Projections from the Rostral Zona Incerta to the Thalamic Paraventricular Nucleus Mediate Nociceptive Neurotransmission in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Stereotaxic Injections
2.3. Histology
2.4. Whole-Cell Patch-Clamp Recordings
2.5. Fiber Photometry
2.6. Optogenetic Manipulation
2.7. Chemogenetic Manipulation
2.8. Von Frey Filament Test
2.9. Hargreaves Test
2.10. Hotplate Test
2.11. Open Field Test (OFT)
2.12. Elevated plus Maze (EPM) Test
2.13. Formalin Test
2.14. Neuropathic Pain Model
2.15. Statistical Analysis
3. Results
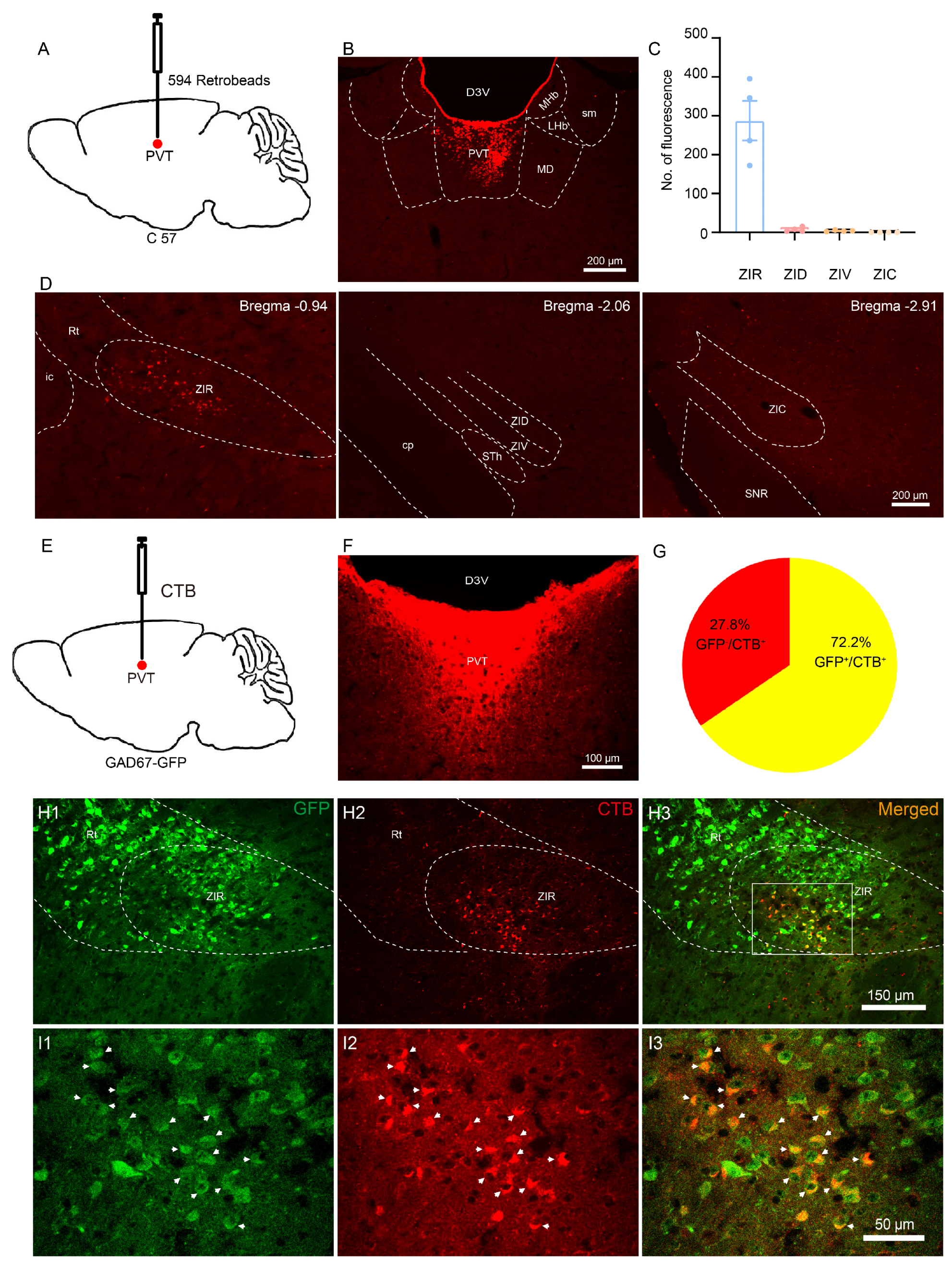
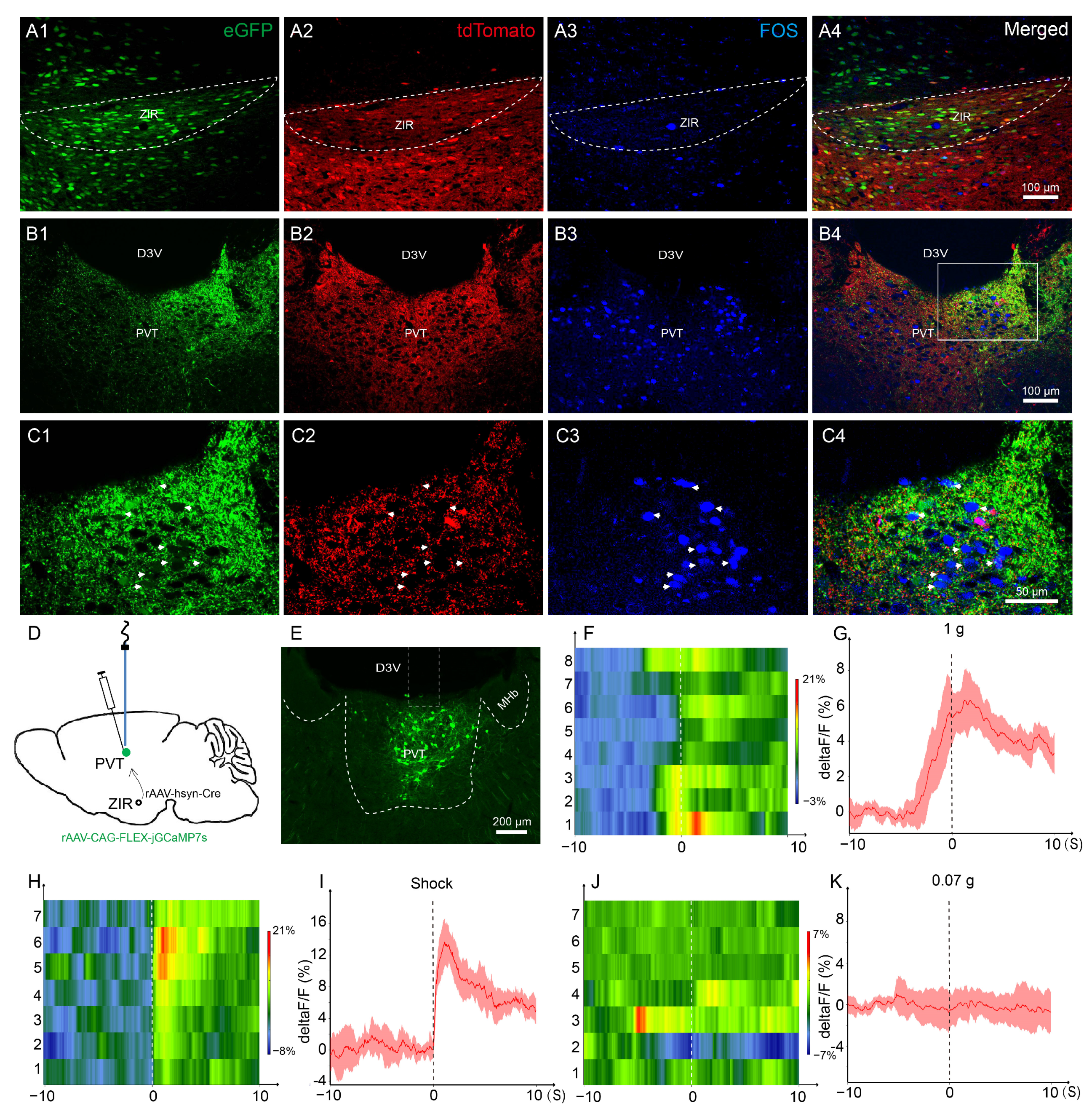
3.1. The ZIRGABA+–PVT Pathway Involvement in Nociception
3.2. In Situ Chemogenetic Activation of the ZIR GABAergic Neurons Attenuates but Inhibition of ZIR Promotes Nociceptive Behaviors
3.3. Optogenetic Activation of the ZIRGABA+–PVT Pathway Alleviates Nociception but Inhibition Induces Hyperalgesia
3.4. Chemogenetic Activation of the ZIRGABA+–PVT Pathway Attenuates Inflammatory Pain and Neuropathic Pain
3.5. The ZIRGABA+–PVT Pathway Modulates Nociception through GABA-A Receptor (GABAAR)-Expressing Neurons in PVT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuner, R.; Kuner, T. Cellular Circuits in the Brain and Their Modulation in Acute and Chronic Pain. Physiol. Rev. 2021, 101, 213–258. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. A functional subdivision within the somatosensory system and its implications for pain research. Neuron 2022, 110, 749–769. [Google Scholar] [CrossRef]
- Mitrofanis, J. Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience 2005, 130, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chou, X.L.; Zhang, L.I.; Tao, H.W. Zona Incerta: An Integrative Node for Global Behavioral Modulation. Trends Neurosci. 2020, 43, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chou, X.; Peng, B.; Shen, L.; Huang, J.J.; Zhang, L.I.; Tao, H.W. A cross-modality enhancement of defensive flight via parvalbumin neurons in zona incerta. eLife 2019, 8, e42728. [Google Scholar] [CrossRef]
