Baseline Tyrosine Level Is Associated with Dynamic Changes in FAST Score in NAFLD Patients under Lifestyle Modification
Abstract
1. Introduction
2. Methods
2.1. Study Participants
2.2. Baseline Clinical and Laboratory Assessments
2.3. Metabolomics and Genotyping
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Risk Factors for Higher FAST Score (>0.35) at Baseline
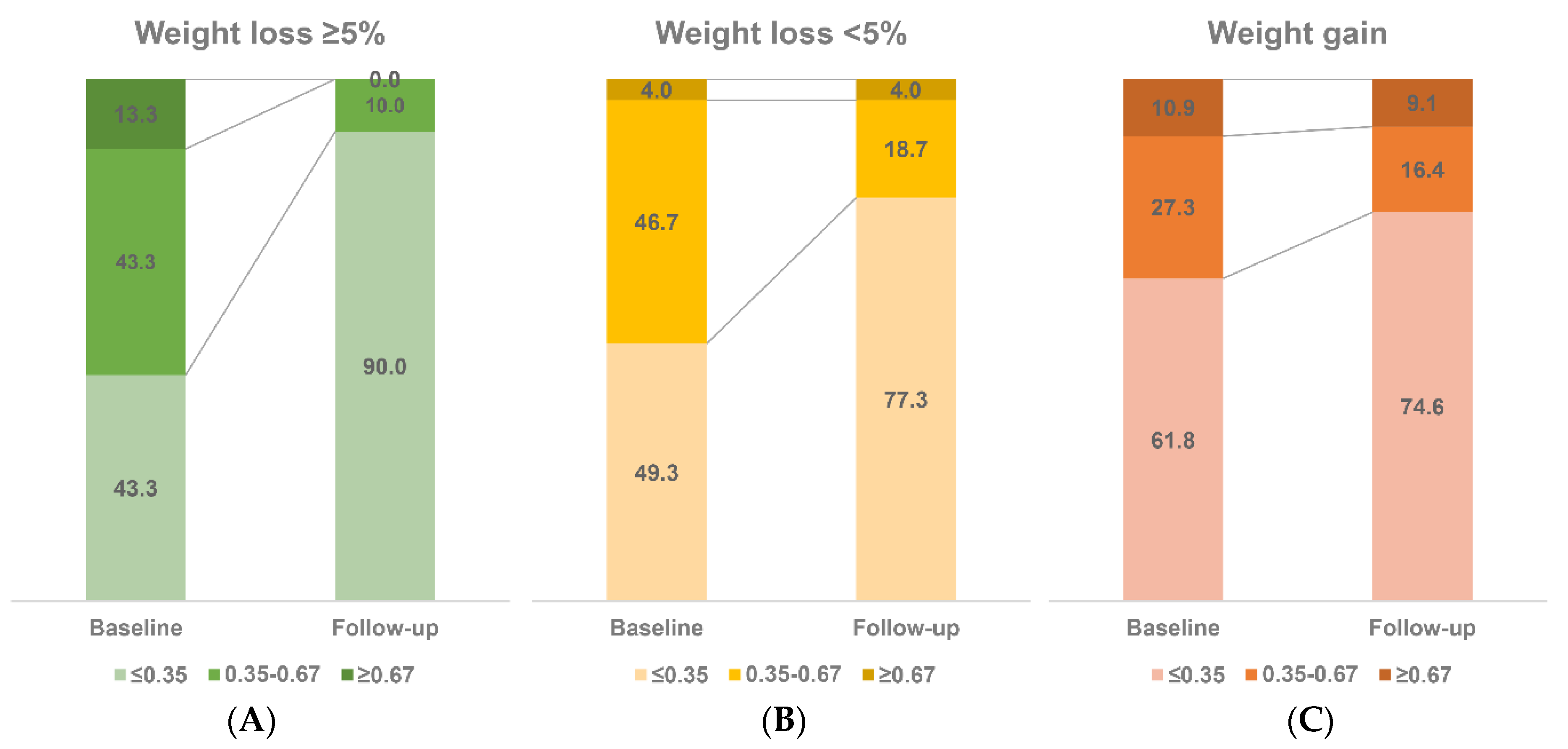
3.3. Follow-Up Sub-Cohort (n = 160)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Sanyal, A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Newsome, P.N. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol. Hepatol. 2018, 3, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.H.; Wong, V.W.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef]
- Zheng, M.; Allington, G.; Vilarinho, S. Genomic medicine for liver disease. Hepatology 2022, 76, 860–868. [Google Scholar] [CrossRef]
- Wong, V.W.S.; Zelber-Sagi, S.; Cusi, K.; Carrieri, P.; Wright, E.; Crespo, J.; Lazarus, J.V. Management of NAFLD in primary care settings. Liver Int. 2022, 42, 2377–2389. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Siddiqui, M.S.; Van Natta, M.L.; Hallinan, E.; Brandman, D.; Kowdley, K.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Abdelmalek, M.; et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology 2018, 67, 134–144. [Google Scholar] [CrossRef]
- Yip, T.C.; Lyu, F.; Lin, H.; Li, G.; Yuen, P.C.; Wong, V.W.; Wong, G.L. Noninvasive biomarkers for liver inflammation in NAFLD: Current and future. Clin. Mol. Hepatol. 2023, 29, S171–S183. [Google Scholar] [CrossRef]
- Puri, P.; Jain, S.; Fuchs, M. Use of FibroScan-AST Score to Stratify High-Risk Nonalcoholic Steatohepatitis in US Veterans. Clin. Gastroenterol. Hepatol. 2020, 18, 3060–3061. [Google Scholar] [CrossRef]
- Ciardullo, S.; Perseghin, G. Reply. Clin. Gastroenterol. Hepatol. 2020, 18, 3061–3062. [Google Scholar] [CrossRef] [PubMed]
- Gawrieh, S.; Wilson, L.A.; Cummings, O.W.; Clark, J.M.; Loomba, R.; Hameed, B.; Abdelmalek, M.F.; Dasarathy, S.; Neuschwander-Tetri, B.A.; Kowdley, K.; et al. Histologic findings of advanced fibrosis and cirrhosis in patients with nonalcoholic fatty liver disease who have normal aminotransferase levels. Am. J. Gastroenterol. 2019, 114, 1626–1635. [Google Scholar] [CrossRef]
