Vascular Damage and Glycometabolic Control in Older Patients with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Arterial Stiffness Measurements
2.3. Laboratory Analyses
2.4. Statistical Analysis
3. Results
3.1. Basic Characteristic
3.2. Vascular Damage Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halter, J.B.; Musi, N.; Horne, F.M.; Crandall, J.P.; Goldberg, A.; Harkless, L.; Hazzard, W.R.; Huang, E.S.; Kirkman, M.S.; Plutzky, J.; et al. Diabetes and cardiovascular disease in older adults: Current status and future directions. Diabetes 2014, 63, 2578–2589. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Assar, M.E.; Angulo, J.; Rodríguez-Mañas, L. Diabetes and ageing-induced vascular inflammation. J. Physiol. 2016, 594, 2125–2146. [Google Scholar] [CrossRef] [PubMed]
- Vatner, S.F.; Zhang, J.; Vyzas, C.; Mishra, K.; Graham, R.M.; Vatner, D.E. Vascular Stiffness in Aging and Disease. Front. Physiol. 2021, 12, 762437. [Google Scholar] [CrossRef]
- Miyoshi, T.; Ito, H. Arterial stiffness in health and disease: The role of cardio-ankle vascular index. J. Cardiol. 2021, 78, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F. Arterial Stiffness in Aging: Does It Have a Place in Clinical Practice? Recent Advances in Hypertension. Hypertension 2021, 77, 768–780. [Google Scholar] [CrossRef]
- Prenner, S.B.; Chirinos, J.A. Arterial stiffness in diabetes mellitus. Atherosclerosis 2015, 238, 370–379. [Google Scholar] [CrossRef]
- Cardoso, C.R.; Salles, G.F. Aortic Stiffness as a Surrogate Endpoint to Micro- and Macrovascular Complications in Patients with Type 2 Diabetes. Int. J. Mol. Sci. 2016, 17, 2044. [Google Scholar] [CrossRef]
- Cecelja, M.; Chowienczyk, P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: A systematic review. Hypertension 2009, 54, 1328–1336. [Google Scholar] [CrossRef]
- Cecelja, M.; Chowienczyk, P. Molecular Mechanisms of Arterial Stiffening. Pulse 2016, 4, 43–48. [Google Scholar] [CrossRef]
- Anderson, T.J. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can. J. Cardiol. 2006, 22, 72B–80B. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; Lamacchia, O.; Ciccarelli, M.; Dattilo, G.; Tricarico, L.; Brunetti, N.D. Vascular and metabolic effects of SGLT2i and GLP-1 in heart failure patients. Heart Fail. