Framework of Methodology to Assess the Link between A Posteriori Dietary Patterns and Nutritional Adequacy: Application to Pregnancy
Abstract
:1. Introduction
2. Results
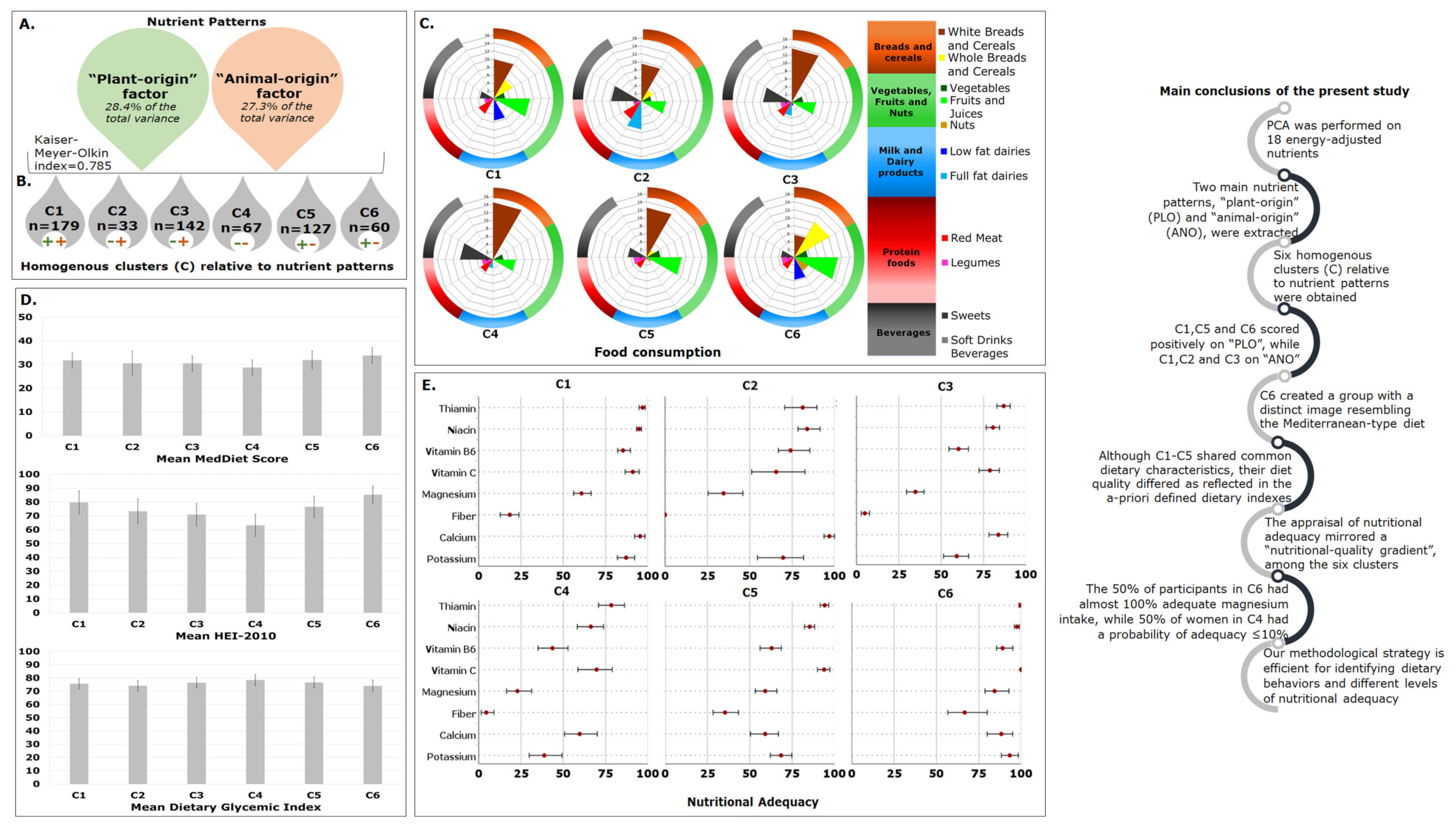
2.1. Identification of Nutrient Patterns
2.2. Homogenous Groups of Participants
2.3. Evaluation of Clusters’ Profile—A Multidimensional Approach
2.3.1. First-level Approach—Mean Nutrient Patterns’ Scores
2.3.2. Second-Level Approach—Demographic/Anthropometric and Lifestyle Characteristics
2.3.3. Third-Level Approach—Food Consumption and Dietary Indexes
2.3.4. Fourth-Level Approach—Nutritional Adequacy
Probability of Adequacy
EAR Cut-Point Method
3. Discussion
3.1. Commentary on Issues of Importance in This Study
3.1.1. Methodological Design
3.1.2. Dietary Patterns and Nutritional Adequacy
3.1.3. Metabolic Aspects
4. Materials and Methods
4.1. Study Population
4.1.1. Participants
4.1.2. Exclusion Criteria
4.2. Data Collection
4.2.1. Demographic/Anthropometric and Lifestyle Characteristics
4.2.2. Dietary Assessment, Nutrient Intake and Dietary Indexes
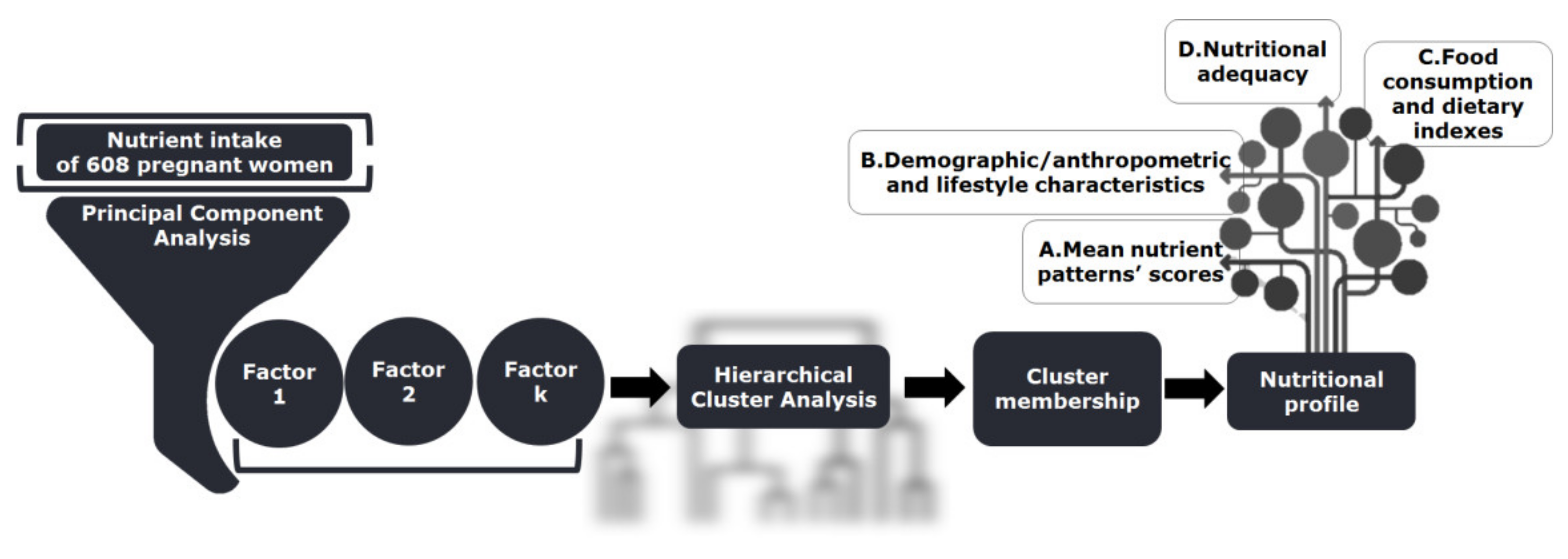
4.3. Methodological and Statistical Design
4.3.1. Principal Component Analysis
4.3.2. Hierarchical Cluster Analysis
4.3.3. Statistical Comparisons among Clusters regarding Demographic/Anthropometric Features and Dietary Quality
4.3.4. Appraisal of Nutritional Adequacy
Probability Approach
EAR Cut-Point Method
Interval Estimates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koletzko, B.; Brands, B.; Poston, L.; Godfrey, K.; Demmelmair, H. Symposium on ‘Metabolic flexibility in animal and human nutrition’ Session I: Early nutrition programming, life performance and cognitive function: Early nutrition programming of long-term health. Proc. Nutr. Soc. 2012, 71, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecorguillé, M.; Teo, S.; Phillips, C.M. Maternal Dietary Quality and Dietary Inflammation Associations with Offspring Growth, Placental Development, and DNA Methylation. Nutrients 2021, 13, 3130. [Google Scholar] [CrossRef] [PubMed]
- Gesteiro, E.; Sánchez-Muniz, F.J.; Bastida, S. Mediterranean diet and pregnancy. In The Mediterranean Diet, 2nd ed.; Preedy, V.R., Ross Watson, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 409–427. [Google Scholar] [CrossRef]
- Cetin, I.; Bühling, K.; Demir, C.; Kortam, A.; Prescott, S.L.; Yamashiro, Y.; Yarmolinskaya, M.; Koletzko, B. Impact of micronutrient status during pregnancy on early nutrition programming. Ann. Nutr. Metab. 2019, 74, 269–278. [Google Scholar] [CrossRef]
- Barker, D.J. Developmental origins of chronic disease. Public Health 2012, 126, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Brands, B.; Chourdakis, M.; Cramer, S.; Grote, V.; Hellmuth, C.; Kirchberg, F.; Prell, C.; Rzehak, P.; Uhl, O.; et al. The Power of Programming and the EarlyNutrition project: Opportunities for health promotion by nutrition during the first thousand days of life and beyond. Ann. Nutr. Metab. 2014, 64, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Conradie, C.; Baumgartner, J.; Malan, L.; Symington, E.A.; Cockeran, M.; Smuts, C.M.; Faber, M. A priori and a posteriori dietary patterns among pregnant women in Johannesburg: South Africa: The nuped study. Nutrients 2021, 13, 565. [Google Scholar] [CrossRef]
- Parisi, F.; Laoreti, A.; Cetin, I. Multiple micronutrient needs in pregnancy in industrialized countries. Ann. Nutr. Metab. 2014, 65, 13–21. [Google Scholar] [CrossRef]
- Hanson, M.A.; Bardsley, A.; De-Regil, L.M.; Moore, S.E.; Oken, E.; Poston, L.; Ma, R.C.; McAuliffe, F.M.; Maleta, K.; Chittaranjan, N.; et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int. J. Gynaecol. Obstet. 2015, 131, S213–S253. [Google Scholar] [CrossRef] [Green Version]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.; Smith, R.; Collins, C.E. Micronutrient intakes during pregnancy in developed countries: Systematic review and meta-analysis. Nutr. Rev. 2013, 71, 118–132. [Google Scholar] [CrossRef]
- Dhonukshe-Rutten, R.A.; Bouwman, J.; Brown, K.A.; Cavelaars, A.E.; Collings, R.; Grammatikaki, E.; de Groot, L.C.; Gurinovic, M.; Harvey, L.J.; Hermoso, M.; et al. EURRECA—Evidence-based methodology for deriving micronutrient recommendations. Crit. Rev. Food Sci. Nutr. 2013, 53, 999–1040. [Google Scholar] [CrossRef]
