Essential Oils from Papaver rhoeas and Their Metabolomic Profiling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. SFME Procedure
2.4. Fractionation and Alkylthiolation of Alkenes
2.5. GC/MS Analysis
2.6. Identification of the Volatile Compounds
2.7. Statistical Analysis
3. Results
3.1. Essential Oil Characterization
3.1.1. Composition of Papaver rhoeas Flower EO from Hill Population
3.1.2. Composition of Papaver rhoeas Leaf EO from Hill Population
3.1.3. Composition of Papaver rhoeas Flower EO from Lowland Population
3.1.4. Composition of Papaver rhoeas Leaf EO from Lowland Population
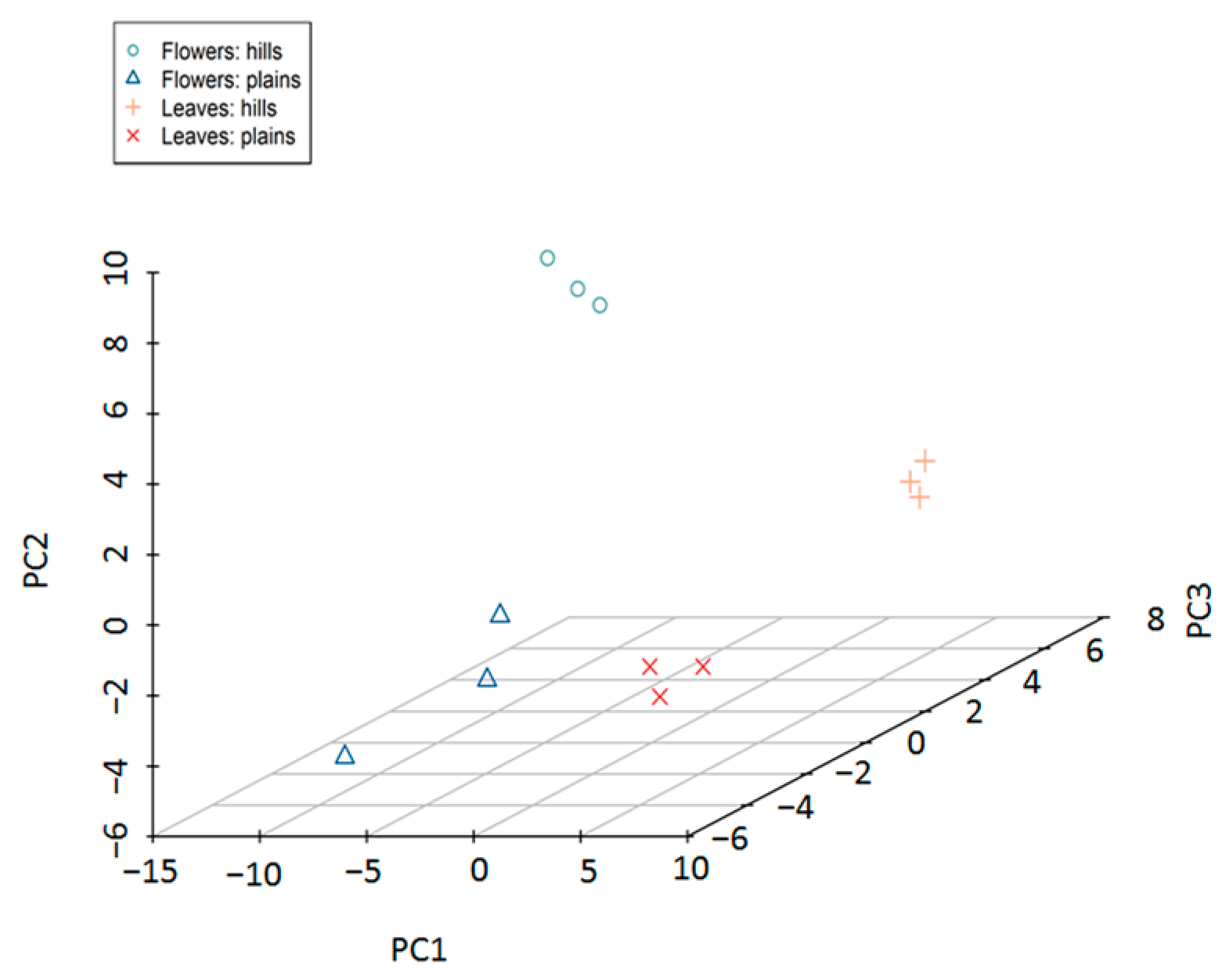
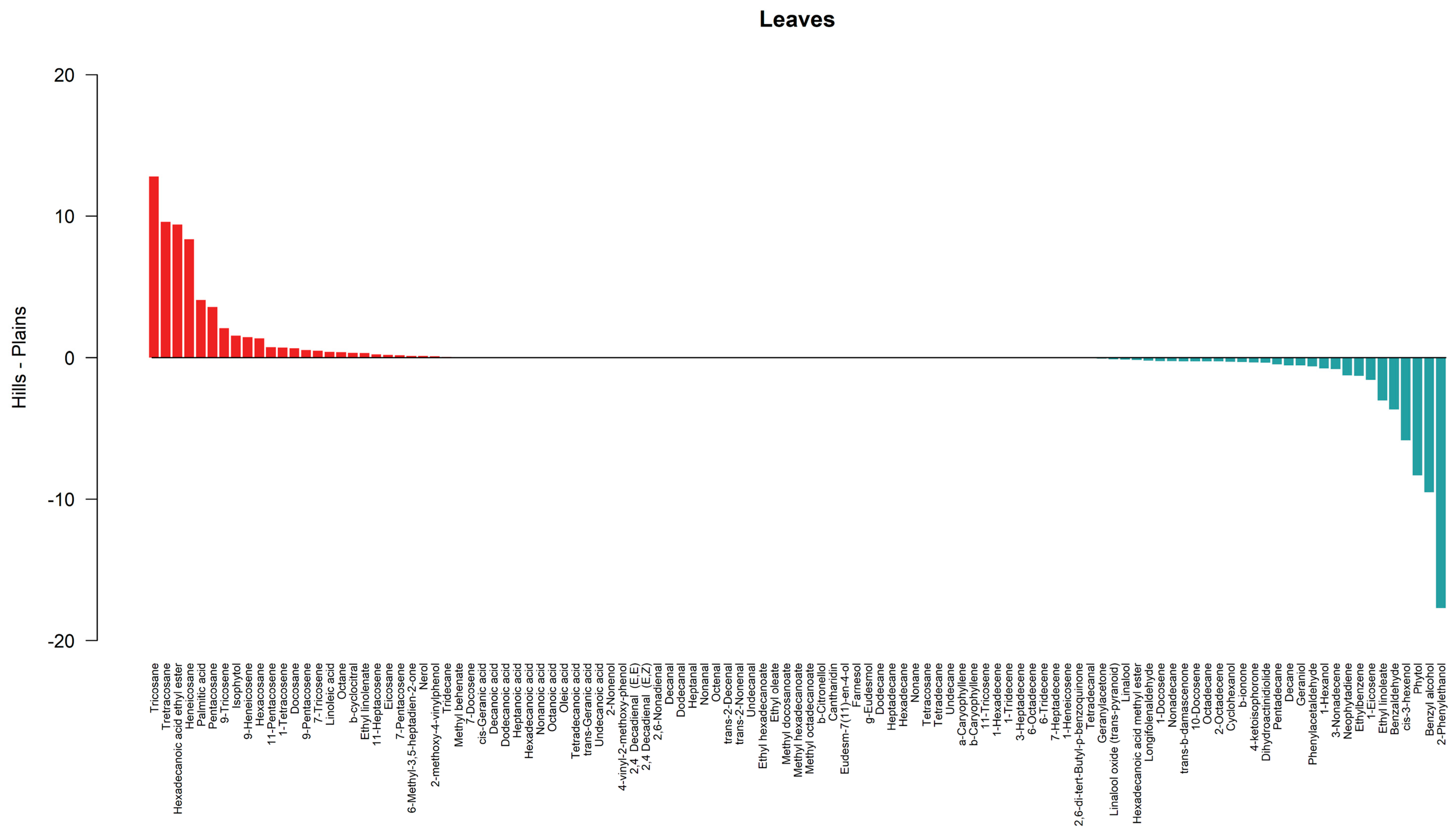
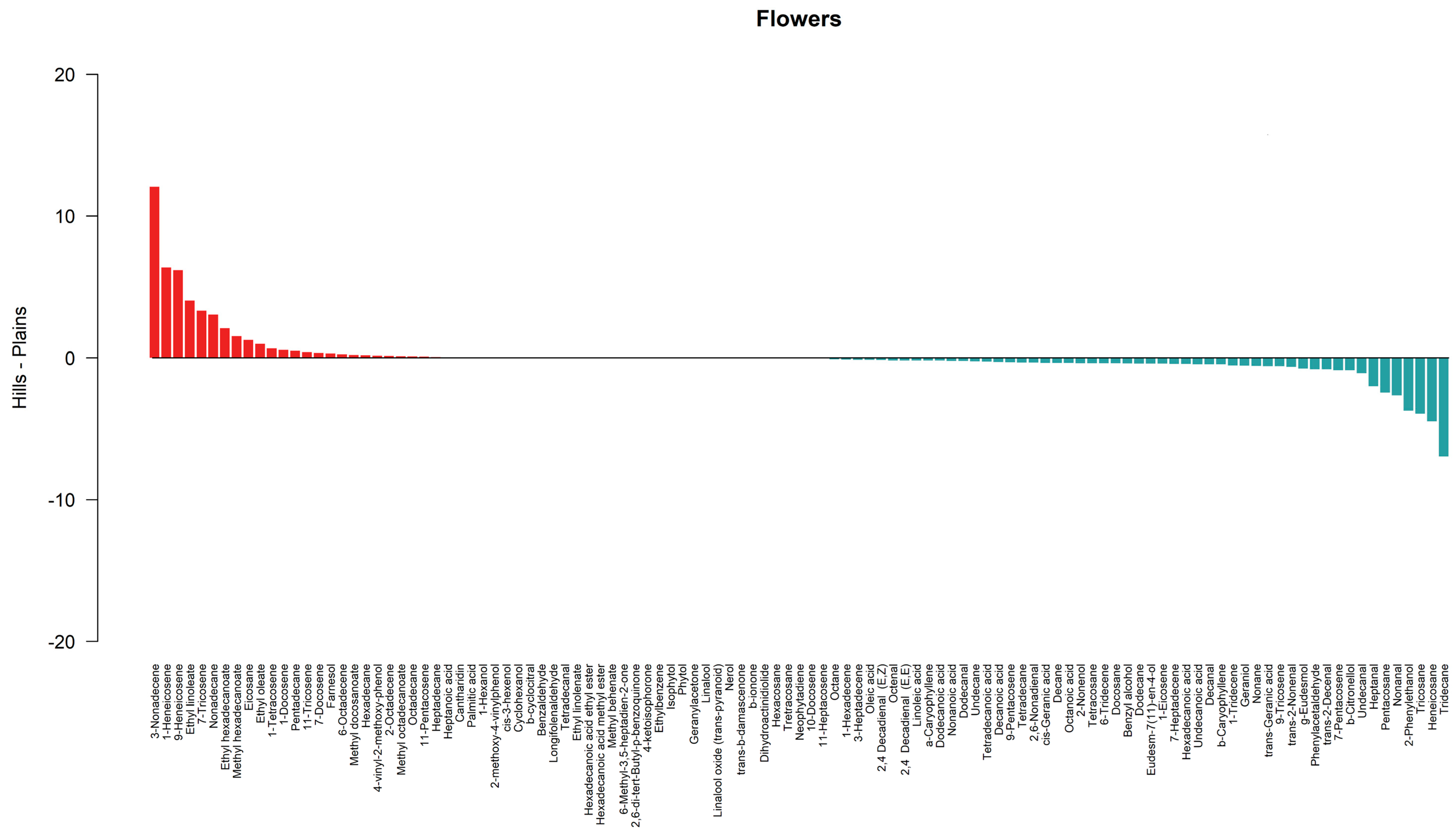
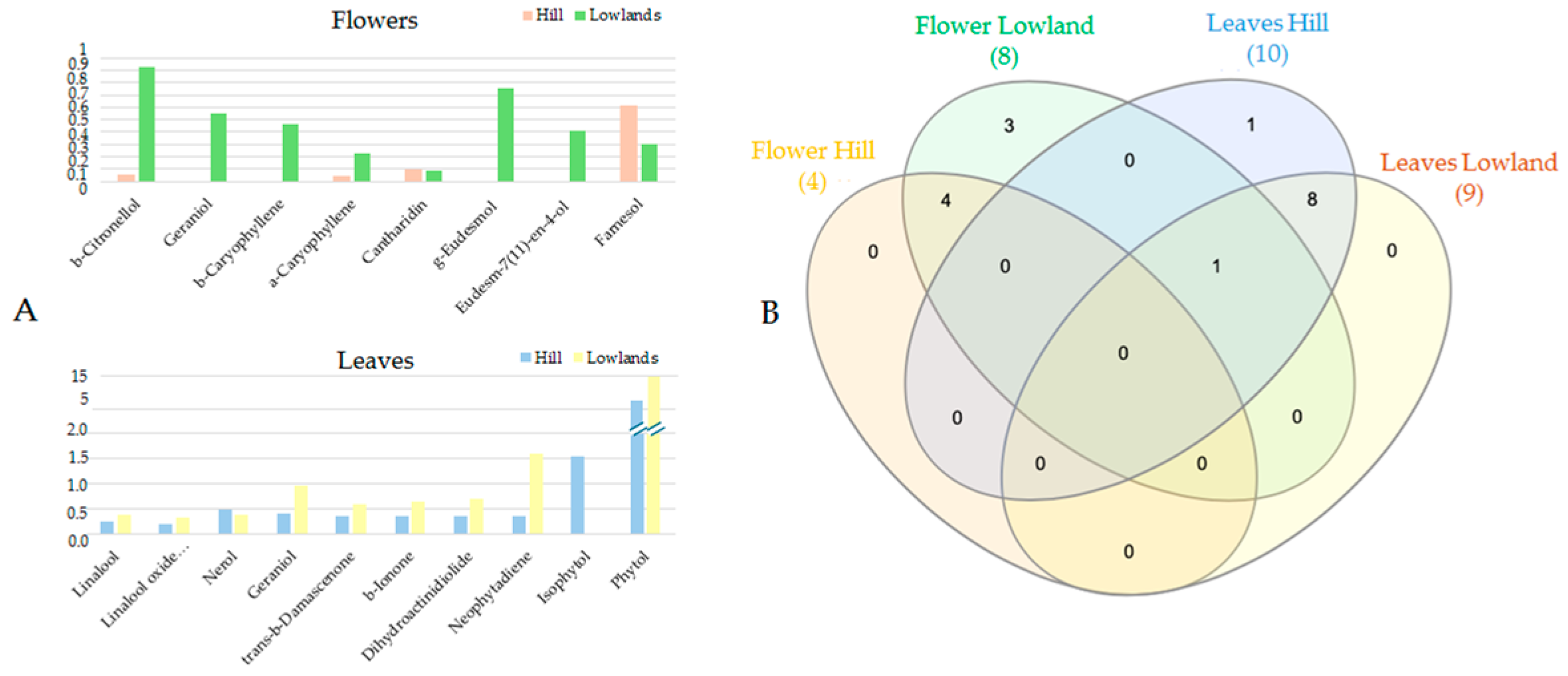
3.2. Similarity Between Lowland and Hill Populations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Durczyńska, Z.; Żukowska, G. Properties and Applications of Essential Oils: A Review. J. Ecol. Eng. 2024, 25, 333–340. [Google Scholar] [CrossRef]
- Cavalloro, V.; Marrubini, G.; Rossino, G.; Martino, E.; Collina, S. Combining DoE and MASE: A winning strategy for the isolation of natural bioactive compounds from plant materials. Green Chem. 2024, 26, 244–258. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia (II Ed.); Edagri: Bologna, Italy, 2017. [Google Scholar]
- Butnariu, M.; Quispe, C.; Herrera-Bravo, J.; Pentea, M.; Sarac, I.; Küşümler, A.S.; Özçelik, B.; Painuli, S.; Semwal, P.; Imran, M.; et al. Papaver Plants: Current Insights on Phytochemical and Nutritional Composition Along with Biotechnological Applications. Oxid. Med. Cell. Longev. 2022, 2022, 2041769. [Google Scholar] [CrossRef] [PubMed]
- Cavalloro, V.; Soddu, F.; Baroni, S.; Robustelli della Cuna, F.S.; Tavazzi, E.; Martino, E.; Collina, S. Teodorico Borgognoni’s Formulary for Thirteenth Century Anesthetic Preparations. Life 2023, 13, 1913. [Google Scholar] [CrossRef] [PubMed]
- Capasso, F.; de Pasquale, R.; Grandolini, G. Farmacognosia Botanica, Chimica e Farmacologia Delle Piante Medicinali, 2nd ed.; Springer: Milan, Italy, 2011. [Google Scholar]
