Supplementing Low-Sodium Bicarbonate–Calcic (Lete)® Water: Effects in Women on Bone and Systemic Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Inclusion and Exclusion Criteria
2.3. Study Design
2.4. Clinical Assessment and Intervention
2.5. Dietary Assessment and Intervention
2.6. Sample Pre-Treatment for NMR Analysis
2.7. NMR Data Acquisition and Processing
2.8. Statistical Analysis
3. Results
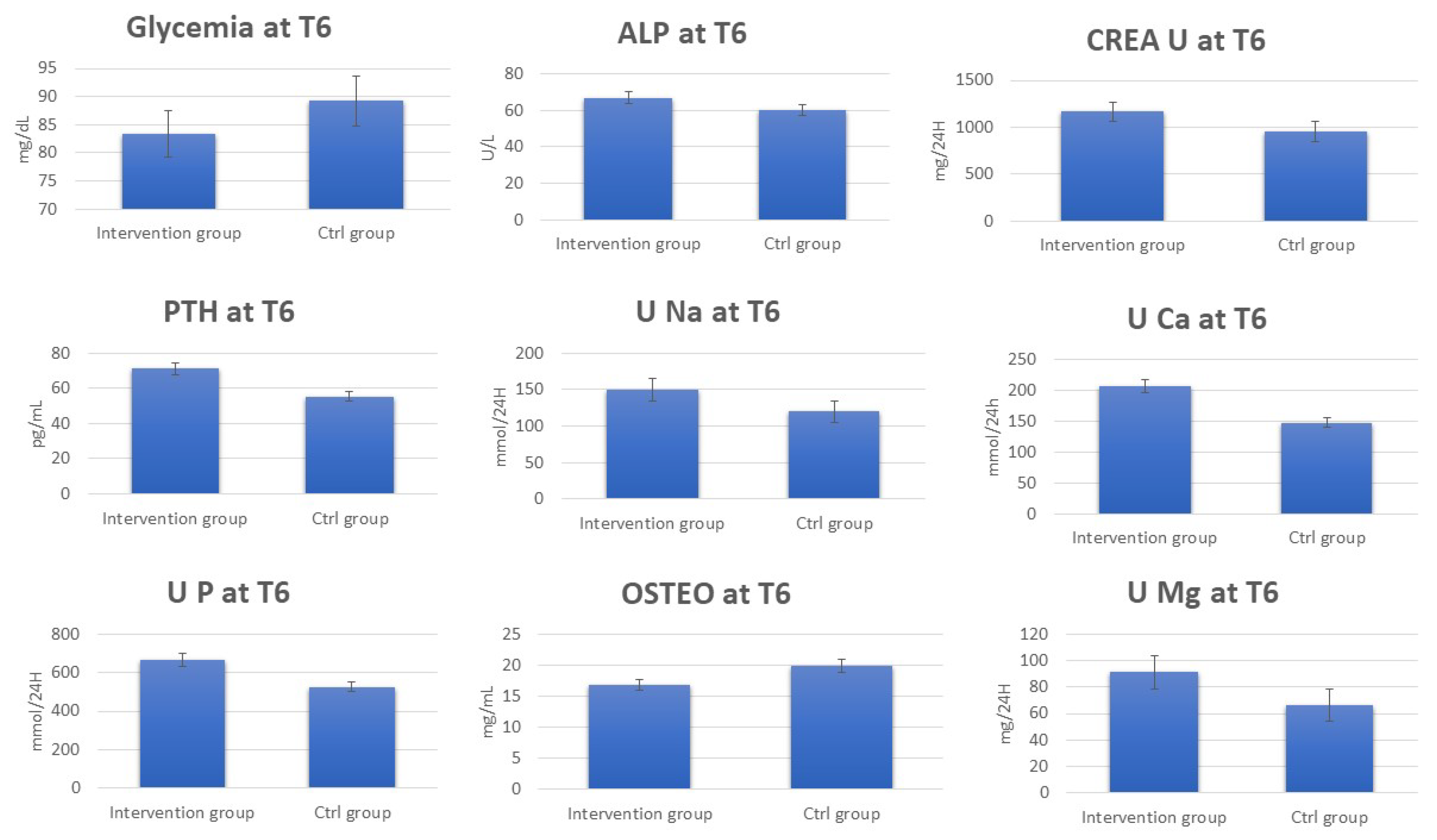
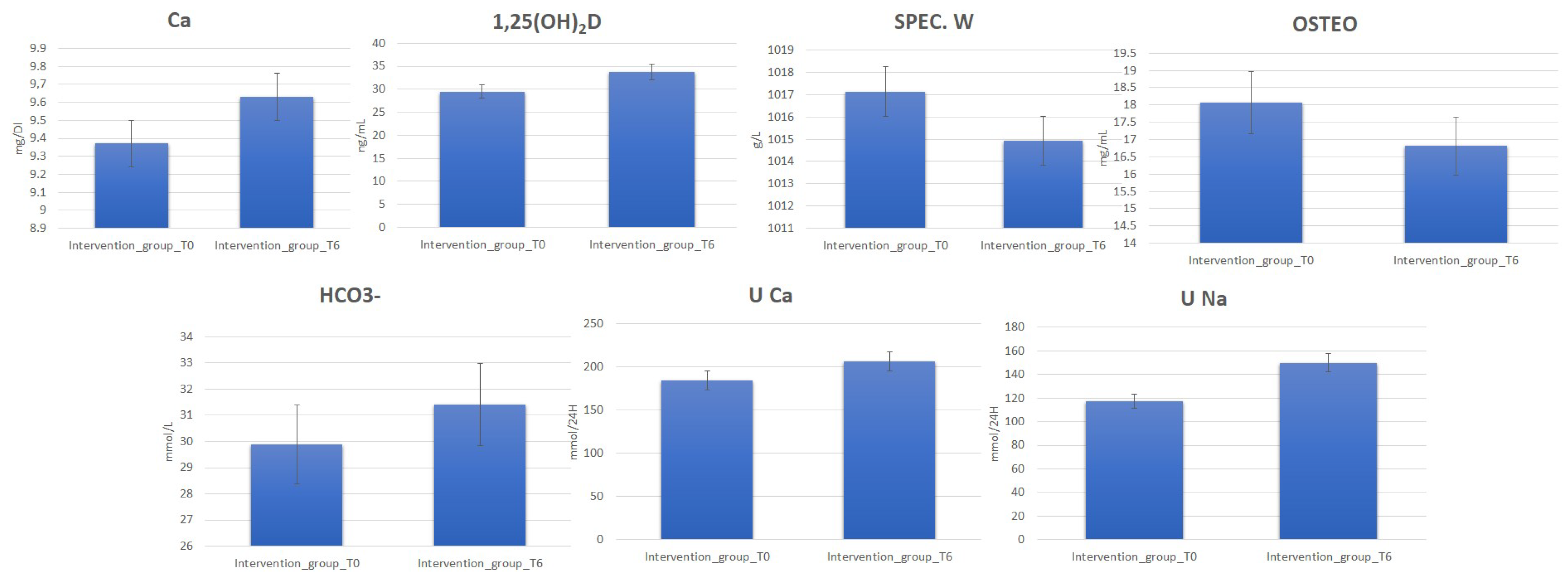
3.1. Clinical Analysis
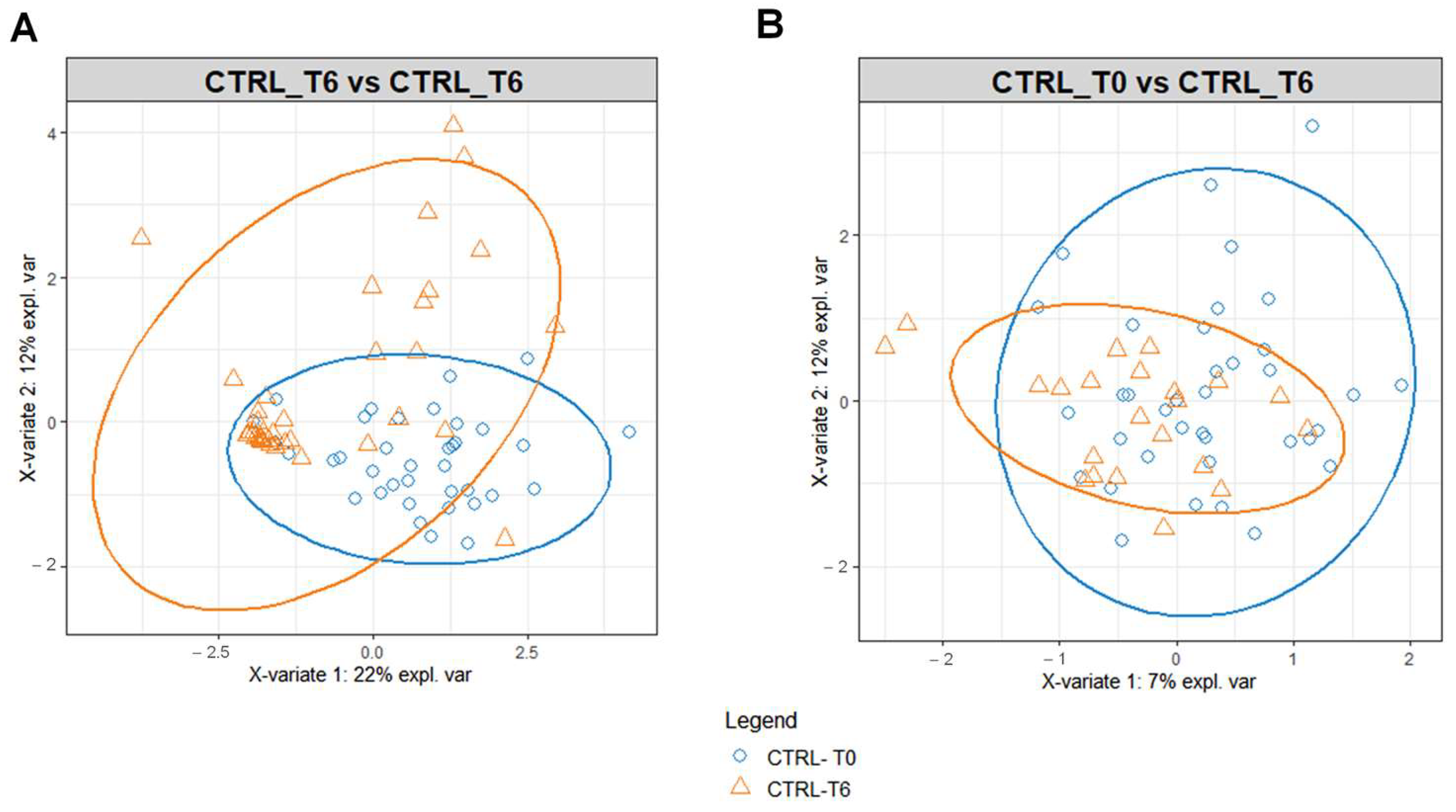
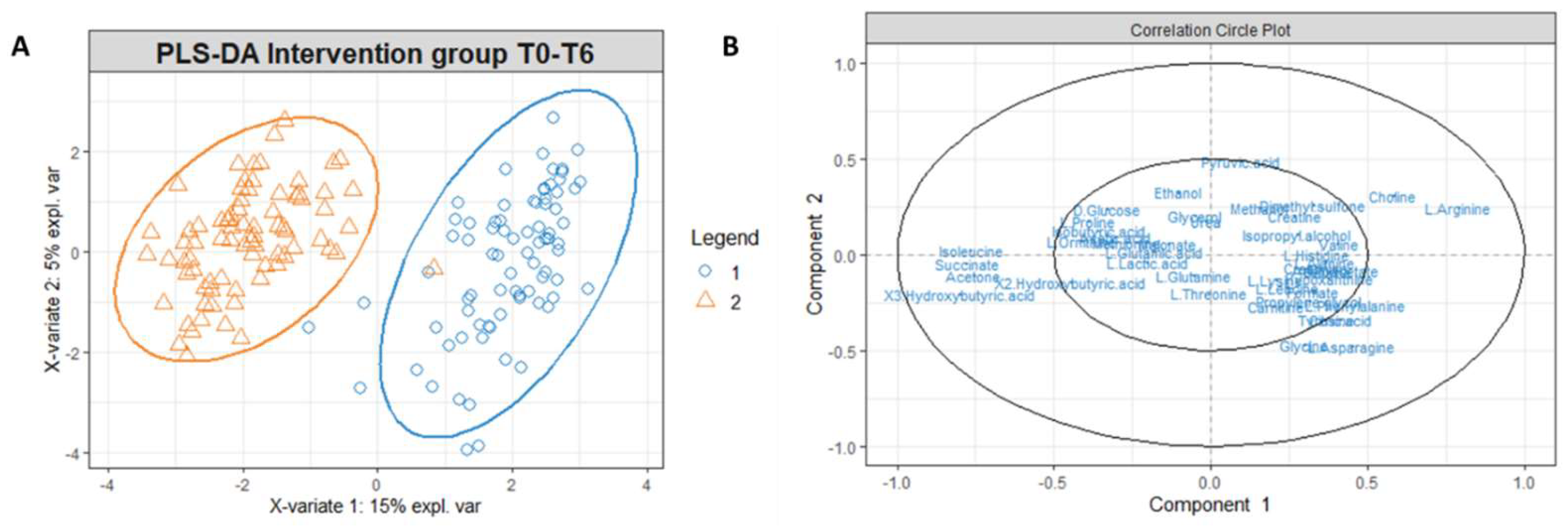
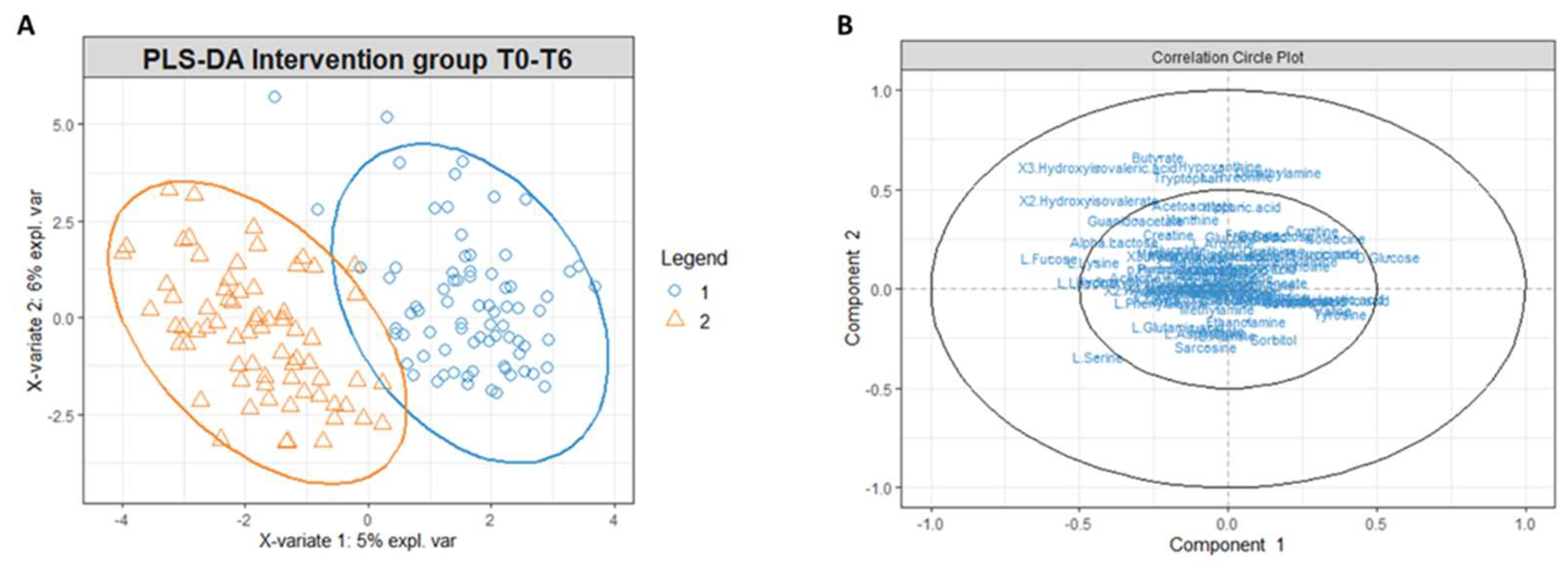
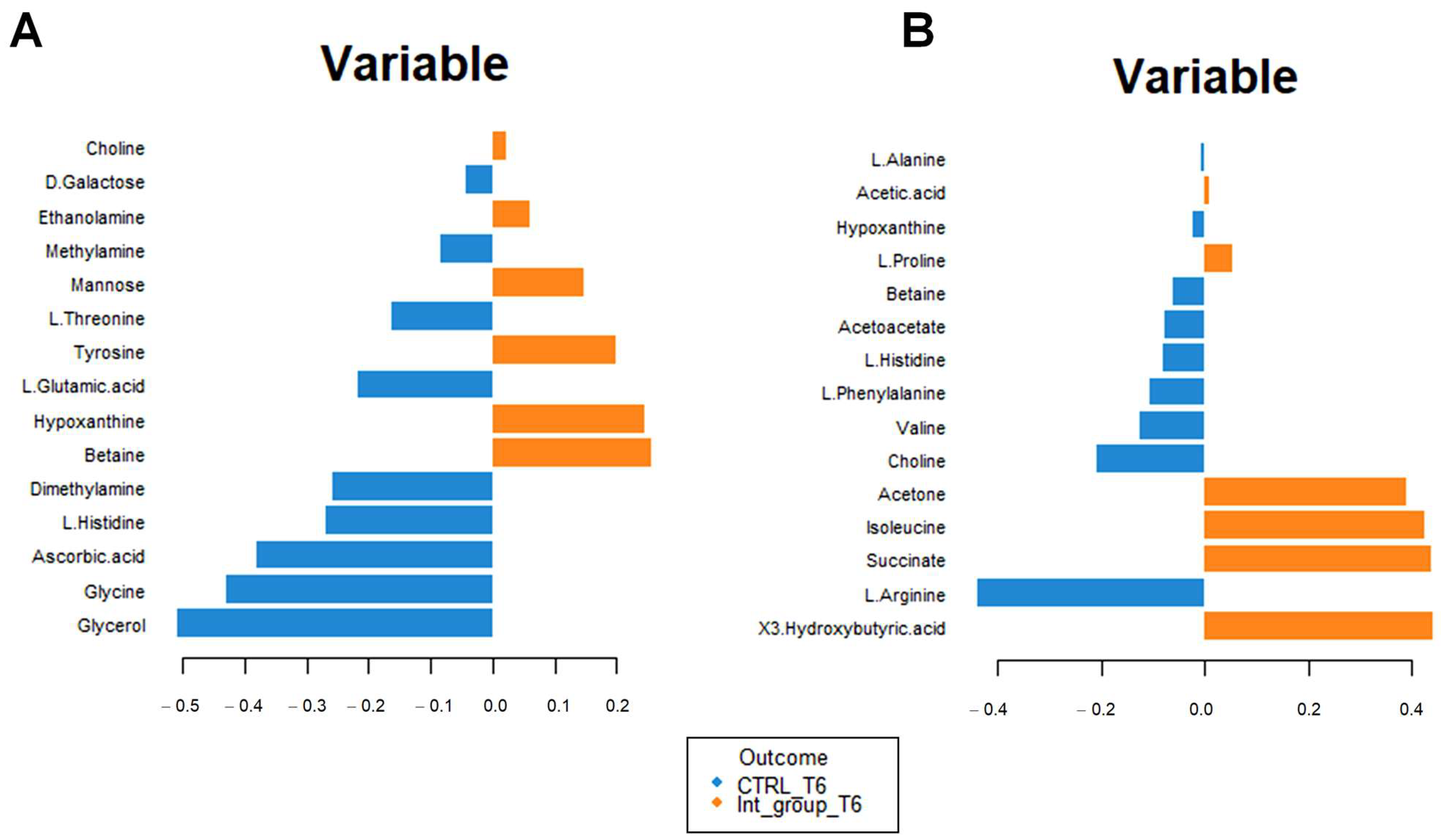
3.2. Multivariate Statistical and Pathway Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, C.M. The role of nutrition on optimizing peak bone mass. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 135–137. [Google Scholar] [PubMed]
- Flynn, A. The role of dietary calcium in bone health. Proc. Nutr. Soc. 2003, 62, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Knoche, M. Calcium and the physiology of sweet cherries: A review. Sci. Hortic. 2019, 245, 107–115. [Google Scholar] [CrossRef]
- Power, M.L.; Heaney, R.P.; Kalkwarf, H.J.; Pitkin, R.M.; Repke, J.T.; Tsang, R.C.; Schulkin, J. The role of calcium in health and disease. Am. J. Obstet. Gynecol. 1999, 181, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Dowell, M.S. Absorbability of the calcium in a high-calcium mineral water. Osteoporos. Int. 1994, 4, 323–324. [Google Scholar] [CrossRef] [PubMed]
- DeGrazia, J.A.; Ivanovich, P.; Fellows, H.; Rich, C. A double isotope method for measurement of intestinal absorption of calcium in man. J. Lab. Clin. Med. 1965, 66, 822–829. [Google Scholar]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Mente, A. High Urinary Calcium Excretion and Familial Aggregation of Hypertension, Kidney Stone Disease, Obesity, Excessive Weight Gain and Type 2 Diabetes in Patients with Calcareous Stones; University of Toronto: Toronto, ON, Canada, 2007. [Google Scholar]
- Shadman, A.; Bastani, B. Kidney calculi: Pathophysiology and as a systemic disorder. Iran. J. Kidney Dis. 2017, 11, 180. [Google Scholar]
