Betaxanthin Profiling in Relation to the Biological Activities of Red and Yellow Beta vulgaris L. Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents and Reference Compounds
2.3. Sample Preparation
2.4. Quantitation and Qualitation of Compounds
2.5. HPLC-MS Analysis
2.6. Antimicrobial Activity
2.7. Cytotoxicity Evaluation and Anticancer Selectivity
2.8. Statistical Evaluation
3. Results and Discussion
3.1. The Profile and Content of Betaxanthins in B. vulgaris
3.2. Antimicrobial Activity
3.3. Correlation between Phytochemical Composition and Antimicrobial Activity
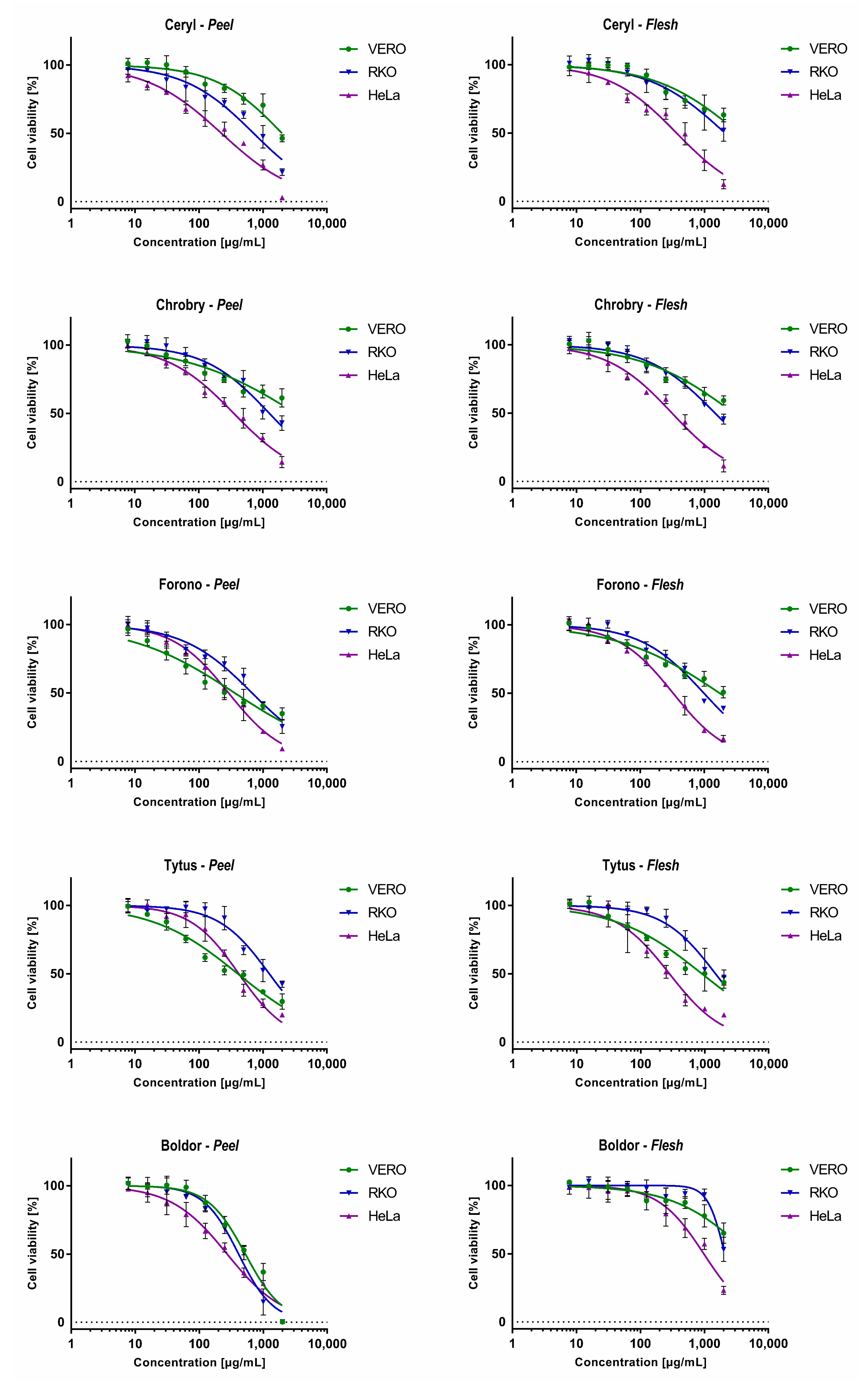
3.4. Cytotoxicity and Anticancer Selectivity
3.5. Correlation between Phytochemical Composition and Anticancer Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, M.L.; Hamid, S.B.S. Beetroot as a potential functional food for cancer chemoprevention, a narrative review. J. Cancer Prev. 2021, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; D’Anneo, A.; Frazzitta, A.; Restivo, I.; Livrea, M.A.; Attanzio, A.; Tesoriere, L. The phytochemical indicaxanthin synergistically enhances cisplatin-induced apoptosis in Hela cells via oxidative stress-dependent P53/P21waf1 axis. Biomolecules 2020, 10, 994. [Google Scholar] [CrossRef]
- Attanzio, A.; Restivo, I.; Tutone, M.; Tesoriere, L.; Allegra, M.; Livrea, M.A. Redox properties, bioactivity and health effects of indicaxanthin, a Tablebioavailable phytochemical from Opuntia ficus indica, L.: A critical review of accumulated evidence and perspectives. Antioxidants 2022, 11, 2364. [Google Scholar] [CrossRef] [PubMed]
- Stücheli, P.; Sieber, S.; Fuchs, D.W.; Scheller, L.; Strittmatter, T.; Saxena, P.; Gademann, K.; Fussenegger, M. Genetically encoded betaxanthin-based small-molecular fluorescent reporter for Mammalian cells. Nucleic Acids Res. 2020, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; Tutone, M.; Tesoriere, L.; Almerico, A.M.; Culletta, G.; Livrea, M.A.; Attanzio, A. Indicaxanthin, a multi-target natural compound from Opuntia ficus-indica fruit: From its poly-pharmacological effects to biochemical mechanisms and molecular modelling studies. Eur. J. Med. Chem. 2019, 179, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Shinde, P.R.; Wagh, K.R.; Patil, P.S.; Bairagi, V.A. Pharmacognostic standardization and antibacterial potential of aerial herbs of Portulaca grandiflora Hooker (Portulaceae). World J. Pharm. Sci. 2014, 2, 1871–1885. [Google Scholar]
- De Oliveira, S.P.A.; de Nascimento, H.M.A.; Sampaio, K.B.; de Souza, E.L. A review on bioactive compounds of beet (Beta vulgaris L. subsp. vulgaris) with special emphasis on their beneficial effects on gut microbiota and gastrointestinal health. Crit. Rev. Food Sci. Nutr. 2021, 61, 2022–2033. [Google Scholar]
- Chavalittumrong, P.; Sriwanthana, B.; Rojanawiwat, A.; Kijphati, R.; Jitjuk, B.; Treesangsri, W.; Phadungpat, S.; Bansiddhi, J.; Bunjob, M. Safety of the aqueous extract of Portulaca grandiflora Hook in healthy volunteers. Safety 2007, 29, 1. [Google Scholar]
- Dos Santos Baião, D.; Silva de Freitas, C.; Paes Gomes, L.; da Silva, D.; Carolina, N.T.F.; Correa, A.; Ribeiro Pereira, P.; Mere del Aguila, E.; Margaret Flosi Paschoalin, V. Polyphenols from root, tubercles and grains cropped in Brazil: Chemical and nutritional characterization and their effects on human health and diseases. Nutrients 2017, 9, 1044. [Google Scholar] [CrossRef]
- Kumorkiewicz-Jamro, A.; Świergosz, T.; Sutor, K.; Spórna-Kucab, A.; Wybraniec, S. Multi-Colored shades of betalains: Recent advances in betacyanin chemistry. Nat. Prod. Rep. 2021, 38, 2315–2346. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Bernaś, K.; Grzegorczyk, A.; Malm, A.; Skalicka-Woźniak, K.; Wybraniec, S. Liquid chromatographic techniques in betacyanin isomers separation from Gomphrena globosa L. flowers for the determination of their antimicrobial activities. J. Pharm. Biomed. Anal. 2018, 161, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Spórna-Kucab, A.; Kumorkiewicz, A.; Szmyr, N.; Szneler, E.; Wybraniec, S. Separation of betacyanins from flowers of Amaranthus cruentus L. in a polar solvent system by high-speed counter-current chromatography. J. Sep. Sci. 2019, 42, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Esquivel, O.; Ortiz-Moreno, A.; Álvarez, V.B.; Dorantes-Álvarez, L.; Giusti, M.M. Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res. Int. 2011, 44, 2160–2168. [Google Scholar] [CrossRef]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef]