- Kim, H.; Han, S.; Song, K.; Lee, M.Y.; Park, B.; Ha, I.J.; Lee, S.-G. Ethyl Acetate Fractions of Papaver rhoeas L. and Papaver nudicaule L. Exert Antioxidant and Anti-Inflammatory Activities. Antioxidants 2021, 10, 1895. [Google Scholar] [CrossRef] [PubMed]
- Boussoum, M.O.; Ali-Nehari, A.; Ouldmokhtar, R.; George, B. Characterization of extracts from Papaver rhoeas and potential valorization of these extracts in dyeing applications. Turkish J. Chem. 2021, 45, 1576–1584. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, K.; Singh, P. Extract of Papaver Rhoeas for Daylight Imaging of Latent Fingerprints. ChemistrySelect 2024, 9, e202402589. [Google Scholar] [CrossRef]
- Slavík, J.; Slavíková, L.; Bochořáková, J. Alkaloids from Papaver rhoeas var. chelidonioides O. KUNTZE, P. confine JORD., and P. dubium L. Collect. Czechoslov. Chem. Commun. 1989, 54, 1118–1125. [Google Scholar] [CrossRef]
- Kalav, Y.; Sariyar, G. Alkaloids from Turkish Papaver rhoeas. Planta Med. 1989, 55, 488. [Google Scholar] [CrossRef]
- Velickovic, J.M.; Mitic, M.N.; Arsic, B.B.; Paunovic, D.Đ.; Stojanovic, B.T.; Veljkovic, J.N.; Dimitrijevic, D.S.; Stevanovic, S.D.; Kostic, D.A. HPLC analysis of extracts of fresh petals of Papaver Rhoeas l. Stud. Univ. Babeș-Bolyai Chem. 2019, 64, 239–247. [Google Scholar] [CrossRef]
- Bautista-Sopelana, L.M.; Bolívar, P.; Gómez-Muñoz, M.T.; Martínez-Díaz, R.A.; Andrés, M.F.; Alonso, J.C.; Bravo, C.; González-Coloma, A. Bioactivity of plants eaten by wild birds against laboratory models of parasites and pathogens. Front. Ecol. Evol. 2022, 10, 1–13. [Google Scholar] [CrossRef]
- Dogan, G.; Bagcı, E. Essential Oil Composition of Papaver rhoeas L. (Corn poppy) (Papaveraceae) from Turkey. HJBC 2014, 42, 545–549. [Google Scholar]
- Villa, C.; Robustelli Della Cuna, F.S.; Russo, E.; Ibrahim, M.F.; Grignani, E.; Preda, S. Microwave-Assisted and Conventional Extractions of Volatile Compounds from Rosa x damascena Mill. Fresh Petals for Cosmetic Applications. Molecules 2022, 27, 3963. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Lucchesi, M.E.; Smadja, J. Solvent-Free Microwave Extraction of Volatile Natural Substances. US Patent Application Publication 20040187340, 30 September 2004. [Google Scholar]
