Comparative Metabolomics of Ligulate and Tubular Flowers of Two Cultivars of Calendula officinalis L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Plant Objects
2.2. UPLC-PDA-HRMS Analysis of Metabolites
2.3. Processing of the MS Data
2.4. Bioinfomatic Analysis of the MS Data
2.5. Characterisation of Metabolites
2.6. Quantitation of Metabolites
2.7. Reagents
3. Results
3.1. UPLC-PDA-HRMS Analysis of Metabolome
3.2. Characterisation of Phenolic Compounds
| Code | RT | UV Maxima | [M-H]− | MS/MS | Mass | Chemical | Error | Metabolite |
|---|---|---|---|---|---|---|---|---|
| (min) | λ (nm) | (m/z) | Fragments (m/z) | (Da) | Formula | (ppm) | Characterisation | |
| P1 | 2.37 | 323 | 353.0875 | 179.0339, 191.0556 | 354.0947 | C16H18O9 | −1.1 | 5-O-Caffeoylquinic acid [5,28] |
| P2 | 2.52 | 310sh, 326 | 353.0874 | 179.0336, 191.0556 | 354.0946 | C16H18O9 | −1.4 | 3-O-Caffeoylquinic acid [5,28] |
| P3 | 2.72 | 310sh *, 326 | 353.0875 | 179.0340, 191.0555 | 354.0947 | C16H18O9 | −1.1 | 4-O-Caffeoylquinic acid [5,28] |
| P4 | 2.94 | 255, 270sh, 354 | 755.2036 | 301.0344, 271.0243 | 756.2108 | C33H40O20 | −0.7 | Quercetin-3-O-rutinosyl-rhamnoside [5,40,41] |
| P5 | 3.10 | 254, 270sh, 355 | 609.1459 | 301.0342, 271.0243 | 610.1531 | C27H30O16 | −0.5 | Quercetin-3-O-β-D-rutinoside [5,40,41] |
| P6 | 3.12 | 254, 270sh, 356 | 769.2185 | 315.0505, 460.1011 | 770.2257 | C34H42O20 | −1.6 | Isorhamnetin-3-O-rutinosyl-rhamnoside [5,41] |
| P7 | 3.25 | 263, 346 | 593.1512 | 285.0399, 431.0977 | 594.1584 | C27H30O15 | −0.1 | Kaempferol-3-O-rutinoside [5,45] |
| P8 | 3.29 | 254, 270sh, 355 | 463.0878 | 301.0348, 271.0276 | 464.0950 | C21H20O12 | −1.0 | Quercetin-3-O-glucoside [5,40,41] |
| P9 | 3.37 | 254, 270sh, 351 | 609.1459 | 301.0343, 271.0244 | 610.1531 | C27H30O16 | −0.5 | Quercetin-3-O-rhamnosyl-glucoside [5,40,41] |
| P10 | 3.43 | 254, 270sh, 356 | 623.1613 | 315.0491, 299.0191 | 624.1685 | C28H32O16 | −0.9 | Isorhamnetin-3-O-rutinoside [5,41] |
| P11 | 3.48 | 254, 270sh, 354 | 623.1612 | 315.0491, 299.0191 | 624.1684 | C28H32O16 | −0.9 | Isorhamnetin 3-O-rhamnopyranosyl-glucopyranoside [5,41] |
| P12 | 3.59 | 300sh, 328 | 515.119 | 179.0336, 191.0557, 353.0874 | 516.1262 | C25H24O12 | −1.1 | 3,5-Di-O-caffeoylquinic acid [28] |
| P13 | 3.76 | 299, 308 | 639.3185 | 119.0497, 519.2606 | 640.3257 | C37H44N4O6 | −0.6 | Tris-trans-p-coumaroyl-spermine |
| P14 | 3.83 | 254, 265sh, 354 | 563.1036 | 315.0506, 299.0195 | 564.1108 | C25H24O15 | −1.3 | Isorhamnetin-malonyl-hexoside |
| P15 | 4.82 | 298, 308 | 785.3546 | 119.0495, 639.3183 | 786.3618 | C46H50N4O8 | −1.4 | Tetra-trans-p-coumaroyl-spermine |
3.3. Characterisation of Triterpenoid Glycosides
3.4. Characterisation of Lipids
3.5. Comparison of Metabolites of Ligulate and Tubular Flowers
3.6. Comparison of the Metabolites of Two C. officinalis Cultivars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khouchlaa, A.; El Baaboua, A.; El Moudden, H.; Lakhdar, F.; Bakrim, S.; El Menyiy, N.; Belmehdi, O.; Harhar, H.; El Omari, N.; Balahbib, A.; et al. Traditional uses, bioactive compounds, and pharmacological investigations of Calendula arvensis L.: A comprehensive review. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 2482544. [Google Scholar] [CrossRef]
- Ashwalayan, V.D.; Kumar, A.; Verma, M.; Garg, V.K.; Gupta, S.K. Therapeutic potential of Calendula officinalis. Pharm. Pharmacol. Int. J. 2018, 6, 149–155. [Google Scholar] [CrossRef]
- Szopa, A.; Klimek-Szczykutowicz, M.; Jafernik, K.; Koc, K.; Ekiert, H. Pot marigold (Calendula officinalis L.)—A position in classical phytotherapy and newly documented activities. Acta Sci. Pol. Hortorum Cultus 2020, 19, 47–61. [Google Scholar] [CrossRef]
- Patil, K.; Sandjay, C.; Doggalli, N.; Devi, K.; Harshitha, N. A review of Calendula officinalis—Magic in science. J. Clin. Diagn. Res. 2022, 16, 23–27. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Marigold metabolites: Diversity and separation methods of Calendula genus phytochemicals from 1891 to 2022. Molecules 2022, 27, 8626. [Google Scholar] [CrossRef] [PubMed]