- Amati, F.; Hassounah, S.; Swaka, A. The impact of Mediterranean dietary patterns during pregnancy on maternal and offspring health. Nutrients 2019, 11, 1098. [Google Scholar] [CrossRef] [Green Version]
- Ancira-Moreno, M.; O’Neill, M.S.; Rivera-Dommarco, J.Á.; Batis, C.; Rodríguez Ramírez, S.; Sánchez, B.N.; Castillo-Castrejón, M.; Vadillo-Ortega, F. Dietary patterns and diet quality during pregnancy and low birthweight: The PRINCESA cohort. Matern. Child. Nutr. 2020, 16, e12972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghavan, R.; Dreibelbis, C.; Kingshipp, B.L.; Wong, Y.P.; Abrams, B.; Gernand, A.D.; Rasmussen, K.M.; Siega-Riz, A.M.; Stang, J.; Casavale, K.O.; et al. Dietary patterns before and during pregnancy and maternal outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109 (Suppl. S1), 705S–728S. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, D.; Mao, X.; Xia, Y.; Baker, P.N.; Zhang, H. Maternal dietary patterns and pregnancy outcome. Nutrients 2016, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.S.; Chen, Q.Z.; He, J.R.; Wei, X.L.; Lu, J.H.; Li, S.H.; Wen, X.X.; Chan, F.F.; Chen, N.N.; Qiu, L.; et al. Maternal dietary patterns and fetal growth: A large prospective cohort study in China. Nutrients 2016, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Coelho, N.D.; Cunha, D.B.; Esteves, A.P.; Lacerda, E.M.; Theme, M.M. Dietary patterns in pregnancy and birth weight. Rev. Saude Publica 2015, 49, 62. [Google Scholar] [CrossRef] [Green Version]
- Chia, A.R.; Chen, L.W.; Lai, J.S.; Wong, C.H.; Neelakantan, N.; van Dam, R.M.; Chong, M.F. Maternal dietary patterns and birth outcomes: A systematic review and meta-analysis. Adv. Nutr. 2019, 10, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Colón-Ramos, U.; Racette, S.B.; Ganiban, J.; Nguyen, T.G.; Kocak, M.; Carroll, K.N.; Völgyi, E.; Tylavsky, F.A. Association between dietary patterns during pregnancy and birth size measures in a diverse population in Southern US. Nutrients 2015, 7, 1318–1332. [Google Scholar] [CrossRef] [Green Version]
- Lovegrove, J.A.; Hodson, L.; Sharma, S.; Lanham-New, S.A. Nutrition Research Methodologies, 1st ed.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2015; p. 66. [Google Scholar]
- Martin, C.L.; Sotres-Alvarez, D.; Siega-Riz, A.M. Maternal dietary patterns during the second trimester are associated with preterm birth. J. Nutr. 2015, 145, 1857–1864. [Google Scholar] [CrossRef]
- Moskal, A.; Pisa, P.T.; Ferrari, P.; Byrnes, G.; Freisling, H.; Boutron-Ruault, M.C.; Cadeau, C.; Nailler, L.; Wendt, A.; Kühn, T.; et al. Nutrient patterns and their food sources in an International Study Setting: Report from the EPIC study. PLoS ONE 2014, 9, e98647. [Google Scholar] [CrossRef] [Green Version]
- Gete, D.G.; Waller, M.; Mishra, G.D. Effects of maternal diets on preterm birth and low birth weight: A systematic review. Br. J. Nutr. 2020, 123, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, S.; Soltani, S.; de Souza, R.J.; Forbes, S.C.; Toupchian, O.; Salehi-Abargouei, A. Associations between maternal dietary patterns and perinatal outcomes: A systematic review and meta-analysis of cohort studies. Adv. Nutr. 2021, 12, 1332–1352. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Di Nunzio, M.; Bordoni, A.; Gori, D.; Lanari, M. Effect of adherence to Mediterranean diet during pregnancy on children’s health: A systematic review. Nutrients 2019, 11, 997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, I.M.; Borrmann, B.; Grosser, A.; Razum, O.; Spallek, J. Determinants of dietary patterns and diet quality during pregnancy: A systematic review with narrative synthesis. Public Health Nutr. 2017, 20, 1009–1028. [Google Scholar] [CrossRef] [PubMed]
- McGowan, C.A.; McAuliffe, F.M. Maternal dietary patterns and associated nutrient intakes during each trimester of pregnancy. Public Health Nutr. 2013, 16, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Okubo, H.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Murakami, K.; Hirota, Y.; Child Health Study Group. Nutritional adequacy of three dietary patterns defined by cluster analysis in 997 pregnant Japanese women: The Osaka Maternal and Child Health Study. Public Health Nutr. 2011, 14, 611–621. [Google Scholar] [CrossRef] [Green Version]
- International Physical Activity Questionnaire (IPAQ). The IPAQ Home Page. Available online: https://sites.google.com/site/theipaq/home (accessed on 15 May 2010).