- Khan, M.I. Plant betalains: Safety, antioxidant activity, clinical efficacy, and bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Pietrzkowski, Z.; Nemzer, B.; Spórna, A.; Stalica, P. Influence of betalain-rich extract on reduction of discomfort associated with osteoarthritis. New Med. 2010, 1, 12–17. [Google Scholar]
- Spórna-Kucab, A.; Wróbel, N.; Kumorkiewicz-Jamro, A.; Wybraniec, S. Separation of betacyanins from Iresine herbstii Hook. Ex Lindl. leaves by high-speed countercurrent chromatography in a polar solvent system. J. Chrom. A 2020, 1626, 461370. [Google Scholar] [CrossRef] [PubMed]
- Spórna-Kucab, A.; Milo, A.; Kumorkiewicz, A.; Wybraniec, S. Studies on polar high-speed counter-current chromatographic systems in separation of amaranthine-type betacyanins from Celosia species. J. Chrom. B 2018, 1073, 96–103. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Jagodzińska, J.; Wybraniec, S. Separation of betacyanins from purple flowers of Gomphrena globosa L. by ion-pair high-speed counter-current chromatography. J. Chrom. A 2017, 1489, 51–57. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Hołda, E.; Wybraniec, S. High-speed counter-current chromatography in separation of betacyanins from flowers of red Gomphrena globosa L. cultivars. J. Chrom. B 2016, 1033, 421–427. [Google Scholar] [CrossRef]
- Wybraniec, S.; Stalica, P.; Sporna, A.; Mizrahi, Y. Profiles of betacyanins in epidermal layers of grafted and light-stressed cacti studied by LC-DAD-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 5347–5354. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Stalica, P.; Jerz, G.; Klose, B.; Gebers, N.; Winterhalter, P.; Spórna, A.; Szaleniec, M.; Mizrahi, Y. Separation of polar betalain pigments from cacti fruits of Hylocereus polyrhizus by ion-pair high-speed countercurrent chromatography. J. Chrom. A 2009, 1216, 6890–6899. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Wybraniec, S.; Nowak-Wydra, B.; Mitka, K.; Kowalski, P.; Mizrahi, Y. Minor betalains in fruits of Hylocereus species. Phytochemistry 2007, 68, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Christinet, L.; Burdet, F.X.; Zaiko, M.; Hinz, U.; Zrÿd, J.-P. Characterization and functional identification of a novel plant 4,5-extradiol dioxygenase involved in betalain pigment biosynthesis in Portulaca grandiflora. Plant Physiol. 2004, 134, 265–274. [Google Scholar] [CrossRef]
- Azeredo, H.M. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Wybraniec, S. High-speed counter-current chromatography in separation and identification of saponins from Beta vulgaris L. cultivar red sphere. Polish J. Food Nutr. Sci. 2020, 70, 67–74. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Tekieli, A.; Skalicka-Woźniak, K.; Grzegorczyk, A.; Świergosz, T.; Wybraniec, S. Characterization of triterpene saponin composition of white, yellow and red beetroot (Beta vulgaris L.). Pol. J. Food Nutr. Sci. 2022, 72, 159–170. [Google Scholar] [CrossRef]
- Spórna-Kucab, A.; Jerz, G.; Kumorkiewicz-Jamro, A.; Tekieli, A.; Wybraniec, S. High-speed countercurrent chromatography for isolation and enrichment of betacyanins from fresh and dried leaves of Atriplex hortensis L. var. “Rubra”. J. Sep. Sci. 2021, 44, 4222–4236. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F.; Escribano, J. Development of a protocol for the semi-synthesis and purification of betaxanthins. Phytochem. Anal. 2006, 17, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, J.; Gandía-Herrero, F.; Escribano, J.; García-Carmona, F.; Jiménez-Atiénzar, M. One-step synthesis of betalains using a novel betalamic acid derivatized support. J. Agric. Food Chem. 2014, 62, 3776–3782. [Google Scholar] [CrossRef] [PubMed]
- Spórna-Kucab, A.; Tekieli, A.; Grzegorczyk, A.; Świątek, Ł.; Rajtar, B.; Skalicka-Woźniak, K.; Starzak, K.; Nemzer, B.; Pietrzkowski, Z.; Wybraniec, S. Metabolite profiling analysis and the correlation with biological activity of betalain-rich Portulaca grandiflora Hook. extracts. Antioxidants 2022, 11, 1654. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Schieber, A.; Carle, R. Identification of betalains from yellow beet (Beta vulgaris L.) and cactus pear [Opuntia ficus-indica (L.) Mill.] by high-performance liquid chromatography−electrospray ionization mass spectrometry. J. Agric. Food Chem. 2002, 50, 2302–2307. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar] [CrossRef]
- EUCAST. European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID): Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. Clin. Microbiol. Infect. Dis. 2003, 40, 1–7. [Google Scholar]