- Zhang, X.; van den Pol, A.N. Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science 2017, 356, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Centurion, C.; Luo, S.; Vidal-Ortiz, A.; Swank, C.; Shiromani, P.J. Activity of a subset of vesicular GABA-transporter neurons in the ventral zona incerta anticipates sleep onset. Sleep 2021, 44, zsaa268. [Google Scholar] [CrossRef]
- Craig, A.D. Distribution of trigeminothalamic and spinothalamic lamina I terminations in the macaque monkey. J. Comp. Neurol. 2004, 477, 119–148. [Google Scholar] [CrossRef]
- Shammah-Lagnado, S.J.; Negrão, N.; Ricardo, J.A. Afferent connections of the zona incerta: A horseradish peroxidase study in the rat. Neuroscience 1985, 15, 109–134. [Google Scholar] [CrossRef]
- Petronilho, A.; Reis, G.M.; Dias, Q.M.; Fais, R.S.; Prado, W.A. Antinociceptive effect of stimulating the zona incerta with glutamate in rats. Pharmacol. Biochem. Behav. 2012, 101, 360–368. [Google Scholar] [CrossRef]
- Moon, H.C.; Park, Y.S. Reduced GABAergic neuronal activity in zona incerta causes neuropathic pain in a rat sciatic nerve chronic constriction injury model. J. Pain. Res. 2017, 10, 1125–1134. [Google Scholar] [CrossRef]
- Farzinpour, Z.; Liu, A.; Cao, P.; Mao, Y.; Zhang, Z.; Jin, Y. Microglial Engulfment of Spines in the Ventral Zona Incerta Regulates Anxiety-Like Behaviors in a Mouse Model of Acute Pain. Front. Cell. Neurosci. 2022, 16, 898346. [Google Scholar] [CrossRef]
- Lu, C.W.; Harper, D.E.; Askari, A.; Willsey, M.S.; Vu, P.P.; Schrepf, A.D.; Harte, S.E.; Patil, P.G. Stimulation of zona incerta selectively modulates pain in humans. Sci. Rep. 2021, 11, 8924. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, P.; He, C.; Feng, X.Y.; Huang, Y.; Yang, W.W.; Gao, H.J.; Shen, X.F.; Lin, S.; Cao, S.X.; et al. Incerta-thalamic Circuit Controls Nocifensive Behavior via Cannabinoid Type 1 Receptors. Neuron 2020, 107, 538–551.e537. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.C.; Pleil, K.E. Circuit and neuropeptide mechanisms of the paraventricular thalamus across stages of alcohol and drug use. Neuropharmacology 2021, 198, 108748. [Google Scholar] [CrossRef]