- Cano-Ibáñez, N.; Martínez-Galiano, J.M.; Luque-Fernández, M.A.; Martín-Peláez, S.; Bueno-Cavanillas, A.; Delgado-Rodríguez, M. Maternal dietary patterns during pregnancy and their association with gestational weight gain and nutrient adequacy. Int. J. Environ. Res. Public Health 2020, 17, 7908. [Google Scholar] [CrossRef]
- Fernández-Gómez, E.; Luque-Vara, T.; Moya-Fernández, P.J.; López-Olivares, M.; Gallardo-Vigil, M.Á.; Enrique-Mirón, C. Factors influencing dietary patterns during pregnancy in a culturally diverse society. Nutrients 2020, 12, 3242. [Google Scholar] [CrossRef]
- Mitku, A.A.; Zewotir, T.; North, D.; Jeena, P.; Naidoo, R.N. The differential effect of maternal dietary patterns on quantiles of Birthweight. BMC Public Health 2020, 20, 976. [Google Scholar] [CrossRef]
- Miura, K.; Takamori, A.; Hamazaki, K.; Tsuchida, A.; Tanaka, T.; Origasa, H.; Inadera, H.; Japan Environment and Children’s Study Group. Dietary patterns during pregnancy and health-related quality of life: The Japan Environment and Children’s Study. PLoS ONE 2020, 15, e0236330. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A review of statistical methods for dietary pattern analysis. Nutr. J. 2021, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Edefonti, V.; De Vito, R.; Dalmartello, M.; Patel, L.; Salvatori, A.; Ferraroni, M. Reproducibility and validity of a posteriori dietary patterns: A systematic review. Adv. Nutr. 2020, 11, 293–326. [Google Scholar] [CrossRef] [PubMed]
- Shakya, P.R.; Melaku, Y.A.; Page, A.J.; Gill, T.K. Nutrient patterns and depressive symptoms among Australian adults. Eur. J. Nutr. 2021, 60, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.B.; Malta, M.B.; Papini, S.J.; Benício, M.H.; Corrente, J.E.; Carvalhaes, M.A. Adherence to dietary patterns during pregnancy and association with maternal characteristics in pregnant Brazilian women. Nutrition 2019, 62, 85–92. [Google Scholar] [CrossRef]
- Hajizadeh, B.; Jessri, M.; Akhoondan, M.; Moasheri, S.M.; Rashidkhani, B. Nutrient patterns and risk of esophageal squamous cell carcinoma: A case-control study. Dis. Esophagus 2012, 25, 442–448. [Google Scholar] [CrossRef]
- Timmermans, S.; Steegers-Theunissen, R.P.; Vujkovic, M.; Bakker, R.; den Breeijen, H.; Raat, H.; Russcher, H.; Lindemans, J.; Hofman, A.; Jaddoe, V.W.; et al. Major dietary patterns and blood pressure patterns during pregnancy: The Generation R Study. Am. J. Obstet. Gynecol. 2011, 205, 337.e1–337.e12. [Google Scholar] [CrossRef] [Green Version]
- Vajdi, M.; Farhangi, M.A.; Nikniaz, L. Diet-derived nutrient patterns and components of metabolic syndrome: A cross-sectional community-based study. BMC Endocr. Disord. 2020, 20, 69. [Google Scholar] [CrossRef]
- Richard, E.L.; Laughlin, G.A.; Kritz-Silverstein, D.; Reas, E.T.; Barrett-Connor, E.; McEvoy, L.K. Dietary patterns and cognitive function among older community-dwelling adults. Nutrients 2018, 10, 1088. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wittert, G.; Taylor, A.W.; Adams, R.; Appleton, S.; Shi, Z. Nutrient patterns and chronic inflammation in a cohort of community dwelling middle-aged men. Clin. Nutr. 2017, 36, 1040–1047. [Google Scholar] [CrossRef]
- Freisling, H.; Pisa, P.T.; Ferrari, P.; Byrnes, G.; Moskal, A.; Dahm, C.C.; Vergnaud, A.C.; Boutron-Ruault, M.C.; Fagherazzi, G.; Cadeau, C.; et al. Main nutrient patterns are associated with prospective weight change in adults from 10 European countries. Eur. J. Nutr. 2016, 55, 2093–2104. [Google Scholar] [CrossRef] [Green Version]
- Melaku, Y.A.; Gill, T.K.; Appleton, S.L.; Taylor, A.W.; Adams, R.; Shi, Z. Prospective associations of dietary and nutrient patterns with fracture risk: A 20-year follow-up study. Nutrients 2017, 9, 1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesołowska, E.; Jankowska, A.; Trafalska, E.; Kałużny, P.; Grzesiak, M.; Dominowska, J.; Hanke, W.; Calamandrei, G.; Polańska, K. Sociodemographic, lifestyle, environmental and pregnancy-related determinants of dietary patterns during pregnancy. Int. J. Environ. Res. Public Health 2019, 16, 754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englund-Ögge, L.; Brantsæter, A.L.; Sengpiel, V.; Haugen, M.; Birgisdottir, B.E.; Myhre, R.; Meltzer, H.M.; Jacobsson, B. Maternal dietary patterns and preterm delivery: Results from large prospective cohort study. BMJ 2014, 348, g1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garay, S.M.; Savory, K.A.; Sumption, L.; Penketh, R.; Janssen, A.B.; John, R.M. The grown in Wales study: Examining dietary patterns, custom birthweight centiles and the risk of delivering a small-for-gestational age (SGA) infant. PLoS ONE 2019, 14, e0213412. [Google Scholar] [CrossRef]
- Völgyi, E.; Carroll, K.N.; Hare, M.E.; Ringwald-Smith, K.; Piyathilake, C.; Yoo, W.; Tylavsky, F.A. Dietary patterns in pregnancy and effects on nutrient intake in the Mid-South: The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study. Nutrients 2013, 5, 1511–1530. [Google Scholar] [CrossRef] [Green Version]
- Karageorgou, D.; Magriplis, E.; Mitsopoulou, A.V.; Dimakopoulos, I.; Bakogianni, I.; Micha, R.; Michas, G.; Chourdakis, M.; Ntouroupi, T.; Tsaniklidou, S.M.; et al. Dietary patterns and lifestyle characteristics in adults: Results from the Hellenic National Nutrition and Health Survey (HNNHS). Public Health 2019, 171, 76–88. [Google Scholar] [CrossRef]
- Costacou, T.; Bamia, C.; Ferrari, P.; Riboli, E.; Trichopoulos, D.; Trichopoulou, A. Tracing the Mediterranean diet through principal components and cluster analyses in the Greek population. Eur. J. Clin. Nutr. 2003, 57, 1378–1385. [Google Scholar] [CrossRef]
- Ni, Y.; Szpiro, A.; Loftus, C.; Tylavsky, F.; Kratz, M.; Bush, N.R.; LeWinn, K.Z.; Sathyanarayana, S.; Enquobahrie, D.A.; Davis, R.; et al. Associations Between Maternal Nutrition in Pregnancy and Child Blood Pressure at 4–6 Years: A Prospective Study in a Community-Based Pregnancy Cohort. J. Nutr. 2021, 151, 949–961. [Google Scholar] [CrossRef]
- Yang, J.; Dang, S.; Cheng, Y.; Qiu, H.; Mi, B.; Jiang, Y.; Qu, P.; Zeng, L.; Wang, Q.; Li, Q.; et al. Dietary intakes and dietary patterns among pregnant women in Northwest China. Public Health Nutr. 2017, 20, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Grieger, J.A.; Grzeskowiak, L.E.; Clifton, V.L. Preconception dietary patterns in human pregnancies are associated with preterm delivery. J. Nutr. 2014, 144, 1075–1080. [Google Scholar] [CrossRef]
