Inter-Day Variation in the Fasting Plasma Lipopolysaccharide Concentration in the Morning Is Associated with Inter-Day Variation in Appetite in Japanese Males: A Short-Term Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
2.2. Participants
2.3. Study Design
2.4. Self-Collection of Blood from Fingertips
2.5. Measurement of Plasma LPS Concentration
2.6. Measurement of Plasma TG Concentration
2.7. Measurement of Breath Acetone Concentration
2.8. Measurement of BMI
2.9. Measurement of Appetite Scores
2.10. Measurement of Nutrient Intake
2.11. Measurement of Physical Activity Level
2.12. Statistical Analysis
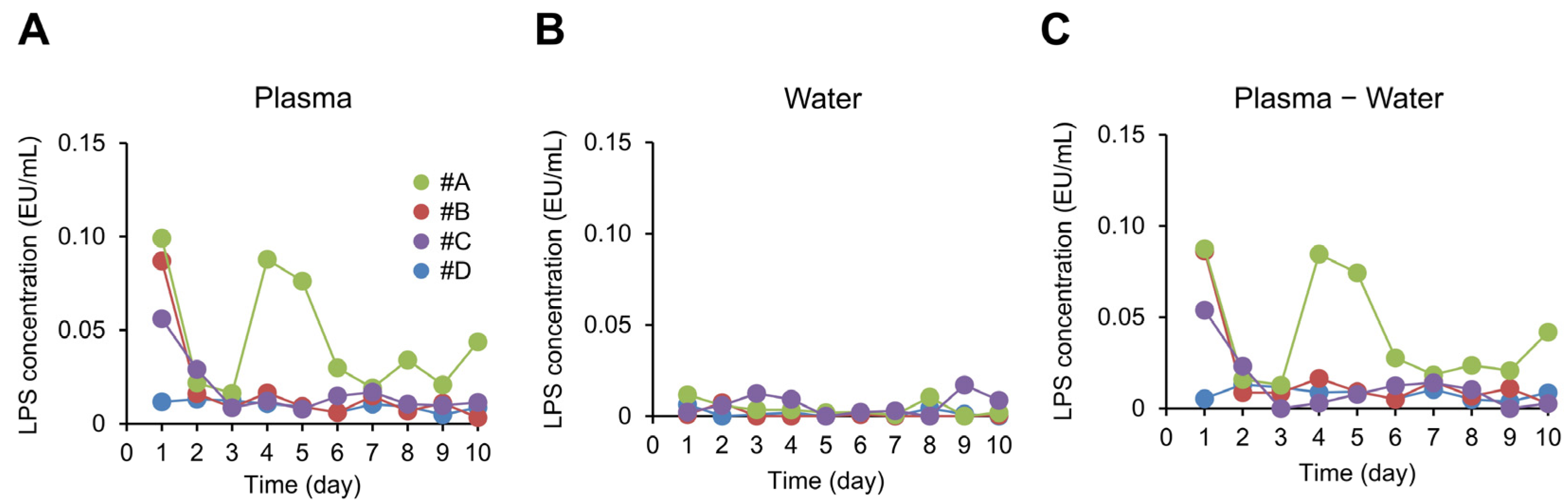
3. Results
3.1. Participant Characteristics
3.2. Correlation between Plasma LPS Concentration and Fat-Burning Markers
3.3. Correlation between Plasma LPS Concentration and Appetite Scores
3.4. Correlation between Plasma LPS Concentration and Nutrient Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Druce, M. The regulation of appetite. Arch. Dis. Child. 2006, 91, 183–187. [Google Scholar] [CrossRef]
- Wakil, S.J.; Abu-Elheiga, L.A. Fatty acid metabolism: Target for metabolic syndrome. J. Lipid Res. 2009, 50, S138–S143. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.J.; Malkova, D. Altered gut and adipose tissue hormones in overweight and obese individuals: Cause or consequence? Int. J. Obes. 2015, 40, 622–632. [Google Scholar] [CrossRef]
- Hue, L.; Taegtmeyer, H. The Randle cycle revisited: A new head for an old hat. Am. J. Physiol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef]
- Miyake, K. Innate recognition of lipopolysaccharide by Toll-like receptor 4–MD-2. Trends Microbiol. 2004, 12, 186–192. [Google Scholar] [CrossRef]
- Liang, H.; Hussey, S.E.; Sanchez-Avila, A.; Tantiwong, P.; Musi, N. Effect of Lipopolysaccharide on Inflammation and Insulin Action in Human Muscle. PLoS ONE 2013, 8, e63983. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.N.; McGillicuddy, F.C.; Anderson, P.D.; Hinkle, C.C.; Shah, R.; Pruscino, L.; Tabita-Martinez, J.; Sellers, K.F.; Rickels, M.R.; Reilly, M.P. Experimental Endotoxemia Induces Adipose Inflammation and Insulin Resistance in Humans. Diabetes 2010, 59, 172–181. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Havulinna, A.S.; Lehto, M.; Sundvall, J.; Salomaa, V. Endotoxemia Is Associated With an Increased Risk of Incident Diabetes. Diabetes Care 2011, 34, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Willment, A.; Patel, S.S.; Sun, X.; Song, M.; Mannery, Y.O.; Kosters, A.; McClain, C.J.; Vos, M.B. Fructose Induced Endotoxemia in Pediatric Nonalcoholic Fatty Liver Disease. Int. J. Hepatol. 2014, 2014, 560620. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Madhulika, A.; Deepika, G.; Rao, G.V.; Reddy, D.N.; Subramanyam, C.; Sasikala, M.; Talukdar, R. Altered intestinal microbiota in patients with chronic pancreatitis: Implications in diabetes and metabolic abnormalities. Sci. Rep. 2017, 7, 43640. [Google Scholar] [CrossRef]
