The Impact of Iron Dyshomeostasis and Anaemia on Long-Term Pulmonary Recovery and Persisting Symptom Burden after COVID-19: A Prospective Observational Cohort Study
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
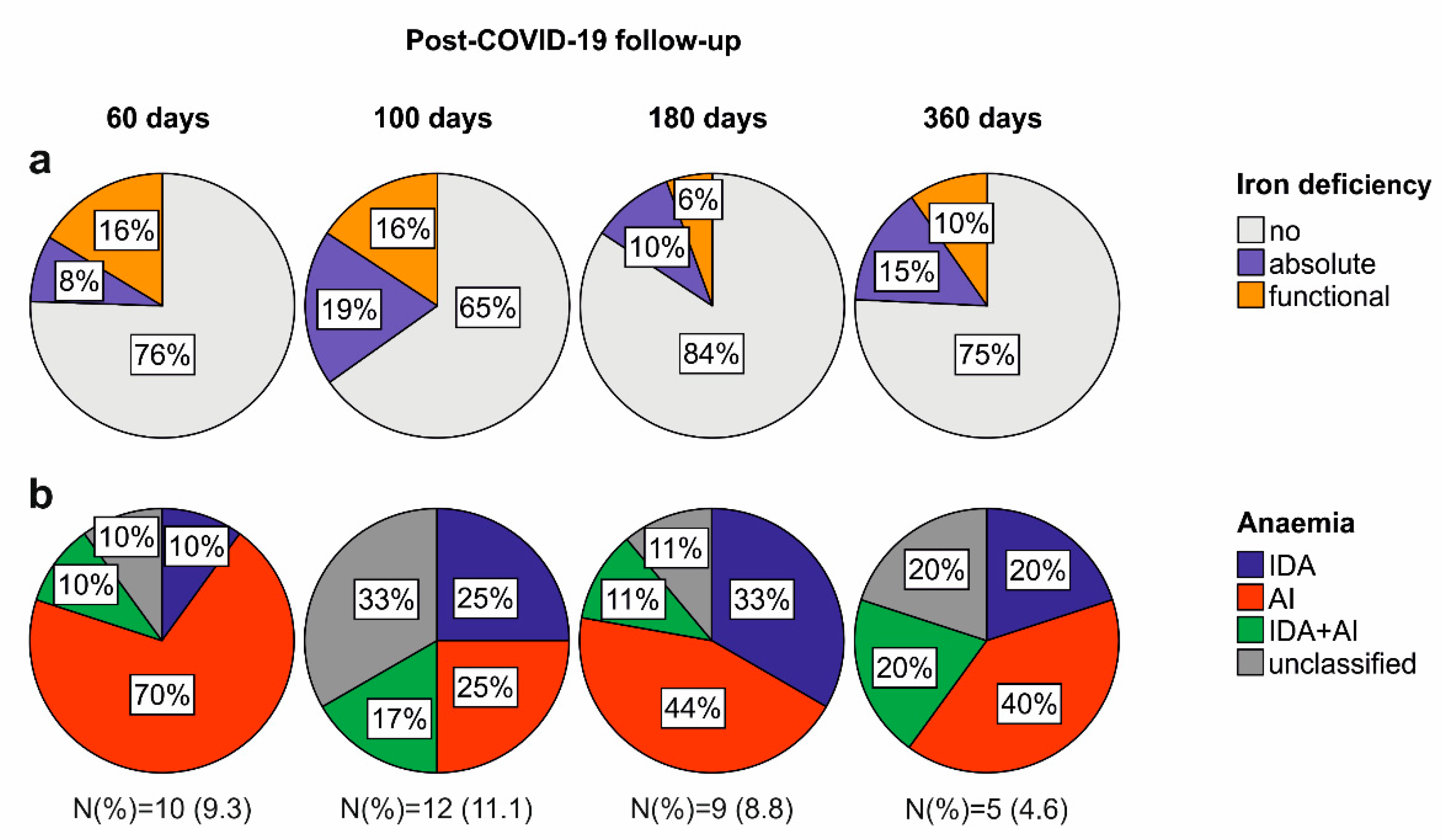
2.2. Iron Deficiency and Anaemia in Patients Recovering from COVID-19
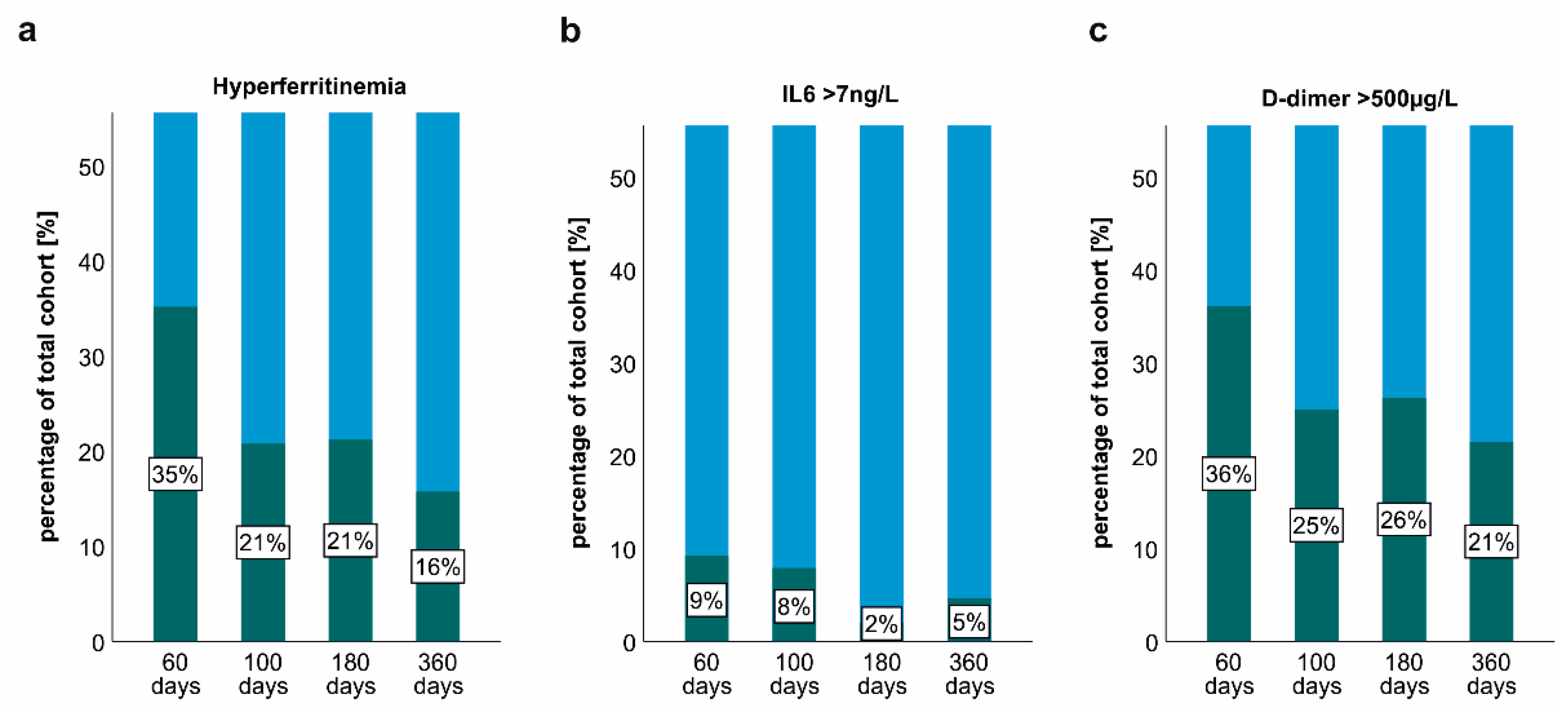
2.3. Persisting Inflammation and Hyperferritinaemia at Post-COVID-19 Follow-Up
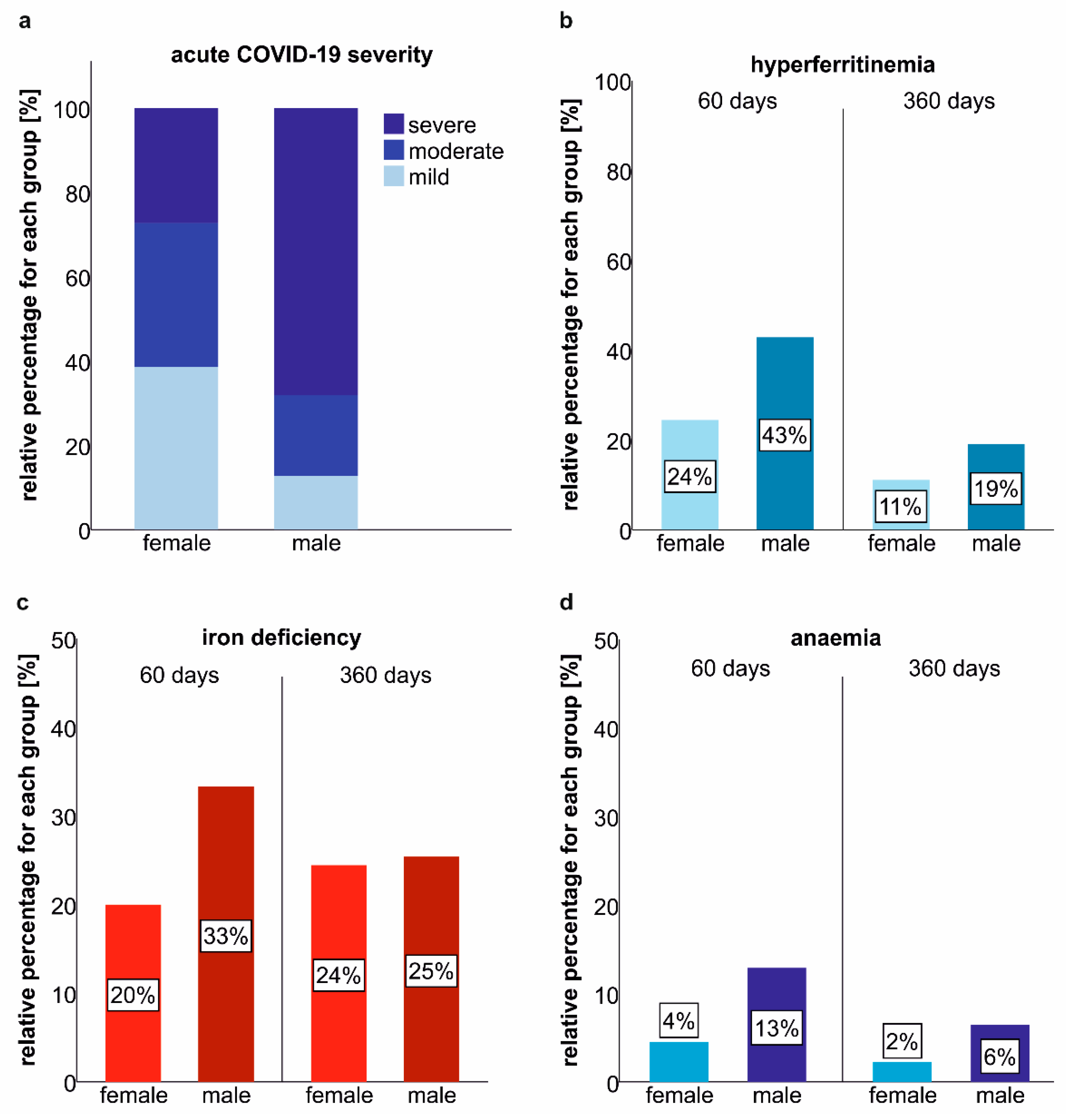
2.4. Gender-Related Differences in the Prevalence of Iron Deficiency and Anaemia at Post-COVID-19 Follow-Up
2.5. Association of Persisting Inflammation, Iron Dyshomeostasis and Anaemia
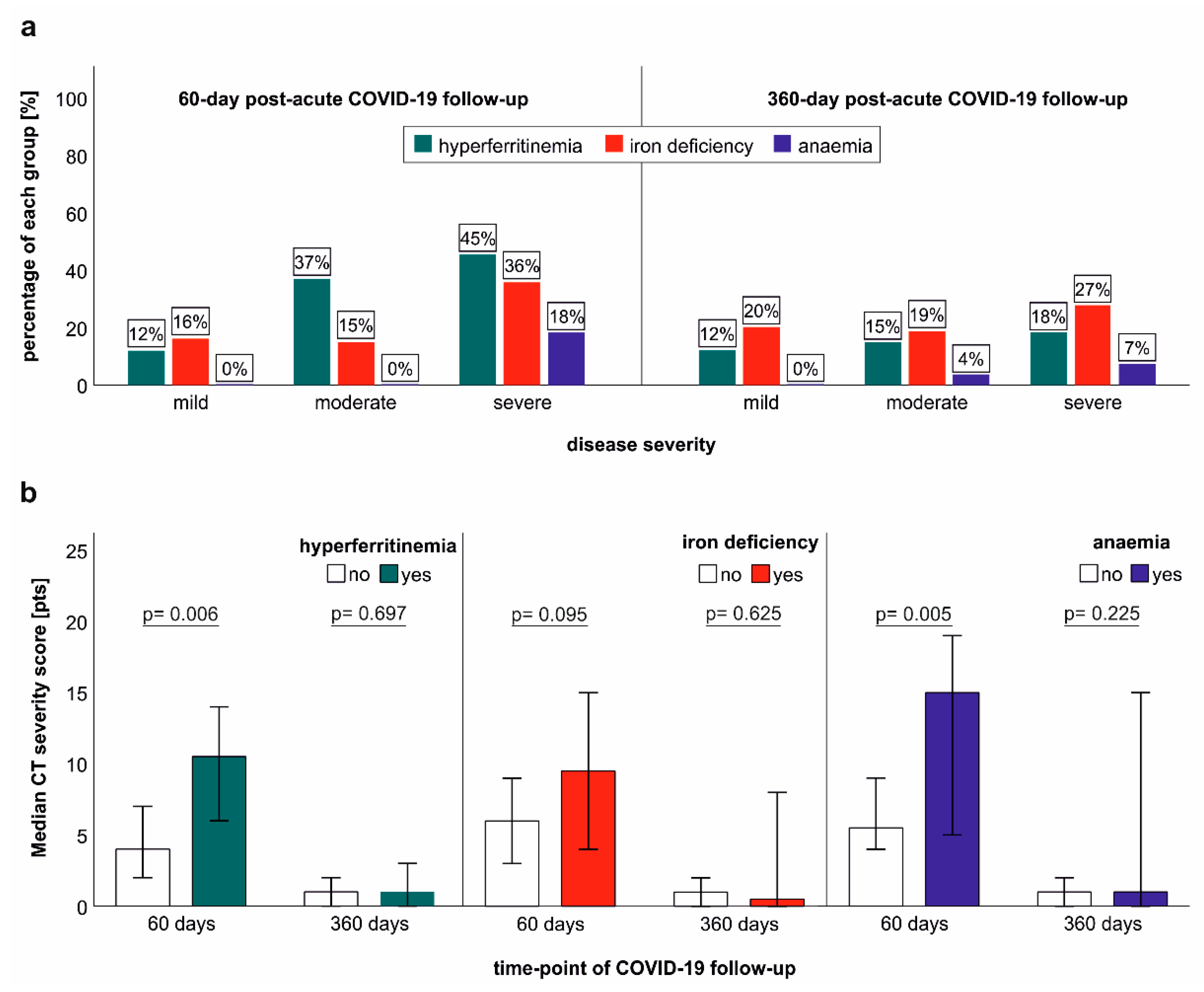
2.6. Association of Iron Dyshomeostasis and Anaemia with Clinical COVID-19 Severity and Structural Lung Recovery
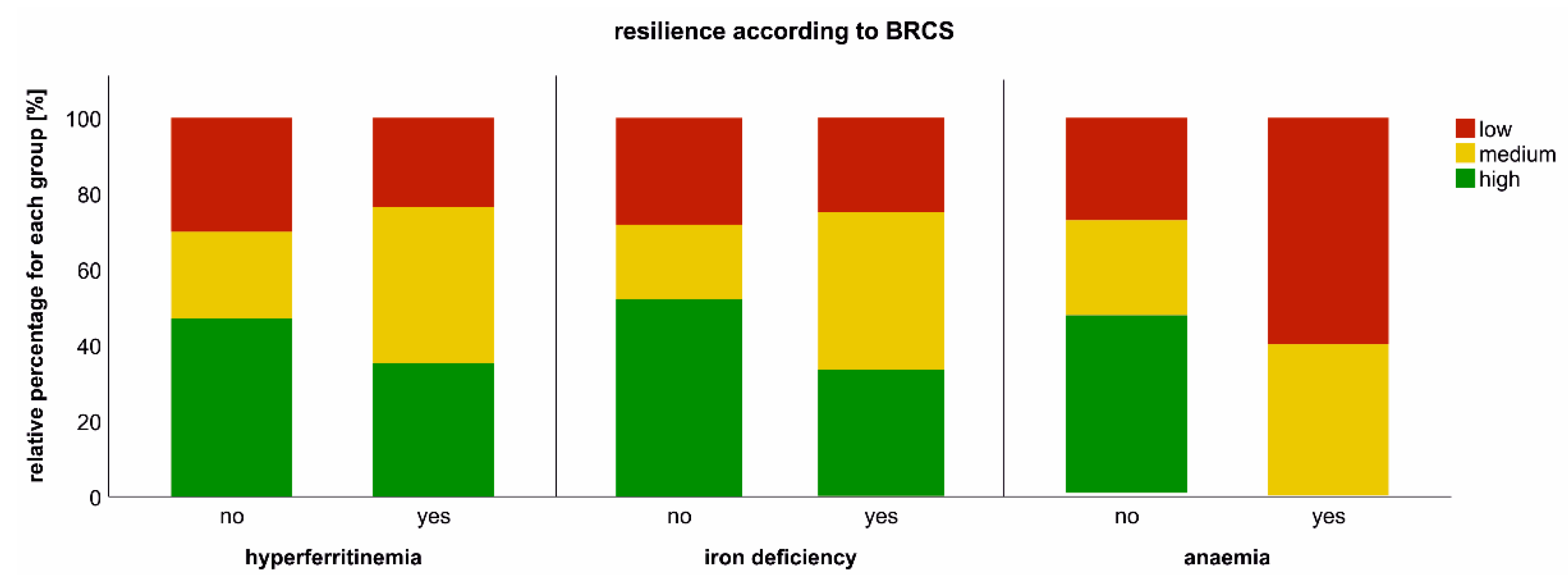
2.7. Impact of Iron Dyshomeostasis and Anaemia on Long-Term Symptom Burden and Exercise Tolerance
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. Laboratory Assessment
4.3. Diagnosis of Anaemia, Iron Deficiency and Hyperferritinaemia
4.4. Structural Lung Evaluation with CT
4.5. Assessment of Exercise Capacity, Resilience, Fatigue and Quality of Life
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonnweber, T.; Nachbaur, D.; Schroll, A.; Nairz, M.; Seifert, M.; Demetz, E.; Haschka, D.; Mitterstiller, A.M.; Kleinsasser, A.; Burtscher, M.; et al. Hypoxia induced downregulation of hepcidin is mediated by platelet derived growth factor BB. Gut 2014, 63, 1951–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Davidoff, O.; Niss, K.; Haase, V.H. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J. Clin. Investig. 2012, 122, 4635–4644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, J.; Yang, Y.; Ma, H.; Li, Z.; Zhang, J.; Cheng, J.; Zhang, X.; Zhao, Y.; Xia, Z.; et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol. Med. 2020, 12, e12421. [Google Scholar] [CrossRef]
- Girelli, D.; Marchi, G.; Busti, F.; Vianello, A. Iron metabolism in infections: Focus on COVID-19. Semin. Hematol. 2021, 58, 182–187. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; Hlh Across Speciality Collaboration, U.K. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Bellmann-Weiler, R.; Lanser, L.; Barket, R.; Rangger, L.; Schapfl, A.; Schaber, M.; Fritsche, G.; Wöll, E.; Weiss, G. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J. Clin. Med. 2020, 9, 2429. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Domenico, I.; Nemeth, E.; Nelson, J.M.; Phillips, J.D.; Ajioka, R.S.; Kay, M.S.; Kushner, J.P.; Ganz, T.; Ward, D.M.; Kaplan, J. The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab. 2008, 8, 146–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta 2009, 1790, 589–599. [Google Scholar] [CrossRef]