- Fitzpatrick, L.A. Secondary causes of osteoporosis. Mayo Clin. Proc. 2002, 77, 453–468. [Google Scholar] [CrossRef]
- Heaney, R.P. Pathophysiology of osteoporosis. Endocrinol. Metab. Clin. N. Am. 1998, 27, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Bacciottini, L.; Tanini, A.; Falchetti, A.; Masi, L.; Franceschelli, F.; Pampaloni, B.; Giorgi, G.; Brandi, M.L. Calcium bioavailability from a calcium-rich mineral water, with some observations on method. J. Clin. Gastroenterol. 2004, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Carpintero, P.; Gil-Garay, E.; Hernández-Vaquero, D.; Ferrer, H.; Munuera, L. Interventions to improve inpatient osteoporosis management following first osteoporotic fracture: The PREVENT project. Arch. Orthop. Trauma Surg. 2009, 129, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Rylander, R.; Arnaud, M.J. Mineral water intake reduces blood pressure among subjects with low urinary magnesium and calcium levels. BMC Public Health 2004, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.J.N. Calcium intake in bone health: A focus on calcium-rich mineral waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Handbook on Indoor Radon: A Public Health Perspective; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Quattrini, S.; Pampaloni, B.; Brandi, M.L. Natural mineral waters: Chemical characteristics and health effects. Clin. Cases Miner. Bone Metab. 2016, 13, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Cotruvo, J.A.; Bartram, J. Calcium and Magnesium in Drinking-Water: Public Health Significance; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- European Union. Directive 54/EC of the European Parliament and of the Council of 18 June 2009 on the exploitation and marketing of natural mineral waters. Off. J. Eur. Union 2009, 164. [Google Scholar]
- Halpern, G.M.; Van de Water, J.; Delabroise, A.-M.; Keen, C.L.; Gershwin, M.E. Comparative uptake of calcium from milk and a calcium-rich mineral water in lactose intolerant adults: Implications for treatment of osteoporosis. Am. J. Prev. Med. 1991, 7, 379–383. [Google Scholar] [CrossRef]
