Dietary Supplement, Containing the Dry Extract of Curcumin, Emblica and Cassia, Counteracts Intestinal Inflammation and Enteric Dysmotility Associated with Obesity
Abstract
1. Introduction
2. Experimental Design
2.1. Animals
2.2. Animal Model of Diet-Induced Obesity and Experimental Design
2.3. Measurement of Body Mass Index and Evaluation of Metabolic Parameters
2.4. Recording of Colonic Contractile Activity
2.5. Evaluation of Plasma Lipopolysaccharide Binding Protein
2.6. Quantification of Plasma Myeloperoxidase and Colonic Interleukin-1β Levels
2.7. Assay of Faecal Calprotectin
2.8. Western Blot Assays
2.9. Statistical Analysis
3. Results
3.1. Plant-Based Food Supplement Counteracts the Body Weight Gain and BMI in Obese Mice
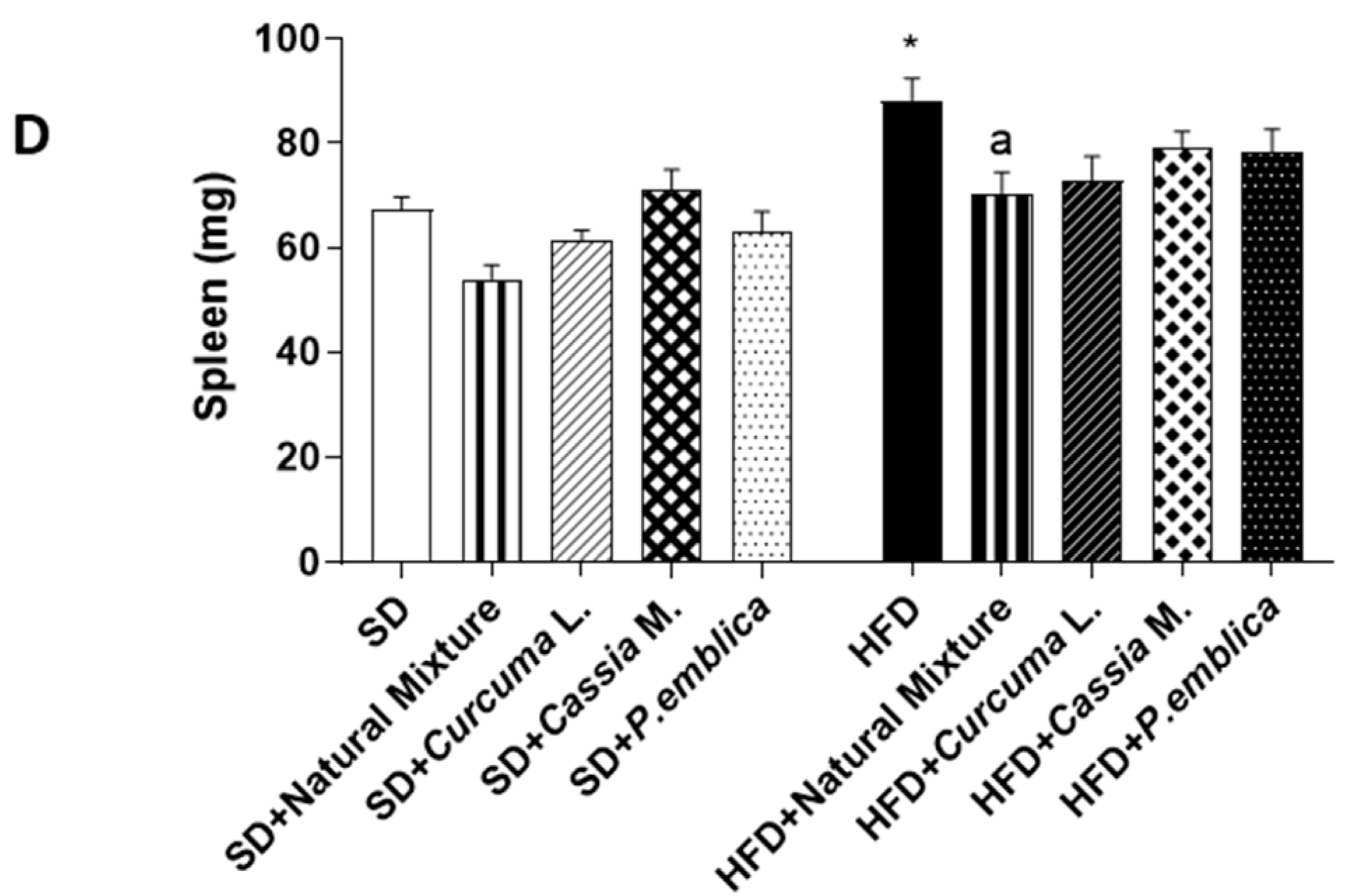
3.2. Dietary Supplementation with Curcumin, Emblica and Cassia Reduces Spleen and Liver Weight
3.3. Plant-Based Food Supplement Ameliorated Metabolic Parameters in HFD Mice


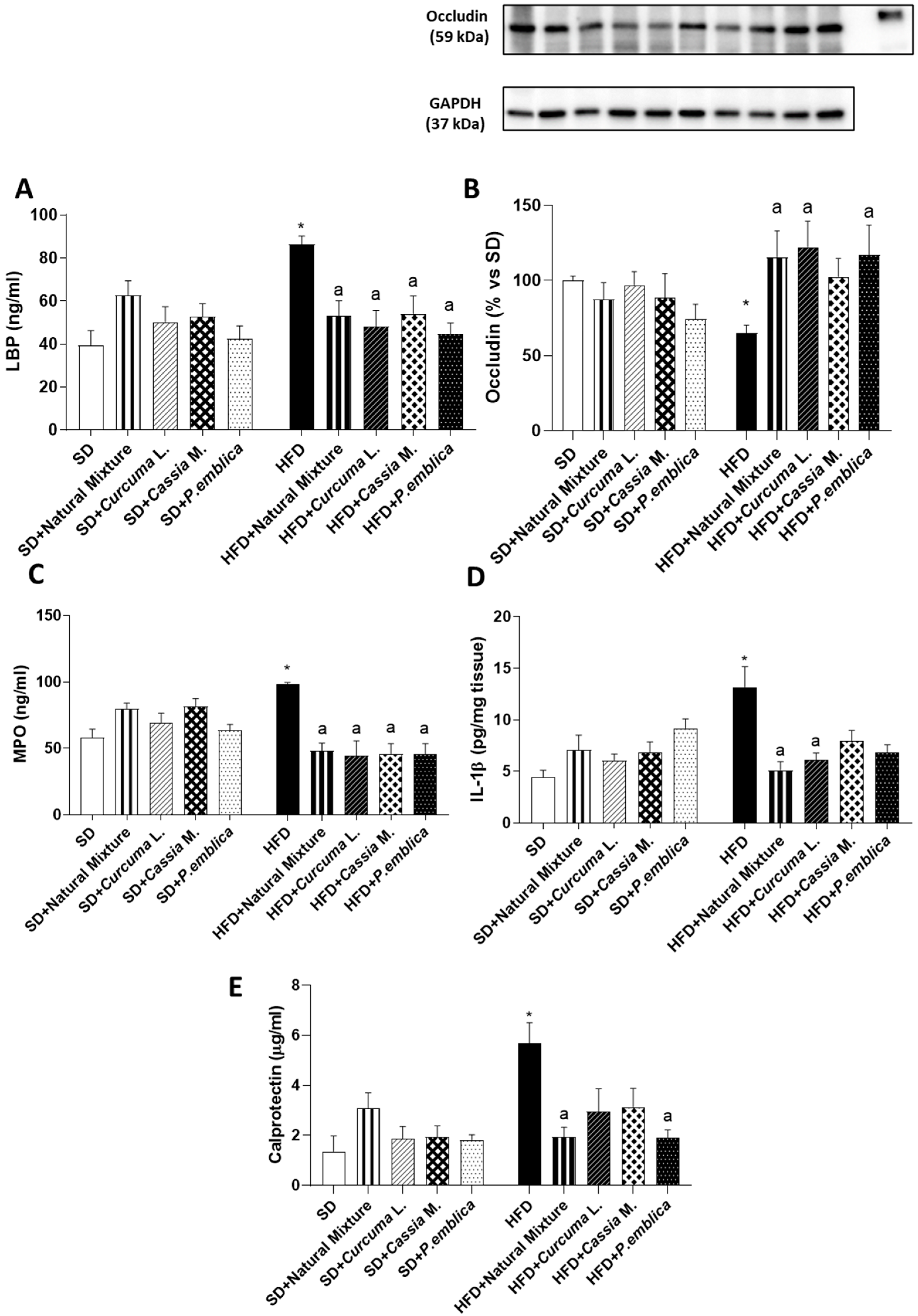
3.4. Plant-Based Food Supplement Ameliorates the Intestinal Barrier Integrity
3.5. Dietary Supplementation with Curcumin, Emblica and Cassia Reduces Plasmatic MPO and Tissutal IL-1β Levels in HFD Mice
3.6. Plant-Based Food Supplement Reduced Faecal Calprotectin Levels in Obese Mice
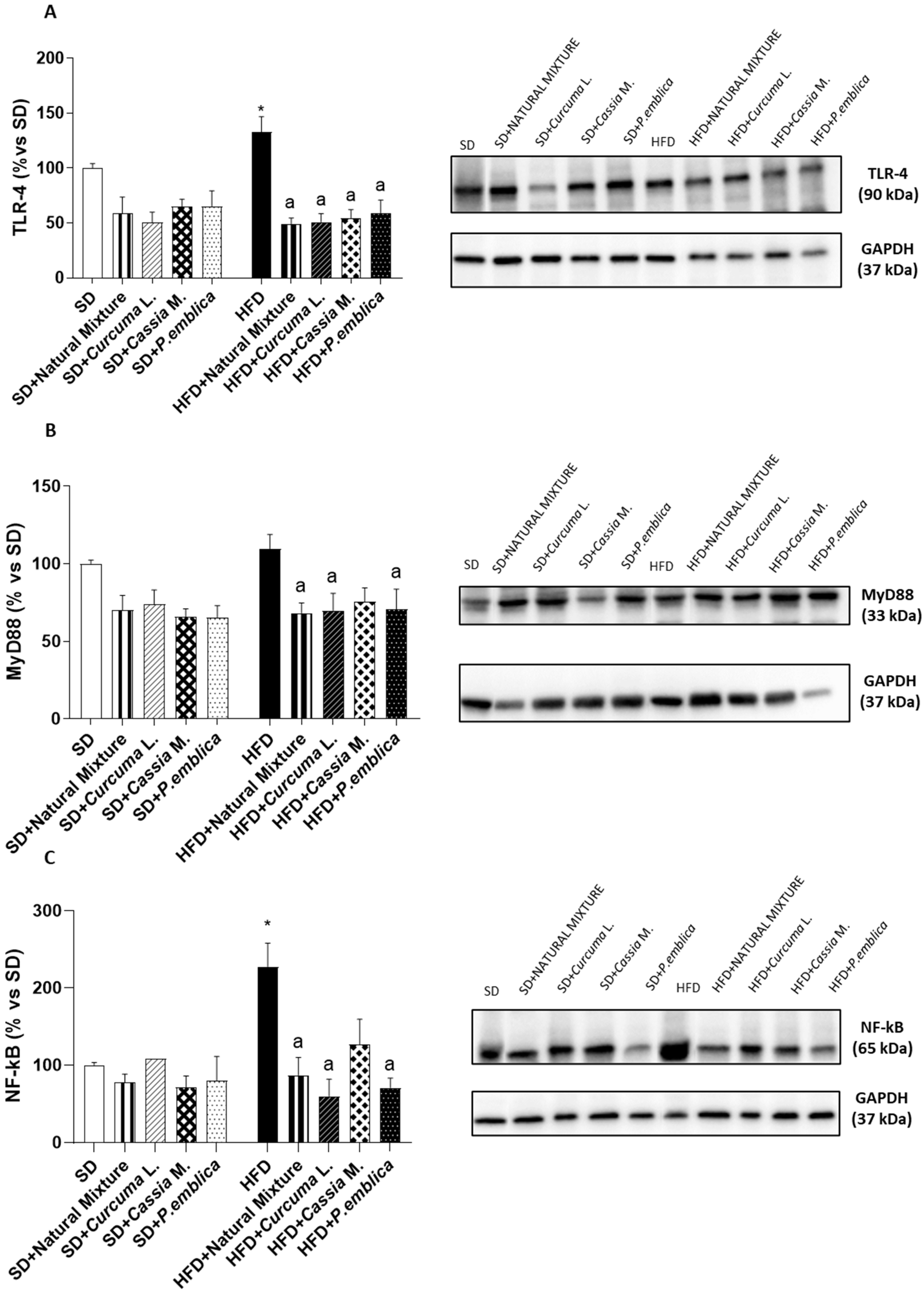
3.7. Dietary Supplementation with Curcumin, Emblica and Cassia Reduces Colonic Expression of TLR-4, MyD88 and NF-κB
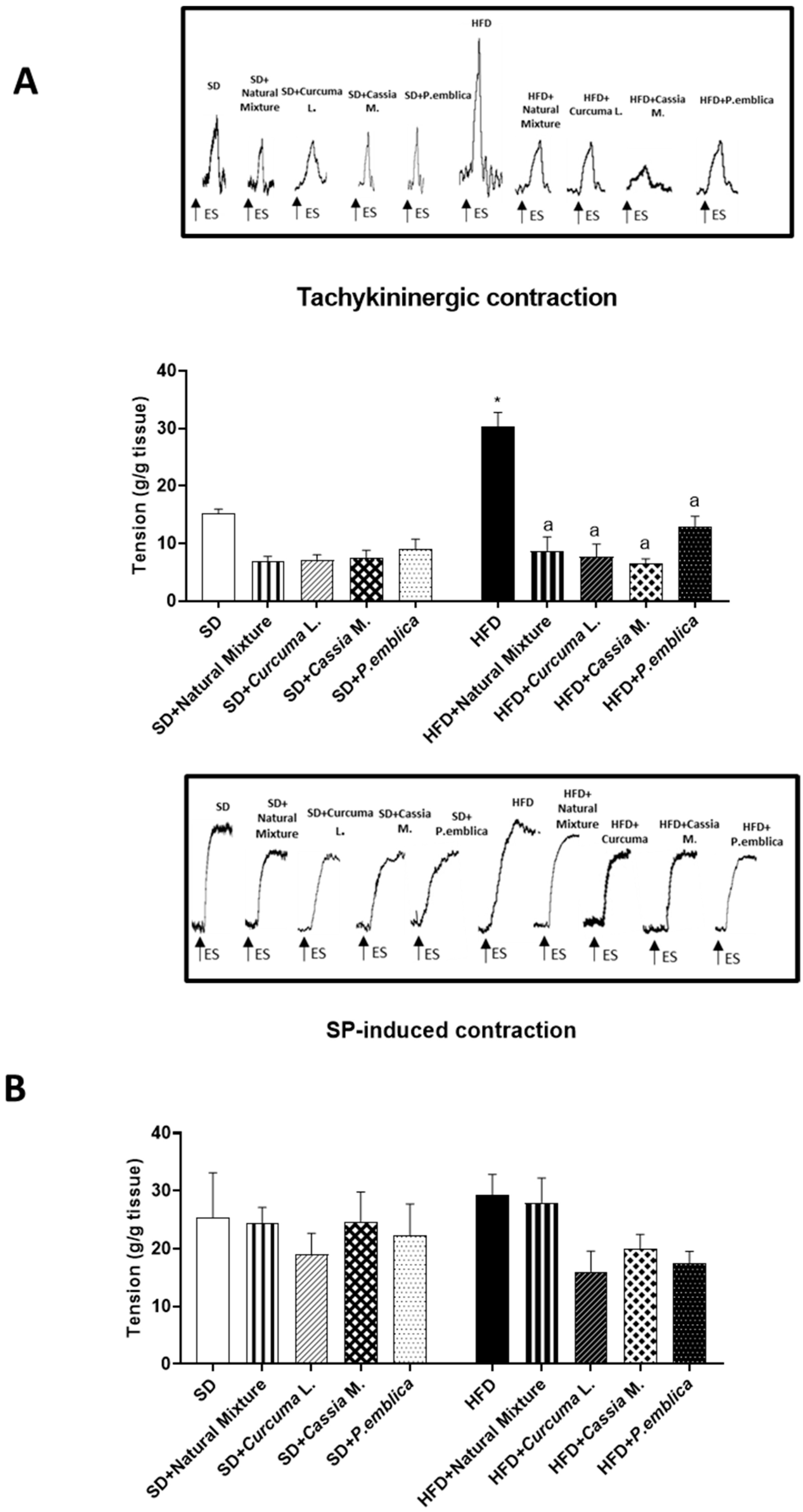
3.8. Plant-Based Food Supplement Counteracted Colonic Dysmotility in Obese Mice