- Northstone, K.; Emmett, P.M.; Rogers, I. Dietary patterns in pregnancy and associations with nutrient intakes. Br. J. Nutr. 2008, 99, 406–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashworth, C.J.; Antipatis, C. Micronutrient programming of development throughout gestation. Reproduction 2001, 122, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Tsakoumaki, F.; Kyrkou, C.; Athanasiadis, A.P.; Menexes, G.; Michaelidou, A.M. Nutritional Inadequacy: Unraveling the Methodological Challenges for the Application of the Probability Approach or the EAR Cut-Point Method—A Pregnancy Perspective. Nutrients 2021, 13, 3473. [Google Scholar] [CrossRef] [PubMed]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and micronutrient intake during pregnancy: An overview of recent evidence. Nutrients 2019, 11, 443. [Google Scholar] [CrossRef] [Green Version]
- Marangoni, F.; Cetin, I.; Verduci, E.; Canzone, G.; Giovannini, M.; Scollo, P.; Corsello, G.; Poli, A. Maternal diet and nutrient requirements in pregnancy and breastfeeding. An Italian consensus document. Nutrients 2016, 8, 629. [Google Scholar] [CrossRef]
- Caradonna, F.; Consiglio, O.; Luparello, C.; Gentile, C. Science and healthy meals in the world: Nutritional epigenomics and nutrigenetics of the mediterranean diet. Nutrients 2020, 12, 1748. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet for type 2 diabetes: Cardiometabolic benefits. Endocrine 2017, 56, 27–32. [Google Scholar] [CrossRef]
- Du, H.; Van Der, A.D.L.; Feskens, E.J. Dietary glycaemic index: A review of the physiological mechanisms and observed health impacts. Acta Cardiol. 2006, 61, 393–397. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Eckel, R.H. The glycemic index at 20 y. Am. J. Clin. Nutr. 2002, 76, 264S–265S. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266S–773S. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H. Hormone-mediated Changes in Nutrient Metabolism in Pregnancy: A Physiological Basis for Normal Fetal Development. Ann. N. Y. Acad. Sci. 1997, 817, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Fotiou, M.; Fotakis, C.; Tsakoumaki, F.; Athanasiadou, E.; Kyrkou, C.; Dimitropoulou, A.; Tsiaka, T.; Chatziioannou, A.C.; Sarafidis, K.; Menexes, G.; et al. H NMR-based metabolomics reveals the effect of maternal habitual dietary patterns on human amniotic fluid profile. Sci. Rep. 2018, 8, 4076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslova, E.; Halldorsson, T.I.; Astrup, A.; Olsen, S.F. Dietary protein-to-carbohydrate ratio and added sugar as determinants of excessive gestational weight gain: A prospective cohort study. BMJ Open 2015, 5, e005839. [Google Scholar] [CrossRef] [Green Version]
- Blumfield, M.L.; Hure, A.J.; MacDonald-Wicks, L.K.; Smith, R.; Simpson, S.J.; Giles, W.B.; Raubenheimer, D.; Collins, C.E. Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am. J. Clin. Nutr. 2012, 96, 1032–1041. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Panel on dietetic products, nutrition and allergies (NDA), scientific opinion on dietary reference values for energy. EFSA J. 2013, 11, 3005. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C. Nutrition Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Athanasiadou, E.; Kyrkou, C.; Fotiou, M.; Tsakoumaki, F.; Dimitropoulou, A.; Polychroniadou, E.; Menexes, G.; Athanasiadis, A.P.; Biliaderis, C.G.; Michaelidou, A.M. Development and validation of a Mediterranean oriented culture-specific semi-quantitative food frequency questionnaire. Nutrients 2016, 8, 522. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation (WHO). 2019 Europe, WHO Body Mass Index-BMI. Available online: http://www.euro.who.int/en/health-topics/diseaseprevention/nutrition/a-healthylifestyle/body-mass-index-bmi (accessed on 16 April 2019).
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Papazian, T.; Serhal, A.; Hout, H.; Younes, H.; Abi Tayeh, G.; Azouri, J.; Lteif, F.H.; Kesrouani, A.; Khabbaz, L.R. Discrepancies among different tools evaluating Mediterranean diet adherence during pregnancy, correlated to maternal anthropometric, dietary and biochemical characteristics. Clin. Nutr. 2019, 38, 1398–1405. [Google Scholar] [CrossRef]
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.; Kuczynski, K.J.; Kahle, L.L.; Krebs-Smith, S.M. Update of the healthy eating index: HEI-2010. J. Acad. Nutr. Diet. 2013, 113, 569–580. [Google Scholar] [CrossRef] [Green Version]
- Bowman, S.A.; Clemens, J.C.; Friday, J.E.; Lynch, K.L.; Moshfegh, A.J. Food Patterns Equivalents Database 2013–14: Methodology and User Guide. 2017. Available online: www.ars.usda.gov/Services/docs.htm?docid=23870 (accessed on 26 May 2017).
- Zhu, Y.; Hedderson, M.M.; Sridhar, S.; Xu, F.; Feng, J.; Ferrara, A. Poor diet quality in pregnancy is associated with increased risk of excess fetal growth: A prospective multi-racial/ethnic cohort study. Int. J. Epidemiol. 2019, 48, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Block, G.; Sternfeld, B.; Sowers, M. Dietary glycemic load, glycemic index, and associated factors in a multiethnic cohort of midline women. JACN 2009, 28, 636–647. [Google Scholar] [CrossRef] [Green Version]
- Foster-Powell, K.; Holt, S.H.A.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilopoulou, A.; Galitsianos, I.; Fotiou, M.; Menexes, G.; Tsakoumaki, F.; Tsitlakidou, P.; Psirropoulos, D.; Michaelidou, A.M. An exploratory study of dietary intake patterns among adults diagnosed with cardiovascular risk factors. Int. J. Food Sci. Nutr. 2015, 66, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis: A Global Perspective. In Canonical Correlation: A Supplement to Multivariate Data Analysis, 7th ed.; Pearson Prentice Hall Publishing: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Sharma, S. Cluster analysis. In Applied Multivariate Techniques; John Wiley & Sons Inc.: New York, NY, USA, 1996. [Google Scholar]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Michos, M.C.; Mamolos, A.P.; Menexes, G.C.; Tsatsarelis, C.A.; Tsirakoglou, V.M.; Kalburtji, K.L. Energy inputs, outputs and greenhouse gas emissions in organic, integrated and conventional peach orchards. Ecol. Indic. 2012, 13, 22–28. [Google Scholar] [CrossRef]
- Cortina, J.M.; Nouri, H. Effect Size for ANOVA Designs; Sage Publications: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Toothaker, L.E. Multiple Comparison Procedures; Sage Publications: Thousand Oaks, CA, USA, 1993; p. 62. [Google Scholar]
- Oria, M.; Harrison, M.; Stallings, V.A. Institute of Medicine (US) Committee to Review the Dietary Reference Intakes for Sodium and Potassium. In Dietary Reference Intakes for Sodium and Potassium; National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- IOM 2000 Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes: Applications in Dietary Assessment; National Academies Press: Washington, DC, USA, 2000.