- Theurl, I.; Theurl, M.; Seifert, M.; Mair, S.; Nairz, M.; Rumpold, H.; Zoller, H.; Bellmann-Weiler, R.; Niederegger, H.; Talasz, H.; et al. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood 2008, 111, 2392–2399. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G. Iron and immunity: A double-edged sword. Eur. J. Clin. Investig. 2002, 32 (Suppl. 1), 70–78. [Google Scholar] [CrossRef]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Lanser, L.; Burkert, F.R.; Bellmann-Weiler, R.; Schroll, A.; Wildner, S.; Fritsche, G.; Weiss, G. Dynamics in Anemia Development and Dysregulation of Iron Homeostasis in Hospitalized Patients with COVID-19. Metabolites 2021, 11, 653. [Google Scholar] [CrossRef]
- Kilercik, M.; Ucal, Y.; Serdar, M.; Serteser, M.; Ozpinar, A.; Schweigert, F.J. Zinc protoporphyrin levels in COVID-19 are indicative of iron deficiency and potential predictor of disease severity. PLoS ONE 2022, 17, e0262487. [Google Scholar] [CrossRef]
- Sonnweber, T.; Boehm, A.; Sahanic, S.; Pizzini, A.; Aichner, M.; Sonnweber, B.; Kurz, K.; Koppelstatter, S.; Haschka, D.; Petzer, V.; et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: A prospective observational cohort study. Respir. Res. 2020, 21, 276. [Google Scholar] [CrossRef]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.H.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free. Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.; Maciorowski, D.; Medernach, B.; Becker, D.P.; Durvasula, R.; Libertin, C.R.; Kempaiah, P. Iron dysregulation in COVID-19 and reciprocal evolution of SARS-CoV-2: Natura nihil frustra facit. J. Cell Biochem. 2022, 123, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.J.; Wagner, S.; Hupf, J.; Evert, K.; Evert, M.; Sossalla, S.; Jungbauer, C.; Maier, L.S.; Neef, S.; Mustroph, J. Cardiac iron overload promotes cardiac injury in patients with severe COVID-19. Infection 2021, 50, 547–552. [Google Scholar] [CrossRef]
- Dorward, D.A.; Russell, C.D.; Um, I.H.; Elshani, M.; Armstrong, S.D.; Penrice-Randal, R.; Millar, T.; Lerpiniere, C.E.B.; Tagliavini, G.; Hartley, C.S.; et al. Tissue-Specific Immunopathology in Fatal COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Hippchen, T.; Altamura, S.; Muckenthaler, M.U.; Merle, U. Hypoferremia is Associated with Increased Hospitalization and Oxygen Demand in COVID-19 Patients. Hemasphere 2020, 4, e492. [Google Scholar] [CrossRef]
