Oral Adelmidrol Administration Up-Regulates Palmitoylethanolamide Production in Mice Colon and Duodenum through a PPAR-γ Independent Action
Abstract
:1. Introduction
2. Results
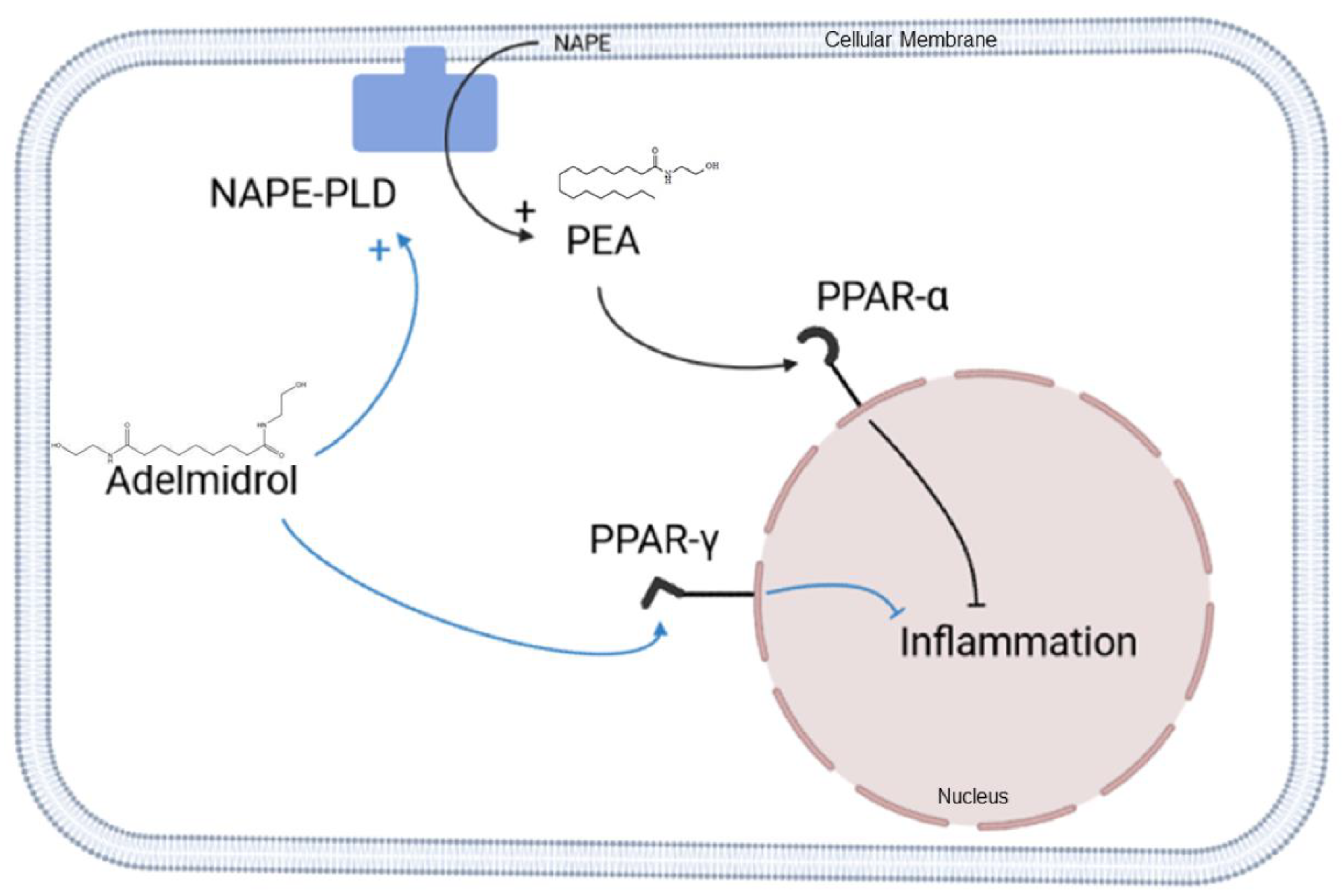
2.1. Adelmidrol Increases Endogenous Levels of PEA in a Dose/Time-Dependent Manner
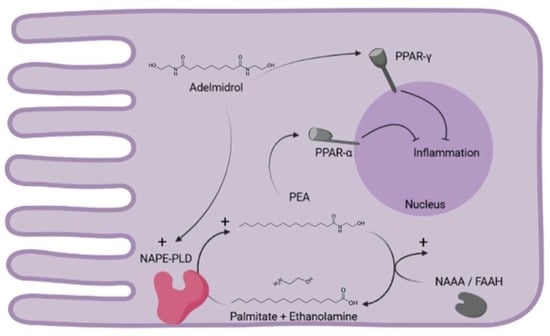
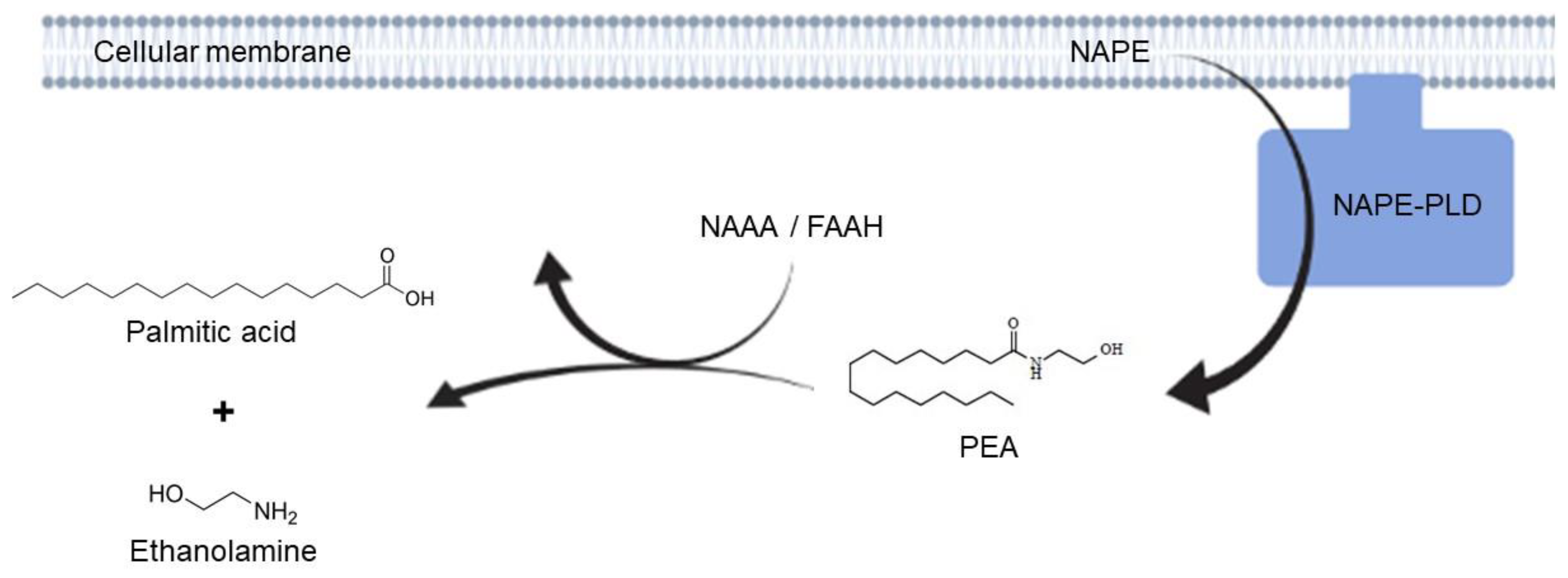
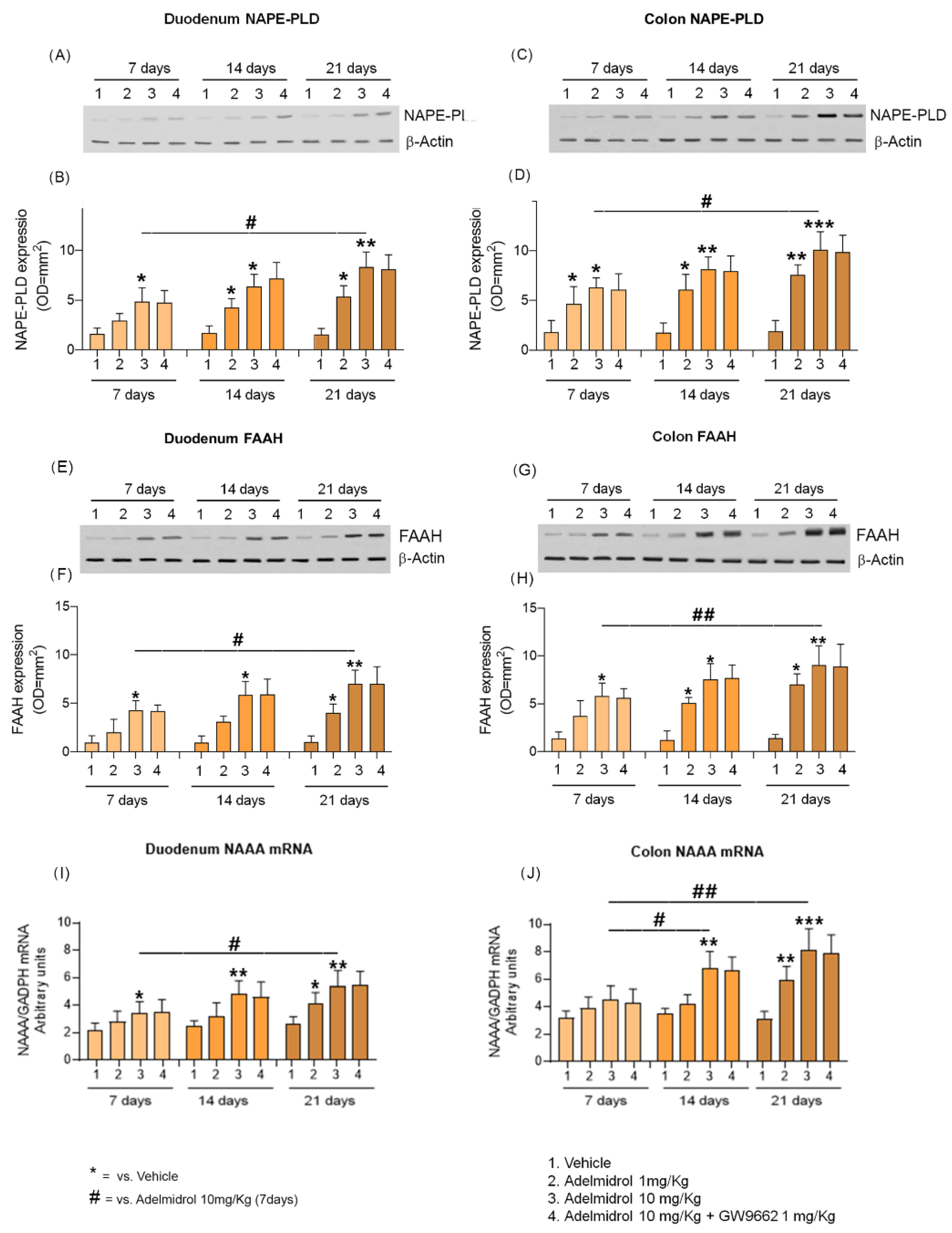
2.2. Adelmidrol Increases NAPE-PLD and FAAH Proteins Expression and NAAA-Coding mRNA with a Dose/Time-Dependent Pattern in Colon and Duodenum
2.3. Adelmidrol Increases PEA Release, Reduces Inflammatory Markers and Cells Viability in Caco-2 Cell Line
3. Discussion
4. Materials and Methods
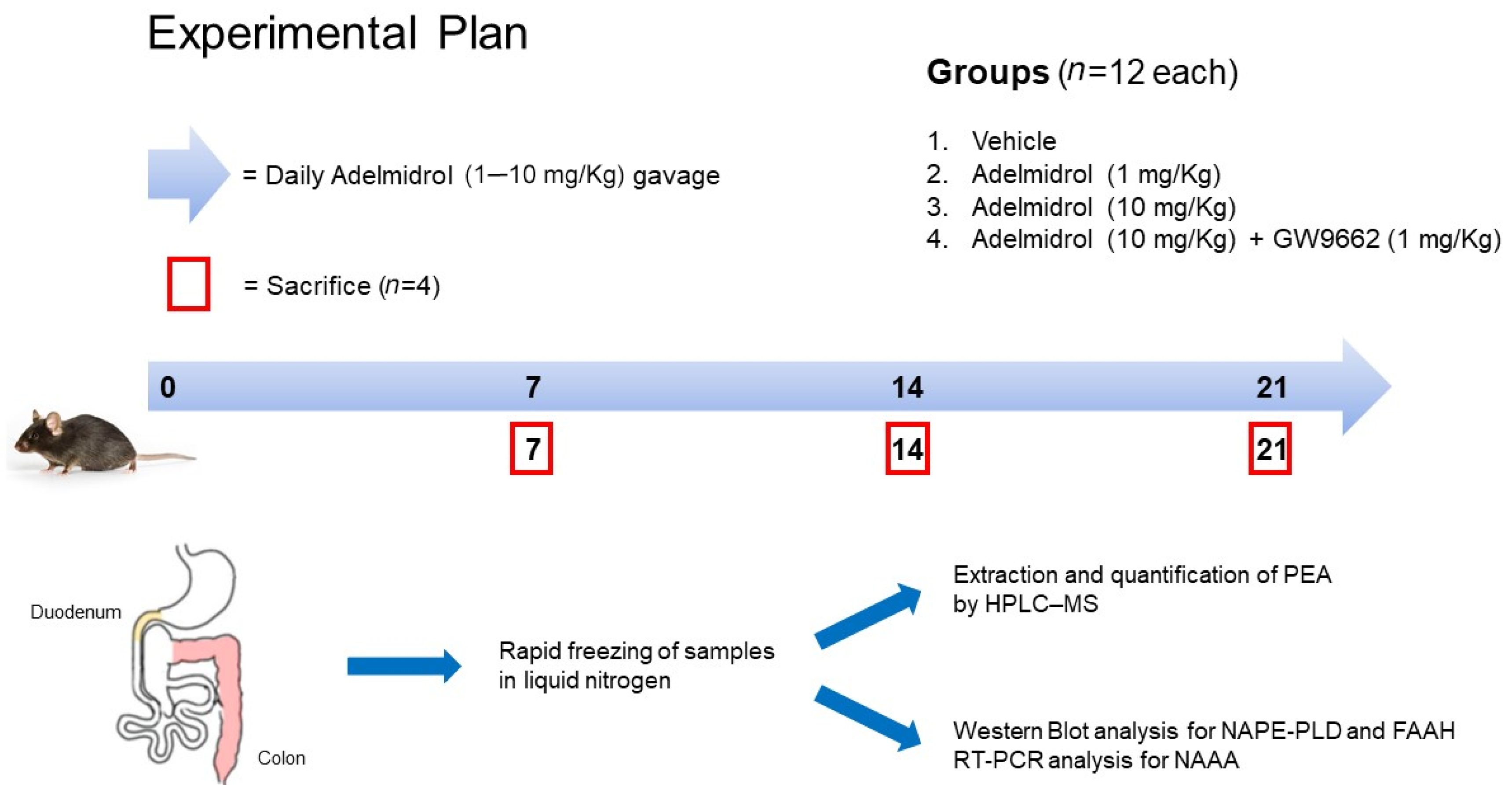
4.1. Animals and Experimental Design
4.2. Extraction and Quantification of In Vivo and In Vitro Produced PEA by HPLC–MS Method
4.3. Western Blot Analysis
4.4. RT-PCR Analysis
4.5. Cells Culture
4.6. Cytotoxicity Assay
4.7. ELISA