- Otten, J.J.; Pitzi Hellwig, J.; Meyers, L.D. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Murphy, S.P.; Poos, M.I. Dietary reference intakes: Summary of applications in dietary assessment. Public Health Nutr. 2002, 5, 843–849. [Google Scholar] [CrossRef] [Green Version]
- Beaton, G.H. Uses and limits of the use of the Recommended Dietary Allowances for evaluating dietary intake data. Am. J. Clin. Nutr. 1985, 41, 155–164. [Google Scholar] [CrossRef]
- Murphy, S.P.; Vorster, H.H. Methods for using nutrient intake values (NIVs) to assess or plan nutrient intakes. Food Nutr. Bull. 2007, 28 (Suppl. S1), S51–S60. [Google Scholar] [CrossRef] [Green Version]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L.; Reidy, K.C.; Catalano, P.M. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). EFSA Panel on Plant Protection Products and their Residues (PPR). Guidance on the use of probabilistic methodology for modelling dietary exposure to pesticide residues. EFSA J. 2012, 10, 2839. [Google Scholar] [CrossRef]
- Efron, B. Better bootstrap confidence intervals. JASA 1987, 82, 171–185. [Google Scholar] [CrossRef]
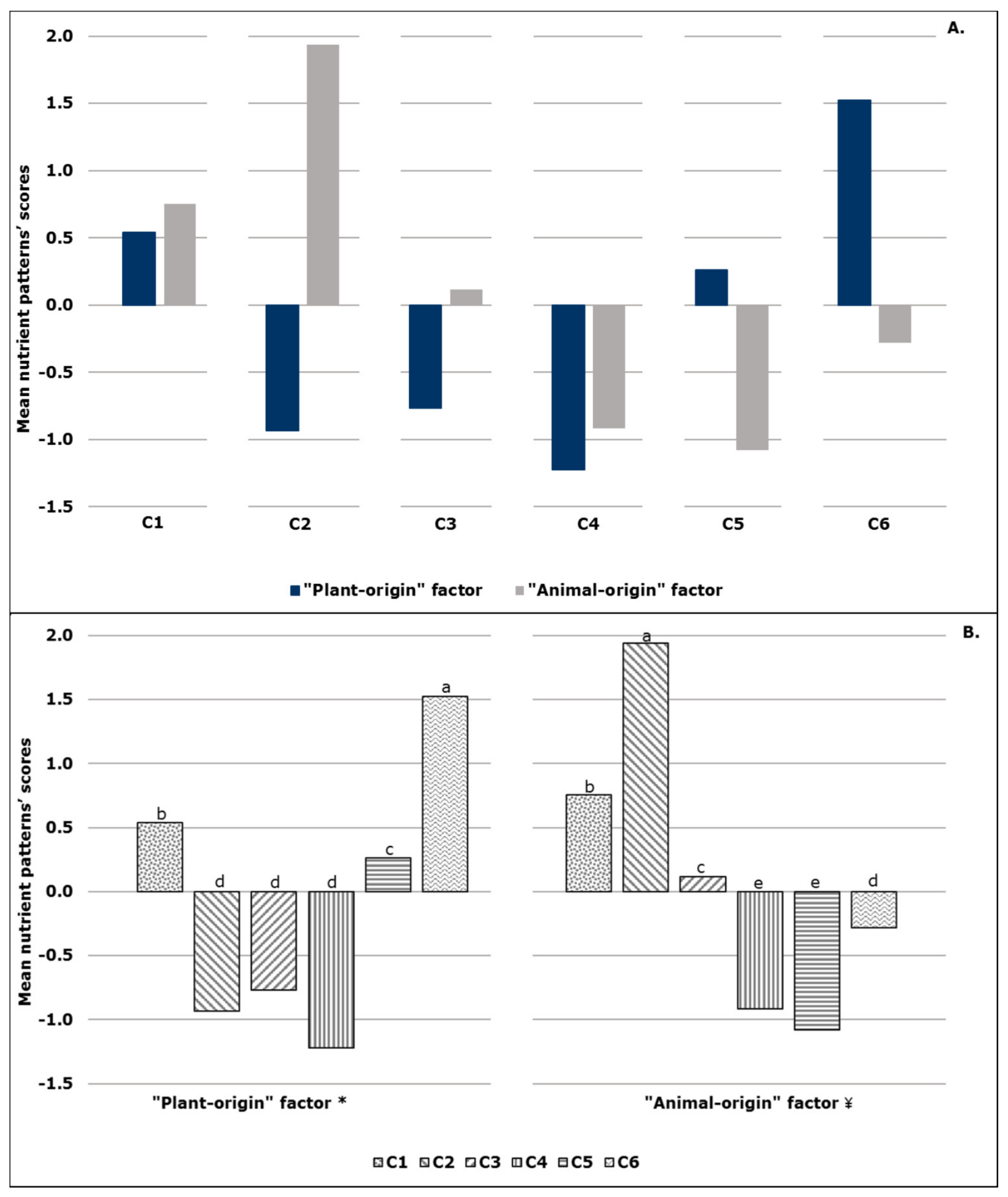
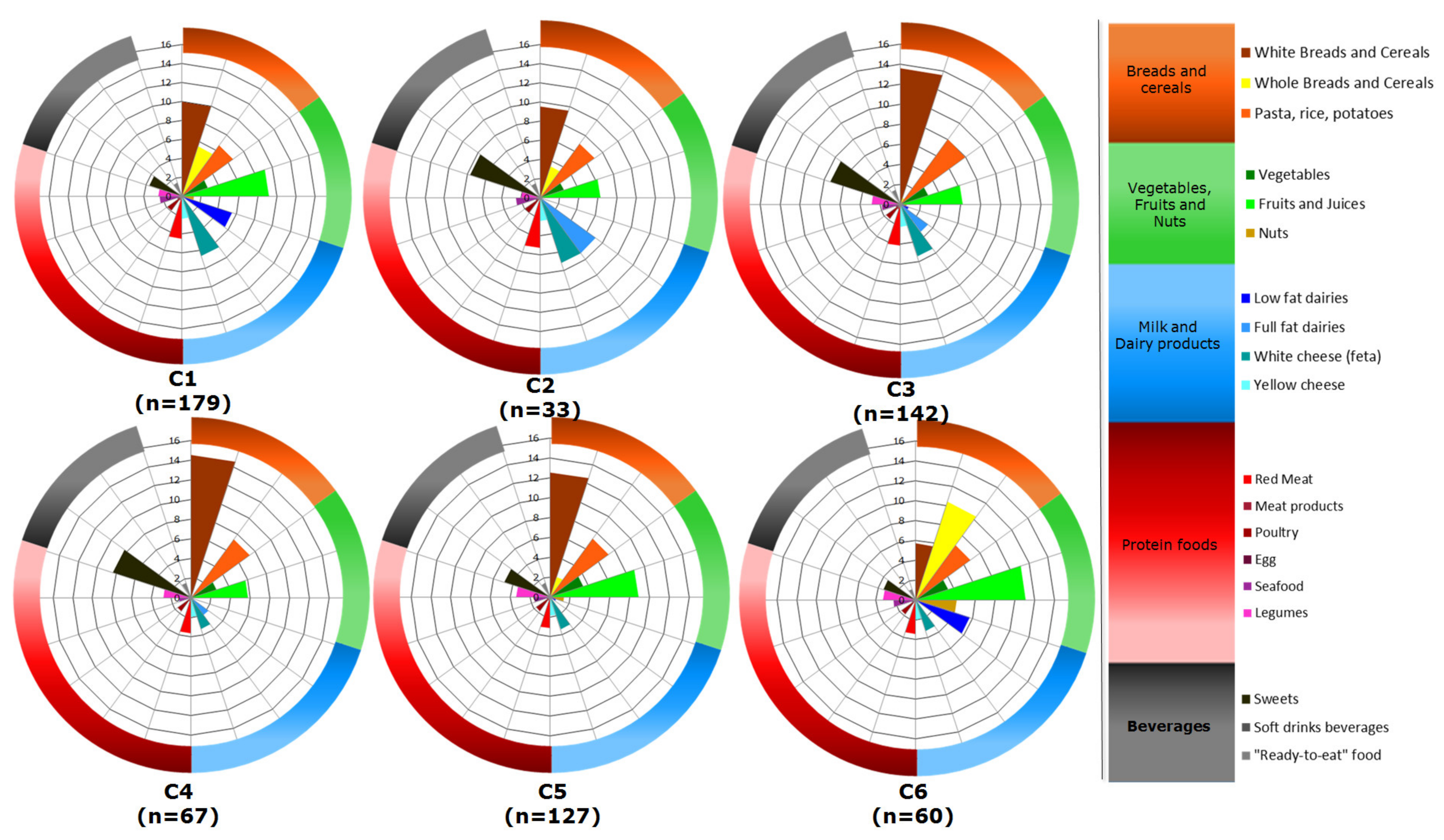
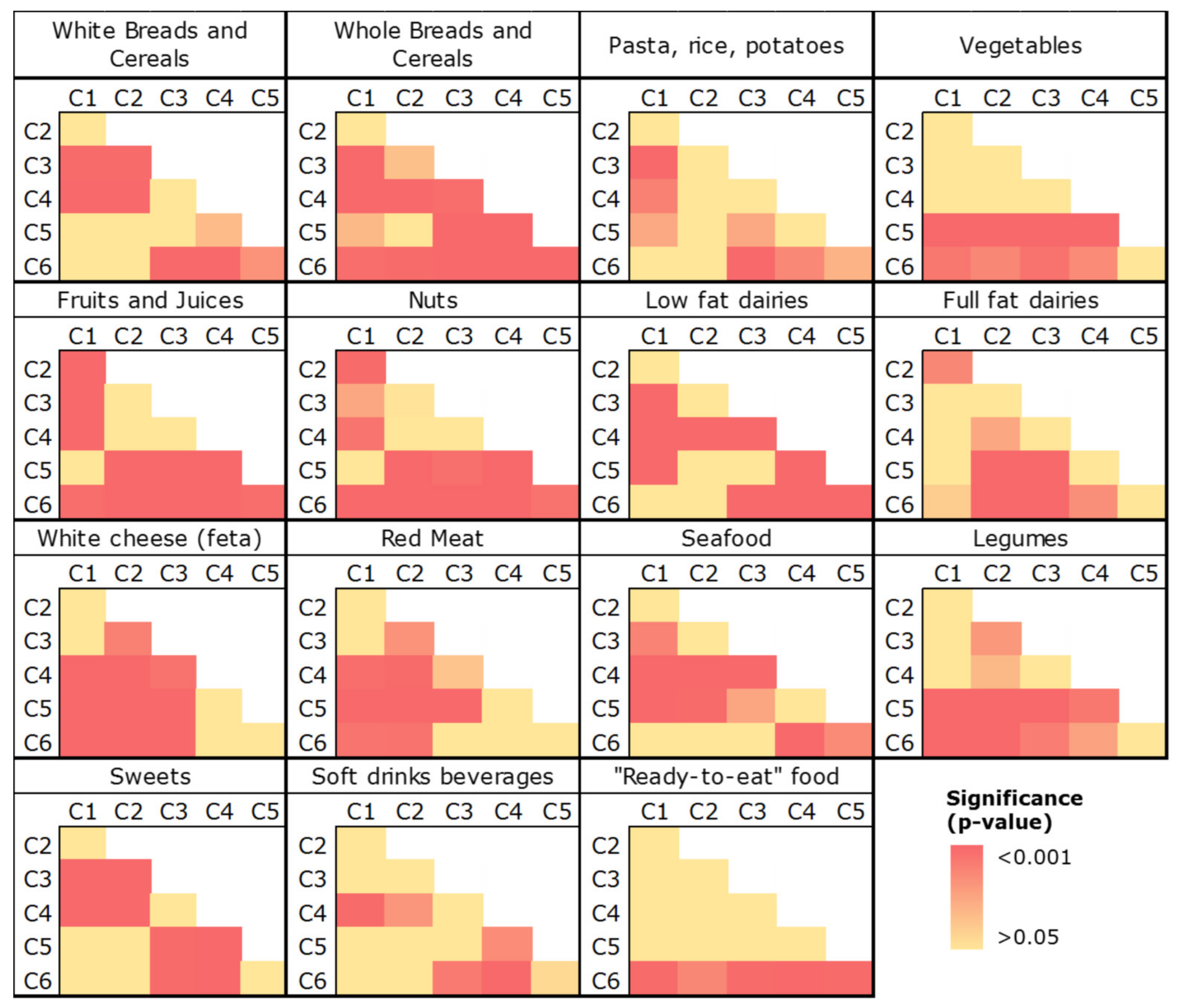
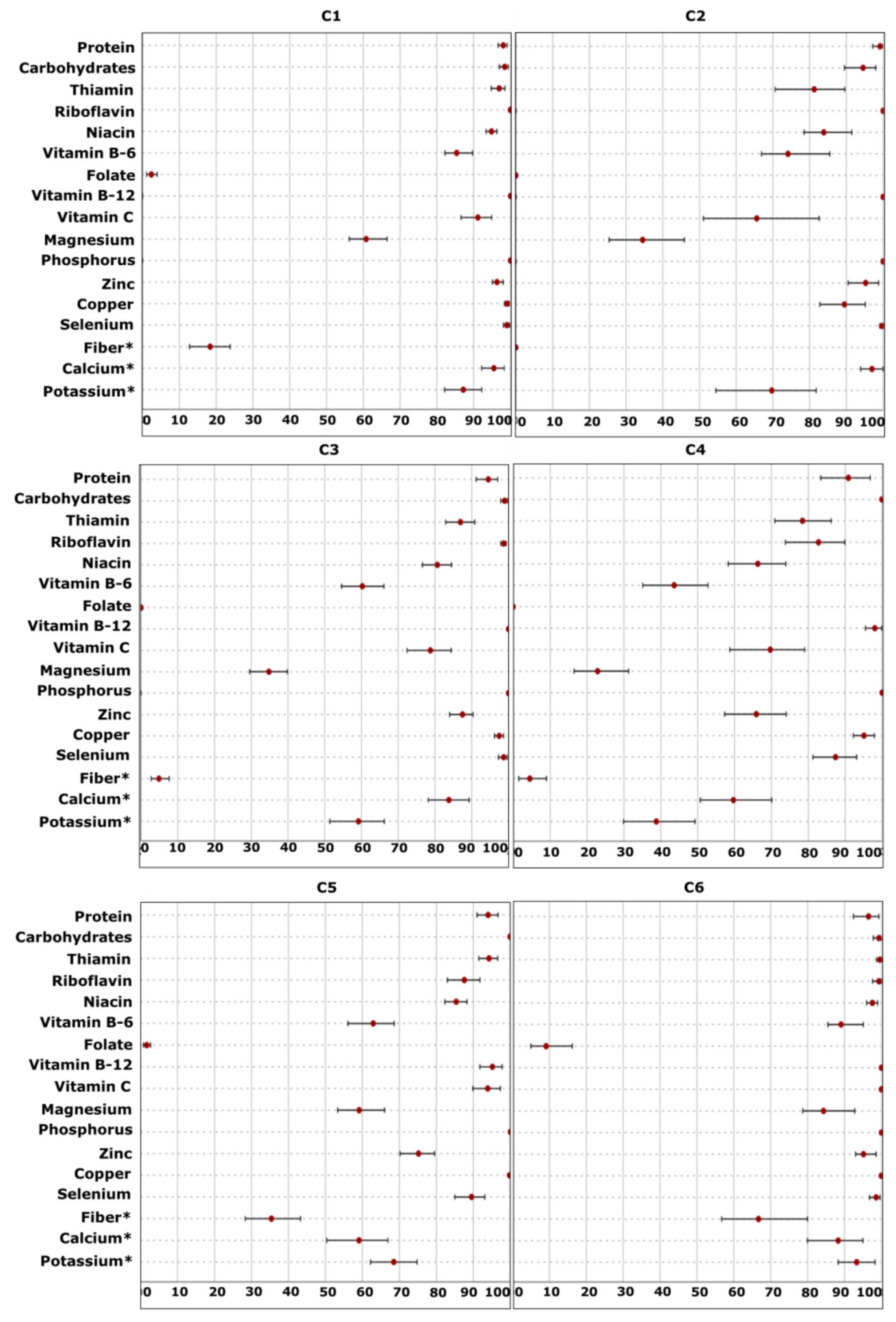
| Plant-Origin Factor | Animal-Origin Factor | |
|---|---|---|
| Folate | 0.858 | |
| Magnesium | 0.789 | |
| Potasium | 0.718 | |
| Carbohydrates/Fiber | −0.707 | |
| Thiamin | 0.698 | |
| Vitamin B-6 | 0.613 | |
| Copper | 0.584 | |
| Niacin | 0.545 | |
| Vitamin C | 0.527 | |
| Phosphorus | 0.813 | |
| Vitamin B-12 | 0.811 | |
| Animal Protein/Plant Protein | 0.772 | |
| Calcium | 0.753 | |
| Riboflavin | 0.726 | |
| Zinc | 0.652 | |
| (MUFA + PUFA)/SFA | −0.622 | |
| Selenium | 0.597 | |
| Cholesterol | 0.581 | |
| Variance explained (%) | 28.4 | 27.3 |
| Eigenvalues | 5.119 | 4.909 |
| C1 (n = 179) | C2 (n = 33) | C3 (n = 142) | C4 (n = 67) | C5 (n = 127) | C6 (n = 60) | p-Value | |
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Maternal age (year) | 36.7 (3.6) | 35.9 (3.7) | 36.4 (3.6) | 36.2 (4.9) | 36.7 (3.5) | 36.4 (3.9) | 0.864 |
| Pre-pregnancy BMI | 23.5 (3.6) | 23.8 (5.2) | 24.1 (5.2) | 23.8 (5) | 24.0 (4.4) | 23.7 (4.3) | 0.889 |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Education | |||||||