- Malm, A.; Grzegorczyk, A.; Biernasiuk, A.; Baj, T.; Rój, E.; Tyśkiewicz, K.; Dębczak, A.; Stolarski, M.J.; Krzyżaniak, M.; Olba-Zięty, E. Could Supercritical Extracts from the Aerial Parts of Helianthus salicifolius A. Dietr. and Helianthus tuberosus L. Be Regarded as Potential Raw Materials for Biocidal Purposes? Agriculture 2020, 11, 10. [Google Scholar] [CrossRef]
- Pecio, Ł.; Kozachok, S.; Saber, F.R.; Garcia-Marti, M.; El-Amier, Y.; Mahrous, E.A.; Świątek, Ł.; Boguszewska, A.; Skiba, A.; Elosaily, A.H.; et al. Metabolic profiling of Ochradenus baccatus Delile. utilizing UHPLC-HRESIMS in relation to the in vitro biological investigations. Food Chem. 2023, 412, 135587. [Google Scholar] [CrossRef]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain Profile, Content and Antioxidant Capacity of Red Beetroot Dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Kugler, F.; Graneis, S.; Stintzing, F.C.; Carle, R. Studies on betaxanthin profiles of vegetables and fruits from the Chenopodiaceae and Cactaceae. Z. Naturforsch. C 2007, 62, 311–318. [Google Scholar] [CrossRef]
- Spórna, A.; Stalica, P.; Jerz, G.; Szaleniec, M.; Wybraniec, S. Liquid chromatographic techniques in separation of betacyanins and their derivatives from red beet roots. Chall. Mod. Technol. 2010, 1, 19–22. [Google Scholar]
- Slatnar, A.; Stampar, F.; Veberic, R.; Jakopic, J. HPLC-MSn identification of betalain profile of different beetroot (Beta vulgaris L. ssp. vulgaris) parts and cultivars. J. Food Sci. 2015, 80, C1952–C1958. [Google Scholar] [CrossRef]
- Sawicki, T.; Topolska, J.; Romaszko, E.; Wiczkowski, W. Profile and content of betalains in plasma and urine of Volunteers after long-term exposure to fermented red beet juice. J. Agric. Food Chem. 2018, 66, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Spórna-Kucab, A.; Ignatova, S.; Garrard, I.; Wybraniec, S. Versatile solvent systems for the separation of betalains from processed Beta vulgaris L. juice using counter-current chromatography. J. Chromatogr. B 2013, 941, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hempel, J.; Böhm, H. Betaxanthin pattern of hairy roots from Beta vulgaris var. Lutea and its alteration by feeding of amino acids. Phytochemistry 1997, 44, 847–852. [Google Scholar] [CrossRef]
- Costa, A.P.D.; Hermes, V.S.; Rios, A.D.O.; Flôres, S.H. Minimally processed beetroot waste as an alternative source to obtain functional ingredients. J. Food Sci. Technol. 2017, 54, 2050–2058. [Google Scholar] [CrossRef]
- Kumar, S.; Brooks, M.S.L. Use of red beet (Beta vulgaris L.) for antimicrobial applications—A critical review. Food Bioprocess Technol. 2018, 11, 17–42. [Google Scholar] [CrossRef]
- Kuete, V. Potential of cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Čanadanović-Brunet, J.M.; Savatović, S.S.; Ćetković, G.S.; Vulić, J.J.; Djilas, S.M.; Markov, S.L.; Cvetković, D.D. Antioxidant and antimicrobial activities of beet root pomace extracts. Czech J. Food Sci. 2011, 29, 575–585. [Google Scholar] [CrossRef]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods. 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Lindsay, J.A.; Holden, M.T.G. Staphylococcus aureus: Superbug, super genome? Trends Microbiol. 2004, 12, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Prasastha Ram, V.; Venkateswara Rao, L.; Srinivasa Rao, T.; Subramanyam, K.V.; Srinivas, K. Prevalence and virulence gene profiles of Proteus mirabilis Isolated from animal, human and water samples in Krishna District, Andhra Pradesh, India. J. Pharm. Innov. 2019, 8, 19–23. [Google Scholar]
- Arivett, B.A.; Ream, D.C.; Fiester, S.E.; Mende, K.; Murray, C.K.; Thompson, M.G.; Kanduru, S.; Summers, A.M.; Roth, A.L.; Zurawski, D.V.; et al. Draft genome sequences of Klebsiella pneumoniae clinical type strain ATCC 13883 and three multidrug-resistant clinical isolates. Genome Announc. 2015, 3, e01385-14. [Google Scholar] [CrossRef] [PubMed]
- Panahi Chegini, P.; Nikokar, I.; Tabarzad, M.; Faezi, S.; Mahboubi, A. Effect of amino acid substitutions on biological activity of antimicrobial peptide: Design, recombinant production, and biological activity. Iran J. Pharm. Res. 2019, 18, 157–168. [Google Scholar] [PubMed]
- Nowak, M.G.; Skwarecki, A.S.; Milewska, M.J. Amino acid based antimicrobial agents—Synthesis and properties. ChemMedChem 2021, 16, 3513–3544. [Google Scholar] [CrossRef]
- Łaska, G.; Sieniawska, E.; Świątek, Ł.; Zjawiony, J.; Khan, S.; Boguszewska, A.; Stocki, M.; Angielczyk, M.; Polz-Dacewicz, M. Phytochemistry and biological activities of Polemonium caeruleum L. Phytochem. Lett. 2019, 30, 314–323. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Shumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Treat. Rep. 1972, 3, 1–103. [Google Scholar]
