The Emerging Therapeutic Role of Prostaglandin E2 Signaling in Pulmonary Hypertension
Abstract
1. Introduction
2. Pathophysiology of PH
3. Pulmonary Vascular Remodeling in PH
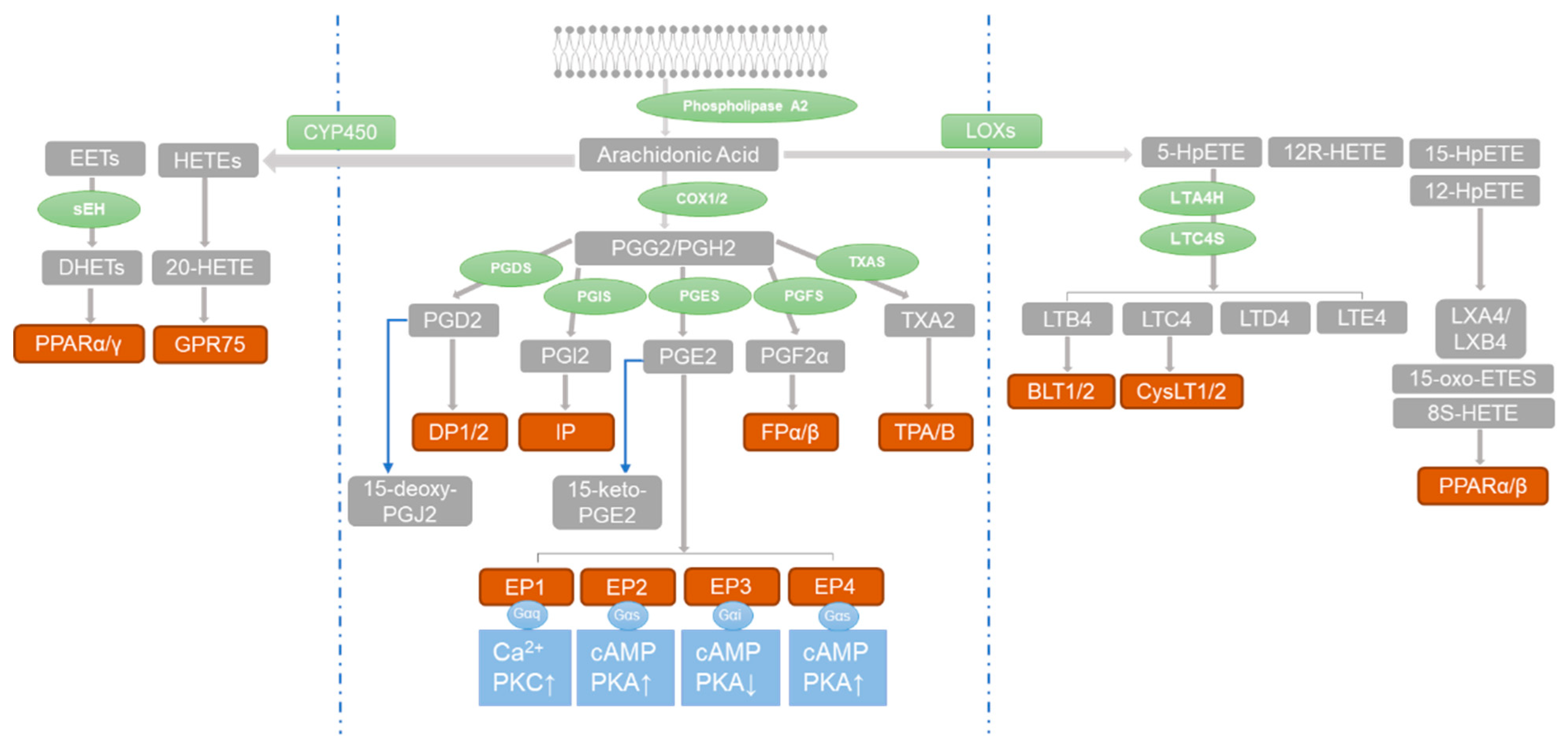
4. Prostaglandins and PH
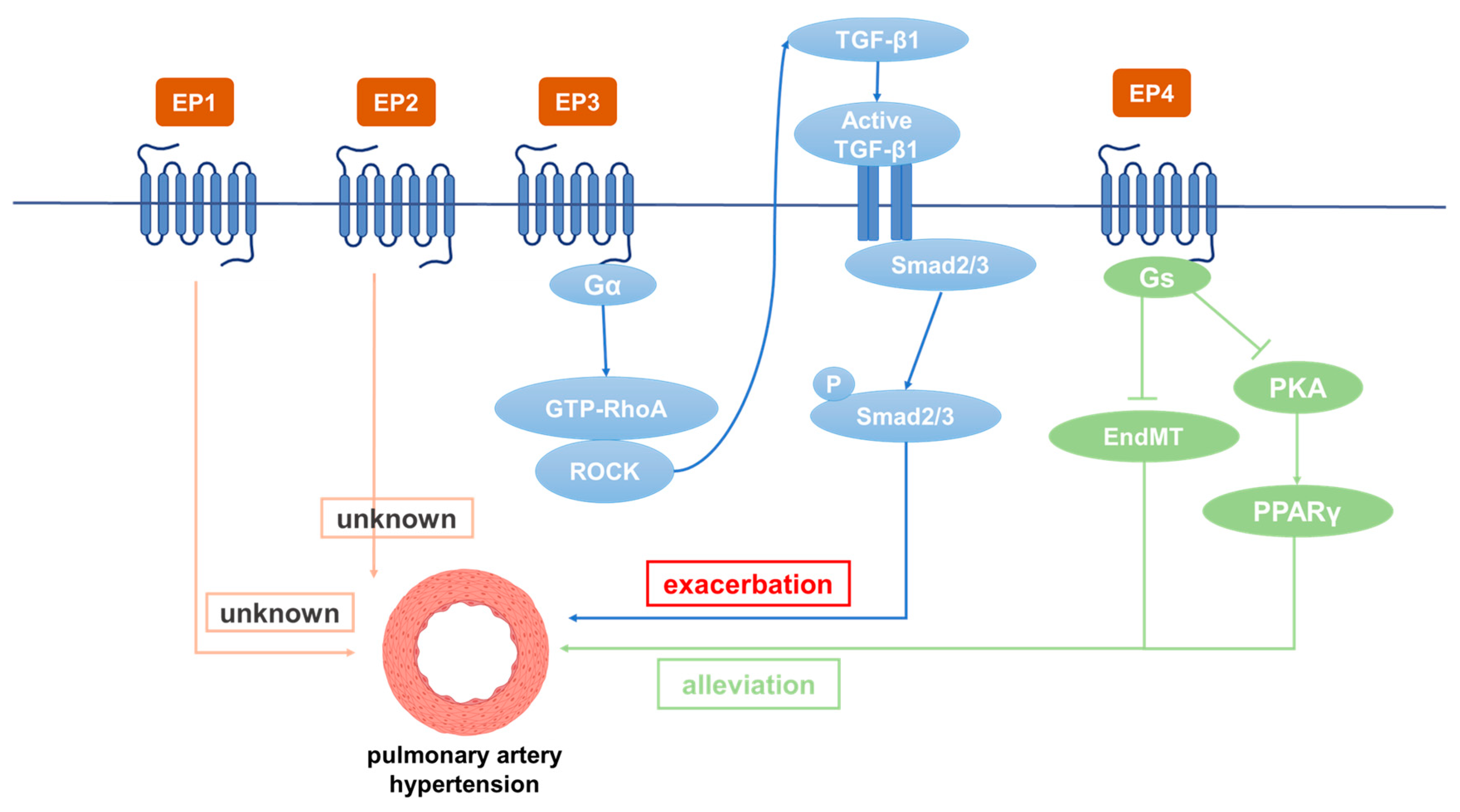
5. Role of PGE2 Receptors in PH
5.1. Role of EP1 in PH
5.2. Role of EP2 in PH
5.3. Role of EP3 in PH
5.4. Role of EP4 in PH
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Chaouat, A.; Naeije, R.; Weitzenblum, E. Pulmonary hypertension in COPD. Eur. Respir. J. 2008, 32, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.; Brida, M. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 61, 2200879. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Richter, M.J.; Rubin, L.J. The physiological basis of pulmonary arterial hypertension. Eur. Respir. J. 2022, 59, 2102334. [Google Scholar] [CrossRef]
- Pregnancy and Abortion in Adolescence. Report of a WHO meeting. World Health Organ. Tech. Rep. Ser. 1975, 583, 1–27.
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and Diagnosis of Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. S25), D42–D50. [Google Scholar] [CrossRef]
- Bourgeois, A.; Omura, J.; Habbout, K.; Bonnet, S.; Boucherat, O. Pulmonary arterial hypertension: New pathophysiological insights and emerging therapeutic targets. Int. J. Biochem. Cell Biol. 2018, 104, 9–13. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef]
- Lau, E.M.T.; Giannoulatou, E.; Celermajer, D.S.; Humbert, M. Epidemiology and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol. 2017, 14, 603–614. [Google Scholar] [CrossRef]
- Chaouat, A.; Bugnet, A.-S.; Kadaoui, N.; Schott, R.; Enache, I.; Ducoloné, A.; Ehrhart, M.; Kessler, R.; Weitzenblum, E. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 172, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Rubin, L.; Hoeper, M.; Jansa, P.; Al-Hiti, H.; Meyer, G.; Chiossi, E.; Kusic-Pajic, A.; Simonneau, G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): A double-blind, randomised controlled trial. Lancet 2008, 371, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, J.; Bauerle, O.; Gomez, A.; Palomar, A.; Guerra, M.L.M.; Furuya, M.E. Primary pulmonary hypertension in children: Clinical characterization and survival. J. Am. Coll. Cardiol. 1995, 25, 466–474. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Kernis, J.T.; et al. Survival in patients with primary pulmonary hypertension. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Ahmetaj-Shala, B.; Kirkby, N.S.; Wright, W.R.; Mackenzie, L.S.; Reed, D.M.; Mohamed, N. Role of prostacyclin in pulmonary hypertension. Glob. Cardiol. Sci. Prac. 2014, 2014, 382–393. [Google Scholar] [CrossRef]
- Safdar, Z. Treatment of pulmonary arterial hypertension: The role of prostacyclin and prostaglandin analogs. Respir. Med. 2011, 105, 818–827. [Google Scholar] [CrossRef]
- Humbert, M.; Sitbon, O.; Simonneau, G. Treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2004, 351, 1425–1436. [Google Scholar] [CrossRef]
- Sitbon, O.; Channick, R.; Chin, K.M.; Frey, A.; Gaine, S.; Galiè, N. Selexipag for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2015, 373, 2522–2533. [Google Scholar] [CrossRef]
- Bubb, K.J.; Trinder, S.L.; Baliga, R.S.; Patel, J.; Clapp, L.H.; MacAllister, R.J.; Hobbs, A.J. Inhibition of phosphodiesterase 2 Augments cgmp and camp signaling to ameliorate pulmonary hypertension. Circulation 2014, 130, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Kirkby, N.S. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br. J. Pharmacol. 2019, 176, 1038–1050. [Google Scholar] [CrossRef]
- Morrell, N.W.; Adnot, S.; Archer, S.L.; Dupuis, J.; Lloyd Jones, P.; MacLean, M.R.; McMurtry, I.F.; Stenmark, K.R.; Thistlethwaite, P.A.; Weissmann, N.; et al. Cellular and molecular basis of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009, 54 (Suppl. S1), S20–S31. [Google Scholar] [CrossRef] [PubMed]