- Noureddin, N.; Alkhouri, N.; Brown, K.A.; Noureddin, M. Driving nonalcoholic steatohepatitis forward using the FibroScan aspartate aminotransferase score, but obey the traffic lights. Hepatology 2020, 72, 2228–2230. [Google Scholar] [CrossRef]
- Feldman, A.; Eder, S.K.; Felder, T.K.; Paulweber, B.; Zandanell, S.; Stechemesser, L.; Schranz, M.; Strebinger, G.; Huber-Schönauer, U.; Niederseer, D.; et al. Clinical and metabolic characterization of obese subjects without non-alcoholic fatty liver: A targeted metabolomics approach. Diabetes Metab. 2019, 45, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Orešič, M.; Hyötyläinen, T.; Kotronen, A.; Gopalacharyulu, P.; Nygren, H.; Arola, J.; Castillo, S.; Mattila, I.; Hakkarainen, A.; Borra, R.J.; et al. Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids. Diabetologia 2013, 56, 2266–2274. [Google Scholar] [CrossRef]
- Cobb, J.; Eckhart, A.; Motsinger-Reif, A.; Carr, B.; Groop, L.; Ferrannini, E. α-Hydroxybutyric acid is a selective metabolite biomarker of impaired glucose tolerance. Diabetes Care 2016, 39, 988–995. [Google Scholar] [CrossRef]
- Heimerl, S.; Fischer, M.; Baessler, A.; Liebisch, G.; Sigruener, A.; Wallner, S.; Schmitz, G. Alterations of plasma lysophosphatidylcholine species in obesity and weight loss. PLoS ONE 2014, 9, e111348. [Google Scholar] [CrossRef] [PubMed]
- Suvitaival, T.; Bondia-Pons, I.; Yetukuri, L.; Pöhö, P.; Nolan, J.J.; Hyötyläinen, T.; Kuusisto, J.; Orešič, M. Lipidome as a predictive tool in progression to type 2 diabetes in Finnish men. Metabolism 2018, 78, 1–12. [Google Scholar] [CrossRef]
- Zalewski, A.; Macphee, C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: Biology, epidemiology, and possible therapeutic target. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y. Recent advances in nonalcoholic fatty liver disease metabolomics. Clin. Mol. Hepatol. 2021, 27, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.G.; Reily, M.D.; Lehman-McKeeman, L.D.; Vaillancourt, R.R.; Cherrington, N.J. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 2015, 47, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef] [PubMed]
- de Mello, V.D.; Sehgal, R.; Männistö, V.; Klåvus, A.; Nilsson, E.; Perfilyev, A.; Kaminska, D.; Miao, Z.; Pajukanta, P.; Ling, C.; et al. Serum aromatic and branched-chain amino acids associated with NASH demonstrate divergent associations with serum lipids. Liver Int. 2021, 41, 754–763. [Google Scholar] [CrossRef]