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Pavlidis, G.; Thymis, J.; Birba, D.; Kalogeris, A.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Endothelial Glycocalyx, Arterial Function, and Myocardial Work Index in Patients with Type 2 Diabetes Mellitus After 12-Month Treatment. J. Am. Heart Assoc. 2020, 9, e015716. [Google Scholar] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Gajdova, J.; Karasek, D.; Goldmannova, D.; Krystynik, O.; Schovanek, J.; Vaverkova, H.; Zadrazil, J. Pulse wave analysis and diabetes mellitus. A systematic review. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2017, 161, 223–233. [Google Scholar] [CrossRef]
- Luttrell, M.; Kim, H.; Shin, S.Y.; Holly, D.; Massett, M.P.; Woodman, C.R. Heterogeneous effect of aging on vasorelaxation responses in large and small arteries. Physiol. Rep. 2020, 8, e14341. [Google Scholar] [CrossRef]
- Lip, G.Y.; Blann, A. von Willebrand factor: A marker of endothelial dysfunction in vascular disorders? Cardiovasc. Res. 1997, 34, 255–265. [Google Scholar] [CrossRef]
- Spiel, A.O.; Gilbert, J.C.; Jilma, B. von Willebrand factor in cardiovascular disease: Focus on acute coronary syndromes. Circulation 2008, 117, 1449–1459. [Google Scholar] [CrossRef]
- Knittel, T.; Neubauer, K.; Armbrust, T.; Ramadori, G. Expression of von Willebrand factor in normal and diseased rat livers and in cultivated liver cells. Hepatology 1995, 21, 470–476. [Google Scholar]
- Baruch, Y.; Neubauer, K.; Shenkar, L.; Sabo, E.; Ritzel, A.; Wilfling, T.; Ramadori, G. Von Willebrand factor in plasma and in liver tissue after partial hepatectomy in the rat. J. Hepatol. 2002, 37, 471–477. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Incalzi, R.A. Plasminogen activator inhibitor-1 (PAI-1): A key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc. Ther. 2010, 28, e72–e91. [Google Scholar] [CrossRef] [PubMed]
- Alessi, M.C.; Poggi, M.; Juhan-Vague, I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr. Opin. Lipidol. 2007, 18, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, X.; Fan, M.; Zhao, J.; Lin, L.; Liu, J. Plasma levels of von Willebrand factor in type 2 diabetes patients with and without cardiovascular diseases: A meta-analysis. Diabetes Metab. Res. Rev. 2020, 36, e3193. [Google Scholar] [CrossRef] [PubMed]