- Sacks, R.S.; Remillard, C.V.; Agange, N.; Auger, W.R.; Thistlethwaite, P.A.; Yuan, J.X.-J. Molecular biology of chronic thromboembolic pulmonary hypertension. Semin. Thorac. Cardiovasc. Surg. 2006, 18, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Clapp, L.H.; Gurung, R. The mechanistic basis of prostacyclin and its stable analogues in pulmonary arterial hypertension: Role of membrane versus nuclear receptors. Prostaglandins Other Lipid Mediat. 2015, 120, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Fishman, A.P. Hypoxia on the pulmonary circulation. How and where it acts. Circ. Res. 1976, 38, 221–231. [Google Scholar] [CrossRef]
- Sajkov, D.; Wang, T.; Saunders, N.A.; Bune, A.J.; Neill, A.M.; Mcevoy, R.D. Daytime pulmonary hemodynamics in patients with obstructive sleep apnea without lung disease. Am. J. Respir. Crit. Care Med. 1999, 159, 1518–1526. [Google Scholar] [CrossRef]
- Southgate, L.; Machado, R.D.; Gräf, S.; Morrell, N.W. Molecular genetic framework underlying pulmonary arterial hypertension. Nat. Rev. Cardiol. 2020, 17, 85–95. [Google Scholar] [CrossRef]
- Zhang, S.; Patel, H.H.; Murray, F.; Remillard, C.V.; Schach, C.; Thistlethwaite, P.A. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L1202–L1210. [Google Scholar] [CrossRef]
- Hassoun, P.M. Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 385, 2361–2376. [Google Scholar] [CrossRef]
- Puetz, S.; Lubomirov, L.T.; Pfitzer, G. Regulation of smooth muscle contraction by small gtpases. Physiology 2009, 24, 342–356. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, H.; Xie, X.-J.; Tao, Y.-K.; He, X.; Roman, R.J.; Aschner, J.L.; Chen, J.-X. Loss of prolyl hydroxylase domain protein 2 in vascular endothelium increases pericyte coverage and promotes pulmonary arterial remodeling. Oncotarget 2016, 7, 58848–58861. [Google Scholar] [CrossRef]
- Ricard, N.; Tu, L.; Le Hiress, M.; Huertas, A.; Phan, C.; Thuillet, R.; Sattler, C.; Fadel, E.; Seferian, A.; Montani, D.; et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle–like cells in pulmonary hypertension. Circulation 2014, 129, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D. Mechanisms of Vascular Remodeling in Hypertension. Am. J. Hypertens. 2021, 34, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [PubMed]
- Adedoyin, O.O.; Loftin, C.D. Microsomal Prostaglandin E Synthase-1 Expression by Aortic Smooth Muscle Cells Attenuates the Differentiated Phenotype. J. Cardiovasc. Pharmacol. 2016, 68, 127–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Humbert, M.; Morrell, N.W.; Archer, S.L.; Stenmark, K.R.; MacLean, M.R.; Lang, I.M.; Christman, B.W.; Weir, E.; Eickelberg, O.; Voelkel, N.F.; et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004, 43 (Suppl. S12), S13–S24. [Google Scholar] [CrossRef]
- Ataya, A.; Patel, S.; Cope, J.; Alnuaimat, H. Pulmonary arterial hypertension and associated conditions. Dis. Mon. 2016, 62, 382–405. [Google Scholar] [CrossRef]
- Price, L.C.; Shao, D.; Meng, C.; Perros, F.; Garfield, B.E.; Zhu, J.; Montani, D.; Dorfmuller, P.; Humbert, M.; Adcock, I.M.; et al. Dexamethasone induces apoptosis in pulmonary arterial smooth muscle cells. Respir. Res. 2015, 16, 114. [Google Scholar] [CrossRef]
- Tang, B.; Chen, G.-X.; Liang, M.-Y.; Yao, J.-P.; Wu, Z.-K. Ellagic acid prevents monocrotaline-induced pulmonary artery hypertension via inhibiting NLRP3 inflammasome activation in rats. Int. J. Cardiol. 2015, 180, 134–141. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef]
- Tozzi, C.A.; Christiansen, D.L.; Poiani, G.J.; Riley, D.J. Excess collagen in hypertensive pulmonary arteries decreases vascular distensibility. Am. J. Respir. Crit. Care Med. 1994, 149, 1317–1326. [Google Scholar] [CrossRef]
- Sommer, N.; Ghofrani, H.A.; Pak, O.; Bonnet, S.; Provencher, S.; Sitbon, O.; Rosenkranz, S.; Hoeper, M.M.; Kiely, D.G. Current and future treatments of pulmonary arterial hypertension. Br. J. Pharmacol. 2021, 178, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Pullamsetti, S.S.; Mamazhakypov, A.; Weissmann, N.; Seeger, W.; Savai, R. Hypoxia-inducible factor signaling in pulmonary hypertension. J. Clin. Investig. 2020, 130, 5638–5651. [Google Scholar] [CrossRef] [PubMed]
