; Garcia Gomez, B.; Campos Juanatey, F.; Touijer, K.A. Prostate Cancer Biomarkers: An Update. Urol. Oncol. 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Shariat, S.F.; Karam, J.A.; Walz, J.; Roehrborn, C.G.; Montorsi, F.; Margulis, V.; Saad, F.; Slawin, K.M.; Karakiewicz, P.I. Improved Prediction of Disease Relapse after Radical Prostatectomy through a Panel of Preoperative Blood-Based Biomarkers. Clin. Cancer Res. 2008, 14, 3785–3791. [Google Scholar] [CrossRef]
- Catto, J.W.F.; Alcaraz, A.; Bjartell, A.S.; De Vere White, R.; Evans, C.P.; Fussel, S.; Hamdy, F.C.; Kallioniemi, O.; Mengual, L.; Schlomm, T.; et al. MicroRNA in Prostate, Bladder, and Kidney Cancer: A Systematic Review. Eur. Urol. 2011, 59, 671–681. [Google Scholar] [CrossRef]
- Adam, B.L.; Vlahou, A.; Semmes, O.J.; Wright, G.L. Proteomic Approaches to Biomarker Discovery in Prostate and Bladder Cancers. Proteomics 2001, 1, 1264–1270. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Carvalho, M.; de Lourdes Bastos, M.; de Pinho, P.G. Biomarkers in Renal Cell Carcinoma: A Metabolomics Approach. Metabolomics 2014, 10, 1210–1222. [Google Scholar] [CrossRef]
- Sagini, K.; Urbanelli, L.; Buratta, S.; Emiliani, C.; Llorente, A. Lipid Biomarkers in Liquid Biopsies: Novel Opportunities for Cancer Diagnosis. Pharmaceutics 2023, 15, 437. [Google Scholar] [CrossRef]
- Engel, K.M.; Prabutzki, P.; Leopold, J.; Nimptsch, A.; Lemmnitzer, K.; Vos, D.R.N.; Hopf, C.; Schiller, J. A New Update of MALDI-TOF Mass Spectrometry in Lipid Research. Prog. Lipid Res. 2022, 86, 101145. [Google Scholar] [CrossRef]
- Tanaka, K.; Waki, H.; Ido, Y.; Akita, S.; Yoshida, Y.; Yoshida, T.; Matsuo, T. Protein and Polymer Analyses up to m/z 100 000 by Laser Ionization Time-of-Flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 1988, 2, 151–153. [Google Scholar] [CrossRef]
- Nizioł, J.; Rode, W.; Zieliński, Z.; Ruman, T. Matrix-Free Laser Desorption–Ionization with Silver Nanoparticle-Enhanced Steel Targets. Int. J. Mass Spectrom. 2013, 335, 22–32. [Google Scholar] [CrossRef]
- Yukihira, D.; Miura, D.; Saito, K.; Takahashi, K.; Wariishi, H. MALDI−MS-Based High-Throughput Metabolite Analysis for Intracellular Metabolic Dynamics. Anal. Chem. 2010, 82, 4278–4282. [Google Scholar] [CrossRef]
- Jurowski, K.; Kochan, K.; Walczak, J.; Barańska, M.; Piekoszewski, W.; Buszewski, B. Analytical Techniques in Lipidomics: State of the Art. Crit. Rev. Anal. Chem. 2017, 47, 418–437. [Google Scholar] [CrossRef]
- Karas, M.; Bachmann, D.; Bahr, U.; Hillenkamp, F. Matrix-Assisted Ultraviolet Laser Desorption of Non-Volatile Compounds. Int. J. Mass Spectrom. Ion Process. 1987, 78, 53–68. [Google Scholar] [CrossRef]
- Hillenkamp, F.; Peter-Katalinic, J. MALDI MS: A Practical Guide to Instrumentation, Methods and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-3-527-67374-2. [Google Scholar]
- Calvano, C.D.; Monopoli, A.; Cataldi, T.R.I.; Palmisano, F. MALDI Matrices for Low Molecular Weight Compounds: An Endless Story? Anal. Bioanal. Chem. 2018, 410, 4015–4038. [Google Scholar] [CrossRef]
- Leopold, J.; Popkova, Y.; Engel, K.M.; Schiller, J. Recent Developments of Useful MALDI Matrices for the Mass Spectrometric Characterization of Lipids. Biomolecules 2018, 8, 173. [Google Scholar] [CrossRef]