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarnelli, G.; D’Alessandro, A.; Iuvone, T.; Capoccia, E.; Gigli, S.; Pesce, M.; Seguella, L.; Nobile, N.; Aprea, G.; Maione, F.; et al. Palmitoylethanolamide Modulates Inflammation-Associated Vascular Endothelial Growth Factor (VEGF) Signaling via the Akt/mTOR Pathway in a Selective Peroxisome Proliferator-Activated Receptor Alpha (PPAR-α)-Dependent Manner. PLoS ONE 2016, 11, e0156198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Couch, D.G.; Tasker, C.; Theophilidou, E.; Lund, J.N.; O’Sullivan, S.E. Cannabidiol and palmitoylethanolamide are anti-inflammatory in the acutely inflamed human colon. Clin. Sci. Lond. Engl. 1979 2017, 131, 2611–2626. [Google Scholar] [CrossRef]
- Couch, D.G.; Cook, H.; Ortori, C.; Barrett, D.; Lund, J.N.; O’Sullivan, S.E. Palmitoylethanolamide and Cannabidiol Prevent Inflammation-induced Hyperpermeability of the Human Gut In Vitro and In Vivo—A Randomized, Placebo-controlled, Double-blind Controlled Trial. Inflamm. Inflamm. Bowel Dis. 2019, 25, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Sarnelli, G.; Pesce, M.; Seguella, L.; Lu, J.; Efficie, E.; Tack, J.; De