- Sang, K.; Zhou, Y.; Li, M.-X. Effect of hypoxia-inducible factor-1α, endothelin-1 and inducible nitric oxide synthase in the pathogenesis of hypoxia-induced pulmonary hypertension of the neonatal rats. Zhonghua Er Ke Za Zhi 2012, 50, 919–924. [Google Scholar] [PubMed]
- Calder, P.C. Eicosanoids. Essays Biochem. 2020, 64, 423–441. [Google Scholar]
- Kelton, J.G.; Blajchman, M.A. Prostaglandin I2 (prostacyclin). Can Med. Assoc. J. 1980, 122, 175–179. [Google Scholar]
- Lee, J.J.; Simmons, D.L. Antipyretic therapy: Clinical pharmacology. Handb. Clin. Neurol. 2018, 157, 869–881. [Google Scholar]
- Hao, C.-M. Breyer Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 2007, 71, 1105–1115. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Zhu, D.; Ran, Y. Role of 15-lipoxygenase/15-hydroxyeicosatetraenoic acid in hypoxia-induced pulmonary hypertension. J. Physiol. Sci. 2012, 62, 163–172. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, L.; Han, W.; Shen, T.; Ma, C.; Liu, Y.; Nie, X.; Liu, M.; Ran, Y.; Zhu, D. Activation of JNK/c-Jun is required for the proliferation, survival, and angiogenesis induced by EET in pulmonary artery endothelial cells. J. Lipid Res. 2012, 53, 1093–1105. [Google Scholar] [CrossRef]
- Loot, A.E.; Fleming, I. Cytochrome P450-Derived Epoxyeicosatrienoic Acids and Pulmonary Hypertension: Central role of transient receptor potential c6 channels. J. Cardiovasc. Pharmacol. 2011, 57, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Jiang, X.; Sung, Y.K.; Qian, J.; Yuan, K.; Nicolls, M.R. Leukotrienes in pulmonary arterial hypertension. Immunol. Res. 2014, 58, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Ruffenach, G.; O’connor, E.; Vaillancourt, M.; Hong, J.; Cao, N.; Sarji, S.; Moazeni, S.; Papesh, J.; Grijalva, V.; Cunningham, C.M.; et al. Oral 15-Hydroxyeicosatetraenoic Acid Induces Pulmonary Hypertension in Mice by Triggering T Cell–Dependent Endothelial Cell Apoptosis. Hypertension 2020, 76, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Qaiser, K.N.; Tonelli, A.R. Novel Treatment Pathways in Pulmonary Arterial Hypertension. Methodist Debakey Cardiovasc. J. 2021, 17, 29–114. [Google Scholar] [CrossRef]
- Somanna, N.K.; Wörner, P.M.; Murthy, S.N.; Pankey, E.A.; Schächtele, D.J.; Hilaire, R.-C.S.; Jansen, D.; Chaffin, A.E.; Nossaman, B.D.; Alt, E.U.; et al. Intratracheal administration of cyclooxygenase-1-transduced adipose tissue-derived stem cells ameliorates monocrotaline-induced pulmonary hypertension in rats. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1187–H1195. [Google Scholar] [CrossRef]
- Chan, P.-C.; Liao, M.-T.; Hsieh, P.-S. The Dualistic Effect of COX-2-Mediated Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2019, 20, 3115. [Google Scholar] [CrossRef]
- Cathcart, M.-C.; Tamosiuniene, R.; Chen, G.; Neilan, T.G.; Bradford, A.; O’Byrne, K.J.; Fitzgerald, D.J.; Pidgeon, G.P. Cyclooxygenase-2-linked attenuation of hypoxia-induced pulmonary hypertension and intravascular thrombosis. J. Pharmacol. Exp. Ther. 2008, 326, 51–58. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, M.; Yu, Y.; Lawson, J.; Funk, C.D.; FitzGerald, G.A. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J. Clin. Investig. 2006, 116, 1391–1399. [Google Scholar] [CrossRef]
- Fedullo, P.; Kerr, K.M.; Kim, N.H.; Auger, W.R. Chronic thromboembolic pulmonary hypertension. N. Engl. J. Med. 2001, 345, 1465–1472. [Google Scholar] [CrossRef]
- Pidgeon, G.P.; Tamosiuniene, R.; Chen, G.; Leonard, I.; Belton, O.; Bradford, A.; Fitzgerald, D.J. Intravascular thrombosis after hypoxia-induced pulmonary hypertension: Regulation by cyclooxygenase-2. Circulation 2004, 110, 2701–2707. [Google Scholar] [CrossRef]