- Couzy, F.; Kastenmayer, P.; Vigo, M.; Clough, J.; Munoz-Box, R.; Barclay, D.V. Calcium bioavailability from a calcium-and sulfate-rich mineral water, compared with milk, in young adult women. Am. J. Clin. Nutr. 1995, 62, 1239–1244. [Google Scholar] [CrossRef]
- Greupner, T.; Schneider, I.; Hahn, A. Calcium bioavailability from mineral waters with different mineralization in comparison to milk and a supplement. J. Am. Coll. Nutr. 2017, 36, 386–390. [Google Scholar] [CrossRef]
- Guillemant, J.; Le, H.; Guillemant, S.; Delabroise, A.; Arnaud, M.J. Acute effects induced by a calcium-rich mineral water on calcium metabolism and on parathyroid function. Osteoporos. Int. 1997, 7, 85–86. [Google Scholar] [CrossRef]
- Wynn, E.; Raetz, E.; Burckhardt, P.J. The composition of mineral waters sourced from Europe and North America in respect to bone health: Composition of mineral water optimal for bone. Br. J. Nutr. 2008, 101, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Van Dokkum, W.; De La Gueronniere, V.; Schaafsma, G.; Bouley, C.; Luten, J.; Latge, C. Bioavailability of calcium of fresh cheeses, enteral food and mineral water. A study with stable calcium isotopes in young adult women. Br. J. Nutr. 1996, 75, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Aptel, I.; Cance-Rouzaud, A.; Grandjean, H.; Epidos Study Group. Association between calcium ingested from drinking water and femoral bone density in elderly women: Evidence from the EPIDOS cohort. J. Bone Miner. Res. 1999, 14, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Guillemant, J.; Le, H.-T.; Accarie, C.; du Montcel, S.T.; Delabroise, A.-M.; Arnaud, M.J.; Guillemant, S. Mineral water as a source of dietary calcium: Acute effects on parathyroid function and bone resorption in young men. Am. J. Clin. Nutr. 2000, 71, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Massey, L.K.; Whiting, S.J. Dietary salt, urinary calcium, and bone loss. J. Bone Miner. Res. 1996, 11, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Modern analytical techniques in metabolomics analysis. Analyst 2012, 137, 293–300. [Google Scholar] [CrossRef]
- Utpott, M.; Rodrigues, E.; de Oliveira Rios, A.; Mercali, G.D.; Flôres, S.H. Metabolomics: An analytical technique for food processing evaluation. Food Chem. 2022, 366, 130685. [Google Scholar] [CrossRef]