- Jurik, A.; Auffenberg, E.; Klein, S.; Deussing, J.M.; Schmid, R.M.; Wotjak, C.T.; Thoeringer, C.K. Roles of prefrontal cortex and paraventricular thalamus in affective and mechanical components of visceral nociception. Pain 2015, 156, 2479–2491. [Google Scholar] [CrossRef]
- Liang, S.H.; Zhao, W.J.; Yin, J.B.; Chen, Y.B.; Li, J.N.; Feng, B.; Lu, Y.C.; Wang, J.; Dong, Y.L.; Li, Y.Q. A Neural Circuit from Thalamic Paraventricular Nucleus to Central Amygdala for the Facilitation of Neuropathic Pain. J. Neurosci. 2020, 40, 7837–7854. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhu, Y. The paraventricular thalamic nucleus: A key hub of neural circuits underlying drug addiction. Pharmacol. Res. 2019, 142, 70–76. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, T.; Jia, X.; Yuan, J.; Li, X.; Gong, H. Whole-Brain Connectome of GABAergic Neurons in the Mouse Zona Incerta. Neurosci. Bull. 2022, 38, 1315–1329. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, X. Serotonin activates paraventricular thalamic neurons through direct depolarization and indirect disinhibition from zona incerta. J. Physiol. 2021, 599, 4883–4900. [Google Scholar] [CrossRef]
- Li, J.N.; Ren, J.H.; He, C.B.; Zhao, W.J.; Li, H.; Dong, Y.L.; Li, Y.Q. Projections from the lateral parabrachial nucleus to the lateral and ventral lateral periaqueductal gray subregions mediate the itching sensation. Pain 2021, 162, 1848–1863. [Google Scholar] [CrossRef]
- Coggeshall, R.E. Fos, nociception and the dorsal horn. Prog. Neurobiol. 2005, 77, 299–352. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.L.; Brito, R.G.; Matos, J.; Quintans, J.S.S.; Quintans-Júnior, L.J. Fos Protein as a Marker of Neuronal Activity: A Useful Tool in the Study of the Mechanism of Action of Natural Products with Analgesic Activity. Mol. Neurobiol. 2018, 55, 4560–4579. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Murray, K.D. Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: Location, location, location. Epilepsia 2012, 53 (Suppl. 1), 45–52. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Kerby, J.; Bonnert, T.P.; Whiting, P.J.; Wafford, K.A. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br. J. Pharmacol. 2002, 136, 965–974. [Google Scholar] [CrossRef]