- Gobeil, É.; Maltais-Payette, I.; Taba, N.; Brière, F.; Ghodsian, N.; Abner, E.; Bourgault, J.; Gagnon, E.; Manikpurage, H.D.; Couture, C.; et al. Mendelian randomization analysis identifies blood tyrosine levels as a biomarker of non-alcoholic fatty liver disease. Metabolites 2022, 12, 440. [Google Scholar] [CrossRef]
- Babu, A.F.; Csader, S.; Männistö, V.; Tauriainen, M.M.; Pentikäinen, H.; Savonen, K.; Klåvus, A.; Koistinen, V.; Hanhineva, K.; Schwab, U. Effects of exercise on NAFLD using non-targeted metabolomics in adipose tissue, plasma, urine, and stool. Sci. Rep. 2022, 12, 6485. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: Expert review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Gray, S.R.; Forrest, E.; Welsh, P.; Sattar, N.; Celis-Morales, C.; Ho, F.K.; Pell, J.P. Associations of muscle mass and grip strength with severe NAFLD: A prospective study of 333,295 UK Biobank participants. J. Hepatol. 2022, 76, 1021–1029. [Google Scholar] [CrossRef]
- McCarney, R.; Warner, J.; Iliffe, S.; van Haselen, R.; Griffin, M.; Fisher, P. The Hawthorne effect: A randomised, controlled trial. BMC Med. Res. Methodol. 2007, 7, 30. [Google Scholar] [CrossRef]
- Farrell, G.C.; Chitturi, S.; Lau, G.K.K.; Sollano, J.D. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia?Pacific region: Executive summary. J. Gastroenterol. Hepatol. 2007, 22, 775–777. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Lee, Y.; Chung, Y.-S.; Lee, D.-J.; Joo, N.-S.; Hong, D.; Song, G.E.; Kim, H.-J.; Choi, Y.J.; Kim, K.-M. Prevalence of Sarcopenia and Sarcopenic Obesity in the Korean Population Based on the Fourth Korean National Health and Nutritional Examination Surveys. J. Gerontol. Ser. A 2012, 67, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lim, S.; Choi, S.H.; Kim, K.M.; Yoon, J.W.; Kim, K.W.; Lim, J.-Y.; Park, K.S.; Jang, H.C.; Kritchevsky, S. Sarcopenia: An Independent Predictor of Mortality in Community-Dwelling Older Korean Men. J. Gerontol. Ser. A 2014, 69, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Kim, D.J.; Cho, E.; Yu, K.-S.; Jang, I.-J.; Yoon, J.-H.; Park, T.; Cho, J.-Y. Comprehensive Metabolomic Search for Biomarkers to Differentiate Early Stage Hepatocellular Carcinoma from Cirrhosis. Cancers 2019, 11, 1497. [Google Scholar] [CrossRef]