- Zhang, R.; Miller, R.G.; Gascon, R.; Champion, S.; Katz, J.; Lancero, M.; Narvaez, A.; Honrada, R.; Ruvalcaba, D.; McGrath, M.S. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2009, 206, 121–124. [Google Scholar] [CrossRef]
- Vakharia, K.; Hinson, J.P. Lipopolysaccharide Directly Stimulates Cortisol Secretion by Human Adrenal Cells by a Cyclooxygenase-Dependent Mechanism. Endocrinology 2005, 146, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.P.; Rassias, A.J.; Pioli, P.A.; Beach, M.L.; Wardwell, K.; Collins, J.E.; Lee, H.-K.; Guyre, P.M. Pretreatment with stress cortisol enhances the human systemic inflammatory response to bacterial endotoxin. Crit. Care Med. 2009, 37, 2727–2732. [Google Scholar] [CrossRef]
- Kox, M.; van Eijk, L.T.; Zwaag, J.; Wildenberg, J.V.D.; Sweep, F.C.G.J.; van der Hoeven, J.G.; Pickkers, P. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 7379–7384. [Google Scholar] [CrossRef]
- Epel, E.; Lapidus, R.; McEwen, B.; Brownell, K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 2001, 26, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Page, K.A.; Seo, D.; Belfort-DeAguiar, R.; Lacadie, C.; Dzuira, J.; Naik, S.; Amarnath, S.; Constable, R.T.; Sherwin, R.S.; Sinha, R. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J. Clin. Investig. 2011, 121, 4161–4169. [Google Scholar] [CrossRef]
- Victorov, A.; Gladkaya, E.; Novikov, D.; Kosykh, V.; Yurkiv, V. Lipopolysaccharide toxin can directly stimulate the intracellular accumulation of lipids and their secretion into medium in the primary culture of rabbit hepatocytes. FEBS Lett. 1989, 256, 155–158. [Google Scholar] [CrossRef]
- Hudgins, L.C.; Parker, T.S.; Levine, D.M.; Gordon, B.R.; Saal, S.D.; Jiang, X.-C.; Seidman, C.E.; Tremaroli, J.D.; Lai, J.; Rubin, A.L. A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. J. Lipid Res. 2003, 44, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Nier, A.; Brandt, A.; Rajcic, D.; Bruns, T.; Bergheim, I. Short-Term Isocaloric Intake of a Fructose- but not Glucose-Rich Diet Affects Bacterial Endotoxin Concentrations and Markers of Metabolic Health in Normal Weight Healthy Subjects. Mol. Nutr. Food Res. 2019, 63, e1800868. [Google Scholar] [CrossRef]
- Vors, C.; Drai, J.; Pineau, G.; Laville, M.; Vidal, H.; Laugerette, F.; Michalski, M.-C. Emulsifying dietary fat modulates postprandial endotoxemia associated with chylomicronemia in obese men: A pilot randomized crossover study. Lipids Health Dis. 2017, 16, 97. [Google Scholar] [CrossRef]