- Barral-Martinez, M.; Garcia-Oliveira, P.; Nuñez-Estevez, B.; Silva, A.; Finimundy, T.C.; Calhelha, R.; Nenadic, M.; Sokovic, M.; Barroso, F.; Simal-Gandara, J.; et al. Plants of the family Asteraceae: Evaluation of biological properties and identification of phenolic compounds. Chem. Proc. 2021, 5, 51. [Google Scholar] [CrossRef]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological applications and health-promoting properties of flavonols: An updated view. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An updated review on the multifaceted therapeutic potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef]
- Ullah, M.A.; Hassan, A.; Hamza, A. Calendula (Calendula officinalis) marigold as medicinal plant. Orthop. Case Rep. 2023, 2, 9. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current advances in naturally occurring caffeoylquinic acids: Structure, bioactivity, and synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Rani, A.; Sharma, A. A review on phytochemistry and ethnopharmacological aspects of genus Calendula. Pharmacogn. Rev. 2013, 7, 179–187. [Google Scholar] [CrossRef]
- Długosz, M.; Wiktorowska, E.; Wiśniewska, A.; Pączkowski, C. Production of oleanolic acid glycosides by hairy root established cultures of Calendula officinalis L. Acta Biochim. Polon. 2013, 60, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.A.; Silva, J.A.T. Biology of Calendula officinalis Linn.: Focus on pharmacology, biological activities and agronomic practices. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 12–27. [Google Scholar]
- Lim, T.K. Calendula officinalis. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2014; Volume 7, pp. 213–244. [Google Scholar] [CrossRef]
- Lehbili, M.; Alabdul Magid, A.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Abedini, A.; Morjani, H.; Sarazin, T.; Gangloff, S.C.; Kabouche, Z. Oleanane-type triterpene saponins from Calendula stellata. Phytochemistry 2017, 144, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.C.; Garcia, P.A.; Castro, M.Á.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of two aromatic plants: Calendula officinalis L. (flowers) and Mentha cervina L. (leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef]
- Dulf, F.V.; Pamfil, D.; Baciu, A.D.; Pintea, A. Fatty acid composition of lipids in pot marigold (Calendula officinalis L.) seed genotypes. Chem. Centr. J. 2013, 7, 8. [Google Scholar] [CrossRef]
- Walisiewicz-Niedbalska, W.; Patkowska-Sokoła, B.; Gwardiak, H.; Szulc, T.; Bodkowski, R.; Rózycki, K. Potential raw materials in the synthesis of bioactive fat derivatives. Przemysł. Chem. 2012, 91, 1058–1063. [Google Scholar]
- Jan, N.; Andrabi, K.I.; John, R. Calendula officinalis—An important medicinal plant with potential biological properties. Proc. Indian Natl. Sci. Acad. 2017, 83, 769–787. [Google Scholar] [CrossRef]
- Hennessy, A.A.; Ross, R.P.; Devery, R.; Stanton, C. The health promoting properties of the conjugated isomers of alpha-linolenic acid. Lipids 2011, 46, 105–119. [Google Scholar] [CrossRef]
- Hennessy, A.A.; Ross, P.R.; Fitzgerald, G.F.; Stanton, C. Sources and bioactive properties of conjugated dietary fatty acids. Lipids 2016, 51, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, H.; Ye, S.H.; Xiao, S.; Xie, Y.P.; Liu, X.; Wang, J.H. Induction of apoptosis and inhibition of invasion in choriocarcinoma JEG-3 cells by α-calendic acid and β-calendic acid. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.F.; Chen, X.E.; Li, D. Conjugated linolenic acids and their bioactivities: A review. Food Funct. 2014, 5, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia. European Union Herbal Monograph on Calendula officinalis L., flos; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2018; 8p. [Google Scholar]
- Król, B. Yield and chemical composition of flower heads of selected cultivars of pot marigold (Calendula officinalis L.). Acta Sci. Pol. Hortic. Cult. 2012, 11, 215–225. [Google Scholar]
- Paim, L.F.; Fontana, M.; Winckler, M.; Grando, A.A.; Muneron, T.L.; Roman Júnior, W.A. Assessment of plant development, morphology and flavonoid content in different cultivation treatments of Calendula officinalis L. Asteraceae. Rev. Bras. Farmacogn. 2010, 20, 974–980. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. New isorhamnetin glycosides and other phenolic compounds from Calendula officinalis. Chem. Nat. Compd. 2013, 49, 833–840. [Google Scholar] [CrossRef]