- Jaschinski, C.; Kirilov, M.; Klimpel, H.; Karck, M.; Gorenflo, M.; Loukanov, T. Cyclooxygenase-2 expression in lung in patients with congenital heart malformations and pulmonary arterial hypertension. Thorac. Cardiovasc. Surg. 2013, 61, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Rudic, R.D.; Brinster, D.; Cheng, Y.; Fries, S.; Song, W.-L.; Austin, S.; Coffman, T.M.; FitzGerald, G.A. Cox-2–derived prostacyclin modulates vascular remodeling. Circ. Res. 2005, 96, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Whelton, A.; Hamilton, C.W. Nonsteroidal anti-inflammatory drugs: Effects on kidney function. J. Clin. Pharmacol. 1991, 31, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Ahmetaj-Shala, B.; Kirkby, N.S.; Knowles, R.; Al’yamani, M.; Mazi, S.; Wang, Z.; Tucker, A.T.; Mackenzie, L.S.; Armstrong, P.C.; Nüsing, R.M.; et al. Reply to letter regarding article, “evidence that links loss of cyclooxygenase-2 with increased asymmetric dimethylarginine: Novel explanation of cardiovascular side effects associated with anti-inflammatory drugs”. Circulation 2015, 13, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ricciotti, E.; Scalia, R.; Tang, S.Y.; Grant, G.; Yu, Z.; Landesberg, G.; Crichton, I.; Wu, W.; Puré, E.; et al. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci. Transl. Med. 2012, 4, 132ra54. [Google Scholar] [CrossRef]
- Rakotoniaina, Z.; Guerard, P.; Lirussi, F.; Rochette, L.; Dumas, M.; Goirand, F.; Bardou, M. Celecoxib but not the combination of celecoxib+atorvastatin prevents the development of monocrotaline-induced pulmonary hypertension in the rat. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 378, 241–251. [Google Scholar] [CrossRef]
- Alqarni, A.A.; Brand, O.J.; Pasini, A.; Alahmari, M.; Alghamdi, A.; Pang, L. Imbalanced prostanoid release mediates cigarette smoke-induced human pulmonary artery cell proliferation. Respir. Res. 2022, 23, 136. [Google Scholar] [CrossRef]
- Walch, L.; Labat, C.; Gascard, J.; De Montpreville, V.; Brink, C.; Norel, X. Prostanoid receptors involved in the relaxation of human pulmonary vessels. Br. J. Pharmacol. 1999, 126, 859–866. [Google Scholar] [CrossRef]
- He, Y.; Zuo, C.; Jia, D.; Bai, P.; Kong, D.; Chen, D.; Liu, G.; Li, J.; Wang, Y.; Chen, G.; et al. Loss of DP1 Aggravates Vascular Remodeling in Pulmonary Arterial Hypertension via mTORC1 Signaling. Am. J. Respir. Crit. Care Med. 2020, 201, 1263–1276. [Google Scholar] [CrossRef]
- Santhosh, K.; Elkhateeb, O.; Nolette, N.; Outbih, O.; Halayko, A.; Dakshinamurti, S. Milrinone attenuates thromboxane receptor-mediated hyperresponsiveness in hypoxic pulmonary arterial myocytes. Br. J. Pharmacol. 2011, 163, 1223–1236. [Google Scholar] [CrossRef]
- Ruopp, N.F.; Cockrill, B.A. Diagnosis and Treatment of Pulmonary Arterial Hypertension: A Review. JAMA 2022, 327, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Tian, H.; Geng, J.; Deng, J.; Liu, Y.; Chen, C.; Zhang, S.; Zhang, Y.; Li, J.; Tian, H.; et al. Mechanism of Beraprost Effects on Pulmonary Hypertension: Contribution of Cross-Binding to PGE2 Receptor 4 and Modulation of O(2) Sensitive Voltage-Gated K(+) Channels. Front. Pharmacol. 2019, 9, 1518. [Google Scholar] [CrossRef] [PubMed]
- Abramovitz, M.; Adam, M.; Boie, Y.; Carrière, M.-C.; Denis, D.; Godbout, C.; Lamontagne, S.; Rochette, C.; Sawyer, N.; Tremblay, N.M.; et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim. Biophys. Acta 2000, 1483, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Whittle, B.J.; Silverstein, A.M.; Mottola, D.M.; Clapp, L.H. Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: Treprostinil is a potent DP1 and EP2 agonist. Biochem. Pharmacol. 2012, 84, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, K.; Hashino, A.; Noda, K.; Kosugi, K.; Kuwabara, K. A Long-acting and highly selective prostacyclin receptor agonist prodrug, 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}-N-(methylsulfonyl)acetamide (NS-304), ameliorates rat pulmonary hypertension with unique relaxant responses of its active form, {4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}acetic acid (MRE-269), on rat pulmonary artery. Experiment 2008, 326, 691–699. [Google Scholar]