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity & inflammation: The linking mechanism & the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, T.K.; Geoghegan, J.G.; Baird, A.W.; Winter, D.C. Implications of altered gastrointestinal motility in obesity. Obes. Surg. 2007, 17, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Mangat, R.; Tsai, S.; Proctor, S.D.; Richard, C. The Interplay of Obesity, Dyslipidemia and Immune Dysfunction: A Brief Overview on Pathophysiology, Animal Models, and Nutritional Modulation. Front. Nutr. 2022, 9, 138. [Google Scholar] [CrossRef]
- D’Antongiovanni, V.; Fornai, M.; Pellegrini, C.; Blandizzi, C.; Antonioli, L. Managing Obesity and Related Comorbidities: A Potential Pharmacological Target in the Adenosine System? Front. Pharmacol. 2021, 11, 621955. [Google Scholar] [CrossRef]
- Massier, L.; Blüher, M.; Kovacs, P.; Chakaroun, R.M. Impaired Intestinal Barrier and Tissue Bacteria: Pathomechanisms for Metabolic Diseases. Front. Endocrinol. 2021, 12, 616506. [Google Scholar] [CrossRef]
- Ely, B.R.; Clayton, Z.S.; McCurdy, C.E.; Pfeiffer, J.; Minson, C.T. Meta-inflammation and cardiometabolic disease in obesity: Can heat therapy help? Temperature 2017, 5, 9–21. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; De Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Antonioli, L.; Caputi, V.; Fornai, M.; Pellegrini, C.; Gentile, D.; Giron, M.C.; Orso, G.; Bernardini, N.; Segnani, C.; Ippolito, C.; et al. Interplay between colonic inflammation and tachykininergic pathways in the onset of colonic dysmotility in a mouse model of diet-induced obesity. Int. J. Obes. 2019, 43, 331–343. [Google Scholar] [CrossRef]
- Pellegrini, C.; Fornai, M.; Benvenuti, L.; Colucci, R.; Caputi, V.; Palazon-Riquelme, P.; Giron, M.C.; Nericcio, A.; Garelli, F.; D’Antongiovanni, V.; et al. NLRP3 at the crossroads between immune/inflammatory responses and enteric neuroplastic remodelling in a mouse model of diet-induced obesity. Br. J. Pharmacol. 2021, 178, 3924–3942. [Google Scholar] [CrossRef]