- Sunner, J.; Dratz, E.; Chen, Y.C. Graphite Surface-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry of Peptides and Proteins from Liquid Solutions. Anal. Chem. 1995, 67, 4335–4342. [Google Scholar] [CrossRef]
- Lim, A.Y.; Ma, J.; Boey, Y.C.F. Development of Nanomaterials for SALDI-MS Analysis in Forensics. Adv. Mater. 2012, 24, 4211–4216. [Google Scholar] [CrossRef]
- Arakawa, R.; Kawasaki, H. Functionalized Nanoparticles and Nanostructured Surfaces for Surface-Assisted Laser Desorption/Ionization Mass Spectrometry. Anal. Sci. 2010, 26, 1229–1240. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Nanoparticle Assisted Laser Desorption/Ionization Mass Spectrometry for Small Molecule Analytes. Microchim. Acta 2018, 185, 200. [Google Scholar] [CrossRef]
- Longuespée, R.; Casadonte, R.; Kriegsmann, M.; Pottier, C.; Picard de Muller, G.; Delvenne, P.; Kriegsmann, J.; De Pauw, E. MALDI Mass Spectrometry Imaging: A Cutting-Edge Tool for Fundamental and Clinical Histopathology. Proteom. Clin. Appl. 2016, 10, 701–719. [Google Scholar] [CrossRef]
- Müller, W.H.; Verdin, A.; De Pauw, E.; Malherbe, C.; Eppe, G. Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging: A Review. Mass Spectrom. Rev. 2022, 41, 373–420. [Google Scholar] [CrossRef]
- Kriegsmann, J.; Kriegsmann, M.; Casadonte, R. MALDI TOF Imaging Mass Spectrometry in Clinical Pathology: A Valuable Tool for Cancer Diagnostics (Review). Int. J. Oncol. 2015, 46, 893–906. [Google Scholar] [CrossRef]
- García-Perdomo, H.A.; Dávila-Raigoza, A.M.; Korkes, F. Metabolomics for the Diagnosis of Bladder Cancer: A Systematic Review. Asian J. Urol. 2023. [Google Scholar] [CrossRef]
- Czétány, P.; Gitta, S.; Balló, A.; Sulc, A.; Máté, G.; Szántó, Á.; Márk, L. Application of Mass Spectrometry Imaging in Uro-Oncology: Discovering Potential Biomarkers. Life 2022, 12, 366. [Google Scholar] [CrossRef]
- Buszewska-Forajta, M.; Pomastowski, P.; Monedeiro, F.; Król-Górniak, A.; Adamczyk, P.; Markuszewski, M.J.; Buszewski, B. New Approach in Determination of Urinary Diagnostic Markers for Prostate Cancer by MALDI-TOF/MS. Talanta 2022, 236, 122843. [Google Scholar] [CrossRef]
- Jirásko, R.; Holčapek, M.; Khalikova, M.; Vrána, D.; Študent, V.; Prouzová, Z.; Melichar, B. MALDI Orbitrap Mass Spectrometry Profiling of Dysregulated Sulfoglycosphingolipids in Renal Cell Carcinoma Tissues. J. Am. Soc. Mass Spectrom. 2017, 28, 1562–1574. [Google Scholar] [CrossRef]
- Arendowski, A.; Ossoliński, K.; Ossolińska, A.; Ossoliński, T.; Nizioł, J.; Ruman, T. Serum and Urine Analysis with Gold Nanoparticle-Assisted Laser Desorption/Ionization Mass Spectrometry for Renal Cell Carcinoma Metabolic Biomarkers Discovery. Adv. Med. Sci. 2021, 66, 326–335. [Google Scholar] [CrossRef]
- Ossoliński, K.; Nizioł, J.; Arendowski, A.; Ossolińska, A.; Ossoliński, T.; Kucharz, J.; Wiechno, P.; Ruman, T. Mass Spectrometry-Based Metabolomic Profiling of Prostate Cancer—A Pilot Study. J. Cancer Metastasis Treat. 2019, 5, 1. [Google Scholar] [CrossRef][Green Version]