- Petrović, L.; Lepojević, Ž.; Sovilj, V.; Adamović, D.; Tešević, V. Composition of essential oil obtained from tubular, head and ligulate flowers of Calendula officinalis L. by steam distillation of plant material and CO2 extracts. J. Essent. Oil Res. 2010, 22, 143–146. [Google Scholar] [CrossRef]
- Zitterl-Eglseer, K.; Reznicek, G.; Jurenitsch, J.; Novak, J.; Zitterl, W.; Franz, C. Morphogenetic variability of faradiol monoesters in marigold Calendula officinalis L. Phytochem. Anal. 2001, 12, 199–201. [Google Scholar] [CrossRef]
- Kuzmenko, I.N.; Kolyasnikova, N.L. The features of seed productivity of Calendula officinalis L. (Asteraceae) in the Preduralye region. Univ. Proc. Volga Reg. Nat. Sci. 2021, 36, 45–55. [Google Scholar] [CrossRef]
- Khazieva, F.M.; Samatadze, T.E.; Korotkikh, I.N. Calendula officinalis. Nauka; Russian Federation: Moscow, Russia, 2023; 141p. [Google Scholar]
- Ossipov, V.; Zubova, M.; Nechaeva, T.; Zagoskina, N.; Salminen, J.-P. The regulating effect of light on the content of flavan-3-ols and derivatives of hydroxybenzoic acids in the callus culture of the tea plant, Camellia sinensis L. Biochem. Syst. Ecol. 2022, 101, 104383. [Google Scholar] [CrossRef]
- Forsberg, E.; Huan, T.; Rinehart, D.; Benton, H.; Warth, B.; Hilmers, B.; Siuzdak, G. Data processing, multi-omic pathway mapping, and metabolite activity analysis using XCMS Online. Nat. Protoc. 2018, 13, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. Clustvis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015, 43, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative analysis of multimodal mass spectrometry data in MZmine-3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E.; et al. METLIN: A technology platform for identifying knowns and unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.F.; Melonib, F.; Borellac, J.C.; Lopesa, N.P. Effect of fertilisation and harvest period on polar metabolites of Calendula officinalis. Rev. Bras. Farmacogn. 2013, 23, 731–735. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Calendosides I-IV, new quercetin and isorhamnetin rhamnoglycosides from Calendula officinalis. Chem. Nat. Compd. 2014, 50, 633–637. [Google Scholar] [CrossRef]
- Faustino, M.V.; Pinto, C.G.A.D.; Gonçalves, M.J.; Salgueiro, L.; Silveira, P.; Silva, A.M.S. Calendula L. species polyphenolic profile and in vitro antifungal activity. J. Funct. Foods 2018, 45, 254–267. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Sa, M.R.; Ji, J.; Vaniya, A.; Wancewicz, B.; Roberts, B.S.; Torbašinović, H.; Lee, T.; Mehta, S.S.; et al. Structure annotation of all mass spectra in untargeted metabolomics. Anal. Chem. 2019, 91, 2155–2162. [Google Scholar] [CrossRef]
- Xue, M.; Shi, H.; Zhang, J.; Liu, Q.Q.; Guan, J.; Zhang, J.Y.; Ma, Q. Stability and degradation of caffeoylquinic acids under different storage conditions studied by high-performance liquid chromatography with photo diode array detection and high-performance liquid chromatography with electrospray ionization collision-induced dissociation tandem mass spectrometry. Molecules 2016, 21, 948. [Google Scholar] [CrossRef]
- Fiorentino, M.; Gravina, C.; Piccolella, S.; Pecoraro, M.T.; Formato, M.; Stinca, A.; Pacifico, S.; Esposito, A. Calendula arvensis (Vaill.) L.: A systematic plant analysis of the polar extracts from its organs by UHPLC-HRMS. Foods 2022, 11, 247. [Google Scholar] [CrossRef]
- Hegazi, N.M.; Khattab, A.R.; Frolov, A.; Wessjohann, L.A.; Farag, M.A. Authentication of saffron spice accessions from its common substitutes via a multiplex approach of UV/VIS fingerprints and UPLC/MS using molecular networking and chemometrics. Food Chem. 2022, 367, 130739. [Google Scholar] [CrossRef] [PubMed]
- Wiktorowska, E.; Długosz, M.; Janiszowska, W. Significant enhancement of oleanolic acid accumulation by biotic elicitors in cell suspension cultures of Calendula officinalis L. Enzyme Microb. Technol. 2010, 46, 14–20. [Google Scholar] [CrossRef]