- Moreira, A.C.; Teles, M.J.; Silva, T.; Bento, C.M.; Alves, I.S.; Pereira, L.; Guimaraes, J.T.; Porto, G.; Oliveira, P.; Gomes, M.S. Iron Related Biomarkers Predict Disease Severity in a Cohort of Portuguese Adult Patients during COVID-19 Acute Infection. Viruses 2021, 13, 2482. [Google Scholar] [CrossRef]
- Claise, C.; Saleh, J.; Rezek, M.; Vaulont, S.; Peyssonnaux, C.; Edeas, M. Low transferrin levels predict heightened inflammation in patients with COVID-19: New insights. Int. J. Infect. Dis. 2021, 116, 74–79. [Google Scholar] [CrossRef]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Shoenfeld, Y. Ferritin - from iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 2022, 126, 102778. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Weigand, M.; Kim, J.; Wu, X.; Strayer, J.; Palmer, A.F.; Zborowski, M.; Yazer, M.; Chalmers, J.J. Hyperferritinemia in critically ill COVID-19 patients—Is ferritin the product of inflammation or a pathogenic mediator? Clin. Chim. Acta 2020, 509, 249–251. [Google Scholar] [CrossRef]
- Edeas, M.; Saleh, J.; Peyssonnaux, C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 2020, 97, 303–305. [Google Scholar] [CrossRef]
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 2020, 10, 1271. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Frost, J.N.; Aaron, L.; Donovan, K.; Drakesmith, H. Systemic hypoferremia and severity of hypoxemic respiratory failure in COVID-19. Crit. Care 2020, 24, 320. [Google Scholar] [CrossRef] [PubMed]
- Kronstein-Wiedemann, R.; Stadtmuller, M.; Traikov, S.; Georgi, M.; Teichert, M.; Yosef, H.; Wallenborn, J.; Karl, A.; Schutze, K.; Wagner, M.; et al. SARS-CoV-2 Infects Red Blood Cell Progenitors and Dysregulates Hemoglobin and Iron Metabolism. Stem Cell Rev. Rep. 2022, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mancilha, E.M.B.; Oliveira, J.S.R. SARS-CoV-2 association with hemoglobin and iron metabolism. Rev. Assoc. Med. Bras. 2021, 67, 1349–1352. [Google Scholar] [CrossRef]
- Kim, Y.M.; Shin, E.C. Type I and III interferon responses in SARS-CoV-2 infection. Exp. Mol. Med. 2021, 53, 750–760. [Google Scholar] [CrossRef]
- Jordan, M.B.; Hildeman, D.; Kappler, J.; Marrack, P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 2004, 104, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Pachlopnik Schmid, J.; Ho, C.H.; Chrétien, F.; Lefebvre, J.M.; Pivert, G.; Kosco-Vilbois, M.; Ferlin, W.; Geissmann, F.; Fischer, A.; de Saint Basile, G. Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol. Med. 2009, 1, 112–124. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Met. Integr. Biometal Sci. 2014, 6, 748–773. [Google Scholar] [CrossRef] [Green Version]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef]