- Seligman, B.G.; Biolo, A.; Polanczyk, C.A.; Gross, J.L.; Clausell, N. Increased plasma levels of endothelin 1 and von Willebrand factor in patients with type 2 diabetes and dyslipidemia. Diabetes Care 2000, 23, 1395–1400. [Google Scholar] [CrossRef]
- Karasek, D.; Spurna, J.; Macakova, D.; Cibickova, L.; Krystynik, O.; Kucerova, V.; Ulehlova, J.; Slavik, L. Hypertriglyceridemic Waist in Patients with Type 2 Diabetes: Its Relationship to Selected Markers of Vascular Damage. Metab. Syndr. Relat. Disord. 2021, 19, 393–400. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takeshita, K.; Saito, H. Plasminogen activator inhibitor-1 in aging. Semin. Thromb. Hemost. 2014, 40, 652–659. [Google Scholar]
- Altalhi, R.; Pechlivani, N.; Ajjan, R.A. PAI-1 in Diabetes: Pathophysiology and Role as a Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 3170. [Google Scholar] [CrossRef]
- Eriksson, P.; Reynisdottir, S.; Lönnqvist, F.; Stemme, V.; Hamsten, A.; Arner, P. Adipose tissue secretion of plasminogen activator inhibitor-1 in non-obese and obese individuals. Diabetologia 1998, 41, 65–71. [Google Scholar] [CrossRef]
- Kaji, H. Adipose Tissue-Derived Plasminogen Activator Inhibitor-1 Function and Regulation. Compr. Physiol. 2016, 6, 1873–1896. [Google Scholar]
- Mertens, I.; Ballaux, D.; Funahashi, T.; Matsuzawa, Y.; Van der Planken, M.; Verrijken, A.; Ruige, J.B.; Van Gaal, L.F. Inverse relationship between plasminogen activator inhibitor-I activity and adiponectin in overweight and obese women. Interrelationship with visceral adipose tissue, insulin resistance, HDL-chol and inflammation. Thromb. Haemost. 2005, 94, 1190–1195. [Google Scholar] [CrossRef]
- Karasek, D.; Vaverkova, H.; Halenka, M.; Jackuliakova, D.; Frysak, Z.; Slavik, L.; Novotny, D. Prothrombotic markers in asymptomatic dyslipidemic subjects. J. Thromb. Thrombolysis 2011, 31, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Mavri, A.; Alessi, M.C.; Bastelica, D.; Geel-Georgelin, O.; Fina, F.; Sentocnik, J.T.; Stegnar, M.; Juhan-Vague, I. Subcutaneous abdominal, but not femoral fat expression of plasminogen activator inhibitor-1 (PAI-1) is related to plasma PAI-1 levels and insulin resistance and decreases after weight loss. Diabetologia 2001, 44, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Mossberg, K.E.; Pournaras, D.J.; Welbourn, R.; le