- Romero, S.A.; Pavan, I.C.B.; Morelli, A.P.; Mancini, M.C.S.; da Silva, L.G.S.; Fagundes, I.; Silva, C.H.R.; Ponte, L.G.S.; Rostagno, M.A.; Bezerra, R.M.N.; et al. Anticancer effects of root and beet leaf extracts (Beta vulgaris L.) in cervical cancer cells (HeLa). Phytother. Res. 2021, 35, 6191–6203. [Google Scholar] [CrossRef]
- Clement, Y.N.; Mahase, V.; Jagroop, A.; Kissoon, K.; Maharaj, A.; Mathura, P.; Quan, C.M.; Ramadhin, D.; Mohammed, C. Herbal remedies and functional foods used by cancer patients attending specialty oncology clinics in Trinidad. BMC Complement Altern. Med. 2016, 16, 399. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonini, E.; Frati, A.; Scarpa, E.-S. C-glycosyl flavonoids from Beta vulgaris Cicla and betalains from Beta vulgaris Rubra: Antioxidant, anticancer and antiinflammatory activities—A review. Phytother. Res. 2017, 31, 871–884. [Google Scholar]
- Kapadia, G.J.; Tokuda, H.; Konoshima, T.; Nishino, H. Chemoprevention of lung and skin cancer by Beta vulgaris (beet) root extract. Cancer Lett. 1996, 100, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.F.; Stoner, G.D. Red beetroot and betalains as cancer chemopreventative agents. Molecules 2019, 24, 1602. [Google Scholar] [CrossRef] [PubMed]
- Henarejos-Escudero, P.; Hernández-García, S.; Guerrero-Rubio, M.A.; García-Carmona, F.; Gandía-Herrero, F. Antitumoral drug potential of tryptophan-betaxanthin and related plant betalains in the caenorhabditis elegans tumoral model. Antioxidants 2020, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Kishimura, A.; Mori, T.; Katayama, Y. Evaluation of a synergistic effect of L-arginine on the anticancer activity of doxorubicin by using a co-culture system. Anal. Sci. 2020, 36, 1279–1283. [Google Scholar] [CrossRef]
- Kudo, S.; Nagasaki, Y. A Novel nitric oxide-based anticancer therapeutics by macrophage-targeted poly(l-arginine)-based nanoparticles. J. Control. Release 2015, 217, 256–262. [Google Scholar] [PubMed]
- Zare-Zardini, H.; Taheri-Kafrani, A.; Amiri, A.; Bordbar, A.K. New Generation of drug delivery systems based on ginsenoside Rh2-, lysine- and arginine-treated highly porous graphene for improving anticancer activity. Sci. Rep. 2018, 8, 586. [Google Scholar] [CrossRef]
- Alsalhi, A.; Ayon, N.J.; Coulibaly, F.M.; Alshamrani, M.; Al-Nafisah, A.; Youan, B.B.C. Enhancing etoposide aqueous solubility and anticancer activity with L-arginine. Assay Drug Dev. Technol. 2021, 19, 508–525. [Google Scholar] [CrossRef]
- Farhangfar, S.D.; Fesahat, F.; Zare-Zardini, H.; Dehghan-Manshadi, M.; Zare, F.; Miresmaeili, S.M.; Vajihinejad, M.; Soltaninejad, H. In vivo study of anticancer activity of ginsenoside Rh2-containing arginine-reduced graphene in a mouse model of breast cancer. Iran. J. Basic Med. Sci. 2022, 25, 1442–1451. [Google Scholar]
- Tutone, M.; Virzì, A.; Almerico, A.M. Reverse screening on indicaxanthin from Opuntia ficus-indica as natural chemoactive and chemopreventive agent. J. Theor. Biol. 2018, 455, 147–160. [Google Scholar] [CrossRef]
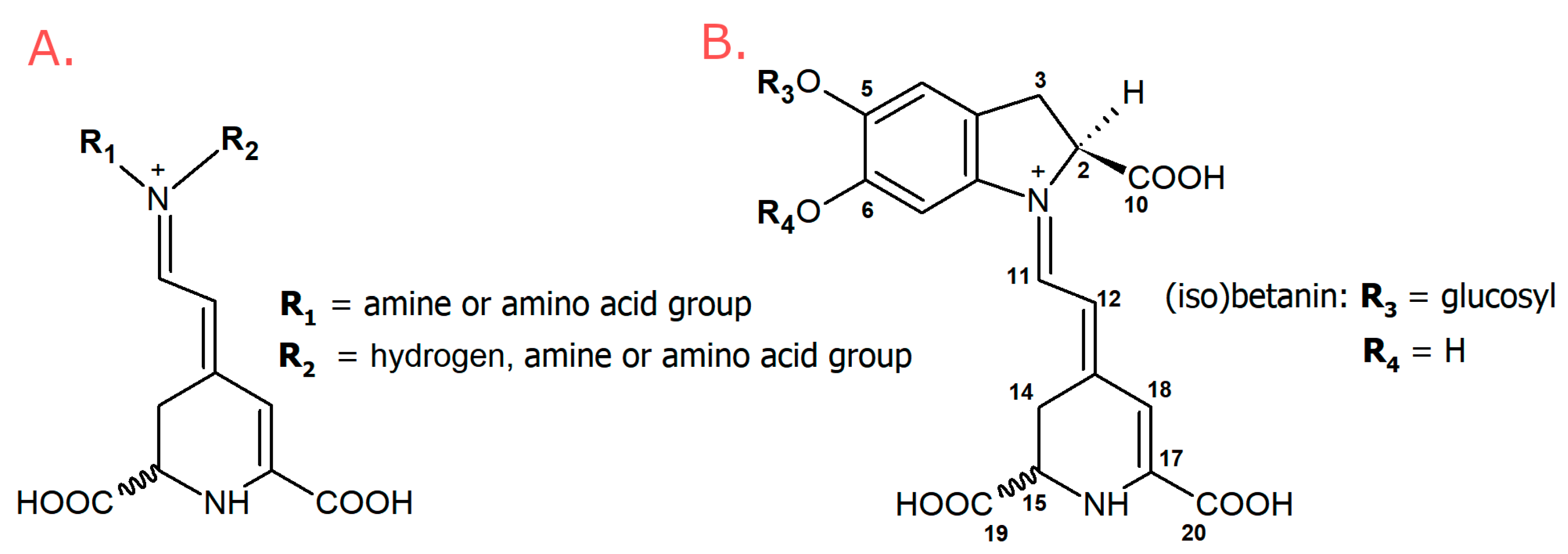
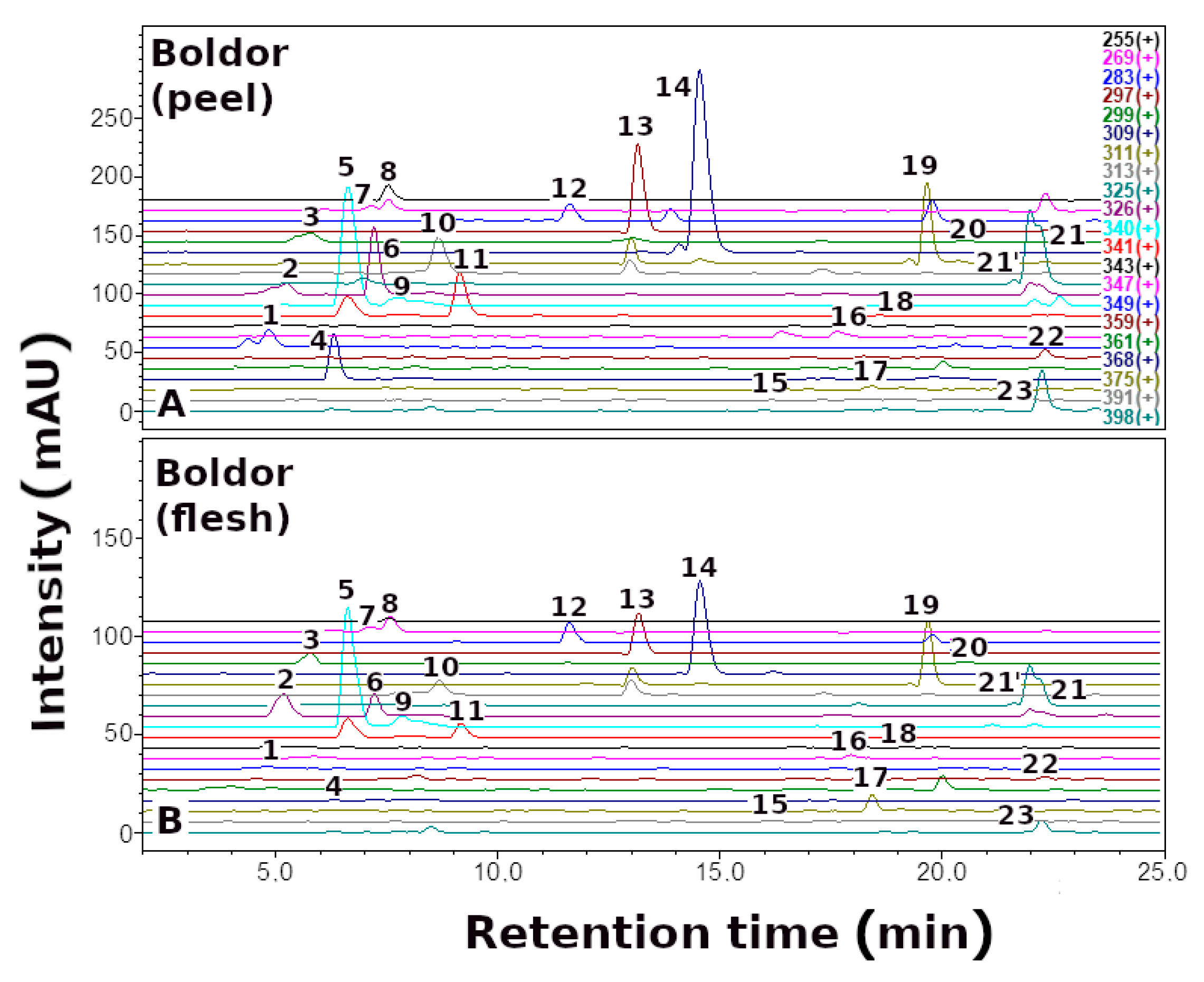
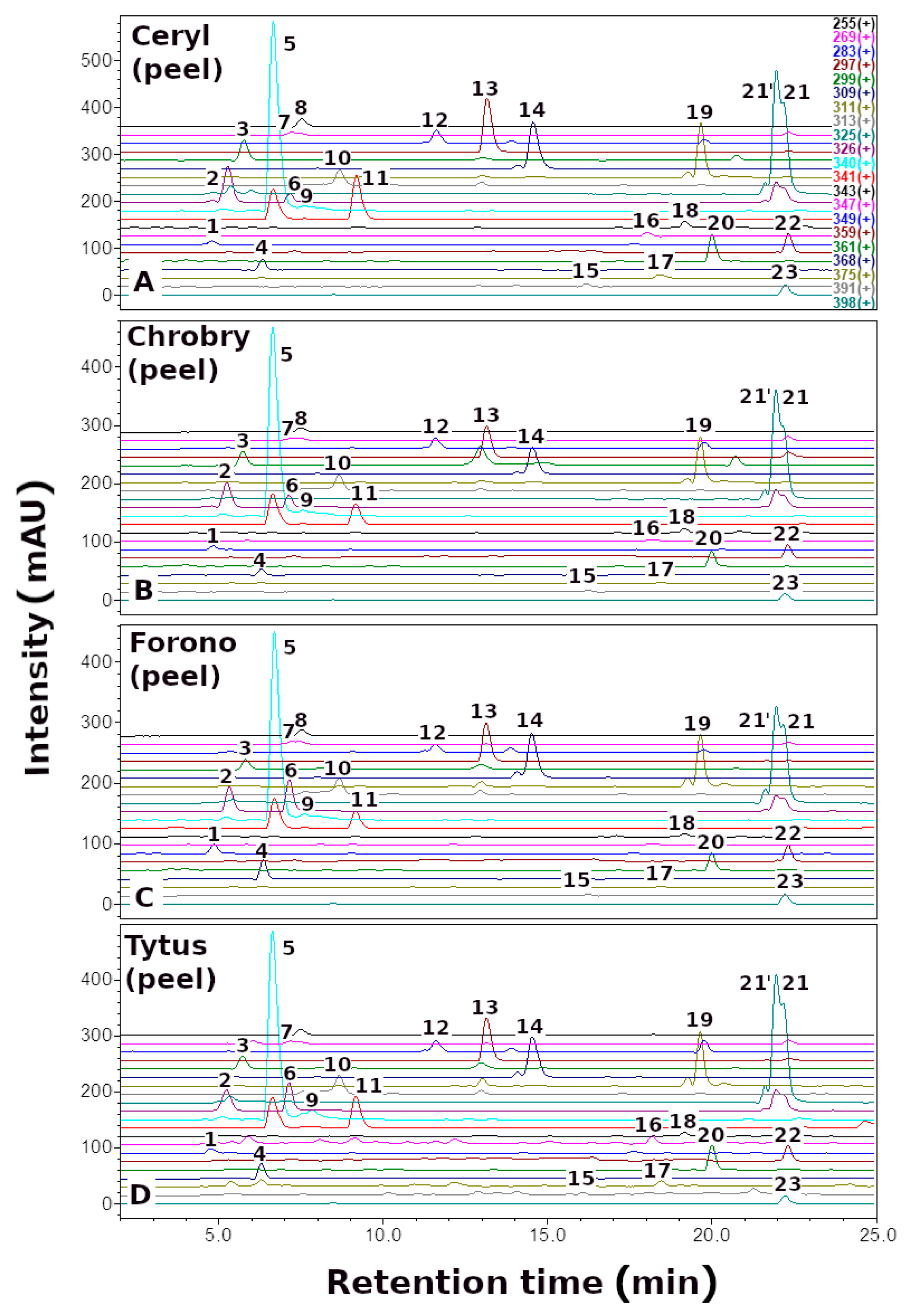
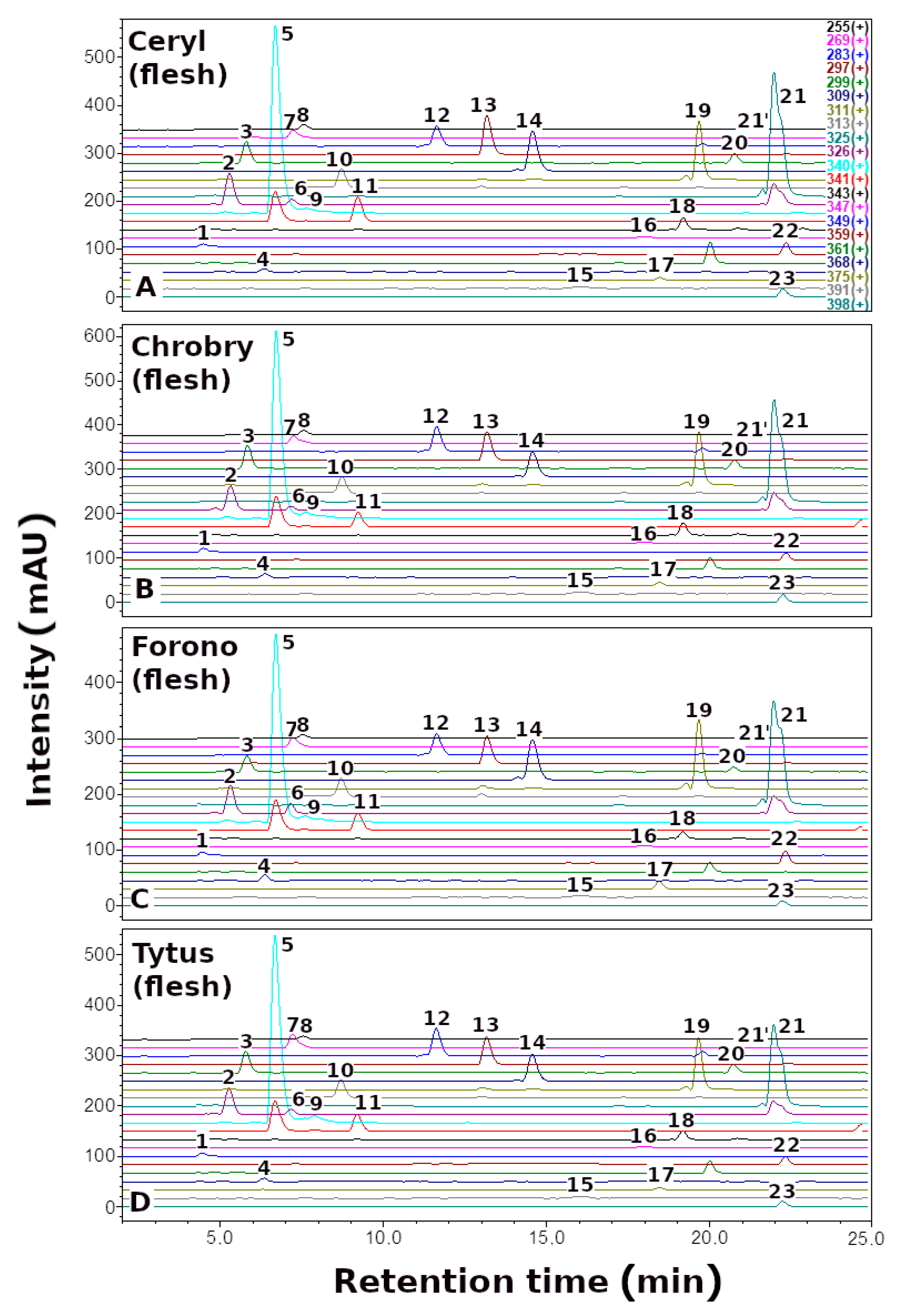
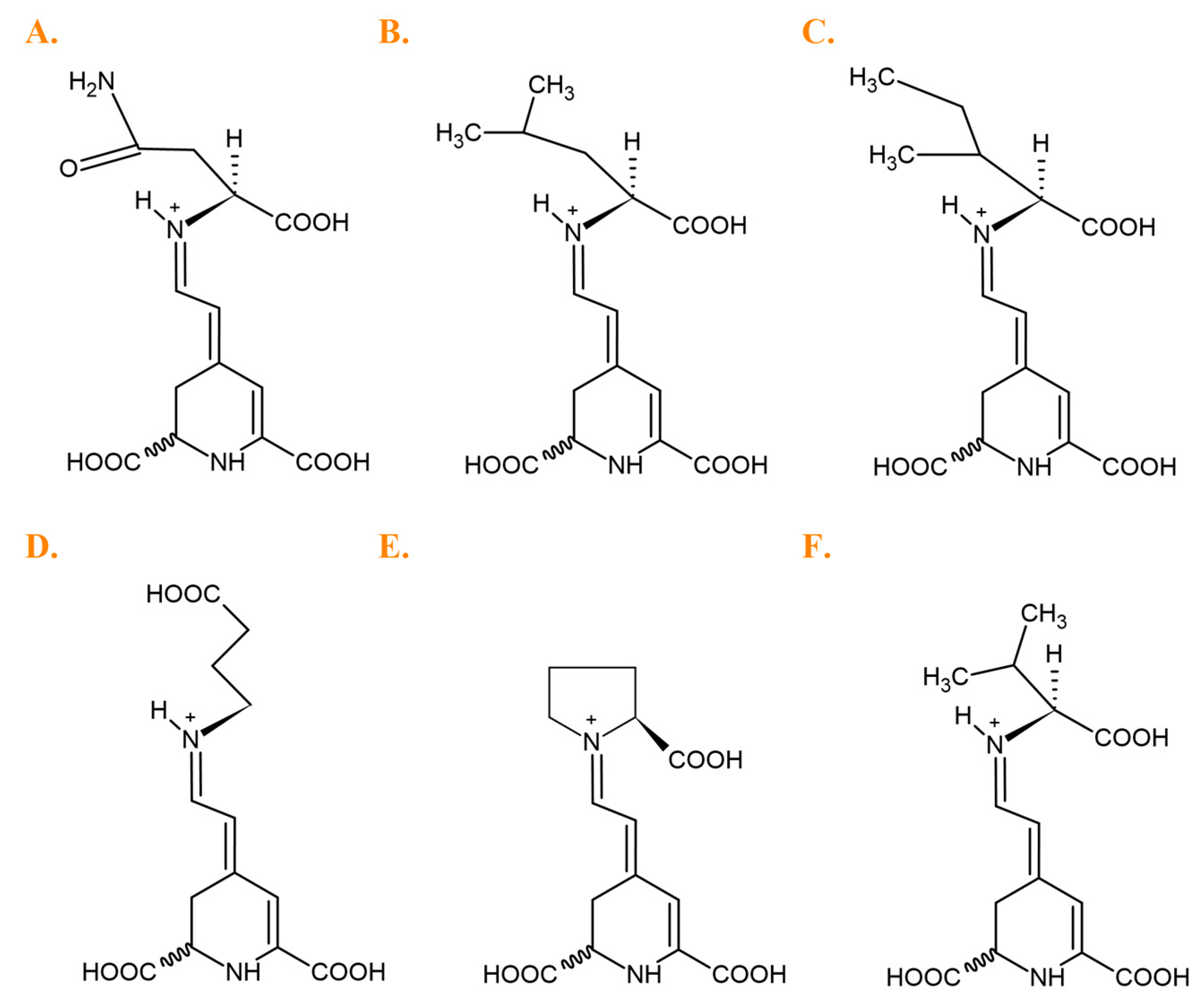
| No. | Betaxanthins | Trivial Name | tR (min) | λmax (nm) | m/z (M + H)+ |
|---|---|---|---|---|---|
| 1 | Histidine-Bx | muscaaurin VII | 4.4 | 470 | 349 |
| 2 | Asparagine-Bx | vulgaxanthin III | 5.1 | 468 | 326 |
| 3 | Serine-Bx | 5.7 | 469 | 299 | |
| 4 | Arginine-Bx | 6.3 | 468 | 368 | |
| 5 | Glutamine-Bx | vulgaxanthin I | 6.6 | 467 | 340 |
| 6 | Ornithine-Bx | 7.1 | 465 | 326 | |
| 7 | Glycine-Bx | 7.2 | 470 | 269 | |
| 8 | Ethanolamine-Bx | 7.5 | 454 | 255 | |
| 9 | Lysine-Bx | 7.6 | 458 | 340 | |
| 10 | Threonine-Bx | 8.7 | 469 | 313 | |
| 11 | Glutamic acid-Bx | vulgaxanthin II | 9.2 | 469 | 341 |
| 12 | Alanine-Bx | 11.5 | 466 | 283 | |
| 13 | γ-Aminobutyric acid-Bx | 11.6 | 454 | 297 | |
| 14 | Proline-Bx | indicaxanthin | 14.6 | 477 | 309 |
| 15 | Dopa-Bx | dopaxanthin | 16.1 | 470 | 391 |
| 16 | Dopamina-Bx | miraxanthin V | 18.2 | 460 | 347 |
| 17 | Tyrosine-Bx | portulacaxanthin II | 18.4 | 471 | 375 |
| 18 | Methionine-Bx | 19.2 | 468 | 343 | |
| 19 | Valine-Bx | 19.6 | 469 | 311 | |
| 20 | 3-methoxytyramine-Bx | 20.0 | 471 | 361 | |
| 21 | Isoleucine-Bx | 22.0 | 469 | 325 | |
| 21 | Leucine-Bx | vulgaxanthin IV | 22.2 | 469 | 325 |
| 22 | Phenylalanine-Bx | 22.3 | 469 | 359 | |
| 23 | Tryptophan-Bx | vulgaxanthin IV | 22.3 | 473 | 398 |