- Kim, D.J.; Oh, J.Y.; Rhee, C.K.; Park, S.J.; Shim, J.J.; Cho, J.-Y. Metabolic Fingerprinting Uncovers the Distinction Between the Phenotypes of Tuberculosis Associated COPD and Smoking-Induced COPD. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Abul-Husn, N.S.; Cheng, X.; Li, A.H.; Xin, Y.; Schurmann, C.; Stevis, P.; Liu, Y.; Kozlitina, J.; Stender, S.; Wood, G.C.; et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N. Engl. J. Med. 2018, 378, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Pirola, C.J.; Garaycoechea, M.; Flichman, D.; Arrese, M.; Martino, J.S.; Gazzi, C.; Castaño, G.O.; Sookoian, S. Splice variant rs72613567 prevents worst histologic outcomes in patients with nonalcoholic fatty liver disease. J. Lipid Res. 2019, 60, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Joo, S.K.; Kim, D.; Lee, S.; Bae, J.M.; Park, J.H.; Kim, J.H.; Chang, M.S.; Kim, W. Development and Validation of a Scoring System, Based on Genetic and Clinical Factors, to Determine Risk of Steatohepatitis in Asian Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 2592–2599.e10. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, H.W.; Yoo, J.-J.; Cho, Y.; Kim, S.U.; Lee, T.H.; Jang, B.K.; Kim, S.G.; Ahn, S.B.; Kim, H.; et al. KASL clinical practice guidelines: Management of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2021, 27, 363–401. [Google Scholar] [CrossRef] [PubMed]
| Total (N= 266) | FAST≤0.35 (n = 126, 47.4%) | FAST > 0.35 (n = 140, 52.6%) | p | ||
|---|---|---|---|---|---|
| Age | 49.6 ± 14.3 | 50.3 ± 12.7 | 49.1 ± 15.7 | 0.478 | |
| Sex (male) | 158 (59.4%) | 77 (61.11%) | 81 (57.8%) | 0.589 | |
| BMI (kg/m2) | 27.2 ± 3.6 | 26.1 ± 3.1 | 28.3 ± 3.8 | <0.001 | |
| Waist circumference (cm) | 93.4 ± 9.9 | 91.5 ± 8.9 | 95.4 ± 10.5 | 0.002 | |
| Metabolic syndrome | 145 (54.5%) | 54 (42.9%) | 91 (65.0%) | 0.001 | |
| Hypertension | 83 (31.2%) | 33 (26.19%) | 50 (35.71%) | 0.094 | |
| Diabetes | 61 (22.93%) | 17 (13.39%) | 44 (31.43%) | 0.001 | |
| Dyslipidemia | 37 (13.9%) | 21 (16.67%) | 16 (11.43%) | 0.228 | |
| AST (IU/L) | 38 (26–60) | 26 (21–32) | 56.5 (43.5–76) | <0.001 | |
| ALT (IU/L) | 52 (31–94) | 31 (20–48) | 88 (57–120.5) | <0.001 | |
| GGT (IU/L) | 44 (27–72) | 32 (22–52) | 59 (36–89) | <0.001 | |
| Glucose (mg/dL) | 103 (96–115) | 100 (95–108) | 105.5 (97.5–123) | 0.002 | |