Roux, C.W.; Brogren, H. Differential response of plasma plasminogen activator inhibitor 1 after weight loss surgery in patients with or without type 2 diabetes. Surg. Obes. Relat. Dis. 2017, 13, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Askarpour, M.; Alizadeh, S.; Hadi, A.; Symonds, M.E.; Miraghajani, M.; Sheikhi, A.; Ghaedi, E. Effect of Bariatric Surgery on the Circulating Level of Adiponectin, Chemerin, Plasminogen Activator Inhibitor-1, Leptin, Resistin, and Visfatin: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2020, 52, 207–215. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Bordin Barbieri, N.; Weinmann, T.; Ziegelmann, P.K.; Duncan, B.B.; Inês Schmidt, M. Plasminogen activator inhibitor-1 and type 2 diabetes: A systematic review and meta-analysis of observational studies. Sci. Rep. 2016, 6, 17714. [Google Scholar] [CrossRef]
| 1. Quartile (n = 39) | 2. Quartile (n = 38) | 3. Quartile (n = 41) | 4. Quartile (n = 42) | Corrected p-Value | |
|---|---|---|---|---|---|
| age (years) | 42.1 ± 4.5 | 51.6 ± 1.4 | 59.2 ± 3.0 | 69.8 ± 3.8 | - |
| female (percentage) | 15 (38%) | 11 (29%) | 12 (29%) | 13 (31%) | n.s. |
| BMI (kg/m2) | 34.1 ± 5.3 | 31.8 ± 5.3 | 32.7 ± 5.2 | 30.9 ± 4.0 | n.s. |
| waist (cm) | 115.3 ± 17.6 | 110.4 ± 14.3 | 113.5 ± 14.2 | 110.0 ± 11.7 | n.s. |
| SBP (mmHg) | 130.2 ± 17.1 d | 124.4 ± 14.5 d | 132.0 ± 14.0 d | 140.3 ± 15.5 a,b,c | 0.002 |
| DBP (mmHg) | 79.3 ± 9.1 | 83.1 ± 10.3 | 79.6 ± 9.1 | 81.1 ± 10.7 | n.s. |
| TC (mmol/L) | 5.0 ± 1.9 | 4.8 ± 1.7 | 4.5 ± 1.0 | 4.2 ± 1.3 | n.s. |
| LDL-C (mmol/L) | 2.6 ± 0.7 d | 2.5 ± 0.8 | 2.5 ± 0.7 | 2.0 ± 1.1 a | 0.020 |
| HDL-C (mmol/L) | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.4 | n.s. |
| TG (mmol/L) | 2.1 (1.6–3.0) | 1.8(1.3–2.6) | 1.7 (1.2–2.5) | 2.0 (1.3–2.9) | n.s. |
| non-HDL-C (mmol/L) | 3.9 ± 1.9 | 3.8 ± 1.8 | 3.5 ± 1.0 | 3.1 ± 1.4 | n.s. |
| apoB (g/L) | 1.1 (0.9–1.3) | 1.0 (0.9–1.2) | 1.0 (0.8–1.3) | 0.9 (0.7–1.0) | n.s. |
| FPG (mmol/L) | 7.8 (6.2–9.9) | 8.3 (6.9–11.0) | 7.4 (6.1–11.2) | 8.1 (7.7–11.0) | n.s. |
| HbA1c (mmol/mol) | 61.5 (43.5–73.3) | 71.5 (49.8–97.5) | 64.0 (44.9–95.5) | 69.3 (49.5–82.9) | n.s. |
| Insulin (mIU/L) | 19.2 (13.0–44.8) | 20.1(12.3–36.1) | 17.5 (11.5–36.8) | 29.7 (13.6–73.6) | n.s. |
| C-peptide (pmol/L) | 846 (565–1199) | 800 (607–1123) | 940 (617–1275) | 746 (423–1083) | n.s. |