- Lyte, J.M.; Gabler, N.K.; Hollis, J.H. Postprandial serum endotoxin in healthy humans is modulated by dietary fat in a randomized, controlled, cross-over study. Lipids Health Dis. 2016, 15, 186. [Google Scholar] [CrossRef]
- Vors, C.; Pineau, G.; Drai, J.; Meugnier, E.; Pesenti, S.; Laville, M.; Laugerette, F.; Malpuech-Brugère, C.; Vidal, H.; Michalski, M.-C. Postprandial Endotoxemia Linked With Chylomicrons and Lipopolysaccharides Handling in Obese Versus Lean Men: A Lipid Dose-Effect Trial. J. Clin. Endocrinol. Metab. 2015, 100, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, B.; Wada, Y.; Grundy, S.M.; Szuszkiewicz-Garcia, M.; Vega, G.L. Fatty acid oxidation in normotriglyceridemic men. J. Clin. Lipidol. 2016, 10, 283–288. [Google Scholar] [CrossRef]
- Prabhakar, A.; Quach, A.; Zhang, H.; Terrera, M.; Jackemeyer, D.; Xian, X.; Tsow, F.; Tao, N.; Forzani, E.S. Acetone as biomarker for ketosis buildup capability—A study in healthy individuals under combined high fat and starvation diets. Nutr. J. 2015, 14, 41. [Google Scholar] [CrossRef]
- Bovey, F.; Cros, J.; Tuzson, B.; Seyssel, K.; Schneiter, P.; Emmenegger, L.; Tappy, L. Breath acetone as a marker of energy balance: An exploratory study in healthy humans. Nutr. Diabetes 2018, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Doucet, E.; Imbeault, P.; St-Pierre, S.; Alméras, N.; Mauriège, P.; Richard, D.; Tremblay, A. Appetite after weight loss by energy restriction and a low-fat diet–exercise follow-up. Int. J. Obes. 2000, 24, 906–914. [Google Scholar] [CrossRef]
- Gorczyca, A.M.; Sjaarda, L.A.; Mitchell, E.M.; Perkins, N.J.; Schliep, K.C.; Wactawski-Wende, J.; Mumford, S.L. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur. J. Nutr. 2016, 55, 1181–1188. [Google Scholar] [CrossRef]
- Hoppler, S.; Walther, A.; La Marca-Ghaemmaghami, P.; Ehlert, U. Lower birthweight and left-/mixed-handedness are associated with intensified age-related sex steroid decline in men. Findings from the Men’s Health 40+ Study. Andrology 2018, 6, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yan, D.; Xu, M.; Li, F.; Ren, M.; Zhang, J.; Wu, M. Interactive association of lipopolysaccharide and free fatty acid with the prevalence of type 2 diabetes: A community-based cross-sectional study. J. Diabetes Investig. 2019, 10, 1438–1446. [Google Scholar] [CrossRef]
- Männistö, V.; Färkkilä, M.; Pussinen, P.; Jula, A.; Männistö, S.; Lundqvist, A.; Valsta, L.; Salomaa, V.; Perola, M.; Åberg, F. Serum lipopolysaccharides predict advanced liver disease in the general population. JHEP Rep. 2019, 1, 345–352. [Google Scholar] [CrossRef]
- Japan Ministry of Health, Labour and Welfare, The National Health and Nutrition Survey, Japan. 2019. Available online: https://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html (accessed on 5 March 2023).