Palma, F.D.E.; D’Alessandro, A.; Esposito, G. Impaired Duodenal Palmitoylethanolamide Release Underlies Acid-Induced Mast Cell Activation in Functional Dyspepsia. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, N.S.; Gumaste, S.; Subah, S.; Bogoda, N.O. Palmitoylethanolamide: Prenatal Developmental Toxicity Study in Rats. Int. J. Toxicol. 2021, 40, 161–170. [Google Scholar] [CrossRef]
- Chirchiglia, D.; Paventi, S.; Seminara, P.; Cione, E.; Gallelli, L. N-Palmitoyl Ethanol Amide Pharmacological Treatment in Patients with Nonsurgical Lumbar Radiculopathy. J. Clin. Pharmacol. 2018, 58, 733–739. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Della Rocca, G.; Gamba, D. Chronic Pain in Dogs and Cats: Is There Place for Dietary Intervention with Micro-Palmitoylethanolamide? Animals 2021, 11, 952. [Google Scholar] [CrossRef]
- Nestmann, E.R. Safety of micronized palmitoylethanolamide (microPEA): Lack of toxicity and genotoxic potential. Food Sci. Nutr. 2016, 5, 292–309. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J. Neuroinflamm. 2014, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrosino, S.; Cordaro, M.; Verde, R.; Schiano Moriello, A.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-hyperalgesic Effect. Front. Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.-S.V.; Barrett, D.A.; Randall, M.D. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br. J. Pharmacol. 2008, 155, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Ramos, J. The entourage effect of the phytocannabinoids. Ann. Neurol. 2015, 77, 1083. [Google Scholar] [CrossRef]
- Nazzaro-Porro, M. Azelaic acid. J. Am. Acad. Dermatol. 1987, 17, 1033–1041. [Google Scholar] [CrossRef]
- Mastrofrancesco, A.; Ottaviani, M.; Aspite, N.; Cardinali, G.; Izzo, E.; Graupe, K.; Zouboulis, C.P.D.; Camera, E.; Picardo, M. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARγ activation. Exp. Dermatol. 2010, 19, 813–820. [Google Scholar] [CrossRef]
- De Filippis, D.; D’Amico, A.; Cinelli, M.P.; Esposito, G.; Di Marzo, V.; Iuvone, T. Adelmidrol, a palmitoylethanolamide analogue, reduces chronic inflammation in a carrageenin-granuloma model in rats. J. Cell. Mol. Med. 2009, 13, 1086–1095. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Di Paola, R.; Cordaro, M.; Gugliandolo, E.; Casili, G.; Morittu, V.M.; Britti, D.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of acute and chronic inflammation. Biochem. Pharmacol. 2016, 119, 27–41. [Google Scholar] [CrossRef]
- Ostardo, E.; Impellizzeri, D.; Cervigni, M.; Porru, D.; Sommariva, M.; Cordaro, M.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; Crupi, R.; et al. Adelmidrol + sodium hyaluronate in IC/BPS or conditions associated to chronic urothelial inflammation. A translational study. Pharmacol. Res. 2018, 134, 16–30. [Google Scholar] [CrossRef]
- Adelmidrol, in Combination with Hyaluronic Acid, Displays Increased Anti-Inflammatory and Analgesic Effects against Monosodium Iodoacetate-Induced Osteoarthritis in Rats—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27955699/ (accessed on 23 February 2022).