- Rosário, C.; Zandman-Goddard, G.; Meyron-Holtz, E.G.; D’Cruz, D.P.; Shoenfeld, Y. The hyperferritinemic syndrome: Macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013, 11, 185. [Google Scholar] [CrossRef] [Green Version]
- Banchini, F.; Cattaneo, G.M.; Capelli, P. Serum ferritin levels in inflammation: A retrospective comparative analysis between COVID-19 and emergency surgical non-COVID-19 patients. World J. Emerg. Surg. 2021, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Haschka, D.; Tymoszuk, P.; Petzer, V.; Hilbe, R.; Heeke, S.; Dichtl, S.; Skvortsov, S.; Demetz, E.; Berger, S.; Seifert, M.; et al. Ferritin H deficiency deteriorates cellular iron handling and worsens Salmonella typhimurium infection by triggering hyperinflammation. JCI Insight 2021, 6, e141760. [Google Scholar] [CrossRef] [PubMed]
- Ruddell, R.G.; Hoang-Le, D.; Barwood, J.M.; Rutherford, P.S.; Piva, T.J.; Watters, D.J.; Santambrogio, P.; Arosio, P.; Ramm, G.A. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology 2009, 49, 887–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonnweber, T.; Tymoszuk, P.; Sahanic, S.; Boehm, A.; Pizzini, A.; Luger, A.; Schwabl, C.; Nairz, M.; Grubwieser, P.; Kurz, K.; et al. Investigating phenotypes of pulmonary COVID-19 recovery - a longitudinal observational prospective multicenter trial. Elife 2022, 11, e72500. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Sahanic, S.; Pizzini, A.; Luger, A.; Schwabl, C.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur. Respir. J. 2021, 57, 2003481. [Google Scholar] [CrossRef]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e820. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Borrelli de Andreis, F.; Aronico, N.; Lenti, M.V.; Barteselli, C.; Merli, S.; Pellegrino, I.; Coppola, L.; Cremonte, E.M.; Croce, G.; et al. Anemia in patients with Covid-19: Pathogenesis and clinical significance. Clin. Exp. Med. 2021, 21, 239–246. [Google Scholar] [CrossRef]
- Lanser, L.; Fuchs, D.; Kurz, K.; Weiss, G. Physiology and Inflammation Driven Pathophysiology of Iron Homeostasis-Mechanistic Insights into Anemia of Inflammation and Its Treatment. Nutrients 2021, 13, 3732. [Google Scholar] [CrossRef]
- Kassebaum, N.J. The Global Burden of Anemia. Hematol. Oncol. Clin. N. Am. 2016, 30, 247–308. [Google Scholar] [CrossRef] [Green Version]