- Lewis, G.F.; O’Meara, N.M.; Soltys, P.A.; Blackman, J.D.; Iverius, P.H.; Pugh, W.L.; Getz, G.S.; Polonsky, K.S. Fasting Hypertriglyceridemia in Non insulin-Dependent Diabetes Mellitus Is an Important Predictor of Postprandial Lipid and Lipoprotein Abnormalities. J. Clin. Endocrinol. Metab. 1991, 72, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, M.C.; Halkes, C.; Meijssen, S.; van Oostrom, A.; Erkelens, D. Diurnal triglyceride profiles: A novel approach to study triglyceride changes. Atherosclerosis 2001, 155, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, E.; Michie, M.; Kawabata, T. Validity of Nutrient Intakes Derived from an Internet Website Dish-Based Dietary Record for Self-Management of Weight among Japanese Women. Nutrients 2017, 9, 1058. [Google Scholar] [CrossRef]
- Cnaan, A.; Laird, N.M.; Slasor, P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat. Med. 1997, 16, 2349–2380. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Kanayama, Y.; Ikegami, S.; Iikuni, T.; Nakajima, K. Evaluation on Basic Characteristics of Non-contact Uroflowmeter and Its Use in Healthy Males. Trans. Jpn. Soc. Med. Biol. Eng. 2018, 56, 252–259. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Diraison, F.; Moulin, P.; Beylot, M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003, 29, 478–485. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Kelley, D.E.; Mandarino, L.J. Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes 2000, 49, 677–683. [Google Scholar] [CrossRef]
- Grunewald, Z.I.; Lee, S.; Kirkland, R.; Ross, M.; de La Serre, C.B. Cannabinoid receptor type-1 partially mediates metabolic endotoxemia-induced inflammation and insulin resistance. Physiol. Behav. 2019, 199, 282–291. [Google Scholar] [CrossRef]
- Kim, K.E.; Heo, J.S.; Han, S.; Kwon, S.-K.; Kim, S.-Y.; Kim, J.H.; Baek, K.-H.; Sheen, Y.H. Blood concentrations of lipopolysaccharide-binding protein, high-sensitivity C-reactive protein, tumor necrosis factor-α, and Interleukin-6 in relation to insulin resistance in young adolescents. Clin. Chim. Acta 2018, 486, 115–121. [Google Scholar] [CrossRef]
- Zampino, R.; Marrone, A.; Rinaldi, L.; Guerrera, B.; Nevola, R.; Boemio, A.; Iuliano, N.; Giordano, M.; Passariello, N.; Sasso, F.C.; et al. Endotoxinemia contributes to steatosis, insulin resistance and atherosclerosis in chronic hepatitis C: The role of pro-inflammatory cytokines and oxidative stress. Infection 2018, 46, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Tanda, N.; Hinokio, Y.; Washio, J.; Takahashi, N.; Koseki, T. Analysis of ketone bodies in exhaled breath and blood of ten healthy Japanese at OGTT using a portable gas chromatograph. J. Breath Res. 2014, 8, 046008. [Google Scholar] [CrossRef] [PubMed]
- Ghimenti, S.; Tabucchi, S.; Lomonaco, T.; Di Francesco, F.; Fuoco, R.; Onor, M.; Lenzi, S.; Trivella, M.G. Monitoring breath during oral glucose tolerance tests. J. Breath Res. 2013, 7, 017115. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Gregersen, N.T.; Gluud, L.L.; Møller, B.K.; Raben, A.; Tetens, I.; Verdich, C.; Astrup, A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: A meta-analysis of test meal studies. Br. J. Nutr. 2007, 98, 17–25. [Google Scholar] [CrossRef]
- Heller, R. Hyperinsulinemic obesity and carbohydrate addiction: The missing link is the carbohydrate frequency factor. Med. Hypotheses 1994, 42, 307–312. [Google Scholar] [CrossRef]