- Panattoni, A.; Calvigioni, M.; Benvenuti, L.; D’Antongiovanni, V.; Pellegrini, C.; Di Salvo, C.; Mazzantini, D.; Celandroni, F.; Fornai, M.; Antonioli, L.; et al. The administration of Enterococcus faecium SF68 counteracts compositional shifts in the gut microbiota of diet-induced obese mice. Front. Microbiol. 2022, 13, 1054097. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Gancarz, M.; Kondracka, A.; Rusinek, R.; Oniszczuk, A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022, 23, 639. [Google Scholar] [CrossRef] [PubMed]
- Nazish, I.; Ansari, S.H. Emblica officinalis—Anti-obesity activity. J. Complement. Integr. Med. 2017, 15, 20160051. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Shimura, S.; Itoh, Y.; Ohsaka, T.; Egawa, M.; Inoue, S. Anti-obesity effects of lipase inhibitor CT-II, an extract from edible herbs, Nomame Herba, on rats fed a high-fat diet. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Smemo, S.; Tena, J.J.; Kim, K.H.; Gamazon, E.R.; Sakabe, N.J.; Gómez-Marín, C.; Aneas, I.; Credidio, F.L.; Sobreira, D.R.; Wasserman, N.F.; et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 2014, 507, 371–375. [Google Scholar] [CrossRef]
- Antonioli, L.; D’Antongiovanni, V.; Pellegrini, C.; Fornai, M.; Benvenuti, L.; di Carlo, A.; van den Wijngaard, R.; Caputi, V.; Cerantola, S.; Giron, M.C.; et al. Colonic dysmotility associated with high-fat diet-induced obesity: Role of enteric glia. FASEB J. 2020, 34, 5512–5524. [Google Scholar] [CrossRef]
- D’Antongiovanni, V.; Benvenuti, L.; Fornai, M.; Pellegrini, C.; van den Wijngaard, R.; Cerantola, S.; Giron, M.C.; Caputi, V.; Colucci, R.; Haskó, G.; et al. Glial A2B Adenosine Receptors Modulate Abnormal Tachykininergic Responses and Prevent Enteric Inflammation Associated with High Fat Diet-Induced Obesity. Cells 2020, 9, 1245. [Google Scholar] [CrossRef]