- Villa, C.; Trucchi, B.; Bertoli, A.; Pistelli, L.; Parodi, A.; Bassi, A.M.; Ruffoni, B. Salvia somalensis essential oil as a potential cosmetic ingredient: Solvent-free microwave extraction, hydrodistillation, GC–MS analysis, odour evaluation and in vitro cytotoxicity assays. Int. J. Cosmet. Sci. 2009, 31, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Roan, C.S.; Yost, R.A.; Hector, J. Dimethyl disulfide derivatives of long chain alkenes, alkadienes, and alkatrienes for gas chromatography/mass spectrometry. Anal. Chem. 1989, 61, 1564–1571. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publ. Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Stein, S.E. NIST/EPA/NIH Mass Spectral Database, Version 2.1; Perkin-Elmer Instrument LLC: Waltham, MA, USA, 2000. [Google Scholar]
- Steyn, J.M.; Hundt, H.K.L. Gas chromatographic—Mass spectrometric method for the quantitation of cantharidin in human serum. J. Chromatogr. B Biomed. Sci. Appl. 1988, 432, 177–184. [Google Scholar] [CrossRef]
- Nikbakhtzadeh, M.; Vahedi, M.; Vatandoost, H.; Mehdinia, A. Origin, transfer and distribution of cantharidin-related compounds in the blister beetle Hycleus scabiosae. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 88–96. [Google Scholar] [CrossRef]
- Lind, H.; Lindeborg, M. Lepidopterans as presumptive pollinators of Anacamptis pyramidalis. Entomol. Tidskr. 1989, 110, 156–160. [Google Scholar]
- Eigenbrode, S.D.; Espelie, K.E. Effects of Plant Epicuticular Lipids on Insect Herbivores. Annu. Rev. Entomol. 1995, 40, 171–194. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Mccormick, J.P.; Carrel, J.E. Cantharidin Biosynthesis and Function in Meloid Beetles. In Pheromone Biochemistry; Elsevier: Amsterdam, The Netherlands, 1987; pp. 307–350. [Google Scholar]
- Wilson, C.R.; Butz, J.K.; Mengel, M.C. Methods for Analysis of Gastrointestinal Toxicants. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Jin, D.; Huang, N.-N.; Wei, J.-X. Hepatotoxic mechanism of cantharidin: Insights and strategies for therapeutic intervention. Front. Pharmacol. 2023, 14, 1201404. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, R.; Tang, W.; Yang, C. Cantharidin-induced toxic injury, oxidative stress, and autophagy attenuated by Astragalus polysaccharides in mouse testis. Reprod. Toxicol. 2024, 123, 108520. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Li, Y.; Liu, J.; Zhang, Y. Toxicity of (S)-(-)-palasonin on various life stages of Plutella xylostella (Lepidoptera: Plutellidae). Ind. Crops Prod. 2023, 201, 116902. [Google Scholar] [CrossRef]