- Kiriyama, M.; Ushikubi, F.; Kobayashi, T.; Hirata, M.; Sugimoto, Y.; Narumiya, S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997, 122, 217–224. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Y.; Wu, J.; Qi, Z.; Yang, G.; Dou, D.; Gao, Y.; Chen, L.; Zhang, X.; Davis, L.S.; et al. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J. Clin. Investig. 2007, 117, 2496–2505. [Google Scholar] [CrossRef]
- Bartlett, C.S.; Boyd, K.L.; Harris, R.C.; Zent, R.; Breyer, R.M. Ep1 disruption attenuates end-organ damage in a mouse model of hypertension. Hypertension 2012, 60, 1184–1191. [Google Scholar] [CrossRef]
- Norel, X. Prostanoid receptors in the human vascular wall. Sci. World J. 2007, 7, 1359–1374. [Google Scholar] [CrossRef]
- Hirata, T.; Narumiya, S. Prostanoid receptors. Chem. Rev. 2011, 111, 6209–6230. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Pullamsetti, S.S.; Breitenbach, S.C.; Weissmann, N.; Ghofrani, H.A.; Grimminger, F.; Nilius, S.M.; Schrör, K.; Meger-Kirchrath, J.; Seeger, W.; et al. Iloprost-induced desensitization of the prostacyclin receptor in isolated rabbit lungs. Respir. Res. 2007, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Wahn, H.; Wolf, J.; Kram, F.; Frantz, S.; Wagner, J.A. The endocannabinoid arachidonyl ethanolamide (anandamide) increases pulmonary arterial pressure via cyclooxygenase-2 products in isolated rabbit lungs. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2491–H2496. [Google Scholar] [CrossRef] [PubMed]
- Masri, F.A.; Xu, W.; Comhair, S.A.; Asosingh, K.; Koo, M.; Vasanji, A.; Drazba, J.; Anand-Apte, B. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L548–L554. [Google Scholar] [CrossRef] [PubMed]
- Rieg, A.D.; Suleiman, S.; Anker, C.; Verjans, E.; Rossaint, R.; Uhlig, S.; Martin, C. PDGF-BB regulates the pulmonary vascular tone: Impact of prostaglandins, calcium, MAPK- and PI3K/AKT/mTOR signalling and actin polymerisation in pulmonary veins of guinea pigs. Respir. Res. 2018, 19, 120. [Google Scholar] [CrossRef]
- Gao, Y.; Raj, J.U. Role of veins in regulation of pulmonary circulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L213–L226. [Google Scholar] [CrossRef]
- Foudi, N.; Kotelevets, L.; Louedec, L.; Leséche, G.; Henin, D.; Chastre, E.; Norel, X. Vasorelaxation induced by prostaglandin E2 in human pulmonary vein: Role of the EP4 receptor subtype. Br. J. Pharmacol. 2008, 154, 1631–1639. [Google Scholar] [CrossRef]
- Lu, A.; Zuo, C.; He, Y.; Chen, G.; Piao, L.; Zhang, J.; Xiao, B.; Shen, Y.; Tang, J.; Kong, D.; et al. EP3 receptor deficiency attenuates pulmonary hypertension through suppression of Rho/TGF-β1 signaling. J. Clin. Investig. 2015, 125, 1228–1242. [Google Scholar] [CrossRef]
- Patel, J.A.; Shen, L.; Hall, S.M.; Benyahia, C.; Norel, X.; McAnulty, R.J.; Moledina, S.; Silverstein, A.M.; Whittle, B.J.; Clapp, L.H. Prostanoid EP2 Receptors Are Up-Regulated in Human Pulmonary Arterial Hypertension: A Key Anti-Proliferative Target for Treprostinil in Smooth Muscle Cells. Int. J. Mol. Sci. 2018, 19, 2372. [Google Scholar] [CrossRef]
- Benyahia, C.; Boukais, K.; Gomez, I.; Silverstein, A.; Clapp, L.; Fabre, A.; Danel, C.; Leséche, G.; Longrois, D.; Norel, X. A comparative study of PGI2 mimetics used clinically on the vasorelaxation of human pulmonary arteries and veins, role of the DP-receptor. Prostaglandins Other Lipid Mediat. 2013, 107, 48–55. [Google Scholar] [CrossRef]
- Yau, L.; Zahradka, P. PGE(2) stimulates vascular smooth muscle cell proliferation via the EP2 receptor. Mol. Cell. Endocrinol. 2003, 203, 77–90. [Google Scholar] [CrossRef]