- Rettberg, N.; Thörner, S.; Garbe, L. Starting hop lipidomics—Isolation and characterization of non-polar, neutral and polar hop lipids. BrewingScience 2013, 66, 176–184. [Google Scholar]
- Sun, D.; Froman, B.E.; Orth, R.G.; MacIsaac, S.A.; Larosa, T.; Dong, F.; Valentin, H.E. Identification of plant sphingolipid desaturases using chromatography and mass spectrometry. J. Chromatogr. Sci. 2009, 47, 895–901. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamura, Y. Headgroup biosynthesis of phosphatidylcholine and phosphatidylethanolamine in seed plants. Prog. Lipid Res. 2021, 82, 101091. [Google Scholar] [CrossRef] [PubMed]
- Negri, G.; Silva, C.C.F.; Coelho, G.R.; Nascimento, R.M.; Mendonça, R.Z. Cardanols detected in non-polar propolis extracts from Scaptotrigona aff. postica (Hymenoptera, Apidae, Meliponini). Braz. J. Food Technol. 2019, 22, e2018265. [Google Scholar] [CrossRef]
- Peng, H.; Meyer, R.S.; Yang, T.; Whitaker, B.D.; Trouth, F.; Shangguan, L.; Huang, I.; Litt, A.; Little, D.P.; Ke, H.; et al. A novel hydroxycinnamoyl transferase for synthesis of hydroxycinnamoyl spermine conjugates in plants. BMC Plant Biol. 2019, 19, 261. [Google Scholar] [CrossRef]
- Edreva, A.M.; Velikova, V.B.; Tsonev, T.D. Phenylamides in plants. Russ. J. Plant Physiol. 2007, 54, 287–301. [Google Scholar] [CrossRef]
- Roumani, M.; Duval, R.E.; Ropars, A.; Risler, A.; Robin, C.; Larbat, R. Phenolamides: Plant specialized metabolites with a wide range of promising pharmacological and health-promoting interests. Biomed. Pharmacother. 2020, 131, 110762. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Snooks, H.; Sang, S. The Chemistry and Health Benefits of Dietary Phenolamides. J. Agric. Food Chem. 2020, 68, 6248–6267. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Nakamura, K.; Furukawa, K.; Konishi, Y.; Ogino, T.; Higashiura, K.; Yago, H.; Okamoto, K.; Otsuka, M. A new nonpeptide tachykinin NK1 receptor antagonist isolated from the plants of Compositae. Chem. Pharm. Bull. 2002, 50, 47–52. [Google Scholar] [CrossRef]
- Rupniak, N.M.J.; Kramer, M.S. NK1 receptor antagonists for depression: Why a validated concept was abandoned. J. Affect. Disord. 2017, 223, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, R.; Lu, Q. Separation and characterization of phenolamines and flavonoids from rape bee pollen, and comparison of their antioxidant activities and protective effects against oxidative stress. Molecules 2020, 25, 1264. [Google Scholar] [CrossRef] [PubMed]
- Bassard, J.-E.; Ullmann, P.; Bernier, F.; Werck-Reichhart, D. Phenolamides: Bridging polyamines to the phenolic metabolism. Phytochemistry 2010, 71, 1808–1824. [Google Scholar] [CrossRef]
- Kite, G.C.; Larsson, S.; Veitch, N.C.; Porter, E.A.; Liang, X.; Simmonds, M.S.J. Acyl spermidines in inflorescence extracts of elder (Sambucus nigra L., Adoxaceae) and elderflower drinks. J. Agric. Food Chem. 2013, 61, 3501–3508. [Google Scholar] [CrossRef]
- Li, S.; Yaermaimaiti, S.; Tian, X.-M.; Wang, Z.-W.; Xu, W.-J.; Luo, J.; Kong, L.-Y. Dynamic metabolic and transcriptomic profiling reveals the biosynthetic characteristics of hydroxycinnamic acid amides (HCAAs) in sunflower pollen. Food Res. Int. 2021, 149, 110678. [Google Scholar] [CrossRef]
- Ischebeck, T. Lipids in pollen—They are different. Biochim. Biophys Acta Mol. Cell Biol. Lipids 2016, 1861, 1315–1328. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cui, M.; Yang, L.; Kim, Y.J.; Zhang, D. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wu, S.; Li, Z.; An, X.; Tian, Y. Lipid metabolism: Critical roles in male fertility and other aspects of reproductive development in plants. Mol. Plant 2020, 13, 955–983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shi, J.; Yang, X. Role of lipid metabolism in plant pollen exine development. In Lipids in Plant and Algae Development. Subcellular Biochemistry; Nakamura, Y., Li-Beisson, Y., Eds.; Springer: Cham, Switzerland, 2016; Volume 86, pp. 315–337. [Google Scholar] [CrossRef]
- Krόl, B.; Paszko, T.; Krόl, A. Conjugated linolenic acid content in seeds of some pot marigold (Calendula officinalis L.) cultivars grown in Poland. Farmacia 2016, 64, 881–886. [Google Scholar]