- Nizioł, J.; Bonifay, V.; Ossoliński, K.; Ossoliński, T.; Ossolińska, A.; Sunner, J.; Beech, I.; Arendowski, A.; Ruman, T. Metabolomic Study of Human Tissue and Urine in Clear Cell Renal Carcinoma by LC-HRMS and PLS-DA. Anal. Bioanal. Chem. 2018, 410, 3859–3869. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Cheng, X.; Liu, X.; Sun, H.; Guo, Z.; Li, J.; Tang, X.; Wang, Z.; Sun, W.; et al. LC-MS-Based Plasma Metabolomics and Lipidomics Analyses for Differential Diagnosis of Bladder Cancer and Renal Cell Carcinoma. Front. Oncol. 2020, 10, 717. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, R.; Ma, C.; Zhou, L.; Liu, X.; Yin, P.; Zhang, Z.; Sun, Y.; Xu, C.; Lu, X.; et al. Discovery and Validation of Potential Urinary Biomarkers for Bladder Cancer Diagnosis Using a Pseudotargeted GC-MS Metabolomics Method. Oncotarget 2017, 8, 20719–20728. [Google Scholar] [CrossRef]
- Pinto, J.; Amaro, F.; Lima, A.R.; Carvalho-Maia, C.; Jerónimo, C.; Henrique, R.; Bastos, M.d.L.; Carvalho, M.; Guedes de Pinho, P. Urinary Volatilomics Unveils a Candidate Biomarker Panel for Noninvasive Detection of Clear Cell Renal Cell Carcinoma. J. Proteome Res. 2021, 20, 3068–3077. [Google Scholar] [CrossRef]
- MacLennan, M.S.; Kok, M.G.M.; Soliman, L.; So, A.; Hurtado-Coll, A.; Chen, D.D.Y. Capillary Electrophoresis-Mass Spectrometry for Targeted and Untargeted Analysis of the Sub-5 kDa Urine Metabolome of Patients with Prostate or Bladder Cancer: A Feasibility Study. J. Chromatogr. B 2018, 1074–1075, 79–85. [Google Scholar] [CrossRef]
- Alberice, J.V.; Amaral, A.F.S.; Armitage, E.G.; Lorente, J.A.; Algaba, F.; Carrilho, E.; Márquez, M.; García, A.; Malats, N.; Barbas, C. Searching for Urine Biomarkers of Bladder Cancer Recurrence Using a Liquid Chromatography-Mass Spectrometry and Capillary Electrophoresis-Mass Spectrometry Metabolomics Approach. J. Chromatogr. A 2013, 1318, 163–170. [Google Scholar] [CrossRef]
- Zira, A.N.; Theocharis, S.E.; Mitropoulos, D.; Migdalis, V.; Mikros, E. (1)H NMR Metabonomic Analysis in Renal Cell Carcinoma: A Possible Diagnostic Tool. J. Proteome Res. 2010, 9, 4038–4044. [Google Scholar] [CrossRef]
- Hasubek, A.-L.; Wang, X.; Zhang, E.; Kobus, M.; Chen, J.; Vandergrift, L.A.; Kurreck, A.; Ehret, F.; Dinges, S.; Hohm, A.; et al. Differentiation of Patients with and without Prostate Cancer Using Urine 1H NMR Metabolomics. Magn. Reson. Chem. 2023, 61, 740–747. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, L.; Chen, H.; Xue, W.; Lin, D. NMR-Based Metabolomic Analysis of Human Bladder Cancer. Anal. Sci. 2012, 28, 451–456. [Google Scholar] [CrossRef]
- Pomastowski, P.; Buszewski, B. Complementarity of Matrix- and Nanostructure-Assisted Laser Desorption/Ionization Approaches. Nanomaterials 2019, 9, 260. [Google Scholar] [CrossRef]
- Kaufman, D.S.; Shipley, W.U.; Feldman, A.S. Bladder Cancer. Lancet 2009, 374, 239–249. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; MSHS, M. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Sanchez-Carbayo, M. Recent Advances in Bladder Cancer Diagnostics. Clin. Biochem. 2004, 37, 562–571. [Google Scholar] [CrossRef]
- Proctor, I.; Stoeber, K.; Williams, G.H. Biomarkers in Bladder Cancer. Histopathology 2010, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Stenzl, A.; Sharma, A.; Vasdev, N. Urinary Biomarkers in Bladder Cancer: A Review of the Current Landscape and Future Directions. Urol. Oncol. 2021, 39, 41–51. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Fan, J.; He, L.; Chen, J.; Liu, H.; Nie, Z. Rapid Screening for Genitourinary Cancers: Mass Spectrometry-Based Metabolic Fingerprinting of Urine. Chem. Commun. 2022, 58, 9433–9436. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Jiang, Y.; Mao, L.; Lai, M.; Jiang, L.; Liu, H.; Nie, Z. TiO2/MXene-Assisted LDI-MS for Urine Metabolic Profiling in Urinary Disease. Adv. Funct. Mater. 2021, 31, 2106743. [Google Scholar] [CrossRef]