- Bellmann-Weiler, R.; Lanser, L.; Burkert, F.; Seiwald, S.; Fritsche, G.; Wildner, S.; Schroll, A.; Koppelstätter, S.; Kurz, K.; Griesmacher, A.; et al. Neopterin Predicts Disease Severity in Hospitalized Patients with COVID-19. Open Forum Infect. Dis. 2021, 8, ofaa521. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 373, 485–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebner, N.; Jankowska, E.A.; Ponikowski, P.; Lainscak, M.; Elsner, S.; Sliziuk, V.; Steinbeck, L.; Kube, J.; Bekfani, T.; Scherbakov, N.; et al. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the Studies Investigating Co-morbidities Aggravating Heart Failure. Int. J. Cardiol. 2016, 205, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, P.A.; Remels, A.H.V.; Zonneveld, M.I.; Rouschop, K.M.A.; Schols, A.; Gosker, H.R. Iron deficiency-induced loss of skeletal muscle mitochondrial proteins and respiratory capacity; the role of mitophagy and secretion of mitochondria-containing vesicles. FASEB J. 2020, 34, 6703–6717. [Google Scholar] [CrossRef] [Green Version]
- Rineau, E.; Gueguen, N.; Procaccio, V.; Geneviève, F.; Reynier, P.; Henrion, D.; Lasocki, S. Iron Deficiency without Anemia Decreases Physical Endurance and Mitochondrial Complex I Activity of Oxidative Skeletal Muscle in the Mouse. Nutrients 2021, 13, 1056. [Google Scholar] [CrossRef]
- Oexle, H.; Gnaiger, E.; Weiss, G. Iron-dependent changes in cellular energy metabolism: Influence on citric acid cycle and oxidative phosphorylation. Biochim. Biophys. Acta 1999, 1413, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.; Valente de Souza, L.; Komlódi, T.; Garcia-Souza, L.F.; Volani, C.; Tymoszuk, P.; Demetz, E.; Seifert, M.; Auer, K.; Hilbe, R.; et al. Mitochondrial Respiration in Response to Iron Deficiency Anemia: Comparison of Peripheral Blood Mononuclear Cells and Liver. Metabolites 2022, 12, 270. [Google Scholar] [CrossRef]
- Fischer, C.; Volani, C.; Komlódi, T.; Seifert, M.; Demetz, E.; Valente de Souza, L.; Auer, K.; Petzer, V.; von Raffay, L.; Moser, P.; et al. Dietary Iron Overload and Hfe(−/−) Related Hemochromatosis Alter Hepatic Mitochondrial Function. Antioxidants 2021, 10, 1818. [Google Scholar] [CrossRef]
- Sumneang, N.; Siri-Angkul, N.; Kumfu, S.; Chattipakorn, S.C.; Chattipakorn, N. The effects of iron overload on mitochondrial function, mitochondrial dynamics, and ferroptosis in cardiomyocytes. Arch. Biochem. Biophys. 2020, 680, 108241. [Google Scholar] [CrossRef]
- Al-Naseem, A.; Sallam, A.; Choudhury, S.; Thachil, J. Iron deficiency without anaemia: A diagnosis that matters. Clin. Med. 2021, 21, 107–113. [Google Scholar] [CrossRef]
- Blomberg, B.; Mohn, K.G.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Rass, V.; Beer, R.; Schiefecker, A.J.; Lindner, A.; Kofler, M.; Ianosi, B.A.; Mahlknecht, P.; Heim, B.; Peball, M.; Carbone, F.; et al. Neurological outcomes 1 year after COVID-19 diagnosis: A prospective longitudinal cohort study. Eur. J. Neurol. 2022, 29, 1685–1696. [Google Scholar] [CrossRef]