- Gajcy, K.; Lochyński, S.; Librowski, T. A role of GABA analogues in the treatment of neurological diseases. Curr. Med. Chem. 2010, 17, 2338–2347. [Google Scholar] [CrossRef]
- Caspary, D.M.; Llano, D.A. Auditory thalamic circuits and GABA(A) receptor function: Putative mechanisms in tinnitus pathology. Hearing Res. 2017, 349, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lavallée, P.; Urbain, N.; Dufresne, C.; Bokor, H.; Acsády, L.; Deschênes, M. Feedforward inhibitory control of sensory information in higher-order thalamic nuclei. J. Neurosci. 2005, 25, 7489–7498. [Google Scholar] [CrossRef]
- Halassa, M.M.; Acsády, L. Thalamic Inhibition: Diverse Sources, Diverse Scales. Trends Neurosci. 2016, 39, 680–693. [Google Scholar] [CrossRef]
- Whitt, J.L.; Masri, R.; Pulimood, N.S.; Keller, A. Pathological activity in mediodorsal thalamus of rats with spinal cord injury pain. J. Neurosci. 2013, 33, 3915–3926. [Google Scholar] [CrossRef]
- Masri, R.; Quiton, R.L.; Lucas, J.M.; Murray, P.D.; Thompson, S.M.; Keller, A. Zona incerta: A role in central pain. J. Neurophysiol. 2009, 102, 181–191. [Google Scholar] [CrossRef]
- Penzo, M.A.; Gao, C. The paraventricular nucleus of the thalamus: An integrative node underlying homeostatic behavior. Trends Neurosci. 2021, 44, 538–549. [Google Scholar] [CrossRef]
- Chang, Y.T.; Chen, W.H.; Shih, H.C.; Min, M.Y.; Shyu, B.C.; Chen, C.C. Anterior nucleus of paraventricular thalamus mediates chronic mechanical hyperalgesia. Pain 2019, 160, 1208–1223. [Google Scholar] [CrossRef]
- Zhang, W.T.; Sha, W.L.; Zhu, Q.; Wu, X.B.; He, C. Plasticity of neuronal excitability and synaptic balance in the anterior nucleus of paraventricular thalamus after nerve injury. Brain Res. Bull. 2022, 188, 1–10. [Google Scholar] [CrossRef]