| >12 years | 86 (48.0) | 15 (45.5) | 76 (53.5) | 34 (50.7) | 68 (53.5) | 25 (41.7) | 0.614 |
| ≤12 years | 93 (52.0) | 18 (54.5) | 66 (46.5) | 33 (49.3) | 59 (46.5) | 35 (58.3) | |
| Physical activity level * | |||||||
| Low activity | 127 (70.9) | 27 (81.8) | 112 (78.9) | 60 (89.6) | 101 (79.5) | 46 (76.7) | 0.194 |
| Moderate activity | 39 (21.8) | 5 (15.2) | 23 (16.2) | 3 (4.5) | 21 (16.5) | 10 (16.7) | |
| High activity | 13 (7.3) | 1 (3.0) | 7 (4.9) | 4 (6.0) | 5 (3.9) | 4 (6.7) | |
| Smoking during pregnancy | |||||||
| Occasional or daily smokers | 20 (11.2) | 5 (15.2) | 27 (19.0) | 15 (22.4) | 21 (16.5) | 3 (5.0) | 0.039 |
| Non-smokers | 159 (88.8) | 28 (84.8) | 115 (81.0) | 52 (77.6) | 106 (83.5) | 57 (95.0) |
| C1 (n = 179) | C2 (n = 33) | C3 (n = 142) | C4 (n = 67) | C5 (n = 127) | C6 (n = 60) | p-Value | |
|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
| White breads and cereals | 9.9 (2–15.5) | 9.5 (1.1–13.6) | 13.6 (9.3–16.7) | 14.5 (11.3–16.7) | 12.5 (1.9–16.9) | 5.7 (0.6–14.4) | <0.001 |
| Whole breads and cereals | 5.5 (0.4–13.1) | 3.4 (0.0–10.9) | 0.7 (0.0–4.4) | 0.0 (0.0–0.6) | 2.1 (0–11.1) | 10.4 (4.9–15.4) | <0.001 |
| Pasta, rice and potatoes | 6.6 (5.2–8.2) | 7 (5.6–9.1) | 8.0 (6.3–9.4) | 7.3 (6.3–9.1) | 7.3 (5.8–8.6) | 6.8 (4.4–8.2) | <0.001 |
| Vegetables | 2.9 (2.2–3.5) | 2.6 (1.8–3.9) | 2.9 (2.1–3.7) | 2.7 (2.1–3.8) | 3.5 (2.9–4.7) | 3.4 (2.5–4.6) | <0.001 |
| Fruits and juices | 9.2 (6.2–12.1) | 6.3 (3.6–8.4) | 6.2 (3.9–8.6) | 5.8 (3.7–8.6) | 8.9 (6.1–12.2) | 11.1 (8.1–15.2) | <0.001 |
| Nuts | 1.2 (0.0–3.5) | 0.0 (0.0–1.1) | 0.6 (0.0–2.2) | 0.0 (0.0–2.0) | 1.4 (0.0–4.3) | 4.1 (0.1–8.4) | <0.001 |
| Low-fat dairy | 5.6 (0.0–8.6) | 0.0 (0.0–8.0) | 0.8 (0.0–5.1) | 0.0 (0.0–0.0) | 0.6 (0.0–4.3) | 5.7 (2.6–8.0) | <0.001 |
| Full-fat dairy | 0.0 (0.0–7.5) | 7.2 (0.0–15.4) | 3.4 (0.0–8.3) | 2.2 (0.0–6.0) | 0.0 (0.0–4.3) | 0.0 (0.0–3.2) | <0.001 |
| White cheese “feta” | 6.6 (3.4–8.1) | 7.2 (3.7–11.5) | 5.5 (3.3–7.3) | 3.3 (2.6–6.5) | 3.4 (1.1–6.1) | 3.3 (1.1–4.9) | <0.001 |
| Yellow cheese | 2.4 (1.1–3.9) | 2.5 (1.7–5.5) | 2.2 (1.4–4.1) | 2.0 (0.8–3.6) | 1.9 (0.7–2.6) | 2.0 (1.0–3.2) | 0.007 |
| Red meat | 4.5 (3.2–6.1) | 5.3 (3.4–7.7) | 4.1 (2.9–5.3) | 3.6 (2.3–5.0) | 3.2 (2.2–4.3) | 3.4 (2.5–4.8) | <0.001 |