- Fusco, R.; Cordaro, M.; Genovese, T.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Peritore, A.; D’Amico, R.; Crupi, R.; Cuzzocrea, S.; et al. Adelmidrol: A New Promising Antioxidant and Anti-Inflammatory Therapeutic Tool in Pulmonary Fibrosis. Antioxidants 2020, 9, 601. [Google Scholar] [CrossRef]
- Cordaro, M.; Impellizzeri, D.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a Palmitoylethanolamide Analogue, as a New Pharmacological Treatment for the Management of Inflammatory Bowel Disease. Mol. Pharmacol. 2016, 90, 549–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulvirenti, N.; Nasca, M.R.; Micali, G. Topical adelmidrol 2% emulsion, a novel aliamide, in the treatment of mild atopic dermatitis in pediatric subjects: A pilot study. Acta Dermatovenerol. Croat. ADC 2007, 15, 80–83. [Google Scholar] [PubMed]
- Abramo, F.; Salluzzi, D.; Leotta, R.; Auxilia, S.; Noli, C.; Miolo, A.; Mantis, P.; Lloyd, D.H. Mast cell morphometry and densitometry in experimental skin wounds treated with a gel containing adelmidrol: A placebo controlled study. Wounds Compend. Clin. Res. Pract. 2008, 20, 149–157. [Google Scholar]
- Petrosino, S.; Puigdemont, A.; della Valle, M.; Fusco, M.; Verde, R.; Allarà, M.; Aveta, T.; Orlando, P.; Di Marzo, V. Adelmidrol increases the endogenous concentrations of palmitoylethanolamide in canine keratinocytes and down-regulates an inflammatory reaction in an in vitro model of contact allergic dermatitis. Vet. J. Lond. Engl. 2016, 207, 85–91. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Verde, R.; Allarà, M.; Imperatore, R.; Ligresti, A.; Mahmoud, A.M.; Peritore, A.F.; Iannotti, F.A.; Di Marzo, V. Palmitoylethanolamide counteracts substance P-induced mast cell activation in vitro by stimulating diacylglycerol lipase activity. J. Neuroinflamm. 2019, 16, 274. [Google Scholar] [CrossRef] [Green Version]
- Smart, D.; Jonsson, K.-O.; Vandevoorde, S.; Lambert, D.M.; Fowler, C.J. ‘Entourage’ effects ofN-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Br. J. Pharmacol. 2002, 136, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Petrosino, S.; Moriello, A.S.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V.; Melck, M.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; De Petrocellis, L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001, 358 Pt 1, 249–255. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Ermund, A.; Movahed, P.; Andersson, D.A.; Simonsen, C.; Jönsson, B.A.G.; Blomgren, A.; Birnir, B.; Bevan, S.; Eschalier, A.; et al. Monoacylglycerols Activate TRPV1—A Link between Phospholipase C and TRPV1. PLoS ONE 2013, 8, e81618. [Google Scholar] [CrossRef] [Green Version]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Ueda, N.; Yamanaka, K.; Terasawa, Y.; Yamamoto, S. An acid amidase hydrolyzing anandamide as an endogenous ligand for cannabinoid receptors. FEBS Lett. 1999, 454, 267–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastelli, M.; Van Hul, M.; Terrasi, R.; Lefort, C.; Régnier, M.; Beiroa, D.; Delzenne, N.M.; Everard, A.; Nogueiras, R.; Luquet, S.; et al. Intestinal NAPE-PLD contributes to short-term regulation of food intake via gut-to-brain axis. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E647–E657. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Watanabe, K.; Tsuduki, T.; Kimura, I.; Kubota, N. NAPE-PLD controls OEA synthesis and fat absorption by regulating lipoprotein synthesis in an in vitro model of intestinal epithelial cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 3167–3179. [Google Scholar] [CrossRef]