- Li, S.; Jiang, X.; Wu, Q.; Jin, Y.; He, R.; Hu, J.; Zheng, Y. Electroacupuncture Suppresses CCI-Induced Neuropathic Pain through GABAA Receptors. Evid. Based Complement. Alternat. Med. 2022, 2022, 4505934. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Du, J.; Xi, D.; Shao, F.; Qiu, M.; Shao, X.; Liang, Y.; Liu, B.; Jin, X.; Fang, J.; et al. Role of GABAAR in the Transition From Acute to Chronic Pain and the Analgesic Effect of Electroacupuncture on Hyperalgesic Priming Model Rats. Front. Neurosci. 2021, 15, 691455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, R.X.; Zhang, Y.; Wang, J.; Liu, F.Y.; Cai, J.; Liao, F.F.; Xu, F.Q.; Yi, M.; Wan, Y. Reduced GABAergic transmission in the ventrobasal thalamus contributes to thermal hyperalgesia in chronic inflammatory pain. Sci. Rep. 2017, 7, 41439. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.B.; Liang, S.H.; Li, F.; Zhao, W.J.; Bai, Y.; Sun, Y.; Wu, Z.Y.; Ding, T.; Sun, Y.; Liu, H.X.; et al. dmPFC-vlPAG projection neurons contribute to pain threshold maintenance and antianxiety behaviors. J. Clin. Investig. 2020, 130, 6555–6570. [Google Scholar] [CrossRef]
- Heise, C.E.; Mitrofanis, J. Evidence for a glutamatergic projection from the zona incerta to the basal ganglia of rats. J. Comp. Neurol. 2004, 468, 482–495. [Google Scholar] [CrossRef]
- Mitrofanis, J.; Ashkan, K.; Wallace, B.A.; Benabid, A.L. Chemoarchitectonic heterogeneities in the primate zona incerta: Clinical and functional implications. J. Neurocytol. 2004, 33, 429–440. [Google Scholar] [CrossRef]
- Kolmac, C.; Mitrofanis, J. Distribution of various neurochemicals within the zona incerta: An immunocytochemical and histochemical study. Anat. Embryol. 1999, 199, 265–280. [Google Scholar] [CrossRef]
- Singh, S.; Wilson, T.D.; Valdivia, S.; Benowitz, B.; Chaudhry, S.; Ma, J.; Adke, A.P.; Soler-Cedeño, O.; Velasquez, D.; Penzo, M.A.; et al. An inhibitory circuit from central amygdala to zona incerta drives pain-related behaviors in mice. eLife 2022, 11, e68760. [Google Scholar] [CrossRef]
- Moriya, S.; Yamashita, A.; Masukawa, D.; Setoyama, H.; Hwang, Y.; Yamanaka, A.; Kuwaki, T. Involvement of A13 dopaminergic neurons located in the zona incerta in nociceptive processing: A fiber photometry study. Mol. Brain 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rizzi, G.; Tan, K.R. Zona incerta subpopulations differentially encode and modulate anxiety. Sci. Adv. 2021, 7, eabf6709. [Google Scholar] [CrossRef] [PubMed]