- Yu, J.H.; Shin, M.S.; Kim, D.J.; Lee, J.R.; Yoon, S.-Y.; Kim, S.G.; Koh, E.H.; Lee, W.J.; Park, J.-Y.; Kim, M.-S. Enhanced carbohydrate craving in patients with poorly controlled Type 2 diabetes mellitus. Diabet. Med. 2013, 30, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Velasquez-Mieyer, P.A.; Cowan, P.A.; Arheart, K.L.; Buffington, C.K.; Spencer, K.A.; Connelly, B.E.; Cowan, G.W.; Lustig, R.H. Suppression of insulin secretion is associated with weight loss and altered macronutrient intake and preference in a subset of obese adults. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 219–226. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Fuke, N.; Yamashita, T.; Shimizu, S.; Matsumoto, M.; Sawada, K.; Jung, S.; Tokuda, I.; Misawa, M.; Suzuki, S.; Ushida, Y.; et al. Association of Plasma Lipopolysaccharide-Binding Protein Concentration with Dietary Factors, Gut Microbiota, and Health Status in the Japanese General Adult Population: A Cross-Sectional Study. Metabolites 2023, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Sakurai, N.; Takahashi, S.; Waki, N.; Suganuma, H.; Aizawa, K.; Matsumura, Y.; Kawada, T.; Shibata, D. TOMATOMET: A metabolome database consists of 7118 accurate mass values detected in mature fruits of 25 tomato cultivars. Plant Direct 2021, 5, e00318. [Google Scholar] [CrossRef]
- Gutsmann, T.; Howe, J.; Zähringer, U.; Garidel, P.; Schromm, A.B.; Koch, M.H.J.; Fujimoto, Y.; Fukase, K.; Moriyon, I.; Martinez-De-Tejada, G.; et al. Structural prerequisites for endotoxic activity in the Limulus test as compared to cytokine production in mononuclear cells. J. Endotoxin Res. 2010, 16, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Howe, J.; Gutsman, T.; Garidel, P. The expression of endotoxic activity in the Limulus test as compared to cytokine production in immune cells. Curr. Med. Chem. 2009, 16, 2653–2660. [Google Scholar] [CrossRef]
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| β | t Value | β | t Value | |
| Change in fasting plasma TG concentration | −144 | −0.6 | −264 | −1.2 |
| Change in fasting breath acetone concentration | 1146 | 7.8 * | 1145 | 7.6 * |
| Change in fasting BMI | 2 | 2.4 * | 2 | 2.2 * |
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | t Value | β | t Value | β | t Value | β | t Value | β | t Value | ||
| Breakfast | Desire to eat | 263 | 0.4 | 142 | 0.2 | 304 | 0.4 | 302 | 0.4 | 76 | 0.1 |
| Hunger | 371 | 0.6 | 356 | 0.5 | 464 | 0.6 | 497 | 0.7 | 196 | 0.3 | |
| Fullness | −2239 | −4.2 * | −2426 | −4.6 * | −2536 | −4.0 * | −2769 | −4.7 * | −2011 | −2.8 * | |
| PFC | −220 | −0.3 | −596 | −0.7 | −207 | −0.2 | −238 | −0.3 | −715 | −1.1 | |
| Lunch | Desire to eat | −438 | −0.9 | −434 | −0.9 | −440 | −0.9 | −532 | −1.0 | −880 | −1.4 |
| Hunger | 426 | 0.6 | 386 | 0.6 | 245 | 0.4 | 163 | 0.2 | −110 | −0.2 | |
| Fullness | −3538 | −3.2 * | −3828 | −3.4 * | −3766 | −3.4 * | −3766 | −3.2 * | −3540 | −3.3 * | |
| PFC | −115 | −0.1 | −88 | −0.1 | 621 | 1.0 | −50 | −0.1 | −1066 | −1.4 | |
| Dinner | Desire to eat | −723 | −1.1 | −386 | −0.6 | −91 | −0.1 | 38 | 0.1 | 310 | 0.5 |
| Hunger | −1095 | −1.5 | −680 | −0.9 | −313 | −0.4 | −234 | −0.3 | 59 | 0.1 | |
| Fullness | 1060 | 1.3 | 336 | 0.4 | −35 | −0.1 | −64 | −0.1 | −310 | −0.4 | |
| PFC | −1346 | −1.3 | −379 | −0.4 | 81 | 0.1 | 207 | 0.2 | 363 | 0.4 | |
| Variable | Breakfast | Lunch | Dinner | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |||||||
| β | t Value | β | t Value | β | t Value | β | t Value | β | t Value | β | t Value | |
| Calories | −1929 | −1.5 | −1937 | −1.5 | 1197 | 0.9 | 492 | 0.4 | 3296 | 2.3 * | 4096 | 3.4 * |
| Protein | −161 | −2.9 * | −164 | −3.0 * | 95 | 1.1 | 49 | 0.6 | 225 | 1.7 | 282 | 2.1 * |
| Fat | −4 | −0.1 | −6 | −0.1 | 84 | 0.7 | 43 | 0.3 | −21 | −0.2 | −9 | −0.1 |