- Dhar Dubey, K.K.; Sharma, G.; Kumar, A. Conjugated linolenic acids: Implication in cancer. J. Agric. Food Chem. 2019, 67, 6091–6101. [Google Scholar] [CrossRef] [PubMed]



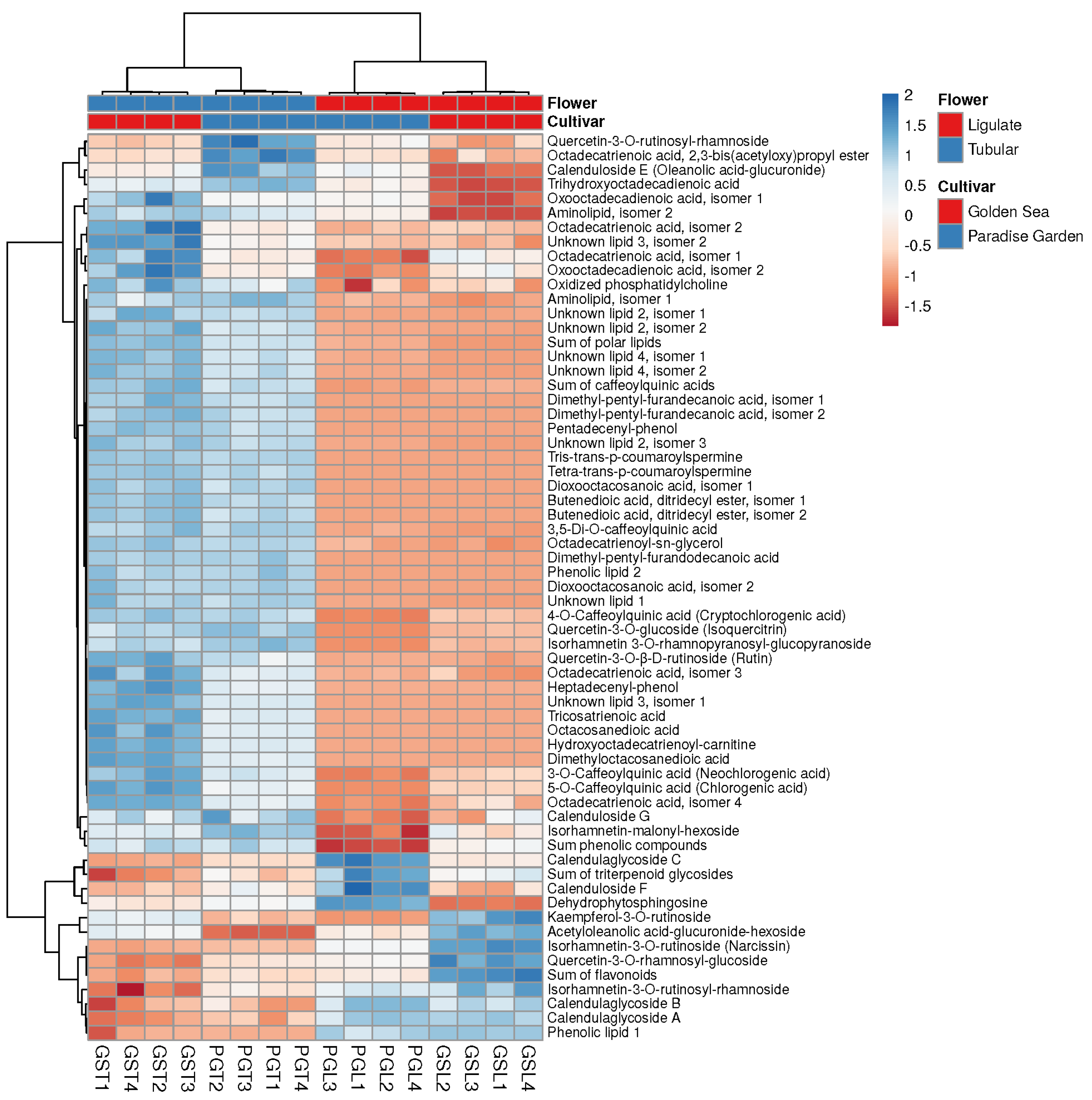
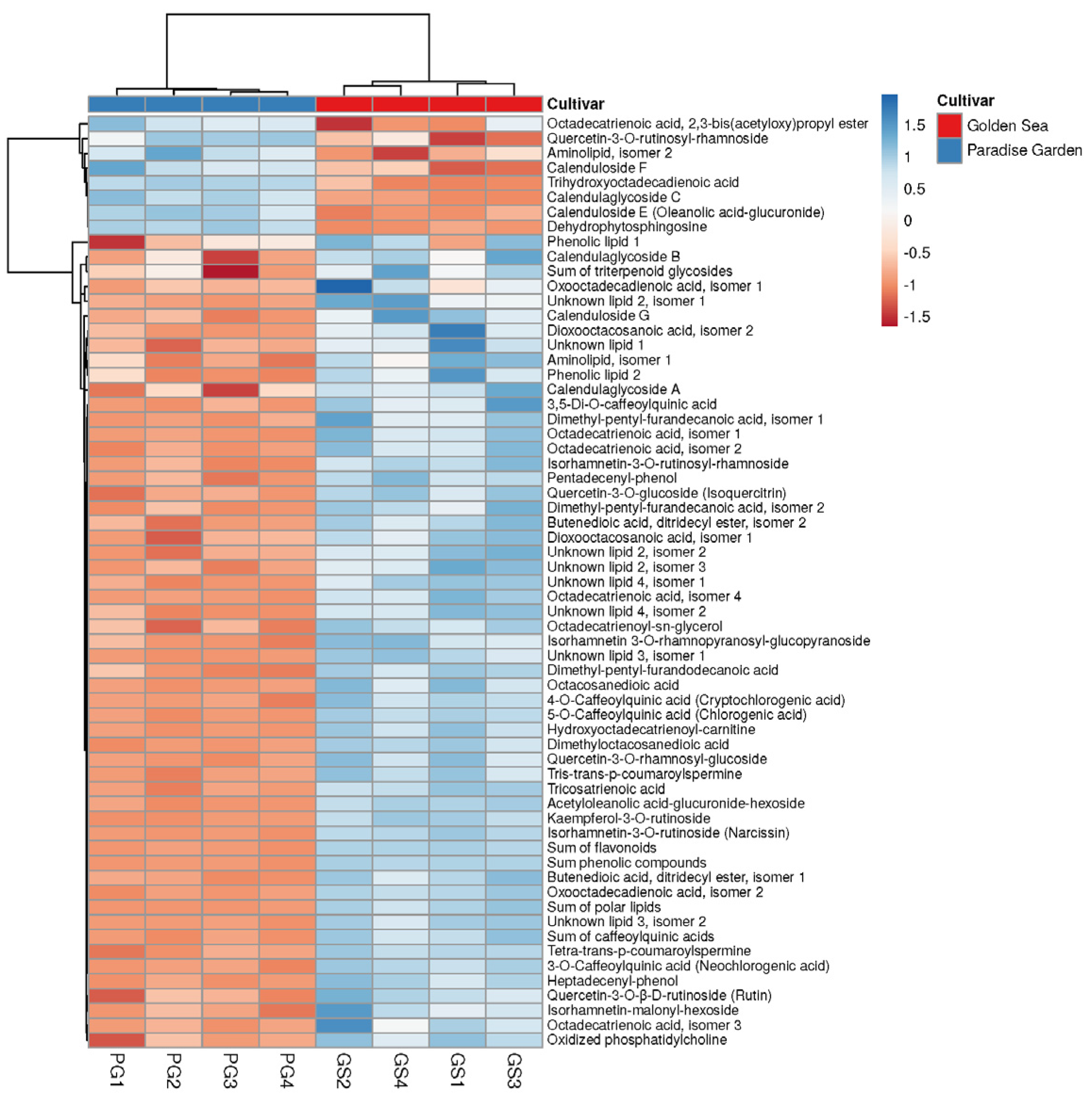
| Cultivar, Flowers | Compared | Negative Ions | Positive Ions | ||
|---|---|---|---|---|---|
| Groups | F | p | F | p | |
| ‘Paradise Garden’ | LF vs. TF | 524.3 | 1.55 × 10−6 | 601.4 | 1.10 × 10−6 |
| ‘Golden Sea’ | LF vs. TF | 527.7 | 1.53 × 10−6 | 675.6 | 8.25 × 10−7 |
| Ligulate flowers | PG vs. GS | 17.1 | 0.036 | 22.7 | 0.014 |
| Tubular flowers | PG vs. GS | 29.2 | 0.004 | 45.4 | 0.002 |
| Code | RT | [M-H]− | MS/MS | Mass | Chemical | Error | Metabolite |
|---|---|---|---|---|---|---|---|
| (min) | (m/z) | Fragments (m/z) | (Da) | Formula | (ppm) | Characterisation | |
| T1 | 4.85 | 1117.5436 | 455.3543, 971.4818 | 1118.5514 | C54H86O24 | 0.4 | Calendulaglycoside A [3,42,47] |
| T2 | 5.01 | 955.4893 | 455.3524, 793.4368 | 956.4971 | C48H76O19 | −0.9 | Calendulaglycoside B [5,45] |
| T3 | 5.23 | 793.4377 | 455.3521, 631.3848 | 794.4455 | C42H66O14 | 0.3 | Calenduloside G [3,42,47] |
| T4 | 5.51 | 835.4481 | 497.3636, 455.3521, 793.4368 | 836.4559 | C44H68O15 | 0.1 | Acetyloleanolic acid-glucuronide-hexoside |
| T5 | 5.78 | 955.4913 | 455.3524, 793.4368 | 956.4991 | C48H76O19 | 1.1 | Calendulaglycoside C [5,45] |
| T6 | 6.06 | 793.4379 | 455.3524, 631.3846 | 794.4457 | C42H66O14 | 0.6 | Calenduloside F [3,42,47] |
| T7 | 6.34 | 631.3843 | 455.3515 | 632.3921 | C36H56O9 | −0.5 | Calenduloside E (Oleanolic acid-glucuronide) [3,47] |
| Code | RT | [M+H]+ | MS/MS | Mass | Chemical | Error | Metabolite |
|---|---|---|---|---|---|---|---|
| (min) | (m/z) | Fragments (m/z) | (Da) | Formula | (ppm) | Characterisation | |
| L1 | 4.67 | 329.2325 | 311.2210, 293.2106, 275.2004 | 328.2247 | C18H32O5 | −0.8 | Trihydroxyoctadecadienoic acid |
| L2 | 4.72 | 279.2319 | 261.2213, 243.2108 | 278.2241 | C18H30O2 | −1.7 | Octadecatrienoic acid, isomer 1 [19,20] |
| L3 | 5.70 | 316.2844 | 298.2736, 280.2826 | 315.2771 | C18H37NO3 | −0.6 | Dehydrophytosphingosine |
| L4 | 6.45 | 295.2274 | 277.2155, 259.2052 | 294.2198 | C18H30O3 | 1.0 | Oxooctadecadienoic acid, isomer 1 |
| L5 | 6.58 | 295.2274 | 277.2155, 259.2052 | 294.2198 | C18H30O3 | 1.0 | Oxooctadecadienoic acid, isomer 2 |
| L6 | 6.64 | 279.2317 | 261.2213, 243.2108 | 278.2244 | C18H30O2 | −0.5 | Octadecatrienoic acid, isomer 2 [19,20] |
| L7 | 6.83 | 337.2738 | 319.2632, 301.2518, 247.2953 | 336.2662 | C21H36O3 | −0.8 | Dimethyl-pentyl-furandecanoic acid, isomer 1 |
| L8 | 6.93 | 337.2738 | 319.2632, 301.2518, 247.2059 | 336.2662 | C21H36O3 | −0.8 | Dimethyl-pentyl-furandecanoic acid, isomer 2 |
| L9 | 7.11 | 279.2316 | 261.2213, 243.2108 | 278.2243 | C18H30O2 | −1.2 | Octadecatrienoic acid, isomer 3 [19,20] |
| L10 | 7.13 | 438.322 | 379.2468 | 437.3147 | C25H43NO5 | 1.4 | Hydroxyoctadecatrienoyl-carnitine |
| L11 | 7.32 | 365.3046 | 347.2946, 329.2472 | 364.2971 | C23H40O3 | −1.8 | Dimethyl-pentyl-furandodecanoic acid |
| L12 | 7.41 | 353.2686 | 335.2579, 261.2210 | 352.2613 | C21H36O4 | −0.2 | Octadecatrienoyl-sn-glycerol |
| L13 | 7.51 | 473.2395 | 225.1846, 207.1740, 189.1634 | 472.2322 | C25H28N8O2 | −2.8 | Aminolipid, isomer 1 |
| L14 | 7.62 | 473.2397 | 225.1846, 207.1740 | 472.2324 | C25H28N8O2 | −2.3 | Aminolipid, isomer 2 |
| L15 | 7.67 | 437.2906 | 247.1692, 233.1534 | 436.2833 | C25H40O6 | 1.8 | Octadecatrienoic acid, 2,3-bis(acetyloxy)propyl ester |
| L16 | 7.77 | 279.2317 | 261.2213, 243.2108 | 278.2244 | C18H30O2 | −0.8 | Octadecatrienoic acid, isomer 4 [19,20] |
| L17 | 7.83 | 455.4093 | 419.3877, 365.3410 | 454.4017 | C28H54O4 | −1.2 | Octacosanedioic acid |
| L18 | 7.90 | 349.3095 | 331.2990, 261.2209 | 348.3022 | C23H40O2 | −1.7 | Tricosatrienoic acid |
| L19 | 8.17 | 453.3935 | 435.3820, 349.3094 | 452.3862 | C28H52O4 | −0.9 | Dioxooctacosanoic acid, isomer 1 |
| L20 | 8.28 | 303.268 | 285.2573, 221.1898 | 302.2604 | C21H34O | −1.9 | Pentadecenyl-phenol |
| L21 | 8.34 | 483.4409 | 465.4303, 393.3723 | 482.4336 | C30H58O4 | 0.2 | Dimethyloctacosanedioic acid |
| L22 | 8.56 | 407.3153 | 207.1377, 179.1064, 161.0959 | 406.308 | C25H42O4 | −0.7 | Phenolic lipid 1 |
