UPLC-ESI-QTRAP-MS/MS Analysis to Quantify Bioactive Compounds in Fennel (Foeniculum vulgare Mill.) Waste with Potential Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. UPLC-ESI-QTRAP-MS/MS Quantitative Analysis
2.2. Method Validation
2.3. Anti-Inflammatory Activity
3. Experimental Design
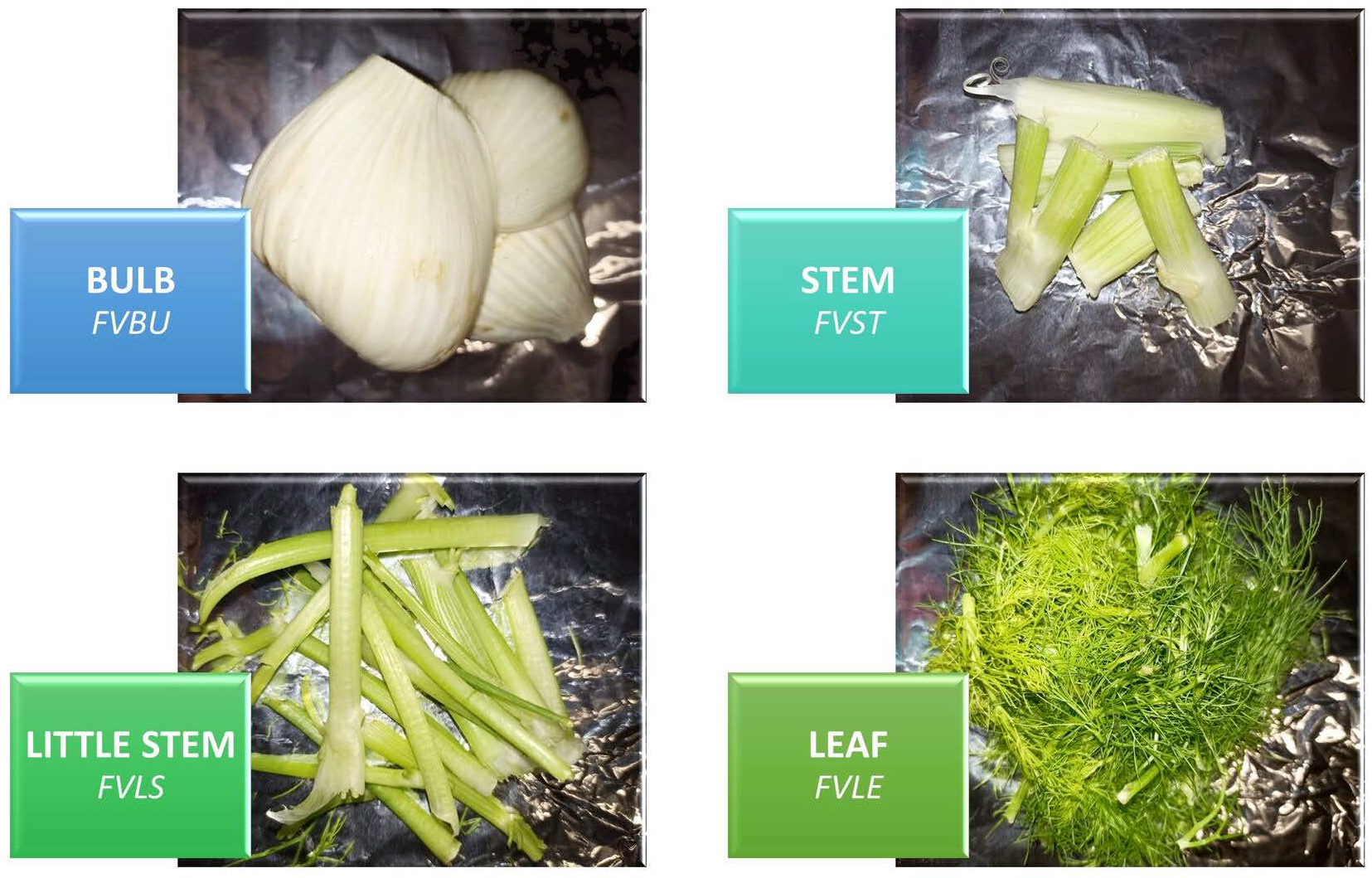
3.1. Raw Materials
3.2. Chemicals
3.3. Sample Preparation
3.4. Quantitative Analysis
3.4.1. ESI-QTRAP-MS and ESI-QTRAP-MS/MS Analyses
3.4.2. UPLC–ESI-QTRAP-MS Analyses in MRM (Multiple Reaction Monitoring) Modality
3.4.3. Method Validation
3.5. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N.; Akram, M.; Daniyal, M.; Koltai, H.; Fridlender, M.; Gorelick, J.; Sparks, D.L. Chapter Four—Antiinflammatory Potential of Medicinal Plants: A Source for Therapeutic Secondary Metabolites. Adv. Agron. 2018, 150, 131–183. [Google Scholar]
- Salih, M.; Osman, W.; Garelnabi, E.; Osman, Z.; Osman, B.; Khalid, H.; Mohamed, M. Secondary metabolites as anti-inflammatory agents. J. Phytopharm. 2014, 3, 275–285. [Google Scholar] [CrossRef]
- Klinge, S.A.; Sawyer, G.A. Effectiveness and safety of topical versus oral nonsteroidal anti-inflammatory drugs: A comprehensive review. Phys. Sportsmed. 2013, 41, 64–74. [Google Scholar] [CrossRef]
- Lim, H.; Heo, M.Y.; Kim, H.P. Flavonoids: Broad Spectrum Agents on Chronic Inflammation. Biomol. Ther. 2019, 27, 241–253. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y. Nutraceuticals of anti-inflammatory activity as complementary therapy for rheumatoid arthritis. Toxicol. Ind. Health 2014, 30, 738–748. [Google Scholar] [CrossRef]
- Yen, F.S.; Qin, C.S.; Xuan, S.T.S.; Ying, P.J.; Le, H.Y.; Darmarajan, T.; Gunasekaran, B.; Salvamani, S. Hypoglycemic Effects of Plant Flavonoids: A Review. Evid. Based Complement. Alternat. Med. 2021, 2021, 2057333. [Google Scholar]