| Carbohydrate | −140 | −1.0 | −139 | −1.1 | 47 | 0.3 | 68 | 0.4 | 648 | 4.2 * | 723 | 3.8 * |
| Dietary fiber | −22 | −1.1 | −23 | −1.1 | 1 | 0.1 | 5 | 0.2 | −31 | −0.9 | −27 | −0.8 |
| Calcium | −2341 | −2.7 * | −2444 | −2.8 * | −871 | −2.2 * | −1015 | −2.3 * | −601 | −0.4 | 382 | 0.3 |
| Iron | −13 | −1.6 | −13 | −1.6 | 5 | 0.7 | 3 | 0.4 | 12 | 0.6 | 22 | 1.1 |
| Vitamin A | 5157 | 1.7 | 6635 | 2.4 * | −4897 | −3.3 * | −5545 | −3.5 * | −1221 | −0.7 | −1314 | −0.7 |
| Vitamin E | −1 | −0.1 | −1 | −0.1 | −15 | −0.9 | −20 | −1.1 | 17 | 1.1 | 21 | 1.2 |
| Vitamin B1 | −1 | −2.2 * | −2 | −3.0 * | 2 | 1.0 | 1 | 0.7 | −3 | −2.7 * | −3 | −2.1 * |
| Vitamin B2 | −1 | −1.1 | −1 | −1.1 | 0 | −0.3 | −1 | −0.7 | 2 | 1.9 | 1 | 1.6 |
| Vitamin C | 554 | 1.5 | 647 | 1.8 | −238 | −1.1 | −251 | −1.1 | −624 | −1.9 | −502 | −1.4 |
| Saturated fatty acids | 1 | 0.0 | 2 | 0.1 | −45 | −1.6 | −65 | −2.1 * | −22 | −0.7 | −26 | −0.8 |
| Salt | −20 | −2.2 * | −20 | −2.1 * | 17 | 1.2 | 15 | 0.9 | 42 | 2.7 * | 48 | 2.9 * |
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| β | t Value | β | t Value | |
| Calories | 2718 | 1.0 | 3107 | 0.9 |
| Protein | 122 | 0.6 | 62 | 0.3 |
| Fat | 61 | 0.3 | 100 | 0.5 |
| Carbohydrate | 524 | 2.4 * | 579 | 2.3 * |
| Dietary fiber | −47 | −0.8 | −65 | −0.9 |
| Calcium | −4448 | −2.1 * | −3374 | −1.5 |
| Iron | −3 | −0.1 | −1 | 0.0 |
| Vitamin A | 1565 | 0.4 | 2693 | 0.6 |
| Vitamin E | −43 | −2.1 * | −42 | −1.8 |
| Vitamin B1 | −4 | −1.5 | −6 | −2.8 * |
| Vitamin B2 | −2 | −1.0 | −3 | −1.8 |
| Vitamin C | −362 | −0.9 | −196 | −0.4 |
| Saturated fatty acids | −30 | −0.6 | −19 | −0.4 |
| Salt | 47 | 2.4 * | 38 | 1.7 |
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| β | t Value | β | t Value | |
| Calories | 3307 | 1.2 | 3795 | 1.2 |
| Protein | 180 | 0.9 | 137 | 0.6 |
| Fat | 99 | 0.5 | 145 | 0.7 |
| Carbohydrate | 501 | 2.5 * | 543 | 2.4 * |
| Dietary fiber | −42 | −0.7 | −60 | −0.9 |
| Calcium | −4326 | −2.0 * | −3143 | −1.4 |
| Iron | 10 | 0.4 | 16 | 0.5 |
| Vitamin A | 1625 | 0.4 | 2737 | 0.6 |
| Vitamin E | −36 | −1.8 | −35 | −1.4 |
| Vitamin B1 | −3 | −1.4 | −6 | −2.6 * |
| Vitamin B2 | −1 | −0.6 | −2 | −1.5 |
| Vitamin C | −363 | −0.9 | −197 | −0.4 |
| Saturated fatty acids | −26 | −0.5 | −18 | −0.3 |
| Salt | 52 | 2.7 * | 43 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuke, N.; Ushida, Y.; Sato, I.; Suganuma, H. Inter-Day Variation in the Fasting Plasma Lipopolysaccharide Concentration in the Morning Is Associated with Inter-Day Variation in Appetite in Japanese Males: A Short-Term Cohort Study. Metabolites 2023, 13, 395. https://doi.org/10.3390/metabo13030395
Fuke N, Ushida Y, Sato I, Suganuma H. Inter-Day Variation in the Fasting Plasma Lipopolysaccharide Concentration in the Morning Is Associated with Inter-Day Variation in Appetite in Japanese Males: A Short-Term Cohort Study. Metabolites. 2023; 13(3):395. https://doi.org/10.3390/metabo13030395
Chicago/Turabian StyleFuke, Nobuo, Yusuke Ushida, Ikuo Sato, and Hiroyuki Suganuma. 2023. "Inter-Day Variation in the Fasting Plasma Lipopolysaccharide Concentration in the Morning Is Associated with Inter-Day Variation in Appetite in Japanese Males: A Short-Term Cohort Study" Metabolites 13, no. 3: 395. https://doi.org/10.3390/metabo13030395
APA StyleFuke, N., Ushida, Y., Sato, I., & Suganuma, H. (2023). Inter-Day Variation in the Fasting Plasma Lipopolysaccharide Concentration in the Morning Is Associated with Inter-Day Variation in Appetite in Japanese Males: A Short-Term Cohort Study. Metabolites, 13(3), 395. https://doi.org/10.3390/metabo13030395