| Mass of Betaxanthins (mg) in 100 g of Dry Extract (DE) Beta vulgaris L. | ||||||
|---|---|---|---|---|---|---|
| Red Cultivar | Yellow Cultivar | |||||
| Ceryl | Chrobry | Forono | Tytus | Boldor | ||
| No. | Betaxanthins | |||||
| 1 | Histidine-Bx | 0.15 ± 0.01 e | 1.06 ± 0.08 c | 0.88 ± 0.05 d | 1.87 ± 0.14 b | 1.93 ± 0.14 a |
| 2 | Asparagine-Bx | 42.52 ± 2.6 a | 26.91 ± 1.8 b | 24.40 ± 1.5 c | 42.52 ± 2.6 a | 11.12 ± 0.80 d |
| 3 | Serine-Bx | 23.81 ± 1.8 a | 16.28 ± 1.3 c | 9.72 ± 0.74 d | 19.64 ± 1.7 b | 5.94 ± 0.47 e |
| 4 | Arginine-Bx | 10.35 ± 0.68 d | 5.64 ± 0.37 e | 15.61 ± 1.0 c | 19.50 ± 1.5 b | 23.71 ± 1.5 a |
| 5 | Glutamine-Bx | 264.95 ± 0.88 b | 257.82 ± 15 c | 208.23 ± 16 d | 350.82 ± 21 a | 93.35 ± 6.1 e |
| 6 | Ornithine-Bx | 8.68 ± 0.57 e | 11.99 ± 0.79 d | 27.87 ± 1.7 c | 39.69 ± 2.7 a | 36.31 ± 2.4 b |
| 7 | Glycine-Bx | 3.28 ± 0.24 b | 1.91 ± 0.12 d | 2.51 ± 0.17 c | 7.72 ± 0.5 a | 1.64 ± 0.14 e |
| 8 | Ethanolamine-Bx | 10.07 ± 0.68 b | 5.26 ± 0.34 e | 6.55 ± 1.1 d | 10.83 ± 0.8 a | 8.53 ± 0.60 c |
| 9 | Lysine-Bx | 1.26 ± 0.11 c | 0.80 ± 0.061 e | 0.96 ± 0.06 d | 12.41 ± 0.87 a | 3.10 ± 0.21 b |
| 10 | Threonine-Bx | 12.17 ± 0.81 e | 20.58 ± 1.4 c | 15.88 ± 1.0 d | 35.43 ± 2.4 a | 20.70 ± 1.5 b |
| 11 | Glutamic acid-Bx | 56.49 ± 4.8 a | 25.46 ± 1.7 c | 18.25 ± 1.3 d | 56.54 ± 3.5 a | 28.09 ± 1.8 b |
| 12 | Alanine-Bx | 14.23 ± 0.91 b | 10.87 ± 0.82 c | 9.40 ± 0.68 e | 21.59 ± 1.8 a | 10.57 ± 0.8 d |
| 13 | γ-Aminobutyric acid-Bx | 66.03 ± 5.1 b | 35.14 ± 2.1 e | 36.88 ± 2.8 d | 70.06 ± 4.8 a | 50.32 ± 3.1 c |
| 14 | Proline-Bx | 65.79 ± 5.4 c | 35.70 ± 2.3 e | 52.97 ± 4.1 d | 82.53 ± 5.2 b | 140.95 ± 10a |
| 15 | Dopa-Bx | 0.21 ± 0.01 a | 0.11 ± 0.008 b | 0.05 ± 0.004 c | 0.30 ± 0.02 a | 0.03 ± 0.002 c |
| 16 | Dopamine-Bx | 0.13 ± 0.009 d | 0.21 ± 0.014 c | 0.04 ± 0.003 e | 2.36 ± 0.17 a | 0.39 ± 0.03 b |
| 17 | Tyrosine-Bx | 0.10 ± 0.006 e | 1.01 ± 0.074 c | 1.08 ± 0.07 b | 1.65 ± 0.12 a | 0.25 ± 0.02 d |
| 18 | Methionine-Bx | 7.81 ± 0.63 a | 3.85 ± 0.25 c | 3.05 ± 0.23 d | 4.57 ± 0.32 b | 0.99 ± 0.06 e |
| 19 | Valine-Bx | 55.61 ± 4.6 b | 45.22 ± 2.8 d | 45.52 ± 2.8 c | 74.29 ± 4.5 a | 40.69 ± 2.8 e |
| 20 | 3-methoxytyramine-Bx | 27.82 ± 1.8 b | 14.52 ± 1.3 d | 15.47 ± 1.3 c | 34.14 ± 2.6 a | 3.40 ± 0.26 e |
| 21′ | Isoleucine-Bx | 115.09 ± 7.1 b | 102.17 ± 6.4 c | 74.83 ± 5.4 d | 159.96 ± 10 a | 34.14 ± 2.3 e |
| 21 | Leucine-Bx | 100.32 ± 6.8 b | 74.91 ± 5.2 c | 74.38 ± 4.8 d | 150.47 ± 9.7 a | 32.62 ± 2.1 e |
| 22 | Phenylalanine-Bx | 20.58 ± 1.5 a | 13.86 ± 0.8 c | 15.00 ± 1.0 b | 20.75 ± 1.5 a | 4.16 ± 0.35 d |
| 23 | Tryptophan-Bx | 12.05 ± 0.83 c | 7.28 ± 0.57 e | 9.75 ± 0.78 d | 12.23 ± 0.78 b | 21.37 ± 1.4 a |
| Total concentration | 919 ± 52 b | 718 ± 36 c | 669 ± 28 d | 1231 ± 64 a | 574 ± 26 e | |
| Mass of Betaxanthins (mg) in 100 g of Dry Extract (DE) Beta vulgaris L. | ||||||
|---|---|---|---|---|---|---|
| Red Cultivar | Yellow Cultivar | |||||
| Ceryl | Chrobry | Forono | Tytus | Boldor | ||
| No. | Betaxanthins | |||||
| 1 | Histidine-Bx | 1.56 ± 0.09 c | 2.84 ± 0.21 a | 1.85 ± 0.16 b | 0.07 ± 0.005 e | 0.38 ± 0.02 d |
| 2 | Asparagine-Bx | 24.64 ± 1.8 a | 2.84 ± 0.18 e | 21.33 ± 1.4 c | 21.37 ± 1.5 b | 13.84 ± 0.90 d |
| 3 | Serine-Bx | 16.92 ± 1.1 c | 22.05 ± 1.7 a | 12.90 ± 0.84 d | 18.19 ± 1.2 b | 7.53 ± 0.55 e |
| 4 | Arginine-Bx | 2.11 ± 0.16 c | 2.80 ± 0.22 b | 3.71 ± 0.25 a | 2.31 ± 0.20 c | 0.51 ± 0.03 d |
| 5 | Glutamine-Bx | 179.79 ± 11 c | 219.64 ± 15 a | 172.67 ± 12 d | 190.90 ± 12 b | 80.10 ± 6.1 e |
| 6 | Ornithine-Bx | 3.84 ± 0.34 c | 3.24 ± 0.24 d | 6.81 ± 0.44 b | 3.91 ± 0.30 c | 11.37 ± 0.80 a |
| 7 | Glycine-Bx | 7.47 ± 0.56 c | 7.23 ± 0.44 d | 8.37 ± 0.68 b | 13.59 ± 0.95 a | 1.25 ± 0.08 e |
| 8 | Ethanolamine-Bx | 4.46 ± 0.27 b | 4.80 ± 0.29 a | 3.28 ± 0.26 c | 2.81 ± 0.21 d | 1.77 ± 0.12 e |
| 9 | Lysine-Bx | 0.61 ± 0.05 c | 0.35 ± 0.02 e | 0.49 ± 0.04 d | 4.91 ± 0.32 a | 4.25 ± 0.32 b |
| 10 | Threonine-Bx | 15.55 ± 1.1 d | 17.62 ± 1.2 a | 16.12 ± 1.4 c | 16.29 ± 1.2 b | 8.41 ± 0.65 e |
| 11 | Glutamic acid-Bx | 21.53 ± 1.4 a | 14.49 ± 0.98 d | 14.95 ± 1.2 c | 15.32 ± 0.97 b | 8.27 ± 0.58 e |
| 12 | Alanine-Bx | 15.90 ± 0.98 d | 24.96 ± 1.6 a | 16.65 ± 1.3 c | 23.46 ± 1.6 b | 11.34 ± 0.76 e |
| 13 | γ-Aminobutyric acid-Bx | 32.88 ± 2.3 a | 29.47 ± 2.0 b | 22.31 ± 1.6 d | 23.93 ± 1.7 c | 22.09 ± 0.22 e |
| 14 | Proline -Bx | 40.13 ± 2.6 c | 29.63 ± 2.0 d | 41.73 ± 2.6 b | 26.82 ± 1.9 e | 62.47 ± 4.2 a |
| 15 | Dopa-Bx | 0.08 ± 0.006 d | 0.21 ± 0.02 c | 0.34 ± 0.03 b | 0.45 ± 0.03 a | 0.20 ± 0.02 c |
| 16 | Dopamine-Bx | 0.12 ± 0.008 c | 0.24 ± 0.02 b | 0.10 ± 0.006 c | 0.12 ± 0.008 c | 0.65 ± 0.04 a |