| L23 | 8.98 | 481.4245 | 463.4138, 409.3671, 377.3409 | 480.4179 | C30H56O4 | 0.1 | Butenedioic acid, ditridecyl ester, isomer 1 |
| L24 | 9.09 | 331.2992 | 313.2887, 239.2366, 109.1015 | 330.2919 | C23H38O | −1.1 | Heptadecenyl-phenol |
| L25 | 9.22 | 453.3935 | 435.3821, 349.3094, 295.2630 | 452.3862 | C28H52O4 | −0.9 | Dioxooctacosanoic acid, isomer 2 |
| L26 | 9.40 | 473.3627 | 273.1848, 247.1324, 245.1532 | 472.3554 | C30H48O4 | 0.2 | Phenolic lipid 2 |
| L27 | 9.50 | 758.5676 | 429.3724, 184.0731 | 757.5604 | C42H80NO8P | −2.4 | Oxidised phosphatidylcholine |
| L28 | 9.54 | 481.4247 | 463.4139, 409.3671, 377.3409 | 480.4179 | C30H56O4 | 0.1 | Butenedioic acid, ditridecyl ester, isomer 2 |
| L29 | 9.56 | 465.3934 | 379.3198, 309.2785, 295.2626 | 464.3862 | C29H52O4 | −0.9 | Unknown lipid 1 |
| L30 | 9.58 | 479.4086 | 393.3354, 375.3250, 323.2941 | 478.4025 | C30H54O4 | 0.6 | Unknown lipid 2, isomer 1 |
| L31 | 9.63 | 429.3723 | 191.1063, 165.0908 | 428.365 | C29H48O2 | −0.9 | Unknown lipid 3, isomer 1 |
| L32 | 9.71 | 479.4098 | 393.3354, 375.3250, 323.2940 | 478.4025 | C30H54O4 | 0.6 | Unknown lipid 2, isomer 2 |
| L33 | 9.84 | 493.4247 | 407.3511, 389.3404, 379.3193 | 492.4174 | C31H56O4 | −1.0 | Unknown lipid 4, isomer 1 |
| L34 | 9.87 | 429.3722 | 191.1068; 165.0909 | 428.3649 | C29H48O2 | −1.2 | Unknown lipid 3, isomer 2 |
| L35 | 10.10 | 479.4096 | 393.3355, 375.3250, 323.2942 | 478.4019 | C30H54O4 | −0.7 | Unknown lipid 2, isomer 3 |
| L36 | 10.56 | 493.4248 | 407.3512, 389.3404, 379.3193 | 492.4175 | C31H56O4 | −0.8 | Unknown lipid 4, isomer 2 |
| Metabolite | ‘Paradise Garden’ | ‘Golden Sea’ | ||||
|---|---|---|---|---|---|---|
| Tubular Flowers/Ligulate Flowers | Tubular Flowers/Ligulate Flowers | |||||
| Ratio a | t-Test | Correlation c | Ratio a | t-Test | Correlation c | |
| (fold) | p b | r | (fold) | p | r | |
| 3-O-Caffeoylquinic acid (Neochlorogenic acid) | 2.77 | *** | 0.99 | 2.12 | *** | 0.97 |
| 5-O-Caffeoylquinic acid (Chlorogenic acid) | 5.23 | *** | 0.93 | 3.21 | *** | 0.96 |
| 4-O-Caffeoylquinic acid (Cryptochlorogenic acid) | 4.33 | *** | 1.00 | 2.61 | *** | 0.98 |
| Quercetin-3-O-rutinosyl-rhamnoside | 1.31 | *** | 0.83 | 1.04 | n.s. | 0.89 |
| Quercetin-3-O-β-D-rutinoside (Rutin) | 2.30 | *** | 0.89 | 3.03 | *** | 0.54 |
| Isorhamnetin-3-O-rutinosyl-rhamnoside | −1.10 | *** | −0.93 | −1.38 | *** | −0.92 |
| Kaempferol-3-O-rutinoside | 1.36 | *** | 0.90 | −1.39 | ** | −0.82 |
| Quercetin-3-O-glucoside (Isoquercitrin) | 7.73 | *** | 0.99 | 3.39 | *** | 0.99 |
| Quercetin-3-O-rhamnosyl-glucoside | −1.08 | ** | −0.80 | −1.78 | *** | −0.99 |
| Isorhamnetin-3-O-rutinoside (Narcissin) | −1.49 | *** | −0.87 | −2.45 | *** | −0.94 |
| Isorhamnetin 3-O-rhamnopyranosyl-glucopyranoside | 4.70 | *** | 0.99 | 2.71 | *** | 0.99 |
| 3,5-Di-O-caffeoylquinic acid | 3.87 | *** | 0.99 | 4.59 | *** | 0.92 |
| Tris-trans-p-coumaroyl-spermine | 88.08 | *** | 1.00 | 82.08 | *** | 1.00 |
| Isorhamnetin-malonyl-hexoside | 1.92 | *** | 0.99 | 1.16 | * | 0.77 |
| Tetra-trans-p-coumaroyl-spermine | 90.50 | *** | 1.00 | 92.76 | *** | 1.00 |
| Calendulaglycoside A | −1.45 | *** | −0.84 | −1.62 | *** | −0.99 |
| Calendulaglycoside B | −1.28 | *** | −0.95 | −1.30 | *** | −0.95 |
| Calenduloside G | 1.37 | *** | 0.87 | 1.15 | n.s. | 0.34 |
| Acetyloleanolic acid-glucuronide-hexoside | −1.53 | *** | −0.93 | −1.27 | *** | −0.50 |
| Calendulaglycoside C | −2.16 | *** | −0.95 | −1.59 | *** | −0.90 |
| Calenduloside F | −1.41 | *** | −0.86 | −1.01 | n.s. | −0.38 |
| Calenduloside E (Oleanolic acid-glucuronide) | 1.42 | *** | 0.89 | 2.01 | *** | 0.87 |
| Trihydroxyoctadecadienoic acid | 1.49 | *** | 0.99 | 2.68 | *** | 1.00 |
| Octadecatrienoic acid, isomer 1 | 1.45 | *** | −0.93 | 1.30 | ** | 0.81 |
| Dehydrophytosphingosine | −1.52 | *** | −0.94 | 1.96 | *** | 0.97 |
| Oxooctadecadienoic acid, isomer 1 | 1.03 | n.s. | 0.61 | 1.91 | *** | 0.98 |
| Oxooctadecadienoic acid, isomer 2 | 1.24 | *** | 0.91 | 1.29 | *** | 0.82 |
| Octadecatrienoic acid, isomer 2 | 1.39 | *** | 0.93 | 2.15 | *** | 0.96 |
| Dimethyl-pentyl-furandecanoic acid, isomer 1 | 38.35 | *** | 0.99 | 52.17 | *** | 0.97 |