| Cholesterol (mg/dL) | 195.8 ± 45.5 | 200 ± 47.97 | 191.99 ± 42.87 | 0.149 | |
| TG (mg/dL) | 142.5 (100–201) | 129 (84–186) | 152 (113–213.5) | 0.005 | |
| HDL (mg/dL) | 47.2 ± 12.2 | 49.7 ± 13.2 | 44.9 ± 10.8 | 0.002 | |
| LDL (mg/dL) | 122.7 ± 40.2 | 124.5 ± 43.4 | 121.0 ± 37.1 | 0.484 | |
| Insulin (μIU/mL) | 11.9 (7.9–18.9) | 9.1 (6.4–13.8) | 15.3 (10.4–25.8) | <0.001 | |
| Uric acid (mg/dL) | 5.9 ± 1.5 | 5.8 ± 1.4 | 6.0 ± 1.6 | 0.261 | |
| FFA (mmol/L) | 819.1 ± 314.5 | 774.8 ± 339.3 | 858.8 ± 286.1 | 0.048 | |
| WBC (103/µL) | 6.73 ± 1.72 | 6.37 ± 1.68 | 7.05 ± 1.70 | 0.002 | |
| Hemoglobin (g/dL) | 14.7 ± 1.6 | 14.7 ± 1.5 | 14.7 ± 1.7 | 0.981 | |
| Platelet (103/µL) | 244.3 ± 64.0 | 245.8 ± 55.8 | 244.9 ± 69.1 | 0.910 | |
| Serum creatinine (mg/dL) | 0.91 ± 0.18 | 0.92 ± 0.19 | 0.90 ± 0.17 | 0.237 | |
| HOMA-IR | 3.2 (2.0–5.4) | 2.3 (1.6–3.6) | 4.2 (2.8–7.2) | <0.001 | |
| SMI_wt | 28.0 ± 3.9 | 28.8 ± 3.6 | 27.2 ± 4.0 | 0.001 | |
| Sarcopenia | 74 (29.8%) | 25 (20.7%) | 49 (38.6%) | 0.002 | |
| Fat% | 32.4 ± 7.9 | 30.6 ± 7.7 | 34.1 ± 7.7 | <0.001 | |
| Handgrip strength (kg) | 34.8 ± 10.9 | 35.1 ± 10.7 | 34.4 ± 11.1 | 0.688 | |
| TSH (mIu/L) | 2.1 (1.3–3.1) | 2.1 (1.4–3.2) | 2.1 (1.3–3.1) | 0.695 | |
| Free T4 (ng/dL) | 1.3 (1.2–1.4) | 1.3 (1.2–1.4) | 1.3 (1.1–1.4) | 0.093 | |
| HbA1c | 5.9 (5.5–6.6) | 5.8 (5.3–6.2) | 6.0 (5.6–6.9) | 0.009 | |
| CAP (dB/min) | 289.3 ± 43.5 | 271.4 ± 39.6 | 305.4 ± 40.6 | <0.001 | |
| LSM (kPa) | 6.2 (4.6–8.6) | 4.6 (3.8–6.1) | 7.7 (6.1–10.2) | <0.001 | |
| FAST score | 0.37 (0.14–0.53) | 0.14 (0.09–0.25) | 0.52 (0.43–0.64) | <0.001 | |
| PNPLA3 rs738409 | C/C | 61 (23.3%) | 36 (28.8%) | 25 (18.3%) | 0.129 |
| C/G | 130 (49.6%) | 58 (46.4%) | 72 (52.5%) | ||
| G/G | 71 (27.1%) | 31 (24.8%) | 40 (29.2%) | ||
| TM6SF2 rs58542926 | C/C | 222 (84.7%) | 108 (86.4%) | 114 (83.2%) | 0.534 |
| C/T | 39 (14.9%) | 17 (13.6%) | 22 (16.1%) | ||
| T/T | 1 (0.4%) | 0 (0%) | 1 (0.7%) | ||
| MBOAT7 rs641738 | C/C | 154 (59.3%) | 75 (60.5%) | 79 (58.1%) | 0.729 |
| C/T | 95 (36.5%) | 45 (36.3%) | 50 (36.8%) | ||
| T/T | 11 (4.2%) | 4 (3.2%) | 7 (5.1%) | ||
| SREBF2 rs133291 | C/C | 84 (32.1%) | 39 (31.2%) | 45 (32.9%) | 0.950 |
| C/T | 142 (54.2%) | 69 (55.2%) | 73 (53.3%) | ||
| T/T | 36 (13.7%) | 17 (13.6%) | 19 (13.9%) | ||