- Battistini, N.; Caselli, D.; Bedogni, G.; Gatti, G. Food intake in university students and its impact on nutritional status. Nutr. Res. 1992, 12, 223–233. [Google Scholar] [CrossRef]
- Bernini, P.; Bertini, I.; Luchinat, C.; Nincheri, P.; Staderini, S.; Turano, P. Standard operating procedures for pre-analytical handling of blood and urine for metabolomic studies and biobanks. J. Biomol. NMR 2011, 49, 231–243. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Ryan, D.; Brennan, L.; Tenori, L.; Luchinat, C.; Gao, X.; Zeri, A.C.; Gowda, G.N.; et al. Recommendations and standardization of biomarker quantification using NMR-based metabolomics with particular focus on urinary analysis. J. Proteome Res. 2016, 15, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, S.; Liu, P.; Bjordahl, T.C.; Mandal, R.; Grant, J.R.; Wilson, M.; Eisner, R.; Sinelnikov, I.; Hu, X.; Luchinat, C. Accurate, fully-automated NMR spectral profiling for metabolomics. PLoS ONE 2015, 10, e0124219. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Nakaya, A. The KEGG databases at GenomeNet. Nucleic Acids Res. 2002, 30, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Pang, Z.; Lu, Y.; Ewald, J.; Xia, J. OmicsNet 2.0: A web-based platform for multi-omics integration and network visual analytics. Nucleic Acids Res. 2022, 50, W527–W533. [Google Scholar] [CrossRef] [PubMed]
- Lipovetsky, S. Book Review: Multivariate Data Integration Using R: Methods and Applications with the mixOmics Package, by Kim-Ahn Lê Cao and Zoe Welham; CRC/CRC Press: Boca Raton, FL, USA; Chapman and Hall: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2022; pp. xxi+308. ISBN 9780367460945. [Google Scholar]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Parsons, L.C. Osteoporosis: Incidence, prevention, and treatment of the silent killer. Nurs. Clin. 2005, 40, 119–133. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Lau, E.M.C.; Woo, J. Nutrition and osteoporosis. Curr. Opin. Rheumatol. 