- Kure, C.; Timmer, J.; Stough, C. The Immunomodulatory Effects of Plant Extracts and Plant Secondary Metabolites on Chronic Neuroinflammation and Cognitive Aging: A Mechanistic and Empirical Review. Front. Pharmacol. 2017, 8, 117. [Google Scholar] [CrossRef]
- Fürst, R.; Zündorf, I. Plant-derived anti-inflammatory compounds: Hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediat. Inflamm. 2014, 2014, 146832. [Google Scholar] [CrossRef]
- Bondy, S.A.-O.; Wu, M.; Prasad, K.A.-O. Attenuation of acute and chronic inflammation using compounds derived from plants. Exp. Biol. Med. 2021, 246, 406–413. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed. Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef]
- Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. LC-ESI/LTQOrbitrap/MS Metabolomic Analysis of Fennel Waste. Foods 2021, 10, 1893. [Google Scholar] [CrossRef]
- Parisi, G.F.; Carota, G.; Castruccio Castracani, C.C.; Spampinato, M.; Manti, S.; Papale, M.; Di Rosa, M.; Barbagallo, I.; Leonardi, S. Nutraceuticals in the Prevention of Viral Infections, including COVID-19, among the Pediatric Population: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 2465. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef]
- Docampo, M.; Olubu, A.; Wang, X.; Pasinetti, G.; Dixon, R.A. Glucuronidated Flavonoids in Neurological Protection: Structural Analysis and Approaches for Chemical and Biological Synthesis. J. Agric. Food Chem. 2017, 65, 7607–7623. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Guideline Q2(R2) on Validation of Analytical Procedures. Available online: europa.eu (accessed on 14 November 2018).