- Heidbreder, A.; Sonnweber, T.; Stefani, A.; Ibrahim, A.; Cesari, M.; Bergmann, M.; Brandauer, E.; Tancevski, I.; Löffler-Ragg, J.; Högl, B. Video-polysomnographic findings after acute COVID-19: REM sleep without atonia as sign of CNS pathology? Sleep Med. 2021, 80, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Chao, H.H.; Huang, W.T.; Chen, S.C.; Yang, H.Y. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: A nationwide database analysis. BMC Psychiatry 2020, 20, 216. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.Q.; Zhang, D.J.; Wang, J.L. A meta-analysis of the trait resilience and mental health. Pers. Indiv. Differ. 2015, 76, 18–27. [Google Scholar] [CrossRef]
- Kim, J.; Wessling-Resnick, M. Iron and mechanisms of emotional behavior. J. Nutr. Biochem. 2014, 25, 1101–1107. [Google Scholar] [CrossRef] [Green Version]
- Haschka, D.; Volani, C.; Stefani, A.; Tymoszuk, P.; Mitterling, T.; Holzknecht, E.; Heidbreder, A.; Coassin, S.; Sumbalova, Z.; Seifert, M.; et al. Association of mitochondrial iron deficiency and dysfunction with idiopathic restless legs syndrome. Mov. Disord. 2019, 34, 114–123. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Looker, A.C. Laboratory methodologies for indicators of iron status: Strengths, limitations, and analytical challenges. Am. J. Clin. Nutr. 2017, 106, 1606S–1614S. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G. Anemia of Chronic Disorders: New Diagnostic Tools and New Treatment Strategies. Semin. Hematol. 2015, 52, 313–320. [Google Scholar] [CrossRef]
- Punnonen, K.; Irjala, K.; Rajamaki, A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 1997, 89, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullis, J.O.; Fitzsimons, E.J.; Griffiths, W.J.; Tsochatzis, E.; Thomas, D.W.; British Society for Haematology. Investigation and management of a raised serum ferritin. Br. J. Haematol. 2018, 181, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Pizzini, A.; Tancevski, I.; Loffler-Ragg, J.; Weiss, G. Anaemia, iron homeostasis and pulmonary hypertension: A review. Intern. Emerg. Med. 2020, 15, 573–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonnweber, T.; Nairz, M.; Theurl, I.; Petzer, V.; Tymoszuk, P.; Haschka, D.; Rieger, E.; Kaessmann, B.; Deri, M.; Watzinger, K.; et al. The crucial impact of iron deficiency definition for the course of precapillary pulmonary hypertension. PLoS ONE 2018, 13, e0203396. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Muller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef] [Green Version]