1998, 10, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, S.; Fazelian, S.; Farahbod, F.; Moradi, F.; Dehghan, M. Effect of Alkaline Drinking Water on Bone Density of Postmenopausal Women with Osteoporosis. J. Menopausal Med. 2021, 27, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wynn, E.; Krieg, M.A.; Aeschlimann, J.M.; Burckhardt, P. Alkaline mineral water lowers bone resorption even in calcium sufficiency: Alkaline mineral water and bone metabolism. Bone 2009, 44, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Pagano, I.; Rastrelli, L. Calcium from a calcium-rich mineral water: Supplementation and bioavailability. Pharmacologyonline 2017, 1, 132–138. [Google Scholar]
- Da, W.; Tao, L.; Zhu, Y. The Role of Osteoclast Energy Metabolism in the Occurrence and Development of Osteoporosis. Front. Endocrinol. 2021, 12, 675385. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Guntur, A.R.; Long, F.; Rosen, C.J. Energy Metabolism of the Osteoblast: Implications for Osteoporosis. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Harada, D.; Nagamachi, S.; Aso, K.; Ikeda, K.; Takahashi, Y.; Furuse, M. Oral administration of l-ornithine increases the content of both collagen constituting amino acids and polyamines in mouse skin. Biochem. Biophys. Res. Commun. 2019, 512, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hu, G.; Karner, C.M. Bioenergetic Metabolism In Osteoblast Differentiation. Curr. Osteoporos. Rep. 2022, 20, 53–64. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, J.; Liu, H.; Wu, Q.; Chen, J.; Chen, G.Q. The mechanism of anti-osteoporosis effects of 3-hydroxybutyrate and derivatives under simulated microgravity. Biomaterials 2014, 35, 8273–8283. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, B.; Shi, Z.; Wu, Q.; Chen, G.-Q. The effect of 3-hydroxybutyrate on the in vitro differentiation of murine osteoblast MC3T3-E1 and in vivo bone formation in ovariectomized rats. Biomaterials 2007, 28, 3063–3073. [Google Scholar] [CrossRef]
- Qiu, C.; Yu, F.; Su, K.; Zhao, Q.; Zhang, L.; Xu, C.; Hu, W.; Wang, Z.; Zhao, L.; Tian, Q.; et al. Multi-omics Data Integration for Identifying Osteoporosis Biomarkers and Their Biological Interaction and Causal Mechanisms. iScience 2020, 23, 100847. [Google Scholar] [CrossRef]



| Demographics and Clinical Information | Intervention Group | Control Group |
|---|---|---|
| Age (years) (average ± SD) | 47.04 ± 4.63 | 47.00 ± 3.81 |