- Pferschy-Wenzig, E.M.; Ortmann, S.; Atanasov, A.G.; Hellauer, K.; Hartler, J.; Kunert, O.; Gold-Binder, M.; Ladurner, A.; Heiß, E.H.; Latkolik, S.; et al. Characterization of Constituents with Potential Anti-Inflammatory Activity in Chinese Lonicera Species by UHPLC-HRMS Based Metabolite Profiling. Metabolites 2022, 12, 288. [Google Scholar] [CrossRef]
- Li, M.; Weigmann, B. A Novel Pathway of Flavonoids Protecting against Inflammatory Bowel Disease: Modulating Enteroendocrine System. Metabolites 2022, 12, 31. [Google Scholar] [CrossRef]
- Chiocchio, I.; Prata, C.; Mandrone, M.; Ricciardiello, F.; Marrazzo, P.; Tomasi, P.; Angeloni, C.; Fiorentini, D.; Malaguti, M.; Poli, F.; et al. Leaves and Spiny Burs of. Metabolites 2020, 10, 408. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.M.; Tomuta, I.; Popa, D.S. Enhanced Recovery of Phenolic and Tocopherolic Compounds from Walnut. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Liu, H.; Ma, S.; Xia, H.; Lou, H.; Zhu, F.; Sun, L. Anti-inflammatory activities and potential mechanisms of phenolic acids isolated from Salvia miltiorrhiza f. alba roots in THP-1 macrophages. J. Ethnopharmacol. 2018, 222, 201–207. [Google Scholar] [CrossRef]
- Chen, M.; Ren, X.; Sun, S.; Wang, X.; Xu, X.; Li, X.; Yan, X.; Li, R.; Wang, Y.; Liu, X.; et al. Structure, Biological Activities and Metabolism of Flavonoid Glucuronides. Mini Rev. Med. Chem. 2022, 22, 322–354. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. IJPR 2011, 10, 655–683. [Google Scholar]