- Nikam, V.S.; Wecker, G.; Schermuly, R.; Rapp, U.; Szelepusa, K.; Seeger, W.; Voswinckel, R. Treprostinil inhibits the adhesion and differentiation of fibrocytes via the cyclic adenosine monophosphate–dependent and ras-proximate protein–dependent inactivation of extracellular regulated kinase. Am. J. Respir. Cell Mol. Biol. 2011, 45, 692–703. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Pullamsetti, S.S.; Dony, E.; Weissmann, N.; Butrous, G.; Banat, G.-A.; Ghofrani, H.A.; Seeger, W.; Grimminger, F.; Schermuly, R.T. Role of the prostanoid ep4 receptor in iloprost-mediated vasodilatation in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2008, 178, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fu, J.-L.; Miao, Y.-F.; Wang, C.-J.; Han, Q.-F.; Li, S.; Huang, S.-Z.; Du, S.-N.; Qiu, Y.-X.; Yang, J.-C.; et al. Prostaglandin E2 receptor EP3 regulates both adipogenesis and lipolysis in mouse white adipose tissue. J. Mol. Cell Biol. 2016, 8, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Jones, R.L.; Chan, K.; Stock, A.I.; Ho, J.K. Potent contractile actions of prostanoid EP3-receptor agonists on human isolated pulmonary artery. Br. J. Pharmacol. 1994, 113, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Miao, Y.; Zhang, Y.; Dou, D.; Liu, L.; Tian, X.; Yang, G.; Pu, D.; Zhang, X.; Kang, J.; et al. Inactivation of the E-prostanoid 3 receptor attenuates the angiotensin ii pressor response via decreasing arterial contractility. Arter. Thromb. Vasc. Biol. 2012, 32, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Iwatsubo, K.; Umemura, M.; Fujita, T.; Ishikawa, Y. The prostanoid EP4 receptor and its signaling pathway. Pharmacol. Rev. 2013, 65, 1010–1052. [Google Scholar]
- El Yafawi, R.; Wirth, J.A. What Is the Role of Oral Prostacyclin Pathway Medications in Pulmonary Arterial Hypertension Management? Curr. Hypertens. Rep. 2017, 19, 97. [Google Scholar] [CrossRef]
- Orie, N.N.; Clapp, L.H. Role of prostanoid IP and EP receptors in mediating vasorelaxant responses to PGI2 analogues in rat tail artery: Evidence for Gi/o modulation via EP3 receptors. Eur. J. Pharmacol. 2011, 654, 258–265. [Google Scholar] [CrossRef]
- Benyahia, C.; Ozen, G.; Orie, N.; Ledwozyw, A.; Louedec, L.; Li, F.; Senbel, A.M.; Silverstein, A.; Danel, C.; Longrois, D.; et al. Ex vivo relaxations of pulmonary arteries induced by prostacyclin mimetics are highly dependent of the precontractile agents. Prostaglandins Other Lipid Mediat. 2015, 121 Pt A, 46–52. [Google Scholar] [CrossRef]
- Morrison, K.; Haag, F.; Ernst, R.; Iglarz, M.; Clozel, M. Selective Prostacyclin Receptor Agonist Selexipag, in Contrast to Prostacyclin Analogs, Does Not Evoke Paradoxical Vasoconstriction of the Rat Femoral Artery. J. Pharmacol. Exp. Ther. 2018, 365, 727–733. [Google Scholar] [CrossRef]
- Shen, L.; Patel, J.A.; Norel, X.; Moledina, S.; Whittle, B.J.; von Kessler, K.; Sista, P.; Clapp, L.H. Pharmacology of the single isomer, esuberaprost (beraprost-314d) on pulmonary vascular tone, IP receptors and human smooth muscle proliferation in pulmonary hypertension. Biochem. Pharmacol. 2019, 166, 242–252. [Google Scholar] [PubMed]
- Zhang, J.; Zou, F.; Tang, J.; Zhang, Q.; Gong, Y.; Wang, Q. Cyclooxygenase-2-derived prostaglandin E2 promotes injury-induced vascular neointimal hyperplasia through the E-prostanoid 3 receptor. Circ. Res. 2013, 113, 104–114. [Google Scholar] [PubMed]
- Cheng, Y.; Austin, S.C.; Rocca, B.; Koller, B.H.; Coffman, T.M.; Grosser, T.; Lawson, J.A.; FitzGerald, G.A. Role of Prostacyclin in the Cardiovascular Response to Thromboxane A2. Science 2002, 296, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Wharton, J.; Davie, N.; Upton, P.D.; Yacoub, M.H.; Polak, J.M.; Morrell, N.W. Prostacyclin analogues differentially inhibit growth of distal and proximal human pulmonary artery smooth muscle cells. Circulation 2000, 102, 3130–3136. [Google Scholar] [CrossRef]
- Desai, S.; April, H.; Nwaneshiudu, C.; Ashby, B. Comparison of agonist-induced internalization of the human ep2 and ep4 prostaglandin receptors: Role of the carboxyl terminus in ep4 receptor sequestration. Mol. Pharmacol. 2000, 58, 1279–1286. [Google Scholar] [PubMed]
- Barst, R.J.; McGoon, M.; McLaughlin, V.; Tapson, V.; Oudiz, R.; Shapiro, S.; Robbins, I.M.; Channick, R.; Badesch, D.; Rayburn, B.K.; et al. Beraprost therapy for pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2003, 41, 2119–2125. [Google Scholar] [PubMed]