- Hemp, C.; Dettner, K. Compilation of canthariphilous insects. Beiträge Zur Entomol. Contrib. Entomol. 2001, 51, 231–245. [Google Scholar] [CrossRef]

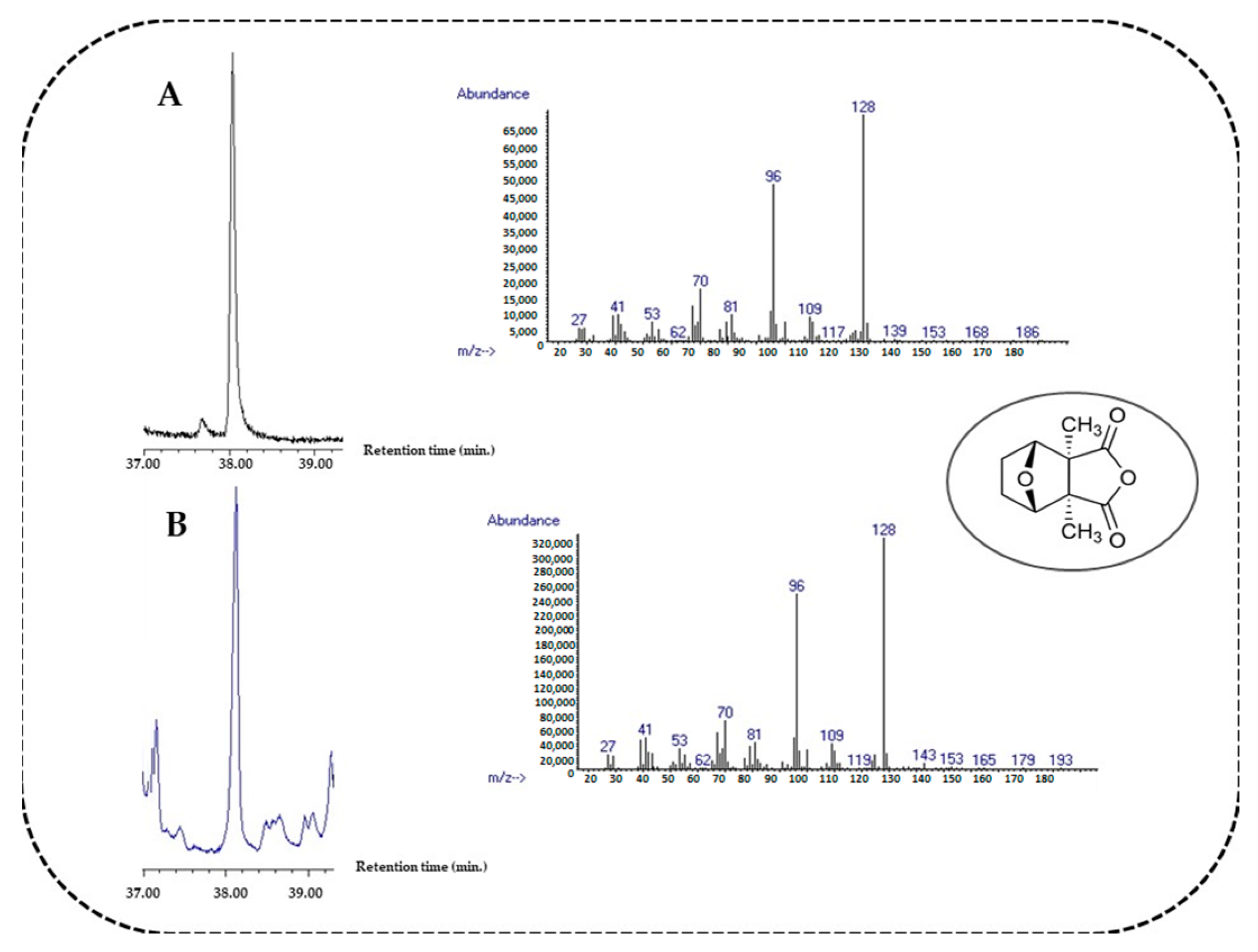
| Flowers | Leaves | Identification (e) | |||||
|---|---|---|---|---|---|---|---|
| Hill | Lowlands | Hill | Lowlands | ||||
| Compound (a) | Ri (b) | Ri (c) | % (d) | % | % | % | |
| Octane | 800 | 801 | 0.14 ± 0.13 | 0.20 ± 0.27 | 0.39 ± 0.28 | - | STD, RI |
| Ethylbenzene | 859 | 861 | - | - | - | 1.29 ± 0.28 | NIST, RI |
| cis-3-Hexenol | 863 | 866 | - | - | - | 5.85 ± 0.29 | NIST, RI |
| 1-Hexanol | 870 | 876 | - | - | - | 0.76 ± 0.24 | NIST, RI |
| Cyclohexanol | 891 | 894 | - | - | - | 0.30 ± 0.24 | NIST, RI |
| Benzaldehyde | 965 | 967 | - | - | - | 3.66 ± 0.34 | NIST, RI |
| Nonane | 900 | 901 | 0.09 ± 0 06 | 0.65 ± 0.60 | - | - | NIST, RI |
| Heptanal | 901 | 907 | - | 2.00 ± 0.20 | - | - | NIST, RI |
| Decane | 1000 | 1000 | 0.09 ± 0.11 | 0.46 ± 0.39 | - | 0.55 ± 0.41 | STD, RI |
| Benzyl alcohol | 1037 | 1041 | 0.08 ± 0.09 | 0.47 ± 0.45 | 2.31 ± 0.37 | 11.83 ± 0.82 | NIST, RI |
| Phenylacetaldehyde | 1048 | 1049 | - | 0.80 ± 0.36 | 1.75 ± 0.23 | 2.36 ± 0.33 | NIST, RI |
| Octenal | 1060 | 1061 | - | 0.18 ± 0.19 | - | - | NIST, RI |
| 2-Nonenol | 1072 | 1072 | - | 0.38 ± 0.25 | - | - | NIST, RI |
| Heptanoic acid | 1091 | 1092 | 0.14 ± 0.10 | 0.09 ± 0.07 | - | - | NIST, RI |
| Undecane | 1100 | 1101 | 0.43 ± 0.71 | 0.68 ± 0.27 | - | - | STD, RI |
| Linalool | 1105 | 1101 | - | - | 0.23 ± 0.24 | 0.37 ± 0.28 | NIST, RI |
| 6-Methyl-3,5-heptadien-2-one | 1106 | 1106 | - | - | 0.49 ± 0.33 | 0.37 ± 0.42 | NIST, RI |
| Nonanal | 1108 | 1111 | 0.45 ± 0.69 | 3.10 ± 0.21 | - | - | NIST, RI |
| 2-Phenylethanol | 1117 | 1120 | - | 3.72 ± 0.64 | 0.85 ± 0.42 | 18.55 ± 0.42 | NIST, RI |
| 4-Ketoisophorone | 1142 | 1149 | - | - | 0.26 ± 0.24 | 0.60 ± 0.39 | NIST, RI |