| 17 | Tyrosine-Bx | 1.87 ± 0.15 d | 3.04 ± 0.24 c | 6.02 ± 0.43 b | 1.71 ± 0.14 d | 7.71 ± 0.52 a |
| 18 | Methionine-Bx | 8.66 ± 0.59 b | 11.50 ± 0.7 a | 5.08 ± 0.37 d | 6.17 ± 0.50 c | 0.14 ± 0.009 e |
| 19 | Valine-Bx | 42.70 ± 2.7 c | 48.81 ± 3.5 b | 50.66 ± 3.7 a | 39.35 ± 2.8 d | 30.69 ± 2.2 e |
| 20 | 3-methoxytyramine-Bx | 16.00 ± 0.98 a | 9.61 ± 0.74 b | 6.70 ± 0.5 d | 9.42 ± 0.57 c | 6.66 ± 0.44 d |
| 21′ | Isoleucine-Bx | 83.54 ± 5.8 a | 80.24 ± 5.4 b | 68.30 ± 4.1 c | 55.96 ± 3.6 d | 17.49 ± 1.3 e |
| 21 | Leucine-Bx | 55.17 ± 3.7 b | 59.86 ± 3.7 a | 55.08 ± 3.5 c | 41.21 ± 3.2 d | 13.17 ± 0.87 e |
| 22 | Phenylalanine-Bx | 8.95 ± 0.77 b | 6.80 ± 0.54 c | 9.30 ± 0.61 a | 6.21 ± 0.43 d | 1.23 ± 0.09 e |
| 23 | Tryptophan-Bx | 6.43 ± 0.55 b | 7.33 ± 0.61 a | 3.95 ± 0.30 e | 4.46 ± 0.31 d | 5.89 ± 0.47 c |
| Total concentration | 590 ± 32 b | 609 ± 40 a | 548 ± 28 c | 528 ± 34 d | 317 ± 22 e | |
| Gram-Positive Bacteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Microorganism/ Extract | Staphylococcus aureus ATCC 29213 | Staphylococcus aureus ATCC 25923 | Staphylococcus aureus ATCC 43300 | Staphylococcus aureus ATCC BAA-1707 | Staphylococcus epidermidis ATCC 12228 | Entarococcus faecalis ATCC 29212 | Micrococcus luteus ATCC 10240 | Bacillus subtilis ATCC 6633 | Bacillus cereus ATCC 10876 | |
| MIC;MBC | ||||||||||
| Ceryl | Peel | 1;4 | 0.06;0.5 | 4;4 | 4;4 | 4;4 | 1;8 | 0.06;0.06 | 0.125;8 | 16;16 |
| Flesh | 16;16 | 16;16 | 16;16 | 16;16 | 4;4 | 4;8 | 4;8 | 16;16 | 16;16 | |
| Chrobry | Peel | 0.125;8 | 0.125;4 | 0.25;4 | 0.25;8 | 0.25;1 | 0.5;8 | 0.125;0.25 | 0.125;8 | 1;8 |
| Flesh | 16;16 | 16;16 | 16;16 | 16;16 | 4;4 | 16;16 | 2;8 | 16;16 | 16;16 | |
| Forono | Peel | 16;16 | 4;4 | 4;8 | 4;4 | 2;4 | 16;16 | 0.06;0.06 | 0.06;8 | 4;8 |
| Flesh | 16;16 | 4;4 | 16;16 | 16;16 | 2;4 | 16;16 | 1;2 | 0.125;8 | 8;8 | |
| Tytus | Peel | 0.25;8 | 0.25;8 | 0.25;4 | 0.5;8 | 0.25;8 | 0.5;8 | 0.125;0.25 | 16;16 | 16;16 |
| Flesh | 16;16 | 16;16 | 16;16 | 16;16 | 4;4 | 4;8 | 0.5;0.5 | 16;16 | 16;16 | |
| Boldor | Peel | 16;16 | 16;16 | 16;16 | 16;16 | 16;16 | 4;8 | 4;8 | 16;16 | 4;8 |
| Flesh | 16;16 | 16;16 | 16;16 | 16;16 | 16;16 | 4;8 | 2;4 | 16;16 | 16;16 | |
| Correlation | ||||||||||
| No. | Betaxanthins | |||||||||
| 1 | Histidine-Bx | 0.274 | 0.274 | 0.307 | 0.308 | −0.306 | 0.597 | 0.328 | 0.127 | 0.223 |
| 2 | Asparagine-Bx | −0.453 | −0.543 | −0.413 | −0.414 | −0.629 | −0.287 | −0.398 | −0.451 | −0.049 |
| 3 | Serine-Bx | −0.086 | 0.009 | 0.078 | 0.077 | −0.592 | 0.109 | −0.131 | −0.029 | 0.362 |
| 4 | Arginine-Bx | −0.368 | −0.587 | −0.646 | −0.645 | −0.259 | 0.026 | −0.349 | −0.363 | −0.459 |
| 5 | Glutamine-Bx | −0.307 | −0.346 | −0.276 | −0.276 | −0.870 | 0.134 | −0.407 | −0.275 | 0.159 |
| 6 | Ornithine-Bx | −0.307 | −0.547 | −0.651 | −0.648 | −0.281 | 0.046 | −0.344 | −0.236 | −0.376 |
| 7 | Glycine-Bx | 0.343 | 0.326 | 0.453 | 0.455 | −0.383 | 0.206 | −0.001 | 0.253 | 0.527 |
| 8 | Ethanolamine-Bx | −0.513 | −0.524 | −0.510 | −0.511 | −0.574 | −0.075 | −0.300 | −0.352 | −0.091 |
| 9 | Lysine-Bx | −0.304 | −0.108 | −0.240 | −0.233 | −0.236 | −0.416 | −0.347 | 0.411 | 0.450 |
| 10 | Threonine-Bx | −0.124 | −0.166 | −0.108 | −0.105 | −0.818 | 0.197 | −0.184 | 0.015 | 0.253 |
| 11 | Glutamic acid-Bx | −0.655 | −0.554 | −0.504 | −0.504 | −0.508 | −0.368 | −0.340 | −0.308 | 0.002 |
| 12 | Alanine-Bx | 0.234 | 0.268 | 0.363 | 0.364 | −0.479 | 0.315 | −0.023 | 0.215 | 0.526 |
| 13 | γ-Aminobutyric acid-Bx | −0.507 | −0.459 | −0.437 | −0.438 | −0.586 | −0.177 | −0.274 | −0.283 | 0.026 |
| 14 | Proline-Bx | −0.178 | −0.314 | −0.172 | −0.171 | −0.223 | −0.019 | 0.113 | −0.240 | −0.235 |
| 15 | Dopa-Bx | 0.135 | 0.065 | 0.296 | 0.297 | −0.380 | 0.182 | −0.329 | 0.028 | 0.373 |
| 16 | Dopamine-Bx | −0.515 | −0.318 | −0.433 | −0.424 | −0.279 | −0.327 | −0.222 | 0.356 | 0.384 |
| 17 | Tyrosine-Bx | 0.451 | 0.123 | 0.518 | 0.519 | −0.219 | 0.643 | 0.067 | −0.091 | 0.247 |
| 18 | Methionine-Bx | 0.171 | 0.248 | 0.306 | 0.305 | −0.426 | 0.288 | 0.153 | 0.130 | 0.443 |
| 19 | Valine-Bx | −0.097 | −0.256 | −0.052 | −0.052 | −0.789 | 0.321 | −0.238 | −0.259 | 0.193 |
| 20 | 3-methoxytyramine-Bx | −0.552 | −0.483 | −0.515 | −0.514 | −0.652 | −0.265 | −0.338 | −0.233 | 0.152 |
| 21′ | Isoleucine-Bx | −0.426 | −0.435 | −0.359 | −0.359 | −0.840 | 0.015 | −0.306 | −0.280 | 0.141 |
| 21 | Leucine-Bx | −0.527 | −0.608 | −0.528 | −0.527 | −0.857 | 0.032 | −0.474 | −0.373 | 0.060 |
| 22 | Phenylalanine-Bx | −0.555 | −0.715 | −0.607 | −0.609 | −0.774 | −0.029 | −0.541 | −0.614 | −0.181 |