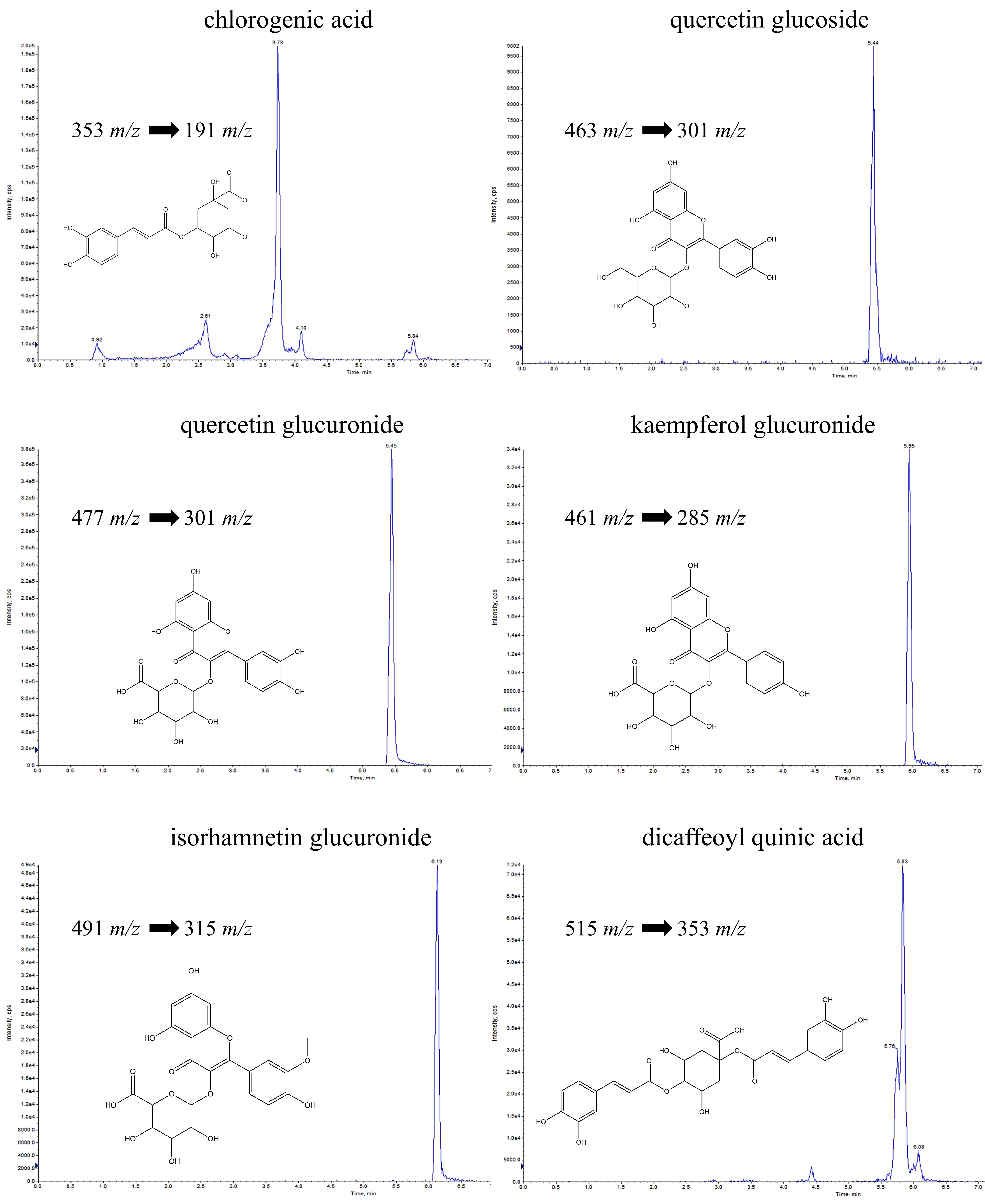
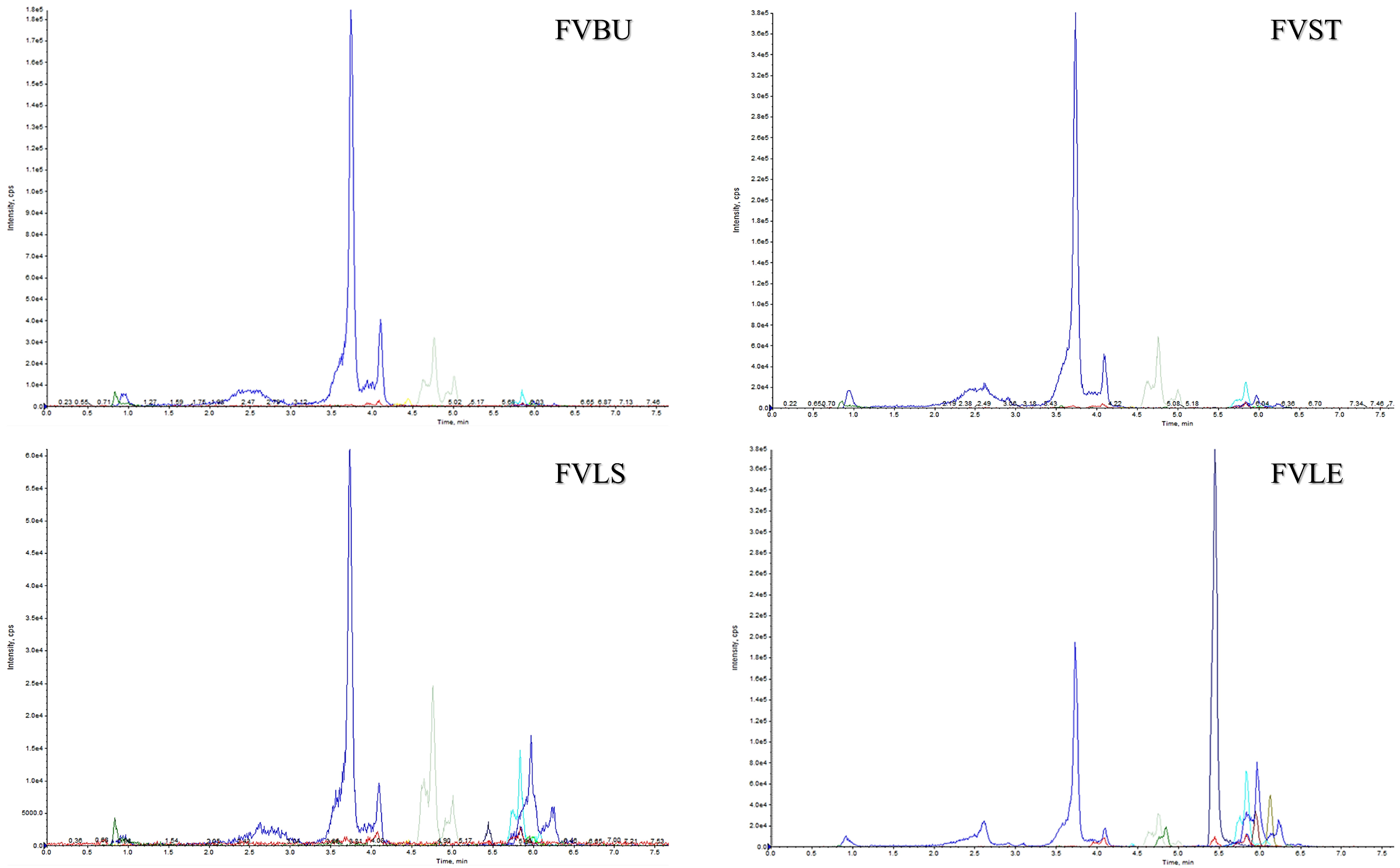
| Compounds | DP | EP | CE | CXP | PI | DI | FVBU | FVST | FVLS | FVLE | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | neochlorogenic acid | −60 | −4 | −24 | −17 | 353 | 191 | 25.39 ± 1.44 | 58.70 ± 0.69 | 6.80 ± 0.06 | 17.10 ± 0.86 |
| 2 | dicaffeoylquinic acid | −61 | −4 | −24 | −38 | 515 | 353 | 6.59 ± 0.09 | 27.82 ± 0.17 | 11.15 ± 0.21 | 41.17 ± 0.00 |
| 3 | quercetin glucoside | −138 | −9 | −28 | −32 | 463 | 301 | nd | nd | 3.16 ± 0.67 | 50.85 ± 0.00 |
| 4 | feruloyl quinic acid | −89 | −4 | −33 | −21 | 367 | 191 | 65.25 ± 0.00 | 152.32 ± 6.86 | 42.47 ± 0.00 | 46.77 ± 1.20 |
| 5 | quercetin glucuronide | −52 | −8 | −35 | −30 | 477 | 301 | nd | nd | 1.14 ± 0.11 | 163.76 ± 2.61 |
| 6 | isorhamnetin glucuronide | −52 | −8 | −35 | −30 | 491 | 315 | 0.67 ± 0.07 | 0.97 ± 0.38 | 0.89 ± 0.13 | 257.90 ± 4.23 |
| 7 | kaempferol glucuronide | −59 | −8 | −35 | −30 | 461 | 285 | nd | nd | 0.14 ± 0.00 | 37.84 ± 1.28 |
| 8 | coumaroyl quinic acid * | −60 | −4 | −24 | −17 | 337 | 191 | 6.42 ± 0.23 | 1.58 ± 0.05 | nd | nd |
| 9 | dicaffeoylquinic acid malonyl * | −61 | −4 | −24 | −38 | 601 | 395 | 4.44 ± 0.24 | 11.73 ± 1.68 | 19.98 ± 1.01 | 48.18 ± 4.65 |
| Compounds | IC50 (µM) | |
|---|---|---|
| COX-1 | COX-2 | |
| Kaempferol glucuronide | 228.38 ± 16.81 | 47.93 ± 3.50 |
| Isorhamnetin glucuronide | 94.72 ± 1.22 | 15.88 ± 1.16 |