- Jiang, X.; Gao, F.; Chen, X.; Yu, Y.; Ding, G.; Wu, J. Ultrasensitive and High Reproducible Detection of Urinary Metabolites Using the Tip-Contact Extraction Method Coupled with Negative LDI-MS. J. Proteome Res. 2021, 20, 4022–4030. [Google Scholar] [CrossRef]
- Ossoliński, K.; Ruman, T.; Copié, V.; Tripet, B.P.; Kołodziej, A.; Płaza-Altamer, A.; Ossolińska, A.; Ossoliński, T.; Nieczaj, A.; Nizioł, J. Targeted and Untargeted Urinary Metabolic Profiling of Bladder Cancer. J. Pharm. Biomed. Anal. 2023, 233, 115473. [Google Scholar] [CrossRef]
- Ossoliński, K.; Ruman, T.; Copié, V.; Tripet, B.P.; Nogueira, L.B.; Nogueira, K.O.P.C.; Kołodziej, A.; Płaza-Altamer, A.; Ossolińska, A.; Ossoliński, T.; et al. Metabolomic and Elemental Profiling of Blood Serum in Bladder Cancer. J. Pharm. Anal. 2022, 12, 889–900. [Google Scholar] [CrossRef]
- Ossoliński, K.; Ruman, T.; Ossoliński, T.; Ossolińska, A.; Arendowski, A.; Kołodziej, A.; Płaza-Altamer, A.; Nizioł, J. Monoisotopic Silver Nanoparticles-Based Mass Spectrometry Imaging of Human Bladder Cancer Tissue: Biomarker Discovery. Adv. Med. Sci. 2023, 68, 38–45. [Google Scholar] [CrossRef]
- Chow, W.-H.; Dong, L.M.; Devesa, S.S. Epidemiology and Risk Factors for Kidney Cancer. Nat. Rev. Urol. 2010, 7, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Moch, H. An Overview of Renal Cell Cancer: Pathology and Genetics. Semin. Cancer Biol. 2013, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cheville, J.C.; Lohse, C.M.; Zincke, H.; Weaver, A.L.; Blute, M.L. Comparisons of Outcome and Prognostic Features Among Histologic Subtypes of Renal Cell Carcinoma. Am. J. Surg. Pathol. 2003, 27, 612. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Bukowski, R.M. Targeted Therapy for Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2006, 24, 5601–5608. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef]
- Nizioł, J.; Copié, V.; Tripet, B.P.; Nogueira, L.B.; Nogueira, K.O.P.C.; Ossoliński, K.; Arendowski, A.; Ruman, T. Metabolomic and Elemental Profiling of Human Tissue in Kidney Cancer. Metabolomics 2021, 17, 30. [Google Scholar] [CrossRef]
- Nizioł, J.; Ossoliński, K.; Tripet, B.P.; Copié, V.; Arendowski, A.; Ruman, T. Nuclear Magnetic Resonance and Surface-Assisted Laser Desorption/Ionization Mass Spectrometry-Based Serum Metabolomics of Kidney Cancer. Anal. Bioanal. Chem. 2020, 412, 5827–5841. [Google Scholar] [CrossRef]
- Nizioł, J.; Ossoliński, K.; Tripet, B.P.; Copié, V.; Arendowski, A.; Ruman, T. Nuclear Magnetic Resonance and Surface-Assisted Laser Desorption/Ionization Mass Spectrometry-Based Metabolome Profiling of Urine Samples from Kidney Cancer Patients. J. Pharm. Biomed. Anal. 2021, 193, 113752. [Google Scholar] [CrossRef]
- Yang, J.; Yin, X.; Zhang, L.; Zhang, X.; Lin, Y.; Zhuang, L.; Liu, W.; Zhang, R.; Yan, X.; Shi, L.; et al. Defective Fe Metal–Organic Frameworks Enhance Metabolic Profiling for High-Accuracy Diagnosis of Human Cancers. Adv. Mater. 2022, 34, 2201422. [Google Scholar] [CrossRef]