| 2,6-Nonadienal | 1153 | 1157 | - | 0.33 ± 0.23 | - | - | NIST, RI |
| trans-2-Nonenal | 1164 | 1164 | - | 0.63 ± 0.36 | - | - | NIST, RI |
| Linalool oxide (trans-pyranoid) | 1177 | 1175 | - | - | 0.19 ± 0.10 | 0.31 ± 0.31 | NIST, RI |
| Octanoic acid | 1181 | 1180 | - | 0.36 ± 0.25 | - | - | NIST, RI |
| Dodecane | 1200 | 1200 | 0.15 ± 0.23 | 0.55 ± 0.46 | - | - | STD, RI |
| Decanal | 1209 | 1210 | 0.23 ± 0.28 | 0.69 ± 0.41 | - | - | NIST, RI |
| β-Cyclocitral | 1222 | 1225 | - | - | 0.51 ± 0.33 | 0.17 ± 0.08 | NIST, RI |
| β-Citronellol | 1228 | 1230 | 0.05 ± 0.03 | 0.93 ± 0.40 | - | - | NIST, RI |
| Nerol | 1232 | 1228 | - | - | 0.49 ± 0.48 | 0.38 ± 0.24 | NIST, RI |
| Geraniol | 1250 | 1255 | - | 0.55 ± 0.37 | 0.41 ± 0.38 | 0.96 ± 0.22 | NIST, RI |
| trans-2-Decenal | 1265 | 1268 | - | 0.80 ± 0.44 | - | - | NIST, RI |
| Nonanoic acid | 1280 | 1284 | - | 0.21 ± 0.17 | - | - | NIST, RI |
| 6-Tridecene | 1286 | 1290 | - | 0.38 ± 0.27 | - | - | NIST, RI |
| 1-Tridecene | 1293 | 1294 | - | 0.53 ± 0.45 | - | - | NIST, RI |
| Tridecane | 1300 | 1300 | 0.05 ± 0.03 | 7.00 ± 0.80 | 0.34 ± 0.23 | 0.29 ± 0.19 | STD, RI |
| Undecanal | 1314 | 1313 | 0.19 ± 0.08 | 1.26 ± 0.38 | - | - | NIST, RI |
| 2-Methoxy-4-vinyl-phenol | 1317 | 1315 | - | - | 0.61 ± 0.19 | 0.50 ± 0.33 | NIST, RI |
| 4-Vinyl-2-methoxy-phenol | 1321 | 1319 | 0.33 ± 0.46 | 0.18 ± 0.24 | - | - | NIST, RI |
| 2,4 Decadienal (E,Z) | 1326 | 1324 | - | 0.15 ± 0.08 | - | - | NIST, RI |
| 2,4 Decadienal (E,E) | 1330 | 1337 | - | 0.18 ± 0.09 | - | - | NIST, RI |
| trans-Geranic acid | 1362 | 1368 | 0.07 ± 0.03 | 0.65 ± 0.29 | - | - | NIST, RI |
| cis-Geranic acid | 1374 | 1371 | - | 0.36 ± 0.27 | - | - | NIST, RI |
| Decanoic acid | 1373 | 1377 | - | 0.30 ± 0.37 | - | - | NIST, RI |
| trans-β-Damascenone | 1385 | 1384 | - | - | 0.34 ± 0.22 | 0.60 ± 0.32 | NIST, RI |
| Tetradecane | 1400 | 1400 | 0.04 ± 0.03 | 0.37 ± 0.28 | - | - | STD, RI |
| Dodecanal | 1411 | 1412 | 0.04 ± 0.02 | 0.26 ± 0.13 | - | - | NIST, RI |
| β-Caryophyllene | 1427 | 1430 | - | 0.46 ± 0.33 | - | - | NIST, RI |
| Geranylacetone | 1451 | 1450 | - | - | 0.29 ± 0.16 | 0.37 ± 0.23 | NIST, RI |
| α-Caryophyllene | 1460 | 1467 | 0.04 ± 0.02 | 0.23 ± 0.12 | - | - | NIST, RI |