- Sugimoto, Y.; Narumiya, S. Prostaglandin E receptors. J. Biol. Chem. 2007, 282, 11613–11617. [Google Scholar]
- Falcetti, E.; Hall, S.M.; Phillips, P.G.; Patel, J.; Morrell, N.W.; Haworth, S.G.; Clapp, L.H. Smooth muscle proliferation and role of the prostacyclin (ip) receptor in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2010, 182, 1161–1170. [Google Scholar] [CrossRef]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar]
- Higuchi, S.; Fujikawa, R.; Ikedo, T.; Hayashi, K.; Yasui, M.; Nagata, M.; Nakatsuji, M.; Yokode, M.; Minami, M. EP4 Receptor–Associated Protein in Macrophages Protects against Bleomycin-Induced Pulmonary Inflammation in Mice. J. Immunol. 2016, 197, 4436–4443. [Google Scholar]
- Li, M.; Riddle, S.R.; Frid, M.G.; El Kasmi, K.C.; McKinsey, T.A.; Sokol, R.J.; Strassheim, D.; Meyrick, B.; Yeager, M.E.; Flockton, A.R.; et al. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J. Immunol. 2011, 187, 2711–2722. [Google Scholar] [PubMed]
- Gill, S.K.; Yao, Y.; Kay, L.J.; Bewley, M.A.; Marriott, H.M.; Peachell, P.T. The anti-inflammatory effects of PGE(2) on human lung macrophages are mediated by the EP(4) receptor. Br. J. Pharmacol. 2016, 173, 3099–3109. [Google Scholar] [CrossRef] [PubMed]
- Birrell, M.A.; Maher, S.A.; Dekkak, B.; Jones, V.; Wong, S.; Brook, P.; Belvisi, M.G. Anti-inflammatory effects of PGE2 in the lung: Role of the EP4 receptor subtype. Thorax 2015, 70, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Du, S.; Fang, B.; Li, C.; Jia, X.; Zheng, S.; Wang, S.; Li, Q.; Su, W.; Wang, N.; et al. VSMC-specific EP4 deletion exacerbates angiotensin II-induced aortic dissection by increasing vascular inflammation and blood pressure. Proc. Natl. Acad. Sci. USA 2019, 116, 8457–8462. [Google Scholar] [CrossRef] [PubMed]
- Harari, S.; Elia, D.; Humbert, M. Pulmonary Hypertension in Parenchymal Lung Diseases: Any Future for New Therapies? Chest 2018, 153, 217–223. [Google Scholar] [CrossRef]
- Ozen, G.; Benyahia, C.; Mani, S.; Boukais, K.; Silverstein, A.M.; Bayles, R.; Nelsen, A.C.; Castier, Y.; Danel, C.; Mal, H.; et al. Bronchodilation induced by PGE(2) is impaired in Group III pulmonary hypertension. Br. J. Pharmacol. 2020, 177, 161–174. [Google Scholar] [CrossRef]
- Aso, H.; Ito, S.; Mori, A.; Suganuma, N.; Morioka, M.; Takahara, N.; Kondo, M.; Hasegawa, Y. Differential regulation of airway smooth muscle cell migration by e-prostanoid receptor subtypes. Am. J. Respir. Cell Mol. Biol. 2013, 48, 322–329. [Google Scholar] [CrossRef]
- Ameshima, S.; Golpon, H.; Cool, C.D.; Chan, D.; Vandivier, R.W.; Gardai, S.J. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ. Res. 2003, 92, 1162–1169. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Chen, I.-C.; Li, H.-H.; Huang, C.-C. EP4 Agonist L-902,688 Suppresses EndMT and Attenuates Right Ventricular Cardiac Fibrosis in Experimental Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2018, 19, 727. [Google Scholar] [CrossRef]
- Li, H.H.; Hsu, H.H.; Chang, G.J.; Chen, I.C.; Ho, W.J.; Hsu, P.C. Prostanoid EP(4) agonist L-902,688 activates PPARγ and attenuates pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L349–L359. [Google Scholar] [CrossRef]
- Li, C.; Mpollo, M.-S.E.M.; Gonsalves, C.S.; Tahara, S.M.; Malik, P.; Kalra, V.K.; Mansour, W.; Nakasone, M.A.; von Delbrueck, M.; Yu, Z.; et al. Peroxisome proliferator-activated receptor-α-mediated transcription of mir-199a2 attenuates endothelin-1 expression via hypoxia-inducible factor-1α. J. Biol. Chem. 2014, 289, 36031–36047. [Google Scholar] [CrossRef] [PubMed]
| Three Classic Drugs for the Treatment of PH | Classification | Drug Name | Administration Method | Target | Therapeutic Features | Advantages | Disadvantages | Preference |
|---|---|---|---|---|---|---|---|---|