| HSD17B13 rs72613567 | −/− | 137 (52.3%) | 69 (55.2%) | 68 (49.64%) | 0.665 |
| −/A | 103 (39.3%) | 46 (36.8%) | 57 (41.61%) | ||
| A/A | 22 (8.4%) | 10 (8%) | 12 (8.76%) |
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Age | 0.99 | 0.98–1.01 | 0.482 |
| Sex (male) | 1.14 | 0.70–1.87 | 0.590 |
| BMI | 1.20 | 1.11–1.30 | <0.001 |
| Waist circumference | 1.04 | 1.02–1.07 | 0.003 |
| Metabolic syndrome | 1.57 | 1.27–1.93 | <0.001 |
| Hypertension | 1.57 | 0.93–2.65 | 0.095 |
| Diabetes | 2.94 | 1.58–5.48 | 0.001 |
| Dyslipidemia | 0.65 | 0.32–1.30 | 0.220 |
| ALT | 1.06 | 1.05–1.08 | <0.001 |
| GGT | 1.02 | 1.01–1.03 | <0.001 |
| Glucose | 1.02 | 1.01–1.04 | 0.002 |
| Cholesterol | 1.00 | 0.99–1.00 | 0.151 |
| TG | 1.00 | 1.00–1.00 | 0.101 |
| HDL | 0.97 | 0.95–0.99 | 0.002 |
| LDL | 1.00 | 0.99–1.00 | 0.483 |
| Insulin | 1.10 | 1.06–1.14 | <0.001 |
| WBC | 1.28 | 1.09–1.50 | 0.003 |
| HOMA-IR | 1.45 | 1.27–1.66 | <0.001 |
| SMI_wt | 0.90 | 0.84–0.96 | 0.001 |
| Fat% | 1.06 | 1.03–1.10 | 0.001 |
| PNPLA3 (ref. C/C) | |||
| C/G | 2.02 | 1.09–3.88 | 0.034 |
| G/G | 2.10 | 1.01–4.37 | 0.047 |
| linear (per 1 risk allele) | 1.35 | 0.96–1.91 | 0.089 |
| C/G+G/G vs. C/C | 1.81 | 1.01–3.24 | 0.045 |
| TM6SF2 (ref. C/C) | |||
| C/T | 1.30 | 0.65–2.60 | 0.464 |
| linear (per 1 risk allele) | 1.33 | 0.69–2.56 | 0.398 |
| C/T, T/T vs. C/C | 1.28 | 0.65–2.53 | 0.474 |
| MBOAT7 (ref. C/C) | |||
| C/T | 1.06 | 0.63–1.76 | 0.838 |
| T/T | 1.66 | 0.47–5.91 | 0.433 |
| linear (per 1 risk allele) | 1.14 | 0.75–1.74 | 0.546 |
| C/T, T/T vs. C/C | 1.10 | 0.67–1.81 | 0.695 |
| SREBF2 (ref. C/C) | |||
| C/T | 0.89 | 0.51–1.56 | 0.695 |
| T/T | 1.06 | 0.48–2.34 | 0.879 |
| linear (per 1 risk allele) | 0.97 | 0.67–1.40 | 0.864 |
| C/T, T/T vs. C/C | 0.93 | 0.55–1.56 | 0.776 |
| HSD17B13 (ref. −/−) | |||
| Heterozygous −/A | 1.26 | 0.75–2.10 | 0.382 |
| Homozygous A/A | 1.22 | 0.49–3.01 | 0.669 |
| Variable | OR | 95% CI | Rawp–Value | Rank | BH Adjustedp–Value |
|---|---|---|---|---|---|
| SM (OH) C22:2 | 0.53 | 0.41–0.709 | 5.76193E–06 | 1 | 0.001198481 |
| SM (OH) C16:1 | 0.57 | 0.43–0.74 | 3.95722E-05 | 2 | 0.004115505 |
| PC ae C40:6 | 0.56 | 0.43–0.74 | 4.39683E-05 | 3 | 0.003048471 |
| SM C16:0 | 0.59 | 0.45–0.77 | 7.63941E-05 | 4 | 0.003972496 |
| PC ae C38:0 | 0.58 | 0.44–0.76 | 9.78989E-05 | 5 | 0.004072592 |