| Height (cm) (average ± SD) | 163.67 ± 6.20 | 161.19 ± 6.31 |
| Weight (Kg) (average ± SD) | 72.56 ± 11.52 | 66.99 ± 9.18 |
| Body Mass Index (kg/m2) (average ± SD) | 27.30 ± 4.20 | 26.04 ± 3.43 |
| Education (%) | ||
| Primary | 26.28% | 28.57% |
| Secondary | 43.47% | 42.85% |
| University | 30.25% | 28.58% |
| Smoking (%) | 21.73% | 33.33% |
| Physical activity (%) | 34.78% | 38.09% |
| Elements | Intervention Group | Control Group |
|---|---|---|
| Calcium | 305 | 86.2 |
| Magnesium | 13.1 | 12.0 |
| Sodium | 5.1 | 3.4 |
| Potassium | 1.9 | 1.0 |
| Bicarbonates | 930 | 310 |
| Chlorides | 10.2 | 5.2 |
| Nitrates | 5.1 | 3.1 |
| Fluorides | 0.3 | 0.1 |
| Silica | 9.2 | 4.0 |
| Serum_Pathway | Hits | Raw p | Holm p | FDR |
| Arginine and Proline Metabolism | 8 | 1.95 × 10−71 | 1.33 × 10−69 | 6.74 × 10−70 |
| Carnitine Synthesis | 4 | 1.58 × 10−55 | 1.06 × 10−53 | 3.63 × 10−54 |
| Glycine and Serine Metabolism | 10 | 9.23 × 10−52 | 6.09 × 10−50 | 1.59 × 10−50 |
| Fatty Acid Biosynthesis | 3 | 4.22 × 10−26 | 2.74 × 10−24 | 5.82 × 10−25 |
| Ketone Body Metabolism | 3 | 5.68 × 10−22 | 3.58 × 10−20 | 5.59 × 10−21 |
| Valine Leucine and Isoleucine Degradation | 6 | 3.10 × 10−17 | 1.92 × 10−15 | 2.67 × 10−16 |
| Warburg Effect | 7 | 2.36 × 10−12 | 1.44 × 10−10 | 1.81 × 10−11 |
| Citric Acid Cycle | 3 | 8.33 × 10−12 | 5.00 × 10−10 | 4.98 × 10−11 |
| Betaine Metabolism | 3 | 6.87 × 10−11 | 3.92 × 10−9 | 3.65 × 10−10 |
| Urea Cycle | 7 | 8.80 × 10−11 | 4.93 × 10−9 | 4.34 × 10−10 |
| Glutamate Metabolism | 6 | 3.36 × 10−10 | 1.85 × 10−8 | 1.49 × 10−9 |
| Transfer of Acetyl Groups into Mitochondria | 3 | 3.83 × 10−10 | 2.07 × 10−8 | 1.49 × 10−9 |
| Propanoate Metabolism | 3 | 1.89 × 10−9 | 9.24 × 10−8 | 6.19 × 10−9 |
| Phenylalanine and Tyrosine Metabolism | 4 | 1.99 × 10−9 | 9.56 × 10−8 | 6.25 × 10−9 |
| Methionine Metabolism | 4 | 2.58 × 10−9 | 1.19 × 10−7 | 7.42 × 10−9 |
| Gluconeogenesis | 3 | 4.86 × 10−9 | 2.19 × 10−7 | 1.34 × 10−8 |
| Glucose–Alanine Cycle | 4 | 5.85 × 10−9 | 2.57 × 10−7 | 1.55 × 10−8 |
| Pyruvate Metabolism | 4 | 3.31 × 10−7 | 1.32 × 10−5 | 7.61 × 10−7 |
| Alanine Metabolism | 4 | 4.13 × 10−7 | 1.61 × 10−5 | 9.18 × 10−7 |
| Ammonia Recycling | 6 | 7.42 × 10−7 | 2.75 × 10−5 | 1.55 × 10−6 |