- Sekuła, J.; Nizioł, J.; Misiorek, M.; Dec, P.; Wrona, A.; Arendowski, A.; Ruman, T. Gold Nanoparticle-Enhanced Target for MS Analysis and Imaging of Harmful Compounds in Plant, Animal Tissue and on Fingerprint. Anal. Chim. Acta 2015, 895, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Arendowski, A.; Ossoliński, K.; Nizioł, J.; Ruman, T. Gold Nanostructures—Assisted Laser Desorption/Ionization Mass Spectrometry for Kidney Cancer Blood Serum Biomarker Screening. Int. J. Mass Spectrom. 2020, 456, 116396. [Google Scholar] [CrossRef]
- Arendowski, A.; Ossolinski, K.; Niziol, J.; Ruman, T. Screening of Urinary Renal Cancer Metabolic Biomarkers with Gold Nanoparticles-Assisted Laser Desorption/Ionization Mass Spectrometry. Anal. Sci. 2020, 36, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Nizioł, J.; Ossoliński, K.; Ossoliński, T.; Ossolińska, A.; Bonifay, V.; Sekuła, J.; Dobrowolski, Z.; Sunner, J.; Beech, I.; Ruman, T. Surface-Transfer Mass Spectrometry Imaging of Renal Tissue on Gold Nanoparticle Enhanced Target. Anal. Chem. 2016, 88, 7365–7371. [Google Scholar] [CrossRef] [PubMed]
- Arendowski, A.; Nizioł, J.; Ossoliński, K.; Ossolińska, A.; Ossoliński, T.; Dobrowolski, Z.; Ruman, T. Laser Desorption/Ionization MS Imaging of Cancer Kidney Tissue on Silver Nanoparticle-Enhanced Target. Bioanalysis 2018, 10, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Nizioł, J.; Sunner, J.; Beech, I.; Ossoliński, K.; Ossolińska, A.; Ossoliński, T.; Płaza, A.; Ruman, T. Localization of Metabolites of Human Kidney Tissue with Infrared Laser-Based Selected Reaction Monitoring Mass Spectrometry Imaging and Silver-109 Nanoparticle-Based Surface Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Anal. Chem. 2020, 92, 4251–4258. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.E.; Powers, T.W.; Neely, B.A.; Cazares, L.H.; Troyer, D.A.; Parker, A.S.; Drake, R.R. MALDI Imaging Mass Spectrometry Profiling of Proteins and Lipids in Clear Cell Renal Cell Carcinoma. Proteomics 2014, 14, 924–935. [Google Scholar] [CrossRef]
- Martín-Saiz, L.; Abad-García, B.; Solano-Iturri, J.D.; Mosteiro, L.; Martín-Allende, J.; Rueda, Y.; Pérez-Fernández, A.; Unda, M.; Coterón-Ochoa, P.; Goya, A.; et al. Using the Synergy between HPLC-MS and MALDI-MS Imaging to Explore the Lipidomics of Clear Cell Renal Cell Carcinoma. Anal. Chem. 2023, 95, 2285–2293. [Google Scholar] [CrossRef]
- Erlmeier, F.; Sun, N.; Shen, J.; Feuchtinger, A.; Buck, A.; Prade, V.M.; Kunzke, T.; Schraml, P.; Moch, H.; Autenrieth, M.; et al. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging: Diagnostic Pathways and Metabolites for Renal Tumor Entities. Oncology 2023, 101, 126–133. [Google Scholar] [CrossRef]
- Erlmeier, F.; Sun, N.; Shen, J.; Feuchtinger, A.; Buck, A.; Prade, V.M.; Kunzke, T.; Schraml, P.; Moch, H.; Autenrieth, M.; et al. MALDI Mass Spectrometry Imaging—Prognostic Pathways and Metabolites for Renal Cell Carcinomas. Cancers 2022, 14, 1763. [Google Scholar] [CrossRef]