| SM C24:0 | 0.59 | 0.45–0.77 | 9.88366E-05 | 6 | 0.003426335 |
| PC ae C40:5 | 0.57 | 0.43–0.76 | 0.000114559 | 7 | 0.003404043 |
| PC aa C38:0 | 0.60 | 0.46–0.78 | 0.000149 | 8 | 0.003874005 |
| SM C24:1 | 0.60 | 0.46–0.78 | 0.000155451 | 9 | 0.003592655 |
| SM (OH) C22:1 | 0.60 | 0.46–0.79 | 0.000179669 | 10 | 0.003737106 |
| SM C16:1 | 0.61 | 0.47–0.79 | 0.000194861 | 11 | 0.003684646 |
| PC ae C38:6 | 0.61 | 0.47–0.79 | 0.000200649 | 12 | 0.003477909 |
| PC ae C36:2 | 0.61 | 0.47–0.79 | 0.000220253 | 13 | 0.003524049 |
| LysoPC a C18:2 | 0.61 | 0.47–0.80 | 0.000289073 | 14 | 0.004294803 |
| PC aa C36:6 | 0.62 | 0.48–0.81 | 0.000311757 | 15 | 0.004323034 |
| PC ae C40:4 | 0.60 | 0.46–0.80 | 0.000394197 | 16 | 0.005124564 |
| PC aa C36:5 | 0.62 | 0.48–0.82 | 0.000557316 | 17 | 0.006818923 |
| Tyrosine | 1.56 | 1.19–2.03 | 0.00108511 | 18 | 0.012539053 |
| PC aa C42:4 | 1.99 | 1.30–3.04 | 0.001440041 | 19 | 0.01576466 |
| PC ae C38:5 | 0.66 | 0.51–0.86 | 0.001646602 | 20 | 0.017124665 |
| DCA | 1.57 | 1.18–2.08 | 0.001794496 | 21 | 0.017774053 |
| GLCA | 2.06 | 1.30–3.27 | 0.002110225 | 22 | 0.019951219 |
| LCA | 1.89 | 1.26–2.83 | 0.002162058 | 23 | 0.019552522 |
| PC ae C36:1 | 0.68 | 0.52–0.87 | 0.002786416 | 24 | 0.024148943 |
| SM (OH) C14:1 | 0.68 | 0.53–0.88 | 0.003112874 | 25 | 0.025899116 |
| TUDCA | 3.10 | 1.46–6.58 | 0.003205053 | 26 | 0.025640423 |
| PC ae C36:5 | 0.68 | 0.53–0.88 | 0.003436294 | 27 | 0.026472192 |
| LysoPC a C18:1 | 0.68 | 0.52–0.89 | 0.004346437 | 28 | 0.032287817 |
| GCDCA | 1.59 | 1.16–2.19 | 0.0044439 | 29 | 0.031873489 |
| SM C18:1 | 0.69 | 0.54–0.99 | 0.004463313 | 30 | 0.030945637 |
| PC aa C36:0 | 0.70 | 0.54–0.90 | 0.005357294 | 31 | 0.035945714 |
| SM C18:0 | 0.70 | 0.54–0.90 | 0.006127907 | 32 | 0.039831397 |
| GDCA | 1.59 | 1.14–2.22 | 0.006191177 | 33 | 0.039023178 |
| LysoPC a C17:0 | 0.69 | 0.52–0.90 | 0.00685643 | 34 | 0.041945216 |
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| PC ae C40:6 | 0.48 | 0.34–0.67 | <0.001 | 0.61 | 0.35–1.04 | 0.071 | |||
| lysoPC a C18:2 | 0.57 | 0.42–0.78 | <0.001 | 0.72 | 0.43–1.20 | 0.201 | |||
| SM C24:0 | 0.64 | 0.46–0.89 | 0.008 | 0.57 | 0.35–0.92 | 0.022 | |||
| Tyrosine | 2.74 | 1.88–4.01 | <0.001 | 2.07 | 1.14–3.78 | 0.018 | |||
| Sex | 1.03 | 0.44–2.43 | 0.945 | 1.30 | 0.50–3.39 | 0.591 | |||
| Age | 1.05 | 1.02–1.09 | 0.002 | 1.04 | 1.00–1.08 | 0.035 | |||