- Pellegrini, C.; Daniele, S.; Antonioli, L.; Benvenuti, L.; D’antongiovanni, V.; Piccarducci, R.; Pietrobono, D.; Citi, V.; Piragine, E.; Flori, L.; et al. Prodromal intestinal events in alzheimer’s disease (Ad): Colonic dysmotility and inflammation are associated with enteric ad-related protein deposition. Int. J. Mol. Sci. 2020, 21, 3523. [Google Scholar] [CrossRef]
- Qaddoumi, M.G.; Alanbaei, M.; Hammad, M.M.; Al Khairi, I.; Cherian, P.; Channanath, A.; Thanaraj, T.A.; Al-Mulla, F.; Abu-Farha, M.; Abubaker, J. Investigating the Role of Myeloperoxidase and Angiopoietin-like Protein 6 in Obesity and Diabetes. Sci. Rep. 2020, 10, 6170. [Google Scholar] [CrossRef]
- Olza, J.; Aguilera, C.M.; Gil-Campos, M.; Leis, R.; Bueno, G.; Martínez-Jiménez, M.D.; Valle, M.; Canẽte, R.; Tojo, R.; Moreno, L.A.; et al. Myeloperoxidase is an early biomarker of inflammation and cardiovascular risk in prepubertal obese children. Diabetes Care 2012, 35, 2373–2376. [Google Scholar] [CrossRef] [PubMed]
- McGuire, V.A.; Arthur, J.S.C. Subverting Toll-Like Receptor Signaling by Bacterial Pathogens. Front. Immunol. 2015, 6, 607. [Google Scholar] [CrossRef] [PubMed]
- D’Antongiovanni, V.; Pellegrini, C.; Antonioli, L.; Benvenuti, L.; Di Salvo, C.; Flori, L.; Piccarducci, R.; Daniele, S.; Martelli, A.; Calderone, V.; et al. Palmitoylethanolamide Counteracts Enteric Inflammation and Bowel Motor Dysfunctions in a Mouse Model of Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 2721. [Google Scholar] [CrossRef]