| hs-CRP (mg/L) | 4.1 (2.2–8.4) d | 4.1 (2.1–8.4) d | 4.4 (1.8–8.8) d | 1.6 (0.8–3.5) a,b,c | 0.007 |
| dd (years) | 6.4 ± 3.5 | 6.0 ± 3.1 | 10.0 ± 6.5 | 9.1 ± 6.1 | n.s. |
| CVD (percentage) | 1 (3%) | 9 (23%) | 12 (29%) | 21 (50%) | 0.001 |
| hypertension (percentage) | 29 (74%) | 35 (92%) | 37 (90%) | 40 (95%) | 0.002 |
| 1. Quartile (n = 39) | 2. Quartile (n = 38) | 3. Quartile (n = 42) | 4. Quartile (n = 41) | Corrected p-Value | |
|---|---|---|---|---|---|
| v-WFa (%) | 121.7 ± 42.5 b,c,d | 134.8 ± 50.0 a,d | 129.0 ± 32.9 a,d | 153.7 ± 51.9 a,b,c | 0.010 |
| PAI-1 (ng/mL) | 90.0 ± 44.9 | 100.9 ± 48.3 d | 88.4 ± 43.9 | 81.8 ± 47.5 b | 0.048 |
| t-PA (ng/mL) | 2.8(2.6–3.1) | 2.7(2.3–3.8) | 3.0(2.5–3.6) | 2.8(2.4–3.7) | n.s. |
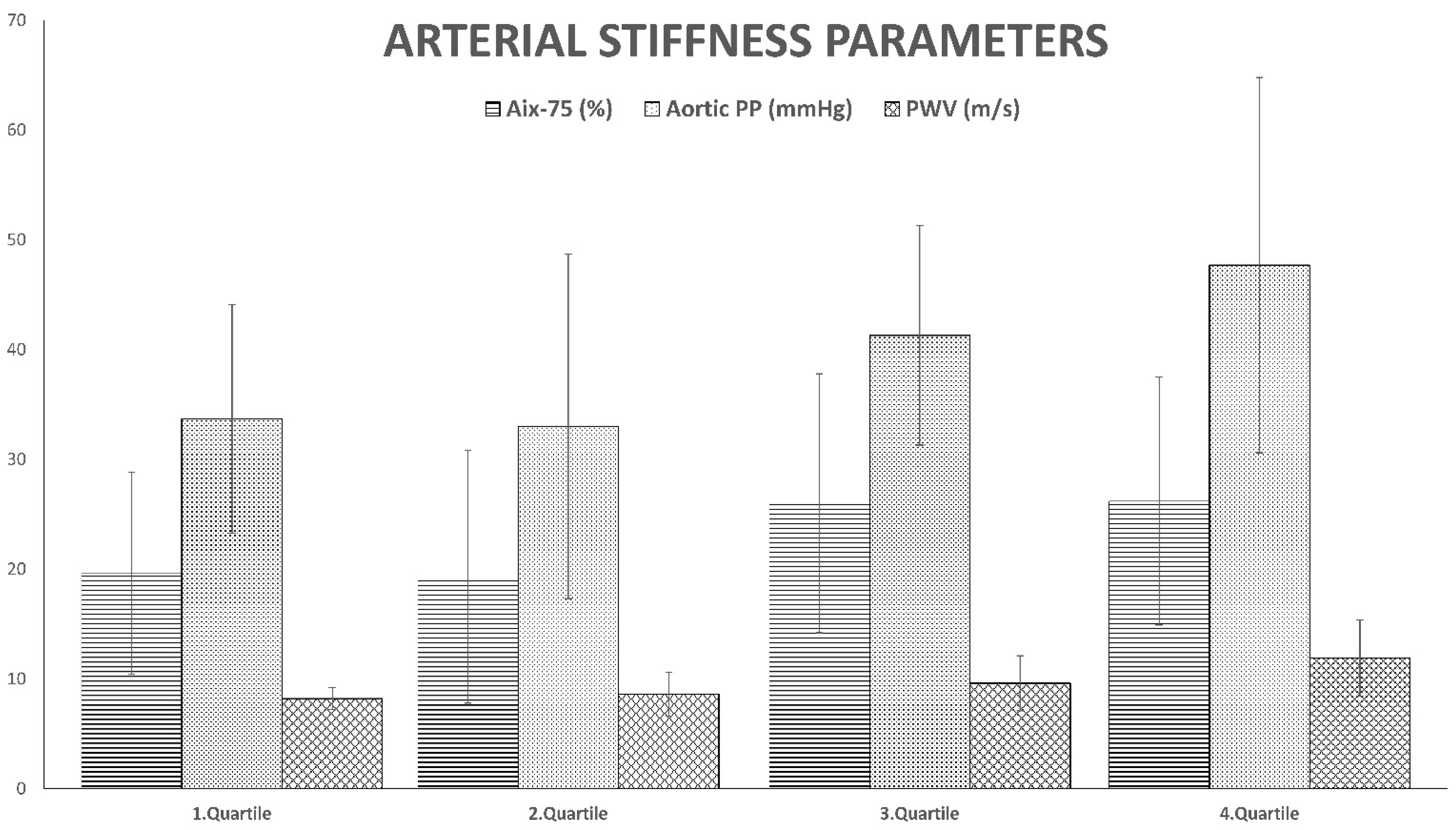
| AP (mmHg) | 6.7 ± 4.4 c,d | 7.0 ± 5.7 c,d | 11.2 ± 6.6 a,b | 13.4 ± 8.6 a,b | <0.001 |
| AIx-75 (%) | 19.6 ± 9.2 c,d | 19.3 ± 11.5 c,d | 26.0 ± 11.8 a,b | 26.2 ± 11.3 a,b | 0.005 |
| aortic SP (mmHg) | 118.0 ± 15.4 b | 108.7 ± 17.2 a,c,d | 123.8 ± 15.3 b | 122.6 ± 18.1 b | 0.001 |
| aortic PP (mmHg) | 33.7 ± 10.4 c,d | 33.0 ± 15.7 c,d | 41.3 ± 10.0 a,b,d | 47.7 ± 17.1 a,b,c | <0.001 |
| PWV (m/s) | 8.2(7.7–9.8) c,d | 8.6(7.0–10.6) d | 9.6(7.9–11.7) d | 11.9(10.1–14.3) a,b,c | <0.001 |
| Pulse Wave Velocity | ||||||
|---|---|---|---|---|---|---|
| Unstandardized coefficients | Standardized coefficients | t | Sig. | |||
| B | SE | Beta | SE | |||
| age | 0.2075 | 0.2473 | 0.0480 | 0.0573 | 0.8391 | 0.4027 |
| SBP | 0.6052 | 0.2484 | 0.0603 | 0.0247 | 2.4365 | 0.0159 |
| insulin | 0.0046 | 0.0604 | 0.0010 | 0.0132 | 0.0755 | 0.9399 |
| Aortic pulse pressure | ||||||
| Unstandardized coefficients | Standardized coefficients | t | Sig. | |||
| B | SE | Beta | SE | |||
| age | 0.0651 | 0.0482 | 0.1366 | 0.1010 | 1.3524 | 0.1782 |
| SBP | 0.9357 | 0.0484 | 0.8439 | 0.0436 | 19.3454 | 0.0000 |
| insulin | −0.0096 | 0.0118 | −0.0191 | 0.0233 | −0.8194 | 0.4138 |
| Aortic systolic pressure | ||||||
| Unstandardized coefficients | Standardized coefficients | t | Sig. | |||
| B | SE | Beta | SE | |||
| age | 0.0804 | 0.0486 | 0.1686 | 0.1020 | 1.6538 | 0.1002 |
| waist | 0.0664 | 0.0615 | 0.0703 | 0.0651 | 1.0799 | 0.2818 |
| SBP | 0.6147 | 0.1081 | 0.5544 | 0.0975 | 5.6880 | 0.0000 |