| Quercetin glucoside | 103.46 ± 8.66 | 9.34 ± 1.11 |
| Dicaffeoylquinic acid | 85.57 ± 10.94 | 14.77 ± 2.39 |
| Quercetin glucuronide | 570.83 ± 40.00 | 26.28 ± 1.55 |
| SC-560 (COX-1) | 0.005 ± 0.001 | / |
| DuP-697 (COX-2) | / | 0.025 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crescenzi, M.A.; D’Urso, G.; Piacente, S.; Montoro, P. UPLC-ESI-QTRAP-MS/MS Analysis to Quantify Bioactive Compounds in Fennel (Foeniculum vulgare Mill.) Waste with Potential Anti-Inflammatory Activity. Metabolites 2022, 12, 701. https://doi.org/10.3390/metabo12080701
Crescenzi MA, D’Urso G, Piacente S, Montoro P. UPLC-ESI-QTRAP-MS/MS Analysis to Quantify Bioactive Compounds in Fennel (Foeniculum vulgare Mill.) Waste with Potential Anti-Inflammatory Activity. Metabolites. 2022; 12(8):701. https://doi.org/10.3390/metabo12080701
Chicago/Turabian StyleCrescenzi, Maria Assunta, Gilda D’Urso, Sonia Piacente, and Paola Montoro. 2022. "UPLC-ESI-QTRAP-MS/MS Analysis to Quantify Bioactive Compounds in Fennel (Foeniculum vulgare Mill.) Waste with Potential Anti-Inflammatory Activity" Metabolites 12, no. 8: 701. https://doi.org/10.3390/metabo12080701
APA StyleCrescenzi, M. A., D’Urso, G., Piacente, S., & Montoro, P. (2022). UPLC-ESI-QTRAP-MS/MS Analysis to Quantify Bioactive Compounds in Fennel (Foeniculum vulgare Mill.) Waste with Potential Anti-Inflammatory Activity. Metabolites, 12(8), 701. https://doi.org/10.3390/metabo12080701