- Pellegrini, C.; D’Antongiovanni, V.; Miraglia, F.; Rota, L.; Benvenuti, L.; Di Salvo, C.; Testa, G.; Capsoni, S.; Carta, G.; Antonioli, L.; et al. Enteric α-synuclein impairs intestinal epithelial barrier through caspase-1-inflammasome signaling in Parkinson’s disease before brain pathology. npj Park. Dis. 2022, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- D’Antongiovanni, V.; Pellegrini, C.; Benvenuti, L.; Fornai, M.; Di Salvo, C.; Natale, G.; Ryskalin, L.; Bertani, L.; Lucarini, E.; Di Cesare Mannelli, L.; et al. Anti-inflammatory Effects of Novel P2X4 Receptor Antagonists, NC-2600 and NP-1815-PX, in a Murine Model of Colitis. Inflammation 2022, 45, 1829–1847. [Google Scholar] [CrossRef]
- Fysekidis, M.; Bouchoucha, M.; Bihan, H.; Reach, G.; Benamouzig, R.; Catheline, J.M. Prevalence and co-occurrence of upper and lower functional gastrointestinal symptoms in patients eligible for bariatric surgery. Obes. Surg. 2012, 22, 403–410. [Google Scholar] [CrossRef]
- Rajindrajith, S.; Devanarayana, N.M.; Benninga, M.A. Obesity and functional gastrointestinal diseases in children. J. Neurogastroenterol. Motil. 2014, 20, 414–416. [Google Scholar] [CrossRef]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Önal, Z.E.; Atasayan, V.; Gürbüz, T.; Hepkaya, E.; Nuhoglu, Ç. Association of glycosylated hemoglobin (HbA1c) levels with Iinsulin resistance in obese children. Afr. Health Sci. 2014, 14, 533–538. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H. Molecular mechanisms involved in hepatic steatosis and insulin resistance. J. Diabetes Investig. 2011, 2, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Kawada, N. The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 3863. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Citro, V.; Conforti, P.; Balsano, C.; Capone, D. Is There a Link between Basal Metabolic Rate, Spleen Volume and Hepatic Growth Factor Levels in Patients with Obesity-Related NAFLD? J. Clin. Med. 2019, 8, 1510. [Google Scholar] [CrossRef] [PubMed]