| ALT | 1.07 | 1.05–1.10 | <0.001 | 1.07 | 1.05–1.09 | <0.001 | |||
| HOMA-IR | 2.94 | 1.20–7.21 | 0.019 | 2.14 | 0.84–5.49 | 0.113 | |||
| Sarcopenia | 3.14 | 1.30–7.59 | 0.011 | 3.85 | 1.45–10.26 | 0.007 | |||
| PNPLA3 | 1.53 | 0.59–3.94 | 0.384 | 1.83 | 0.62–5.40 | 0.272 |
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| PC ae C40:6 | 2.54 | 0.91–7.07 | 0.074 | 2.47 | 0.81–7.53 | 0.111 |
| LysoPC a C18:2 | 0.97 | 0.40–2.36 | 0.940 | 0.98 | 0.38–2.54 | 0.973 |
| SM C24:0 | 1.10 | 0.44–2.79 | 0.835 | 1.25 | 0.41–3.80 | 0.693 |
| Tyrosine | 0.36 | 0.15–0.88 | 0.025 | 0.36 | 0.13–0.97 | 0.044 |
| Sex | 0.86 | 0.20–3.76 | 0.836 | 0.31 | 0.05–2.07 | 0.227 |
| Age | 0.92 | 0.86–0.98 | 0.010 | 0.88 | 0.81–0.97 | 0.007 |
| ALT | 0.97 | 0.95–0.99 | 0.001 | 0.96 | 0.94–0.99 | 0.002 |
| HOMA-IR | 0.84 | 0.11–6.55 | 0.867 | 1.26 | 0.13–11.99 | 0.841 |
| Sarcopenia | 0.42 | 0.10–1.84 | 0.247 | 0.90 | 0.13–6.23 | 0.915 |
| PNPLA3 | 0.93 | 0.13–6.65 | 0.938 | 0.98 | 0.11–8.61 | 0.983 |
| Baseline weight | 0.93 | 0.86–1.01 | 0.099 | |||
| Weight change (ref.: weight loss <5%) | ||||||
| weight loss ≥ 5% | 6.25 | 0.55–70.64 | 0.138 | |||
| weight gain | 0.65 | 0.12–3.46 | 0.612 | |||
| c–index | 0.845 (95% CI 0.815-0.989) * | 0.861 (95% CI 0.858-1.000) * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.Y.; Kim, D.J.; Lee, H.A.; Cho, J.-Y.; Kim, W. Baseline Tyrosine Level Is Associated with Dynamic Changes in FAST Score in NAFLD Patients under Lifestyle Modification. Metabolites 2023, 13, 444. https://doi.org/10.3390/metabo13030444
Kim HY, Kim DJ, Lee HA, Cho J-Y, Kim W. Baseline Tyrosine Level Is Associated with Dynamic Changes in FAST Score in NAFLD Patients under Lifestyle Modification. Metabolites. 2023; 13(3):444. https://doi.org/10.3390/metabo13030444
Chicago/Turabian StyleKim, Hwi Young, Da Jung Kim, Hye Ah Lee, Joo-Youn Cho, and Won Kim. 2023. "Baseline Tyrosine Level Is Associated with Dynamic Changes in FAST Score in NAFLD Patients under Lifestyle Modification" Metabolites 13, no. 3: 444. https://doi.org/10.3390/metabo13030444
APA StyleKim, H. Y., Kim, D. J., Lee, H. A., Cho, J.-Y., & Kim, W. (2023). Baseline Tyrosine Level Is Associated with Dynamic Changes in FAST Score in NAFLD Patients under Lifestyle Modification. Metabolites, 13(3), 444. https://doi.org/10.3390/metabo13030444