| Meat products | 0.5 (0.0–1.0) | 0.2 (0.0–1.6) | 0.5 (0.0–0.9) | 0.4 (0.0–0.7) | 0.4 (0.0–0.8) | 0.1 (0.0–0.6) | 0.282 |
| Poultry | 1.9 (1.6–2.5) | 2.0 (1.1–2.8) | 1.8 (1.4–2.7) | 1.7 (1.1–2.4) | 1.7 (1.2–2.3) | 1.8 (1.3–2.4) | 0.383 |
| Egg | 0.5 (0.2–1.4) | 0.5 (0.3–1.6) | 0.5 (0.2–1.4) | 0.2 (0.0–0.5) | 0.4 (0.0–1.1) | 0.4 (0.0–1.4) | <0.001 |
| Seafood | 2.4 (1.4–3.4) | 2.5 (1.4–4.1) | 1.9 (1.2–2.9) | 1.3 (0.0–2.2) | 1.6 (0.8–2.5) | 2.3 (1.4–3.0) | <0.001 |
| Legumes | 2.5 (1.6–3.4) | 2.0 (0.0–3.1) | 2.9 (1.9–3.6) | 2.8 (2.1–3.9) | 3.4 (2.5–4.6) | 3.3 (2.3–5.0) | <0.001 |
| Sweets | 3.6 (1.6–7.7) | 7.7 (1.4–8.8) | 7.3 (4.6–9.6) | 8.3 (4.0–12.0) | 4.8 (2.4–7.7) | 3.4 (1.4–5.3) | <0.001 |
| Soft drink beverages | 0.0 (0.0–0.5) | 0.0 (0.0–0.5) | 0.0 (0.0–1.0) | 0.4 (0.0–2.9) | 0.0 (0.0–0.9) | 0.0 (0.0–0.1) | 0.002 |
| “Ready-to-eat” | 1.5 (0.8–1.8) | 1.6 (0.0–2.8) | 1.5 (0.0–3.0) | 1.6 (1.3–3.2) | 1.5 (0.0–2.7) | 1.1 (0.0–1.6) | 0.002 |
| MedDiet Score | HEI-2010 | Dietary GI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Mean (SD) | p-Value | Median | Mean (SD) | p-Value | Median | Mean (SD) | p-Value | |
| C1 | 32.0 | 31.8 (3.2) b | <0.001 | 79.6 | 79.7 (8.4) b | <0.001 | 76.0 | 75.6 (3.9) b,c | <0.001 |
| C2 | 31.0 | 30.6 (5.1) b | 72.8 | 73.3 (8.9) c,d | 73.8 | 74.1 (4.0) c | |||
| C3 | 30.0 | 30.5 (3.1) b | 71.2 | 70.9 (7.9) d | 76.4 | 76.4 (3.9) b | |||
| C4 | 29.0 | 28.7 (3.2) c | 63.7 | 63.2 (8.0) e | 78.6 | 78.5 (4.2) a | |||
| C5 | 32.0 | 31.9 (3.7) b | 75.8 | 76.6 (7.6) b,c | 76.7 | 76.6 (4.0) a,b | |||
| C6 | 34.0 | 33.8 (3.3) a | 86.0 | 85.2 (6.3) a | 74.6 | 74.0 (4.4) c | |||
| Magnesium | Zinc | Copper | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P10 | P25 | P50 | P75 | P90 | P10 | P25 | P50 | P75 | P90 | P10 | P25 | P50 | P75 | P90 | |
| C1 | 12 | 32 | 65 | 95 | 100 | 90 | 98 | 100 | 100 | 100 | 99 | 100 | 100 | 100 | 100 |
| C2 | 1 | 8 | 28 | 60 | 83 | 85 | 96 | 100 | 100 | 100 | 53 | 84 | 98 | 100 | 100 |
| C3 | 1 | 4 | 17 | 60 | 96 | 67 | 81 | 95 | 100 | 100 | 93 | 98 | 100 | 100 | 100 |
| C4 | 0 | 2 | 10 | 34 | 79 | 12 | 40 | 77 | 97 | 100 | 80 | 98 | 100 | 100 | 100 |
| C5 | 5 | 19 | 69 | 95 | 100 | 30 | 62 | 90 | 99 | 100 | 100 | 100 | 100 | 100 | 100 |
| C6 | 34 | 78 | 97 | 100 | 100 | 83 | 97 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Selenium | Thiamin | Riboflavin | |||||||||||||