- EuroQol. EQ-5D-5L. Available online: https://euroqol.org/eq-5d-instruments/eq-5d-5l-available-modes-of-administration/ (accessed on 18 May 2022).
- Sinclair, V.G.; Wallston, K.A. The development and psychometric evaluation of the Brief Resilient Coping Scale. Assessment 2004, 11, 94–101. [Google Scholar] [CrossRef]
| Demographics | |
| Mean age–years (SD) | 56 (14) |
| Female sex–no. (%) | 45 (42) |
| Median body mass index (SD) 1 | 26.4 (4.5) |
| Smoking history–no. (%) | 39 (36) |
| Comorbidities–No. (%) | |
| None | 27 (25) |
| Cardiovascular disease | 42 (39) |
| Hypertension | 29 (27) |
| Pulmonary disease | 19 (18) |
| Endocrine disease | 63 (58) |
| Diabetes mellitus, type 2 | 17 (16) |
| Chronic kidney disease | 7 (6) |
| Chronic liver disease | 6 (6) |
| Malignancy | 10 (9) |
| Immunodeficiency 2 | 5 (5) |
| Treatment during acute COVID-19 3 | |
| Hospitalization–no. (%) | 81 (75) |
| Oxygen supply–no. (%) | 54 (50) |
| Non-invasive ventilation–no. (%) | 2 (2) |
| Invasive ventilation–no. (%) | 25 (23) |
| Time after COVID-19 Onset | 60 Days | 100 Days | 180 Days | 360 Days | p-Value | Effect Size |
|---|---|---|---|---|---|---|
| serum iron–µmol/L (SD) | 16.0 (6.0) | 15.2 (5.3) | 17.7 (5.6) | 17.3 (5.9) | 0.055 | 0.187 |
| TSAT–% (SD) 1 | 26 (11) | 24 (10) | 27 (9) | 26 (9) | 0.804 | 0.240 |
| serum ferritin–µmol/L (SD) | 269 (251) | 198 (197) | 183 (153) | 198 (191) | <0.001 | −0.443 |
| hepcidin-25–µg/L (SD) | 18.9 (13.8) | 15.7 (13.4) | 18.2 (14.1) | 13.0 (10.2) | <0.001 | −0.526 |
| sTFR–mg/L (SD) 2 | 3.4 (1.1) | 3.2 (1.0) | 2.9 (1.1) | 3.0 (0.8) | <0.001 | −0.477 |
| ferritin index–value (SD) 3 | 1.6 (0.7) | 1.7 (1.0) | 1.5 (1.3) | 1.5 (0.6) | 0.100 | −0.161 |
| haemoglobin–g/L (SD) | 139 (14) | 141 (16) | 144 (15) | 146 (14) | <0.001 | −0.783 |
| leucocytes–G/L (SD) | 6.44 (2.73) | 6.28 (2.07) | 6.13 (1.65) | 6.07 (1.61) | 0.011 | −0.250 |
| thrombocytes–G/L (SD) | 264 (74) | 247 (64) | 240 (61) | 243 (54) | <0.001 | −0.338 |
| Time after COVID-19 Onset | 60 Days | 100 Days | 180 Days | 360 Days | p-Value | Effect Size |
|---|---|---|---|---|---|---|
| CRP–mg/dL (SD) 1 | 0.37 (1.12) | 0.29 (0.68) | 0.21 (0.50) | 0.36 (0.90) | 0.847 | −0.019 |
| Procalcitonin–µg/L (SD) | 0.07 (0.02) | 0.07 (0.03) | 0.02 (0.04) | 0.02 (0.06) | <0.001 | −1.376 |
| IL6–ng/L (SD) | 3.4 (5.5) | 3.2 (2.6) | 1.9 (2.3) | 1.3 (2.7) | <0.001 | −0.410 |
| Neopterin–nmol/L (SD) | 9.7 (4.5) | 8.4 (2.9) | 9.0 (3.7) | 10.2 (6.7) | 0.360 | 0.089 |
| D-dimer–µg/L (SD) | 575 (541) | 572 (891) | 470 (513) | 363 (202) | <0.001 | −0.443 |