| 1 | PGI2 analogues | Epoprostenol | Intravenous | IP | Slow, incremental and individualized dosing where the patient is closely monitored for tolerability. In most case, PGI2 analogues are reserved for patients with severe PH. | Exercise tolerance, hemodynamics, long-term survival and mortality of patients with PH has improved. | The half-life at room temperature is very short, requiring permanent intravenous catheter continuous infusion, causing infection and pain at the site of injection. It is complicated, uncomfortable for patients, and very costly. Common side effects: systemic hypotension, flushing, jaw pain and nausea. Its serious side effects: catheter associated sepsis. | Preferred drug; In North America and in some European countries since the mid-1990s |
| Treprostinil | Subcutaneous, intravenous, inhalation and oral | A stable PGI2 analogue; Indexes of dyspnea, signs, symptoms and exercise capacity of PH, and hemodynamic measures significantly improve. | Causing infection and pain at the site of injection. Common side effects: systemic hypotension, flushing, jaw pain and nausea. | Alternative drug; In the United States since 2002 | ||||
| Iloprost | Inhalation | A chemically stable PGI2 analogue; Hemodynamic values were significantly improved. | Its relatively short duration of action; It must be inhaled as many as 6 to 12 times a day; Side effects included cough and symptoms linked to systemic vasodilatation; It makes patients with PH have a higher rate of syncope. | In Japan | ||||
| Beraprost | Oral | The first biologically stable and orally PGI2 analogue which is absorbed rapidly; The peak concentration was reached 30 minutes after oral administration; With a half-life of 35–40 min. | There was no significant change in cardiovascular hemodynamics. | For treating primary PH in Europe. | ||||
| IP selective agonist | Selexipag | Oral | Specific for IP, it has little or no effect on other prostanoid receptors; The risk of the primary composite end point of death or a complication related to PAH was significantly improved. | Side effects: headache, diarrhea, systemic hypotension, flushing, jaw pain and nausea. | ||||
| 2 | Endothelin receptor antagonists | Bosentan | Oral | ETAR and ETBR | 125 mg/bid Monthly monitoring of liver function tests is mandatory. | Significant improvements in PAP, cardiac output, and PVR | Development of abnormal hepatic function; It is contraindicated during pregnancy because of its teratogenic potential; Its long-term requires further evaluation. | For the treatment of PAH in North America in 2001 and in Europe in 2002. |
| 3 | Phosphodiesterase inhibitors | Sildenafil | Oral | PDE5 | long-term adjunctive treatment can improve exercise capacity and pulmonary hemodynamics. | The experience with sildenafil is preliminary, and controlled studies are in progress to determine its efficacy, side effects, and safety. |
| PGI2 Analogues | IP | EP1 | EP2 | EP3 | EP4 | |
|---|---|---|---|---|---|---|
| Iloprost | Human | 4 | 1 | 1172 | 203 | 212 |
| Mouse | 11 | 21 | 1600 | 27 | 2300 | |
| Treprostinil | Human | 32 | 212 | 3.6 | 2505 | 826 |
| Mouse | YES | ND | YES | ND | ND | |
| Beraprost | Human | 39 | 680 | |||
| Mouse | 16 | 110 | ||||
| Cicaprost | Human | 17 | >1340 | >1340 | 255 | 44 |
| Mouse | 10 | 1300 | 170 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Wang, B.; Xu, H.; Zhang, X. The Emerging Therapeutic Role of Prostaglandin E2 Signaling in Pulmonary Hypertension. Metabolites 2023, 13, 1152. https://doi.org/10.3390/metabo13111152
Ye L, Wang B, Xu H, Zhang X. The Emerging Therapeutic Role of Prostaglandin E2 Signaling in Pulmonary Hypertension. Metabolites. 2023; 13(11):1152. https://doi.org/10.3390/metabo13111152
Chicago/Turabian StyleYe, Lan, Bing Wang, Hu Xu, and Xiaoyan Zhang. 2023. "The Emerging Therapeutic Role of Prostaglandin E2 Signaling in Pulmonary Hypertension" Metabolites 13, no. 11: 1152. https://doi.org/10.3390/metabo13111152
APA StyleYe, L., Wang, B., Xu, H., & Zhang, X. (2023). The Emerging Therapeutic Role of Prostaglandin E2 Signaling in Pulmonary Hypertension. Metabolites, 13(11), 1152. https://doi.org/10.3390/metabo13111152