| 2,6-di-tert-Butyl-p-benzoquinone | 1468 | 1467 | - | - | 0.44 ± 0.22 | 0.48 ± 0.22 | NIST, RI |
| Undecanoic acid | 1468 | 1477 | - | 0.45 ± 0.06 | - | - | NIST, RI |
| β-Ionone | 1486 | 1484 | - | - | 0.34 ± 0.23 | 0.65 ± 0.49 | NIST, RI |
| Pentadecane | 1500 | 1500 | 0.49 ± 0.57 | - | - | 0.48 ± 0.19 | STD, RI |
| Dihydroactinidiolide | 1538 | 1542 | - | - | 0.34 ± 0.36 | 0.69 ± 0.42 | NIST, RI |
| Cantharidin | 1546 | 1546 | 0.10 ± 0.06 | 0.09 ± 0.03 | - | - | STD, RI |
| Dodecanoic acid | 1566 | 1568 | - | 0.19 ± 0.10 | - | - | NIST, RI |
| 1-Hexadecene | 1592 | 1593 | 0.06 ± 0.03 | 0.18 ± 0.05 | - | - | NIST, RI |
| Hexadecane | 1600 | 1600 | 0.32 ± 0.47 | 0.13 ± 0.09 | - | - | STD, RI |
| Tetradecanal | 1611 | 1615 | - | - | 0.45 ± 0.33 | 0.71 ± 0.40 | NIST, RI |
| γ-Eudesmol | 1641 | 1644 | - | 0.75 ± 0.22 | - | - | NIST, RI |
| 7-Heptadecene | 1673 | 1670 | - | 0.42 ± 0.42 | - | - | NIST, RI |
| 3-Heptadecene | 1687 | 1681 | 0.45 ± 0.23 | 0.58 ± 0.34 | - | - | NIST, RI |
| Heptadecane | 1700 | 1700 | 0.45 ± 0.37 | 0.40 ± 0.20 | - | - | STD, RI |
| Eudesm-7(11)-en-4-ol | 1709 | 1712 | - | 0.41 ± 0.40 | - | - | NIST, RI |
| Farnesol | 1722 | 1718 | 0.61 ± 0.39 | 0.30 ± 0.19 | - | - | NIST, RI |
| Tetradecanoic acid | 1762 | 1763 | - | 0.26 ± 0.10 | - | - | NIST, RI |
| 6-Octadecene | 1775 | 1778 | 0.76 ± 0.33 | 0.52 ± 0.34 | - | - | NIST, RI |
| 2-Octadecene | 1798 | 1793 | 0.44 ± 0.47 | 0.30 ± 0.29 | 0.21 ± 0.20 | 0.48 ± 0.08 | NIST, RI |
| Octadecane | 1800 | 1800 | 0.31 ± 0.14 | 0.21 ± 0.17 | - | 0.27 ± 0.23 | STD, RI |
| Neophytadiene | 1837 | 1836 | - | - | 0.36 ± 0.21 | 1.61 ± 0.36 | NIST, RI |
| 3-Nonadecene | 1881 | 1880 | 15.54 ± 1.32 | 3.47 ± 0.34 | 0.73 ± 0.29 | 1.54 ± 0.12 | NIST, RI |
| Nonadecane | 1900 | 1900 | 13.10 ± 0.66 | 10.05 ± 0.20 | 2.09 ± 0.40 | 2.34 ± 0.32 | STD, RI |
| Methyl hexadecanoate | 1933 | 1934 | 1.69 ± 0.29 | 0.16 ± 0.11 | 0.68 ± 0.19 | 0.86 ± 0.39 | NIST, RI |
| Isophytol | 1947 | 1947 | - | - | 1.54 ± 0.34 | - | NIST, RI |
| Palmitic acid | 1975 | 1974 | 2.12 ± 0.37 | 2.54 ± 0.36 | 4.06 ± 0.72 | - | NIST, RI |
| 1-Eicosene | 1992 | 1994 | - | 0.41 ± 0.17 | 1.00 ± 0.38 | 2.58 ± 0.24 | NIST, RI |