| Serum Marker | Iron | TSAT 3 | Ferritin | sTFR 4 | sTFRF Index 5 | Hepcidin |
|---|---|---|---|---|---|---|
| 60 days post-COVID-19 follow-up | ||||||
| CRP–ρ 1 | −0.198 * | −0.272 ** | 0.040 | 0.175 | 0.095 | −0.006 |
| Procalcitonin–ρ | −0.085 | −0.069 | 0.089 | 0.185 | 0.060 | −0.012 |
| IL6–ρ 2 | −0.202 * | −0.161 | 0.067 | 0.272 ** | 0.213 * | −0.065 |
| Neopterin–ρ | −0.185 | −0.158 | 0.235 * | 0.242 * | 0.064 | 0.035 |
| D-dimer–ρ | −0.248 * | −0.242 * | 0.028 | 0.177 | 0.131 | −0.191 |
| 360 days post−COVID−19 follow−up | ||||||
| CRP–ρ 1 | −0.335 ** | −0.334 ** | 0.055 | 0.092 | 0.049 | 0.035 |
| Procalcitonin–ρ | −0.196 * | −0.141 | 0.213 * | 0.192 * | 0.023 | 0.156 |
| IL6–ρ 2 | −0.329 ** | −0.321 ** | 0.001 | 0.030 | 0.052 | 0.056 |
| Neopterin–ρ | −0.235 * | −0.178 | 0.140 | 0.238 * | 0.097 | 0.111 |
| D-dimer–ρ | −0.121 | −0.071 | 0.006 | −0.049 | −0.034 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonnweber, T.; Grubwieser, P.; Sahanic, S.; Böhm, A.K.; Pizzini, A.; Luger, A.; Schwabl, C.; Koppelstätter, S.; Kurz, K.; Puchner, B.; et al. The Impact of Iron Dyshomeostasis and Anaemia on Long-Term Pulmonary Recovery and Persisting Symptom Burden after COVID-19: A Prospective Observational Cohort Study. Metabolites 2022, 12, 546. https://doi.org/10.3390/metabo12060546
Sonnweber T, Grubwieser P, Sahanic S, Böhm AK, Pizzini A, Luger A, Schwabl C, Koppelstätter S, Kurz K, Puchner B, et al. The Impact of Iron Dyshomeostasis and Anaemia on Long-Term Pulmonary Recovery and Persisting Symptom Burden after COVID-19: A Prospective Observational Cohort Study. Metabolites. 2022; 12(6):546. https://doi.org/10.3390/metabo12060546
Chicago/Turabian StyleSonnweber, Thomas, Philipp Grubwieser, Sabina Sahanic, Anna Katharina Böhm, Alex Pizzini, Anna Luger, Christoph Schwabl, Sabine Koppelstätter, Katharina Kurz, Bernhard Puchner, and et al. 2022. "The Impact of Iron Dyshomeostasis and Anaemia on Long-Term Pulmonary Recovery and Persisting Symptom Burden after COVID-19: A Prospective Observational Cohort Study" Metabolites 12, no. 6: 546. https://doi.org/10.3390/metabo12060546
APA StyleSonnweber, T., Grubwieser, P., Sahanic, S., Böhm, A. K., Pizzini, A., Luger, A., Schwabl, C., Koppelstätter, S., Kurz, K., Puchner, B., Sperner-Unterweger, B., Hüfner, K., Wöll, E., Nairz, M., Widmann, G., Tancevski, I., Löffler-Ragg, J., & Weiss, G. (2022). The Impact of Iron Dyshomeostasis and Anaemia on Long-Term Pulmonary Recovery and Persisting Symptom Burden after COVID-19: A Prospective Observational Cohort Study. Metabolites, 12(6), 546. https://doi.org/10.3390/metabo12060546