- Andersen, M.K.; Krossa, S.; Høiem, T.S.; Buchholz, R.; Claes, B.S.R.; Balluff, B.; Ellis, S.R.; Richardsen, E.; Bertilsson, H.; Heeren, R.M.A.; et al. Simultaneous Detection of Zinc and Its Pathway Metabolites Using MALDI MS Imaging of Prostate Tissue. Anal. Chem. 2020, 92, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kruithof-de Julio, M.; Economides, K.D.; Walker, D.; Yu, H.; Halili, M.V.; Hu, Y.-P.; Price, S.M.; Abate-Shen, C.; Shen, M.M. A Luminal Epithelial Stem Cell That Is a Cell of Origin for Prostate Cancer. Nature 2009, 461, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Buszewska-Forajta, M.; Pomastowski, P.; Monedeiro, F.; Walczak-Skierska, J.; Markuszewski, M.; Matuszewski, M.; Markuszewski, M.J.; Buszewski, B. Lipidomics as a Diagnostic Tool for Prostate Cancer. Cancers 2021, 13, 2000. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nakayama, K.; Goto, T.; Kimura, H.; Akamatsu, S.; Hayashi, Y.; Fujita, K.; Kobayashi, T.; Shimizu, K.; Nonomura, N.; et al. High Level of Phosphatidylcholines/Lysophosphatidylcholine Ratio in Urine Is Associated with Prostate Cancer. Cancer Sci. 2021, 112, 4292–4302. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Terada, N.; Inoue, T.; Nakayama, K.; Okada, Y.; Yoshikawa, T.; Miyazaki, Y.; Uegaki, M.; Sumiyoshi, S.; Kobayashi, T.; et al. The Expression Profile of Phosphatidylinositol in High Spatial Resolution Imaging Mass Spectrometry as a Potential Biomarker for Prostate Cancer. PLoS ONE 2014, 9, e90242. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, J.; Hardie, D.B.; Yang, J.; Pan, J.; Borchers, C.H. Metabolomic Profiling of Prostate Cancer by Matrix Assisted Laser Desorption/Ionization-Fourier Transform Ion Cyclotron Resonance Mass Spectrometry Imaging Using Matrix Coating Assisted by an Electric Field (MCAEF). Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 755–767. [Google Scholar] [CrossRef]
- Butler, L.M.; Mah, C.Y.; Machiels, J.; Vincent, A.D.; Irani, S.; Mutuku, S.M.; Spotbeen, X.; Bagadi, M.; Waltregny, D.; Moldovan, M.; et al. Lipidomic Profiling of Clinical Prostate Cancer Reveals Targetable Alterations in Membrane Lipid Composition. Cancer Res. 2021, 81, 4981–4993. [Google Scholar] [CrossRef]
- Leopold, J.; Prabutzki, P.; Engel, K.M.; Schiller, J. A Five-Year Update on Matrix Compounds for MALDI-MS Analysis of Lipids. Biomolecules 2023, 13, 546. [Google Scholar] [CrossRef]
- Defossez, E.; Bourquin, J.; von Reuss, S.; Rasmann, S.; Glauser, G. Eight Key Rules for Successful Data-Dependent Acquisition in Mass Spectrometry-Based Metabolomics. Mass Spectrom. Rev. 2023, 42, 131–143. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arendowski, A. Matrix- and Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Methods for Urological Cancer Biomarker Discovery—Metabolomics and Lipidomics Approaches. Metabolites 2024, 14, 173. https://doi.org/10.3390/metabo14030173
Arendowski A. Matrix- and Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Methods for Urological Cancer Biomarker Discovery—Metabolomics and Lipidomics Approaches. Metabolites. 2024; 14(3):173. https://doi.org/10.3390/metabo14030173
Chicago/Turabian StyleArendowski, Adrian. 2024. "Matrix- and Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Methods for Urological Cancer Biomarker Discovery—Metabolomics and Lipidomics Approaches" Metabolites 14, no. 3: 173. https://doi.org/10.3390/metabo14030173
APA StyleArendowski, A. (2024). Matrix- and Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Methods for Urological Cancer Biomarker Discovery—Metabolomics and Lipidomics Approaches. Metabolites, 14(3), 173. https://doi.org/10.3390/metabo14030173