- Rankin, L.; Fowler, C.J. The basal pharmacology of palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Izzo, A.A. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pr. Res. Clin. Endocrinol. Metab. 2009, 23, 33–49. [Google Scholar] [CrossRef]
- Alhouayek, M.; Bottemanne, P.; Subramanian, K.V.; Lambert, D.M.; Makriyannis, A.; Cani, P.D.; Muccioli, G.G. N -Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 650–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carta, G.; Murru, E.; Vargiu, R.; Collu, M.; Carta, M.; Banni, S.; Stancampiano, R. Essential fatty acids deficient diet modulates N-Acylethanolamide profile in rat’s tissues. Prostaglandins, Leukot. Essent. Fat. Acids 2020, 153, 102053. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; A Izzo, A.; Fezza, F.; Pinto, A.; Capasso, F.; Mascolo, N.; Di Marzo, V. Inhibitory effect of palmitoylethanolamide on gastrointestinal motility in mice. Br. J. Pharmacol. 2001, 134, 945–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fichna, J.; Wood, J.T.; Papanastasiou, M.; Vadivel, S.K.; Oprocha, P.; Sałaga, M.; Sobczak, M.; Mokrowiecka, A.; Cygankiewicz, A.I.; Zakrzewski, P.K.; et al. Endocannabinoid and Cannabinoid-Like Fatty Acid Amide Levels Correlate with Pain-Related Symptoms in Patients with IBS-D and IBS-C: A Pilot Study. PLoS ONE 2013, 8, e85073. [Google Scholar] [CrossRef] [Green Version]
- Bonello, D.; Squarzoni, P. Effect of a mucoadhesive gel and dental scaling on gingivitis in dogs. J. Vet. Dent. 2008, 25, 28–32. [Google Scholar] [CrossRef]
- Gachet, M.S.; Rhyn, P.; Bosch, O.G.; Quednow, B.B.; Gertsch, J. A quantitiative LC-MS/MS method for the measurement of arachidonic acid, prostanoids, endocannabinoids, N-acylethanolamines and steroids in human plasma. J. Chromatogr. B Analyt Technol. Biomed. Life. Sci. 2015, 976–977, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; Chen, L.; Zhu, C.; Huang, R.; Zheng, X.; Qiu, Y.; Fu, J. Design and Synthesis of Potent N-Acylethanolamine-hydrolyzing Acid Amidase (NAAA) Inhibitor as Anti-Inflammatory Compounds. PLoS ONE 2012, 7, e43023. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Re, A.; Palenca, I.; Seguella, L.; Pesce, M.; Corpetti, C.; Steardo, L.; Rurgo, S.; Sarnelli, G.; Esposito, G. Oral Adelmidrol Administration Up-Regulates Palmitoylethanolamide Production in Mice Colon and Duodenum through a PPAR-γ Independent Action. Metabolites 2022, 12, 457. https://doi.org/10.3390/metabo12050457
Del Re A, Palenca I, Seguella L, Pesce M, Corpetti C, Steardo L, Rurgo S, Sarnelli G, Esposito G. Oral Adelmidrol Administration Up-Regulates Palmitoylethanolamide Production in Mice Colon and Duodenum through a PPAR-γ Independent Action. Metabolites. 2022; 12(5):457. https://doi.org/10.3390/metabo12050457
Chicago/Turabian StyleDel Re, Alessandro, Irene Palenca, Luisa Seguella, Marcella Pesce, Chiara Corpetti, Luca Steardo, Sara Rurgo, Giovanni Sarnelli, and Giuseppe Esposito. 2022. "Oral Adelmidrol Administration Up-Regulates Palmitoylethanolamide Production in Mice Colon and Duodenum through a PPAR-γ Independent Action" Metabolites 12, no. 5: 457. https://doi.org/10.3390/metabo12050457
APA StyleDel Re, A., Palenca, I., Seguella, L., Pesce, M., Corpetti, C., Steardo, L., Rurgo, S., Sarnelli, G., & Esposito, G. (2022). Oral Adelmidrol Administration Up-Regulates Palmitoylethanolamide Production in Mice Colon and Duodenum through a PPAR-γ Independent Action. Metabolites, 12(5), 457. https://doi.org/10.3390/metabo12050457