- Chou, X.L.; Wang, X.; Zhang, Z.G.; Shen, L.; Zingg, B.; Huang, J.; Zhong, W.; Mesik, L.; Zhang, L.I.; Tao, H.W. Inhibitory gain modulation of defense behaviors by zona incerta. Nat. Commun. 2018, 9, 1151. [Google Scholar] [CrossRef]
- Nussel, M.; Zhao, Y.; Knorr, C.; Regensburger, M.; Stadlbauer, A.; Buchfelder, M.; Del Vecchio, A.; Kinfe, T. Deep Brain Stimulation, Stereotactic Radiosurgery and High-Intensity Focused Ultrasound Targeting the Limbic Pain Matrix: A Comprehensive Review. Pain Ther. 2022, 11, 459–476. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.-L.; Chen, S.-H.; Li, J.-N.; Zhao, L.-J.; Wu, X.-M.; Hong, J.; Zhu, K.-H.; Sun, H.-X.; Shi, S.-J.; Mao, E.; et al. Projections from the Rostral Zona Incerta to the Thalamic Paraventricular Nucleus Mediate Nociceptive Neurotransmission in Mice. Metabolites 2023, 13, 226. https://doi.org/10.3390/metabo13020226
Wu F-L, Chen S-H, Li J-N, Zhao L-J, Wu X-M, Hong J, Zhu K-H, Sun H-X, Shi S-J, Mao E, et al. Projections from the Rostral Zona Incerta to the Thalamic Paraventricular Nucleus Mediate Nociceptive Neurotransmission in Mice. Metabolites. 2023; 13(2):226. https://doi.org/10.3390/metabo13020226
Chicago/Turabian StyleWu, Feng-Ling, Si-Hai Chen, Jia-Ni Li, Liu-Jie Zhao, Xue-Mei Wu, Jie Hong, Ke-Hua Zhu, Han-Xue Sun, Su-Juan Shi, E Mao, and et al. 2023. "Projections from the Rostral Zona Incerta to the Thalamic Paraventricular Nucleus Mediate Nociceptive Neurotransmission in Mice" Metabolites 13, no. 2: 226. https://doi.org/10.3390/metabo13020226
APA StyleWu, F.-L., Chen, S.-H., Li, J.-N., Zhao, L.-J., Wu, X.-M., Hong, J., Zhu, K.-H., Sun, H.-X., Shi, S.-J., Mao, E., Zang, W.-D., Cao, J., Kou, Z.-Z., & Li, Y.-Q. (2023). Projections from the Rostral Zona Incerta to the Thalamic Paraventricular Nucleus Mediate Nociceptive Neurotransmission in Mice. Metabolites, 13(2), 226. https://doi.org/10.3390/metabo13020226