| Aspartate Metabolism | 5 | 1.71 × 10−6 | 6.17 × 10−5 | 3.48 × 10−6 |
| Tyrosine Metabolism | 3 | 3.34 × 10−6 | 0.000107 | 6.07 × 10−6 |
| Amino Sugar Metabolism | 4 | 5.58 × 10−6 | 0.000173 | 9.87 × 10−6 |
| Glutathione Metabolism | 3 | 7.06 × 10−5 | 0.001766 | 0.000108 |
| Purine Metabolism | 4 | 0.000228 | 0.00524 | 0.000334 |
| Tryptophan Metabolism | 3 | 0.000626 | 0.013152 | 0.000882 |
| Urinary_Pathway | Hits | Raw p | Holm p | FDR |
| Amino Sugar Metabolism | 5 | 7.64 × 10−30 | 6.03 × 10−28 | 6.03 × 10−28 |
| Galactose Metabolism | 6 | 1.07 × 10−25 | 8.33 × 10−24 | 4.22 × 10−24 |
| Fructose and Mannose Degradation | 4 | 2.90 × 10−25 | 2.23 × 10−23 | 7.00 × 10−24 |
| Glycine and Serine Metabolism | 16 | 3.54 × 10−25 | 2.69 × 10−23 | 7.00 × 10−24 |
| Urea Cycle | 11 | 3.73 × 10−17 | 2.76 × 10−15 | 4.92 × 10−16 |
| Ammonia Recycling | 9 | 1.10 × 10−16 | 8.04 × 10−15 | 1.24 × 10−15 |
| Arginine and Proline Metabolism | 13 | 4.19 × 10−16 | 3.02 × 10−14 | 4.14 × 10−15 |
| Glutamate Metabolism | 10 | 1.17 × 10−15 | 8.28 × 10−14 | 1.02 × 10−14 |
| Aspartate Metabolism | 9 | 4.30 × 10−14 | 3.01 × 10−12 | 3.39 × 10−13 |
| Alanine Metabolism | 6 | 2.64 × 10−13 | 1.80 × 10−11 | 1.74 × 10−12 |
| Cysteine Metabolism | 4 | 6.98 × 10−12 | 4.68 × 10−10 | 4.24 × 10−11 |
| Valine Leucine and Isoleucine Degradation | 10 | 7.53 × 10−12 | 4.97 × 10−10 | 4.25 × 10−11 |
| Propanoate Metabolism | 5 | 8.90 × 10−11 | 5.52 × 10−9 | 3.91 × 10−10 |
| Methionine Metabolism | 8 | 9.57 × 10−11 | 5.84 × 10−9 | 3.98 × 10−10 |
| Purine Metabolism | 7 | 1.27 × 10−10 | 7.64 × 10−9 | 5.03 × 10−10 |
| Glutathione Metabolism | 5 | 4.09 × 10−9 | 2.41 × 10−7 | 1.54 × 10−8 |
| Carnitine Synthesis | 6 | 1.11 × 10−8 | 6.42 × 10−7 | 3.98 × 10−8 |
| Warburg Effect | 10 | 1.71 × 10−8 | 9.73 × 10−7 | 5.86 × 10−8 |
| Citric Acid Cycle | 6 | 3.46 × 10−8 | 1.87 × 10−6 | 1.04 × 10−7 |
| Glucose–Alanine Cycle | 5 | 3.55 × 10−8 | 1.88 × 10−6 | 1.04 × 10−7 |
| Gluconeogenesis | 5 | 1.71 × 10−7 | 8.88 × 10−6 | 4.82 × 10−7 |
| Malate–Aspartate Shuttle | 4 | 1.10 × 10−6 | 5.40 × 10−5 | 2.81 × 10−6 |
| Phosphatidylethanolamine Biosynthesis | 3 | 1.41 × 10−6 | 6.77 × 10−5 | 3.48 × 10−6 |