- Konrad, D.; Wueest, S. The gut-adipose-liver axis in the metabolic syndrome. Physiology 2014, 29, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.; Heckmann, V.; Tóth, D.; Pauka, D.; Petrus, K.; Molnár, T.F. The effect of hepatic steatosis and fibrosis on liver weight and dimensions. Leg. Med. 2020, 47, 101781. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Pan, Z.; Wu, S.; Zheng, M.; Zhong, C.; Xin, X.; Lan, S.; Zhu, Z.; Liu, M.; Wu, H.; et al. Emodin palliates high-fat diet-induced nonalcoholic fatty liver disease in mice via activating the farnesoid X receptor pathway. J. Ethnopharmacol. 2021, 279, 114340. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef]
- Chatterton, D.E.W.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef]
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, A.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients 2019, 11, 2325. [Google Scholar] [CrossRef]
- Saikh, K.U. MyD88 and beyond: A perspective on MyD88-targeted therapeutic approach for modulation of host immunity. Immunol. Res. 2021, 69, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Guo, R.; Wei, S.; Kong, Y.; Wei, X.; Wang, W.; Shi, X.; Jiang, H. Curcumin protects against the intestinal ischemia-reperfusion injury: Involvement of the tight junction protein ZO-1 and TNF-α related mechanism. Korean J. Physiol. Pharmacol. 2016, 20, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, H. Curcumin Improved Intestinal Epithelial Barrier Integrity by Up-Regulating ZO-1/Occludin/Claudin-1 in Septic Rats. Evid. Based. Complement. Alternat. Med. 2022, 2022, 2884522. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice--role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.I.; Collins, S.M. Gut motor function: Immunological control in enteric infection and inflammation. Clin. Exp. Immunol. 2006, 143, 389–397. [Google Scholar] [CrossRef]