| Dimethyl-pentyl-furandecanoic acid, isomer 2 | 384.21 | *** | 0.89 | 867.96 | *** | 0.95 |
| Octadecatrienoic acid, isomer 3 | 1.98 | *** | 0.93 | 2.74 | *** | 0.85 |
| Hydroxyoctadecatrienoyl-carnitine | 38.15 | *** | 0.99 | 78.97 | *** | 1.00 |
| Dimethyl-pentyl-furandodecanoic acid | 50.86 | *** | −0.99 | 38.49 | *** | 0.91 |
| Octadecatrienoyl-sn-glycerol | 1.99 | *** | 0.94 | 2.27 | *** | 0.93 |
| Aminolipid, isomer 1 | 1.87 | *** | 0.88 | 1.87 | *** | 0.98 |
| Aminolipid, isomer 2 | 1.38 | *** | 0.93 | 5.46 | *** | 0.97 |
| Octadecatrienoic acid, 2,3-bis(acetyloxy)propyl ester | 1.59 | *** | 0.72 | 1.13 | n.s. | 0.62 |
| Octadecatrienoic acid, isomer 4 | 1.67 | *** | 0.99 | 1.67 | *** | 0.88 |
| Octacosanedioic acid | 91.93 | *** | 1.00 | 200.32 | *** | 0.99 |
| Tricosatrienoic acid | 92.91 | *** | 0.98 | 128.77 | *** | 0.99 |
| Dioxooctacosanoic acid, isomer 1 | 87.02 | *** | 0.99 | 131.44 | *** | 0.99 |
| Pentadecenyl-phenol | 220.92 | *** | 0.98 | 329.84 | *** | 0.98 |
| Dimethyloctacosanedioic acid | 113.35 | *** | 1.00 | 248.82 | *** | 1.00 |
| Phenolic lipid 1 | −3.57 | *** | −0.98 | −5.02 | *** | −0.99 |
| Butenedioic acid, ditridecyl ester, isomer 1 | 54.63 | *** | 1.00 | 62.60 | *** | 1.00 |
| Heptadecenyl-phenol | 221.76 | *** | 0.98 | 346.67 | *** | 0.94 |
| Dioxooctacosanoic acid, isomer 2 | 51.79 | *** | 0.99 | 96.63 | *** | 0.99 |
| Phenolic lipid 2 | 39.70 | *** | 0.99 | 90.10 | *** | 0.99 |
| Oxidised phosphatidylcholine | 1.20 | *** | 0.88 | 1.21 | *** | 0.81 |
| Butenedioic acid, ditridecyl ester, isomer 2 | 38.21 | *** | 1.00 | 47.35 | *** | 0.99 |
| Unknown lipid 1 | 36.68 | *** | 0.99 | 163.95 | *** | 1.00 |
| Unknown lipid 2, isomer 1 | 4.89 | *** | 0.99 | 5.46 | *** | 0.98 |
| Unknown lipid 3, isomer 1 | 25.37 | *** | 1.00 | 36.95 | *** | 0.99 |
| Unknown lipid 2, isomer 2 | 18.59 | *** | 0.99 | 102.85 | *** | 1.00 |
| Unknown lipid 4, isomer 1 | 18.93 | *** | 0.99 | 185.42 | *** | 1.00 |
| Unknown lipid 3, isomer 2 | 1.32 | *** | 0.81 | 2.13 | *** | 0.91 |
| Unknown lipid 2, isomer 3 | 29.14 | *** | 0.98 | 160.46 | *** | 0.99 |
| Unknown lipid 4, isomer 2 | 24.66 | *** | 0.99 | 184.04 | *** | 1.00 |
| Sum of caffeoylquinic acids | 4.05 | *** | 3.79 | *** | ||
| Sum of flavonoids | −1.04 | n.s. | −1.46 | *** | ||
| Sum of phenolic compounds | 1.42 | *** | 1.09 | n.s. | ||
| Sum of triterpenoid glycosides | −1.20 | *** | −1.31 | ** | ||
| Sum of lipids | 3.03 | *** | 4.50 | *** | ||
| Metabolites | Inflorescences | Tubular Flowers | Ligulate Flowers | |||
|---|---|---|---|---|---|---|
| GS/PG | GS/PG | GS/PG | ||||
| Ratio a | t-Test b | Ratio | t-Test | Ratio | t-Test | |
| (fold) | p | (fold) | p | (fold) | p | |
| 3-O-Caffeoylquinic acid (Neochlorogenic acid) | 1.68 | *** | 1.67 | *** | 1.69 | ** |
| 5-O-Caffeoylquinic acid (Chlorogenic acid) | 2.35 | *** | 2.22 | *** | 2.80 | *** |
| 4-O-Caffeoylquinic acid (Cryptochlorogenic acid) | 1.56 | *** | 1.45 | *** | 1.86 | *** |
| Quercetin-3-O-rutinosyl-rhamnoside | −1.07 | n.s. | −1.05 | n.s. | −1.08 | n.s. |
| Quercetin-3-O-β-D-rutinoside (Rutin) | 1.42 | *** | 1.71 | ** | 1.01 | n.s. |
| Isorhamnetin-3-O-rutinosyl-rhamnoside | 1.14 | n.s. | 1.16 | n.s. | 1.13 | n.s. |
| Kaempferol-3-O-rutinoside | 3.36 | *** | 2.69 | *** | 3.93 | *** |
| Quercetin-3-O-glucoside (Isoquercitrin) | 1.41 | *** | 1.24 | ** | 2.20 | *** |
| Quercetin-3-O-rhamnosyl-glucoside | 1.29 | *** | 1.10 | n.s. | 1.40 | *** |
| Isorhamnetin-3-O-rutinoside (Narcissin) | 1.49 | *** | 1.25 | ** | 1.59 | *** |
| Isorhamnetin 3-O-rhamnopyranosyl-glucopyranoside | 1.35 | *** | 1.24 | ** | 1.67 | *** |
| 3,5-Di-O-caffeoylquinic acid | 1.24 | ** | 1.38 | ** | −1.10 | n.s. |
| Tris-trans-p-coumaroyl-spermine | 1.44 | *** | 1.45 | ** | 1.21 | n.s. |
| Isorhamnetin-malonyl-hexoside | 1.36 | ** | 1.20 | ** | 1.54 | ** |
| Tetra-trans-p-coumaroyl-spermine | 1.51 | *** | 1.52 | *** | 1.15 | n.s. |
| Calendulaglycoside A | 1.11 | n.s. | 1.23 | ** | 1.06 | n.s. |
| Calendulaglycoside B | 1.11 | n.s. | 1.29 | ** | 1.02 | n.s. |
| Calenduloside G | 1.26 | ** | 1.31 | ** | 1.21 | * |
| Acetyloleanolic acid-glucuronide-hexoside | 1.79 | *** | 2.38 | *** | 1.54 | *** |
| Calendulaglycoside C | −1.53 | *** | −1.02 | n.s. | −1.79 | ** |