| Sphingolipid Metabolism | 4 | 1.51 × 10−6 | 7.10 × 10−5 | 3.62 × 10−6 |
| Phenylalanine and Tyrosine Metabolism | 6 | 1.68 × 10−6 | 7.72 × 10−5 | 3.90 × 10−6 |
| Tyrosine Metabolism | 10 | 3.59 × 10−6 | 0.000162 | 8.11 × 10−6 |
| Lysine Degradation | 3 | 4.10 × 10−6 | 0.000181 | 9.00 × 10−6 |
| Tryptophan Metabolism | 5 | 5.89 × 10−6 | 0.000253 | 1.26 × 10−5 |
| Beta−Alanine Metabolism | 6 | 2.18 × 10−5 | 0.000915 | 4.53 × 10−5 |
| Phytanic Acid Peroxisomal Oxidation | 3 | 2.71 × 10−5 | 0.001111 | 5.49 × 10−5 |
| Transfer of Acetyl Groups into Mitochondria | 4 | 3.15 × 10−5 | 0.00126 | 6.22 × 10−5 |
| Oxidation of Branched Chain Fatty Acids | 4 | 8.88 × 10−5 | 0.003462 | 0.000171 |
| Pyruvate Metabolism | 4 | 0.00036 | 0.012612 | 0.000633 |
| Histidine Metabolism | 4 | 0.018303 | 0.45758 | 0.02629 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, C.; Pagano, I.; Castaldo, G.; Grimaldi, M.; D’Elia, M.; Santoro, A.; Conte, A.; Molettieri, P.; Parisella, C.; Buonocore, M.; et al. Supplementing Low-Sodium Bicarbonate–Calcic (Lete)® Water: Effects in Women on Bone and Systemic Metabolism. Metabolites 2023, 13, 1109. https://doi.org/10.3390/metabo13111109
Marino C, Pagano I, Castaldo G, Grimaldi M, D’Elia M, Santoro A, Conte A, Molettieri P, Parisella C, Buonocore M, et al. Supplementing Low-Sodium Bicarbonate–Calcic (Lete)® Water: Effects in Women on Bone and Systemic Metabolism. Metabolites. 2023; 13(11):1109. https://doi.org/10.3390/metabo13111109
Chicago/Turabian StyleMarino, Carmen, Imma Pagano, Giuseppe Castaldo, Manuela Grimaldi, Maria D’Elia, Angelo Santoro, Aurelio Conte, Paola Molettieri, Chiara Parisella, Michela Buonocore, and et al. 2023. "Supplementing Low-Sodium Bicarbonate–Calcic (Lete)® Water: Effects in Women on Bone and Systemic Metabolism" Metabolites 13, no. 11: 1109. https://doi.org/10.3390/metabo13111109
APA StyleMarino, C., Pagano, I., Castaldo, G., Grimaldi, M., D’Elia, M., Santoro, A., Conte, A., Molettieri, P., Parisella, C., Buonocore, M., D’Ursi, A. M., & Rastrelli, L. (2023). Supplementing Low-Sodium Bicarbonate–Calcic (Lete)® Water: Effects in Women on Bone and Systemic Metabolism. Metabolites, 13(11), 1109. https://doi.org/10.3390/metabo13111109