| 23 | Tryptophan-Bx | −0.273 | −0.241 | −0.312 | −0.313 | −0.307 | −0.017 | 0.015 | −0.192 | −0.212 |
| Gram-Negative Bacteria | |||||||
|---|---|---|---|---|---|---|---|
| Microorganism/ Extract | Salmonella Typhimurium ATCC 14028 | Proteus mirabilis ATCC 12453 | Bordetella bronchiseptica ATCC 4617 | Escherichia coli ATCC 25922 | Klebsiella pneumoniae ATCC 13883 | Pseudomonas aeruginosa ATCC 27853 | |
| MIC;MBC | |||||||
| Ceryl | Peel | 16;16 | 16;16 | 2;8 | 4;8 | 16;16 | 4;8 |
| Flesh | 16;16 | 16;16 | 16;16 | 16;16 | 16;16 | 4;8 | |
| Chrobry | Peel | 4;8 | 8;8 | 2;4 | 4;4 | 16;16 | 2;8 |
| Flesh | 16;16 | 16;16 | 4;8 | 16;16 | 16;16 | 4;8 | |
| Forono | Peel | 16;16 | 16;16 | 2;4 | 4;4 | 16;16 | 4;4 |
| Flesh | 16;16 | 16;16 | 8;8 | 16;16 | 16;16 | 4;8 | |
| Tytus | Peel | 16;16 | 16;16 | 4;4 | 4;8 | 16;16 | 4;8 |
| Flesh | 16;16 | 16;16 | 4;8 | 4;8 | 16;16 | 4;8 | |
| Boldor | Peel | 16;16 | 16;16 | 8;8 | 16;16 | 16;16 | 4;8 |
| Flesh | 16;16 | 32;32 | 16;16 | 16;16 | 32;32 | 4;8 | |
| Correlation | |||||||
| No. | Betaxanthins | ||||||
| 1 | Histidine-Bx | 0.096 | −0.34 | 0.092 | 0.540 | −0.343 | 0.096 |
| 2 | Asparagine-Bx | −0.025 | −0.46 | −0.228 | −0.538 | −0.462 | −0.025 |
| 3 | Serine-Bx | 0.068 | −0.51 | −0.246 | −0.129 | −0.515 | 0.068 |
| 4 | Arginine-Bx | 0.137 | −0.48 | −0.585 | −0.531 | −0.477 | 0.137 |
| 5 | Glutamine-Bx | −0.084 | −0.67 | −0.474 | −0.403 | −0.673 | −0.084 |
| 6 | Ornithine-Bx | 0.041 | −0.34 | −0.452 | −0.495 | −0.344 | 0.041 |
| 7 | Glycine-Bx | 0.279 | −0.36 | 0.012 | 0.023 | −0.359 | 0.279 |
| 8 | Ethanolamine-Bx | 0.099 | −0.63 | −0.501 | −0.442 | −0.632 | 0.099 |
| 9 | Lysine-Bx | 0.194 | −0.14 | −0.233 | −0.508 | −0.138 | 0.194 |
| 10 | Threonine-Bx | −0.055 | −0.73 | −0.320 | −0.205 | −0.731 | −0.055 |
| 11 | Glutamic acid-Bx | 0.042 | −0.48 | −0.380 | −0.488 | −0.485 | 0.042 |
| 12 | Alanine-Bx | 0.232 | −0.45 | −0.140 | 0.053 | −0.450 | 0.232 |
| 13 | γ-Aminobutyric acid -Bx | 0.102 | −0.61 | −0.414 | −0.440 | −0.609 | 0.102 |
| 14 | Proline-Bx | 0.345 | −0.66 | −0.202 | −0.091 | −0.660 | 0.345 |
| 15 | Dopa-Bx | 0.205 | −0.28 | −0.240 | −0.146 | −0.281 | 0.205 |
| 16 | Dopamine-Bx | 0.094 | −0.10 | −0.145 | −0.263 | −0.103 | 0.094 |
| 17 | Tyrosine-Bx | 0.202 | −0.02 | 0.252 | 0.516 | −0.021 | 0.202 |
| 18 | Methionine-Bx | 0.188 | −0.42 | −0.019 | 0.182 | −0.421 | 0.188 |
| 19 | Valine-Bx | 0.117 | −0.68 | −0.310 | −0.147 | −0.678 | 0.117 |
| 20 | 3-methoxytyramine-Bx | 0.051 | −0.47 | −0.299 | −0.511 | −0.470 | 0.051 |
| 21′ | Isoleucine-Bx | −0.082 | −0.65 | −0.348 | −0.335 | −0.645 | −0.082 |
| 21 | Leucine-Bx | −0.011 | −0.65 | −0.499 | −0.481 | −0.647 | −0.011 |
| 22 | Phenylalanine-Bx | −0.082 | −0.58 | −0.509 | −0.576 | −0.576 | −0.082 |
| 23 | Tryptophan-Bx | 0.155 | −0.69 | −0.449 | −0.264 | −0.694 | 0.155 |
| Fungal (Yeasts) Strains | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Microorganism/ Extract | Candida parapsilosis ATCC 22019 | Candida albicans ATCC 10231 | Candida albicans ATCC 2091 | Candida glabrata ATCC 90030 | Candida krusei ATCC 14243 | Candida auris CDC B11903 | Candida lusitaniae ATCC 3449 | Candida tropicalis ATCC 1369 | |
| MIC;MFC | |||||||||
| Ceryl | Peel | 0.25;1 | 0.25;1 | 0.25;1 | 0.5;2 | 0.5;4 | 0.5;1 | 1;4 | 1;4 |
| Flesh | 4;8 | 4;8 | 4;8 | 4;8 | 4;8 | 4;8 | 4;4 | 4;4 | |
| Chrobry | Peel | 2;8 | 1;8 | 1;8 | 4;8 | 2;8 | 1;8 | 2;8 | 2;8 |
| Flesh | 4;4 | 4;8 | 4;8 | 4;8 | 4;8 | 4;8 | 4;4 | 4;4 | |
| Forono | Peel | 0.5;2 | 1;4 | 1;4 | 4;4 | 1;4 | 2;4 | 2;4 | 4;4 |
| Flesh | 4;8 | 4;8 | 4;8 | 8;8 | 4;4 | 8;8 | 8;4 | 8;4 | |
| Tytus | Peel | 0.25;2 | 0.25;2 | 0.25;2 | 1;4 | 0.25;0.5 | 0.5;2 | 0.125;4 | 0.25;4 |
| Flesh | 8;8 | 4;4 | 4;4 | 8;8 | 4;4 | 8;8 | 4;4 | 4;4 | |
| Boldor | Peel | 4;8 | 4;8 | 4;8 | 4;8 | 4;8 | 4;8 | 4;8 | 2;4 |
| Flesh | 4;4 | 4;4 | 4;4 | 4;8 | 4;4 | 4;4 | 4;4 | 4;4 | |
| Correlation | |||||||||
| No. | Betaxanthins | ||||||||
| 1 | Histidine-Bx | 0.066 | 0.066 | 0.344 | 0.149 | 0.335 | 0.194 | 0.405 | 0.410 |
| 2 | Asparagine-Bx | −0.279 | −0.494 | −0.494 | −0.165 | −0.497 | −0.168 | −0.260 | −0.126 |
| 3 | Serine-Bx | 0.181 | 0.019 | 0.019 | 0.059 | 0.024 | 0.140 | 0.020 | 0.091 |
| 4 | Arginine-Bx | −0.719 | −0.717 | −0.717 | −0.513 | −0.780 | −0.563 | −0.572 | −0.470 |
| 5 | Glutamine-Bx | −0.109 | −0.308 | −0.308 | −0.033 | −0.299 | −0.062 | −0.164 | 0.010 |
| 6 | Ornithine-Bx | −0.653 | −0.648 | −0.648 | −0.378 | −0.706 | −0.497 | −0.520 | −0.381 |
| 7 | Glycine-Bx | 0.678 | 0.441 | 0.441 | 0.621 | 0.411 | 0.703 | 0.418 | 0.451 |
| 8 | Ethanolamine-Bx | −0.590 | −0.631 | −0.631 | −0.588 | −0.661 | −0.526 | −0.517 | −0.418 |