| Calenduloside F | −1.28 | *** | 1.13 | n.s. | −1.59 | ** |
| Calenduloside E (Oleanolic acid-glucuronide) | −1.46 | *** | −1.11 | n.s. | −2.03 | *** |
| Trihydroxyoctadecadienoic acid | −1.28 | *** | 1.11 | n.s. | −2.10 | *** |
| Octadecatrienoic acid, isomer 1 | 1.75 | *** | 1.89 | ** | 1.63 | ** |
| Dehydrophytosphingosine | −1.86 | *** | 1.13 | n.s. | −3.41 | *** |
| Oxooctadecadienoic acid, isomer 1 | 1.09 | n.s. | 1.69 | ** | −1.42 | *** |
| Oxooctadecadienoic acid, isomer 2 | 1.50 | *** | 1.75 | *** | 1.31 | ** |
| Octadecatrienoic acid, isomer 2 | 1.66 | *** | 2.27 | *** | 1.13 | n.s. |
| Dimethyl-pentyl-furandecanoic acid, isomer 1 | 1.54 | ** | 1.57 | ** | −1.12 | n.s. |
| Dimethyl-pentyl-furandecanoic acid, isomer 2 | 1.57 | *** | 1.58 | ** | −1.85 | ** |
| Octadecatrienoic acid, isomer 3 | 1.43 | ** | 1.77 | ** | −1.01 | n.s. |
| Hydroxyoctadecatrienoyl-carnitine | 2.03 | *** | 2.08 | *** | −1.28 | * |
| Dimethyl-pentyl-furandodecanoic acid | 1.37 | *** | 1.37 | *** | 1.40 | *** |
| Octadecatrienoyl-sn-glycerol | 1.22 | *** | 1.42 | *** | −1.04 | n.s. |
| Aminolipid, isomer 1 | 1.13 | n.s. | 1.26 | ** | −1.03 | n.s. |
| Aminolipid, isomer 2 | −1.18 | ** | 1.49 | ** | −3.42 | *** |
| Octadecatrienoic acid, 2,3-bis(acetyloxy)propyl ester | −1.13 | n.s. | −1.18 | ** | −1.09 | n.s. |
| Octadecatrienoic acid, isomer 4 | 1.48 | *** | 1.66 | *** | 1.29 | * |
| Octacosanedioic acid | 2.15 | ** | 2.18 | ** | −1.29 | n.s. |
| Tricosatrienoic acid | 2.09 | *** | 2.11 | *** | 1.18 | n.s. |
| Dioxooctacosanoic acid, isomer 1 | 1.48 | *** | 1.49 | ** | −1.31 | * |
| Pentadecenyl-phenol | 1.56 | *** | 1.57 | *** | −1.23 | n.s. |
| Dimethyloctacosanedioic acid | 2.19 | *** | 2.21 | *** | −1.28 | * |
| Phenolic lipid 1 | 1.13 | n.s. | 1.05 | n.s. | 1.14 | n.s. |
| Butenedioic acid, ditridecyl ester, isomer 1 | 1.52 | *** | 1.53 | *** | 1.04 | n.s. |
| Heptadecenyl-phenol | 2.38 | *** | 2.39 | *** | 1.18 | n.s. |
| Dioxooctacosanoic acid, isomer 2 | 1.35 | ** | 1.37 | ** | −1.75 | ** |
| Phenolic lipid 2 | 1.32 | ** | 1.36 | *** | −2.16 | ** |
| Oxidised phosphatidylcholine | 1.26 | *** | 1.44 | *** | 1.11 | n.s. |
| Butenedioic acid, ditridecyl ester, isomer 2 | 1.52 | *** | 1.55 | ** | −1.03 | n.s. |
| Unknown lipid 1 | 1.38 | ** | 1.43 | ** | −4.04 | ** |
| Unknown lipid 2, isomer 1 | 1.39 | * | 1.51 | ** | 1.05 | n.s. |
| Unknown lipid 3, isomer 1 | 1.94 | *** | 1.99 | *** | 1.06 | n.s. |
| Unknown lipid 2, isomer 2 | 1.66 | *** | 1.78 | ** | −4.00 | *** |
| Unknown lipid 4, isomer 1 | 1.58 | *** | 1.71 | *** | −7.41 | ** |
| Unknown lipid 3, isomer 2 | 1.47 | *** | 2.06 | ** | −1.01 | n.s. |
| Unknown lipid 2, isomer 3 | 1.45 | *** | 1.52 | *** | −4.68 | ** |
| Unknown lipid 4, isomer 2 | 1.56 | *** | 1.65 | *** | −5.84 | *** |
| Sum of caffeoylquinic acids | 1.50 | *** | 1.58 | *** | 1.31 | ** |
| Sum of flavonoids | 1.32 | *** | 1.25 | *** | 1.37 | *** |
| Sum phenolic compounds | 1.36 | *** | 1.36 | *** | 1.36 | *** |
| Sum of triterpenoid glycosides | 1.12 | n.s. | 1.24 | ** | 1.05 | n.s. |
| Sum of lipids | 1.33 | *** | 1.59 | *** | −1.20 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossipov, V.; Khazieva, F.; Baleev, D.; Salminen, J.-P.; Sidelnikov, N. Comparative Metabolomics of Ligulate and Tubular Flowers of Two Cultivars of Calendula officinalis L. Metabolites 2024, 14, 140. https://doi.org/10.3390/metabo14030140
Ossipov V, Khazieva F, Baleev D, Salminen J-P, Sidelnikov N. Comparative Metabolomics of Ligulate and Tubular Flowers of Two Cultivars of Calendula officinalis L. Metabolites. 2024; 14(3):140. https://doi.org/10.3390/metabo14030140
Chicago/Turabian StyleOssipov, Vladimir, Firdaus Khazieva, Dmitry Baleev, Juha-Pekka Salminen, and Nikolay Sidelnikov. 2024. "Comparative Metabolomics of Ligulate and Tubular Flowers of Two Cultivars of Calendula officinalis L." Metabolites 14, no. 3: 140. https://doi.org/10.3390/metabo14030140
APA StyleOssipov, V., Khazieva, F., Baleev, D., Salminen, J.-P., & Sidelnikov, N. (2024). Comparative Metabolomics of Ligulate and Tubular Flowers of Two Cultivars of Calendula officinalis L. Metabolites, 14(3), 140. https://doi.org/10.3390/metabo14030140