| DBP | 0.2354 | 0.0986 | 0.3469 | 0.1452 | 2.3883 | 0.0181 |
| Augmentation index-75 | ||||||
| Unstandardized coefficients | Standardized coefficients | t | Sig. | |||
| B | SE | Beta | SE | |||
| age | 0.3732 | 0.0756 | 0.2501 | 0.0507 | 4.9361 | 0.0000 |
| SBP | 0.1092 | 0.0756 | 0.0481 | 0.0333 | 1.4442 | 0.1507 |
| Von Willebrand factor | ||||||
| Unstandardized coefficients | Standardized coefficients | t | Sig. | |||
| B | SE | Beta | SE | |||
| TG | 0.2278 | 0.0383 | 7.4126 | 1.2450 | 5.9537 | 0.0000 |
| HDL-C | 0.7713 | 0.0383 | 101.644 | 5.0416 | 20.1609 | 0.0000 |
| Plasminogen activator inhibitor-1 | ||||||
| Unstandardized coefficients | Standardized coefficients | t | Sig. | |||
| B | SE | Beta | SE | |||
| BMI | 0.5258 | 0.1442 | 1.5399 | 0.4222 | 3.6474 | 0.0004 |
| waist | −0.0881 | 0.4868 | −0.0746 | 0.4122 | −0.1809 | 0.8567 |
| hs-CRP | 0.0099 | 0.0503 | 0.1427 | 0.7236 | 0.1972 | 0.8440 |
| TG | 0.0923 | 0.0700 | 1.9695 | 1.4931 | 1.3190 | 0.1891 |
| HDL-C | −0.1693 | 0.1014 | −14.6352 | 8.7653 | −1.6697 | 0.0970 |
| non-HDL-C | 0.0584 | 0.1791 | 1.4434 | 4.4267 | 0.3261 | 0.7448 |
| apo-B | 0.1866 | 0.2026 | 17.1592 | 18.6299 | 0.9211 | 0.3585 |
| FPG | 0.1331 | 0.0941 | 1.3535 | 0.9562 | 1.4155 | 0.1590 |
| C-peptide | 0.2081 | 0.0697 | 0.0187 | 0.0063 | 2.9859 | 0.0033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karasek, D.; Spurna, J.; Macakova, D.; Krystynik, O.; Kucerova, V. Vascular Damage and Glycometabolic Control in Older Patients with Type 2 Diabetes. Metabolites 2023, 13, 382. https://doi.org/10.3390/metabo13030382
Karasek D, Spurna J, Macakova D, Krystynik O, Kucerova V. Vascular Damage and Glycometabolic Control in Older Patients with Type 2 Diabetes. Metabolites. 2023; 13(3):382. https://doi.org/10.3390/metabo13030382
Chicago/Turabian StyleKarasek, David, Jaromira Spurna, Dominika Macakova, Ondrej Krystynik, and Veronika Kucerova. 2023. "Vascular Damage and Glycometabolic Control in Older Patients with Type 2 Diabetes" Metabolites 13, no. 3: 382. https://doi.org/10.3390/metabo13030382
APA StyleKarasek, D., Spurna, J., Macakova, D., Krystynik, O., & Kucerova, V. (2023). Vascular Damage and Glycometabolic Control in Older Patients with Type 2 Diabetes. Metabolites, 13(3), 382. https://doi.org/10.3390/metabo13030382