| 9 | Lysine-Bx | 0.105 | −0.240 | −0.240 | −0.056 | −0.299 | 0.012 | −0.386 | −0.391 |
| 10 | Threonine-Bx | 0.096 | −0.065 | −0.065 | 0.198 | −0.069 | 0.156 | 0.029 | 0.128 |
| 11 | Glutamic acid-Bx | −0.495 | −0.627 | −0.627 | −0.573 | −0.641 | −0.474 | −0.511 | −0.475 |
| 12 | Alanine-Bx | 0.500 | 0.338 | 0.338 | 0.402 | 0.316 | 0.497 | 0.297 | 0.343 |
| 13 | γ-Aminobutyric acid-Bx | −0.460 | −0.558 | −0.558 | −0.517 | −0.583 | −0.432 | −0.473 | −0.391 |
| 14 | Proline-Bx | −0.395 | −0.299 | −0.299 | −0.338 | −0.362 | −0.208 | −0.143 | −0.201 |
| 15 | Dopa-Bx | 0.575 | 0.267 | 0.267 | 0.604 | 0.248 | 0.675 | 0.393 | 0.413 |
| 16 | Dopamine-Bx | −0.358 | −0.412 | −0.412 | −0.459 | −0.465 | −0.381 | −0.489 | −0.506 |
| 17 | Tyrosine-Bx | 0.387 | 0.538 | 0.538 | 0.666 | 0.522 | 0.709 | 0.836 | 0.883 |
| 18 | Methionine-Bx | 0.258 | 0.255 | 0.255 | 0.110 | 0.245 | 0.224 | 0.187 | 0.236 |
| 19 | Valine-Bx | −0.025 | −0.104 | −0.104 | 0.116 | −0.123 | 0.149 | 0.116 | 0.255 |
| 20 | 3-methoxytyramine-Bx | −0.483 | −0.611 | −0.611 | −0.535 | −0.636 | −0.481 | −0.553 | −0.402 |
| 21′ | Isoleucine-Bx | −0.298 | −0.400 | −0.400 | −0.236 | −0.395 | −0.234 | −0.243 | −0.097 |
| 21 | Leucine-Bx | −0.488 | −0.593 | −0.593 | −0.362 | −0.611 | −0.362 | −0.382 | −0.200 |
| 22 | Phenylalanine-Bx | −0.577 | −0.693 | −0.693 | −0.381 | −0.697 | −0.423 | −0.406 | −0.201 |
| 23 | Tryptophan-Bx | −0.447 | −0.426 | −0.426 | −0.534 | −0.458 | −0.463 | −0.447 | −0.444 |
| Extract | VERO | RKO | HeLa | |||
|---|---|---|---|---|---|---|
| CC50 | CC50 | SI | CC50 | SI | ||
| Ceryl | Peel | 1992.00 ± 249.10 | 717.20 ± 79.32 | 2.78 | 225.30 ± 21.28 | 8.84 |
| Flesh | 3432.04 ± 631.00 | 2100.23 ± 352.70 | 1.63 | 367.92 ± 33.30 | 9.33 | |
| Chrobry | Peel | 3330.00 ± 865.15 | 1275.75 ± 124.20 | 2.61 | 354.80 ± 22.64 | 9.39 |
| Flesh | 2896.15 ± 533.50 | 1470.35 ± 139.70 | 1.97 | 312.21 ± 19.80 | 9.28 | |
| Forono | Peel | 361.10 ± 42.17 | 674.20 ± 51.81 | 0.54 | 290.23 ± 20.58 | 1.24 |
| Flesh | 1754.31 ± 253.72 | 993.10 ± 76.91 | 1.77 | 328.10 ± 18.56 | 5.35 | |
| Tytus | Peel | 405.40 ± 36.47 | 1259.00 ± 101.90 | 0.32 | 420.90 ± 29.37 | 0.96 |
| Flesh | 908.45 ± 110.90 | 1479.66 ± 155.60 | 0.61 | 282.38 ± 25.24 | 3.22 | |
| Boldor | Peel | 527.70 ± 35.85 | 413.80 ± 26.25 | 1.28 | 274.74 ± 22.14 | 1.92 |
| Flesh | 4948.60 ± 1440.00 | 2090.00 ± 98.99 | 2.37 | 980.70 ± 74.37 | 5.05 | |
| RKO | HeLa | ||
|---|---|---|---|
| No. | Betaxanthins | ||
| 1 | Histidine-Bx | 0.177 | −0.242 |
| 2 | Asparagine-Bx | −0.080 | −0.453 |
| 3 | Serine-Bx | 0.211 | −0.520 |
| 4 | Arginine-Bx | −0.771 | −0.490 |
| 5 | Glutamine-Bx | 0.044 | −0.628 |
| 6 | Ornithine-Bx | −0.575 | −0.274 |
| 7 | Glycine-Bx | 0.320 | −0.334 |
| 8 | Ethanolamine-Bx | −0.357 | −0.650 |
| 9 | Lysine-Bx | 0.068 | −0.044 |
| 10 | Threonine-Bx | 0.113 | −0.593 |
| 11 | Glutamic acid-Bx | −0.221 | −0.499 |
| 12 | Alanine-Bx | 0.282 | −0.430 |
| 13 | γ-Aminobutyric acid-Bx | −0.220 | −0.622 |
| 14 | Proline-Bx | −0.574 | −0.689 |
| 15 | Dopa-Bx | 0.089 | −0.300 |
| 16 | Dopamine-Bx | 0.075 | 0.074 |
| 17 | Tyrosine-Bx | 0.242 | 0.030 |
| 18 | Methionine-Bx | 0.318 | −0.416 |
| 19 | Valine-Bx | 0.010 | −0.636 |
| 21′ | Isoleucine-Bx | 0.059 | −0.571 |
| 21 | Leucine-Bx | −0.130 | −0.585 |
| 22 | Phenylalanine-Bx | −0.265 | −0.573 |
| 23 | Tryptophan-Bx | −0.498 | −0.753 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spórna-Kucab, A.; Tekieli, A.; Grzegorczyk, A.; Świątek, Ł.; Boguszewska, A.; Skalicka-Woźniak, K. Betaxanthin Profiling in Relation to the Biological Activities of Red and Yellow Beta vulgaris L. Extracts. Metabolites 2023, 13, 408. https://doi.org/10.3390/metabo13030408
Spórna-Kucab A, Tekieli A, Grzegorczyk A, Świątek Ł, Boguszewska A, Skalicka-Woźniak K. Betaxanthin Profiling in Relation to the Biological Activities of Red and Yellow Beta vulgaris L. Extracts. Metabolites. 2023; 13(3):408. https://doi.org/10.3390/metabo13030408
Chicago/Turabian StyleSpórna-Kucab, Aneta, Anna Tekieli, Agnieszka Grzegorczyk, Łukasz Świątek, Anastazja Boguszewska, and Krystyna Skalicka-Woźniak. 2023. "Betaxanthin Profiling in Relation to the Biological Activities of Red and Yellow Beta vulgaris L. Extracts" Metabolites 13, no. 3: 408. https://doi.org/10.3390/metabo13030408
APA StyleSpórna-Kucab, A., Tekieli, A., Grzegorczyk, A., Świątek, Ł., Boguszewska, A., & Skalicka-Woźniak, K. (2023). Betaxanthin Profiling in Relation to the Biological Activities of Red and Yellow Beta vulgaris L. Extracts. Metabolites, 13(3), 408. https://doi.org/10.3390/metabo13030408