| Ethyl hexadecanoate | 1997 | 1997 | 3.57 ± 0.49 | 1.49 ± 0.26 | 11.79 ± 0.68 | 2.39 ± 0.32 | NIST, RI |
| Eicosane | 2000 | 2000 | 1.26 ± 0.30 | - | 0.75 ± 0.40 | 0.55 ± 0.19 | STD, RI |
| 9-Heneicosene | 2073 | 2074 | 10.84 ± 0.81 | 4.67 ± 0.22 | 3.80 ± 0.22 | 2.38 ± 0.23 | NIST, RI |
| 1-Heneicosene | 2087 | 2088 | 6.37 ± 0.47 | - | 0.36 ± 0.25 | 0.40 ± 0.53 | NIST, RI |
| Heneicosane | 2100 | 2100 | 12.95 ± 0.80 | 17.43 ± 0.74 | 13.92 ± 0.49 | 5.55 ± 0.59 | STD, RI |
| Phytol | 2113 | 2116 | - | - | 5.82 ± 0.68 | 14.14 ± 0.41 | NIST, RI |
| Linoleic acid | 2130 | 2133 | 0.48 ± 0.38 | 0.66 ± 0.39 | 0.41 ± 0.34 | - | NIST, RI |
| Methyl octadecanoate | 2133 | 2136 | 0.34 ± 0.39 | 0.23 ± 0.17 | - | - | NIST, RI |
| Ethyl linolenate | 2145 | 2140 | - | - | 1.06 ± 0.46 | 0.73 ± 0.45 | NIST, RI |
| Oleic acid | 2152 | 2152 | 0.61 ± 0.22 | 0.75 ± 0.52 | - | - | NIST, RI |
| 10-Docosene | 2160 | 2162 | - | - | 1.15 ± 0.60 | 1.41 ± 0.43 | NIST, RI |
| Ethyl linoleate | 2171 | 2173 | 4.57 ± 0.67 | 0.54 ± 0.36 | 1.20 ± 0.35 | 4.23 ± 0.20 | NIST, RI |
| Ethyl oleate | 2175 | 2178 | 1.17 ± 0.89 | 0.19 ± 0.17 | - | - | NIST, RI |
| 7-Docosene | 2179 | 2181 | 0.34 ± 0.23 | - | 0.33 ± 0.42 | 0.31 ± 0.07 | NIST, RI |
| 1-Docosene | 2192 | 2188 | 0.56 ± 0.51 | - | - | 0.25 ± 0.15 | NIST, RI |
| Docosane | 2200 | 2200 | 0.67 ± 0.52 | 1.05 ± 0.62 | 1.00 ± 0.38 | 0.36 ± 0.17 | STD, RI |
| 11-Tricosene | 2261 | 2258 | 0.39 ± 0.52 | - | - | - | NIST, RI |
| 9-Tricosene | 2279 | 2278 | 1.15 ± 0.11 | 1.74 ± 0.46 | 2.24 ± 0.14 | 0.18 ± 0.14 | NIST, RI |
| 7-Tricosene | 2286 | 2281 | 3.32 ± 0.31 | - | 0.48 ± 0.21 | - | NIST, RI |
| Tricosane | 2300 | 2300 | 9.36 ± 0.64 | 13.30 ± 0.56 | 15.58 ± 0.46 | 2.78 ± 0.26 | STD, RI |
| 1-Tetracosene | 2390 | 2382 | 0.66 ± 0.54 | - | 0.70 ± 0.45 | - | NIST, RI |
| Tetracosane | 2400 | 2400 | 0.29 ± 0.17 | 0.67 ± 0.49 | 9,92 ± 0.30 | 0.34 ± 0.24 | STD, RI |
| 11-Pentacosene | 2469 | 2466 | 0.35 ± 0.47 | 0.25 ± 0.30 | 0.72 ± 0.35 | - | NIST, RI |
| 9-Pentacosene | 2474 | 2472 | 0.20 ± 0.20 | 0.52 ± 0.52 | 0.52 ± 0.16 | - | NIST, RI |
| 7-Pentacosene | 2480 | 2495 | - | 0.87 ± 0.61 | 0.16 ± 0.12 | - | NIST, RI |
| Pentacosane | 2500 | 2500 | 1.30 ± 0.45 | 3.76 ± 0.83 | 4.15 ± 0.16 | 0.59 ± 0.75 | STD, RI |