- D’Antongiovanni, V.; Pellegrini, C.; Fornai, M.; Colucci, R.; Blandizzi, C.; Antonioli, L.; Bernardini, N. Intestinal epithelial barrier and neuromuscular compartment in health and disease. World J. Gastroenterol. 2020, 26, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Le Pluart, D.; Sabaté, J.-M.; Bouchoucha, M.; Hercberg, S.; Benamouzig, R.; Julia, C. Functional gastrointestinal disorders in 35,447 adults and their association with body mass index. Aliment. Pharmacol. Ther. 2015, 41, 758–767. [Google Scholar] [CrossRef]
- Sebai, H.; Rtibi, K.; Selmi, S.; Jridi, M.; Balti, R.; Marzouki, L. Modulating and opposite actions of two aqueous extracts prepared from Cinnamomum cassia L. bark and Quercus ilex L. on the gastrointestinal tract in rats. RSC Adv. 2019, 9, 21695–21706. [Google Scholar] [CrossRef]
- Micucci, M.; Aldini, R.; Cevenini, M.; Colliva, C.; Spinozzi, S.; Roda, G.; Montagnani, M.; Camborata, C.; Camarda, L.; Chiarini, A.; et al. Curcuma longa L. as a therapeutic agent in intestinal motility disorders. 2: Safety profile in mouse. PLoS ONE 2013, 8, e80925. [Google Scholar] [CrossRef]
- Yao, Y.; Luo, R.; Xiong, S.; Zhang, C.; Zhang, Y. Protective effects of curcumin against rat intestinal inflammation-related motility disorders. Mol. Med. Rep. 2021, 23, 391. [Google Scholar] [CrossRef]
- Aldini, R.; Budriesi, R.; Roda, G.; Micucci, M.; Ioan, P.; D’Errico-Grigioni, A.; Sartini, A.; Guidetti, E.; Marocchi, M.; Cevenini, M.; et al. Curcuma longa extract exerts a myorelaxant effect on the ileum and colon in a mouse experimental colitis model, independent of the anti-inflammatory effect. PLoS ONE 2012, 7, e44650. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Lim, D.Y.; Yeo, W.