| Methyl docosanoate | 2527 | 2530 | 0.26 ± 0.16 | 0.05 ± 0.04 | 0.13 ± 0.09 | 0.10 ± 0.08 | NIST, RI |
| 11-Heptacosene | 2670 | 2671 | - | - | 0.24 ± 0.21 | - | NIST, RI |
| Heptacosane | 2700 | 2700 | - | - | 1.88 ± 0.81 | 0.54 ± 0.73 | STD, RI |
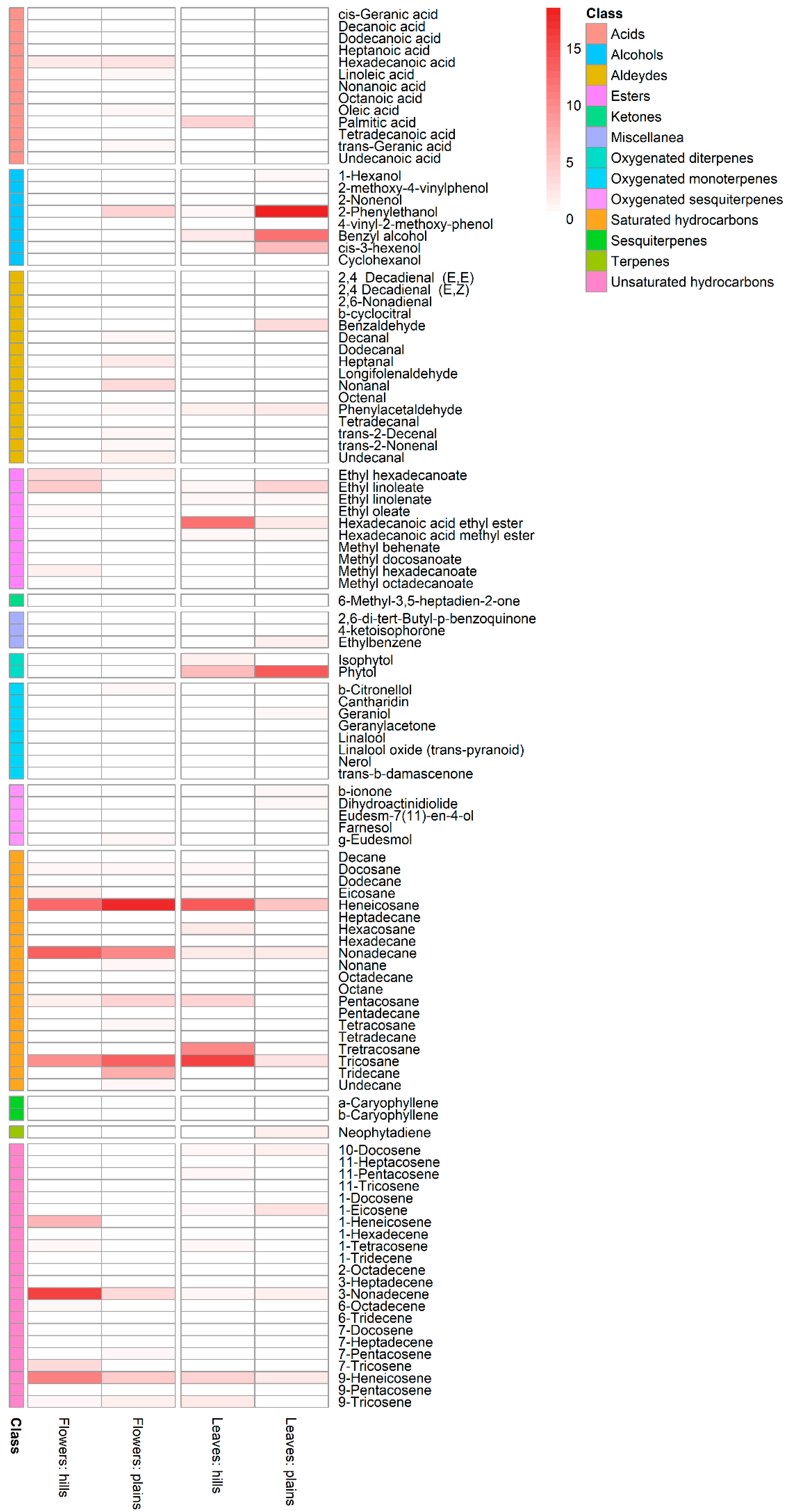
| Flowers % | Leaves % | ||||
|---|---|---|---|---|---|
| Hill | Lowlands | Hill | Lowlands | ||
| Acids | 3.41 | 6.82 | 4.47 | - | |
| Alcohols | 0.42 | 4.75 | 3.76 | 37.79 | |
| Aldehydes | 0.92 | 10.38 | 2.71 | 6.91 | |
| Esters | 11.60 | 2.65 | 14.87 | 8.31 | |
| Ketones | - | - | 0.49 | 0.37 | |
| Saturated hydrocarbons | 41.50 | 56.91 | 50.02 | 14.64 | |
| Unsaturated hydrocarbons | 41.40 | 14.84 | 12.64 | 9.52 | |
| Terpenes | - | - | 0.36 | 1.61 | |
| Oxygenated monoterpenes | 0.15 | 1.57 | 1.95 | 2.98 | |
| Sesquiterpenes | 0.004 | 0.69 | - | - | |
| Oxygenated sesquiterpenes | 0.61 | 1.45 | 0.68 | 1.35 | |
| Miscellanea | - | - | 0.70 | 2.38 | |
| Oxygenated diterpenes | - | - | 7.36 | 14.14 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalloro, V.; Robustelli della Cuna, F.S.; Malovini, A.; Villa, C.; Sottani, C.; Balestra, M.; Bracco, F.; Martino, E.; Collina, S. Essential Oils from Papaver rhoeas and Their Metabolomic Profiling. Metabolites 2024, 14, 664. https://doi.org/10.3390/metabo14120664
Cavalloro V, Robustelli della Cuna FS, Malovini A, Villa C, Sottani C, Balestra M, Bracco F, Martino E, Collina S. Essential Oils from Papaver rhoeas and Their Metabolomic Profiling. Metabolites. 2024; 14(12):664. https://doi.org/10.3390/metabo14120664
Chicago/Turabian StyleCavalloro, Valeria, Francesco Saverio Robustelli della Cuna, Alberto Malovini, Carla Villa, Cristina Sottani, Matteo Balestra, Francesco Bracco, Emanuela Martino, and Simona Collina. 2024. "Essential Oils from Papaver rhoeas and Their Metabolomic Profiling" Metabolites 14, no. 12: 664. https://doi.org/10.3390/metabo14120664
APA StyleCavalloro, V., Robustelli della Cuna, F. S., Malovini, A., Villa, C., Sottani, C., Balestra, M., Bracco, F., Martino, E., & Collina, S. (2024). Essential Oils from Papaver rhoeas and Their Metabolomic Profiling. Metabolites, 14(12), 664. https://doi.org/10.3390/metabo14120664