-S. The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 2018, 11, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Antonelli, E.; Villanacci, V.; Nascimbeni, R.; Dore, M.P.; Pes, G.M.; Maconi, G. Abnormal gut motility in inflammatory bowel disease: An update. Tech. Coloproctol. 2020, 24, 275–282. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Antongiovanni, V.; Fornai, M.; Benvenuti, L.; Di Salvo, C.; Pellegrini, C.; Cappelli, F.; Masi, S.; Antonioli, L. Dietary Supplement, Containing the Dry Extract of Curcumin, Emblica and Cassia, Counteracts Intestinal Inflammation and Enteric Dysmotility Associated with Obesity. Metabolites 2023, 13, 410. https://doi.org/10.3390/metabo13030410
D’Antongiovanni V, Fornai M, Benvenuti L, Di Salvo C, Pellegrini C, Cappelli F, Masi S, Antonioli L. Dietary Supplement, Containing the Dry Extract of Curcumin, Emblica and Cassia, Counteracts Intestinal Inflammation and Enteric Dysmotility Associated with Obesity. Metabolites. 2023; 13(3):410. https://doi.org/10.3390/metabo13030410
Chicago/Turabian StyleD’Antongiovanni, Vanessa, Matteo Fornai, Laura Benvenuti, Clelia Di Salvo, Carolina Pellegrini, Federica Cappelli, Stefano Masi, and Luca Antonioli. 2023. "Dietary Supplement, Containing the Dry Extract of Curcumin, Emblica and Cassia, Counteracts Intestinal Inflammation and Enteric Dysmotility Associated with Obesity" Metabolites 13, no. 3: 410. https://doi.org/10.3390/metabo13030410
APA StyleD’Antongiovanni, V., Fornai, M., Benvenuti, L., Di Salvo, C., Pellegrini, C., Cappelli, F., Masi, S., & Antonioli, L. (2023). Dietary Supplement, Containing the Dry Extract of Curcumin, Emblica and Cassia, Counteracts Intestinal Inflammation and Enteric Dysmotility Associated with Obesity. Metabolites, 13(3), 410. https://doi.org/10.3390/metabo13030410