| P10 | P25 | P50 | P75 | P90 | P10 | P25 | P50 | P75 | P90 | P10 | P25 | P50 | P75 | P90 | |
| C1 | 100 | 100 | 100 | 100 | 100 | 96 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| C2 | 100 | 100 | 100 | 100 | 100 | 38 | 69 | 99 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| C3 | 98 | 100 | 100 | 100 | 100 | 48 | 88 | 99 | 100 | 100 | 98 | 100 | 100 | 100 | 100 |
| C4 | 39 | 87 | 100 | 100 | 100 | 27 | 56 | 97 | 100 | 100 | 25 | 77 | 99 | 100 | 100 |
| C5 | 54 | 97 | 100 | 100 | 100 | 74 | 99 | 100 | 100 | 100 | 40 | 95 | 100 | 100 | 100 |
| C6 | 97 | 99 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Niacin | Vitamin B-6 | Vitamin C | |||||||||||||
| P10 | P25 | P50 | P75 | P90 | P10 | P25 | P50 | P75 | P90 | P10 | P25 | P50 | P75 | P90 | |
| C1 | 82 | 95 | 99 | 100 | 100 | 38 | 88 | 98 | 100 | 100 | 68 | 100 | 100 | 100 | 100 |
| C2 | 51 | 66 | 95 | 99 | 100 | 20 | 59 | 83 | 99 | 100 | 0 | 1 | 100 | 100 | 100 |
| C3 | 44 | 71 | 90 | 98 | 100 | 17 | 32 | 63 | 93 | 100 | 3 | 76 | 100 | 100 | 100 |
| C4 | 13 | 32 | 80 | 94 | 99 | 2 | 7 | 35 | 81 | 99 | 0 | 35 | 100 | 100 | 100 |
| C5 | 56 | 79 | 93 | 99 | 100 | 11 | 32 | 73 | 99 | 100 | 98 | 100 | 100 | 100 | 100 |
| C6 | 91 | 99 | 100 | 100 | 100 | 65 | 82 | 99 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Fiber Intake (AI = 28 g/d) | Percentage of “Adequate” Population * | |||||
|---|---|---|---|---|---|---|
| P10 | P25 | P50 | P75 | P90 | ||
| C1 | 18 | 21 | 24 | 26 | 29 | 18.4 |
| C2 | 11 | 16 | 17 | 19 | 24 | 0.0 |
| C3 | 15 | 18 | 20 | 22 | 25 | 4.9 |
| C4 | 16 | 17 | 20 | 23 | 25 | 4.5 |
| C5 | 20 | 22 | 25 | 30 | 33 | 35.4 |
| C6 | 24 | 27 | 30 | 33 | 38 | 66.7 |
| Potassium Intake (AI = 2.9 g/d) | ||||||
| P10 | P25 | P50 | P75 | P90 | ||
| C1 | 2.8 | 3.0 | 3.3 | 3.6 | 3.9 | 87.2 |
| C2 | 2.5 | 2.7 | 3.0 | 3.3 | 3.5 | 69.7 |
| C3 | 2.4 | 2.7 | 2.9 | 3.3 | 3.6 | 59.2 |
| C4 | 2.3 | 2.4 | 2.7 | 3.0 | 3.3 | 38.8 |
| C5 | 2.6 | 2.8 | 3.2 | 3.6 | 3.9 | 68.5 |
| C6 | 2.9 | 3.1 | 3.5 | 3.8 | 4.2 | 93.3 |
| Calcium Intake (EAR = 800 mg/d) | ||||||
| P10 | P25 | P50 | P75 | P90 | ||
| C1 | 884 | 985 | 1134 | 1274 | 1441 | 95.5 |
| C2 | 908 | 1108 | 1272 | 1492 | 1657 | 97.0 |
| C3 | 718 | 868 | 1005 | 1127 | 1303 | 83.8 |
| C4 | 600 | 701 | 832 | 947 | 1038 | 59.7 |
| C5 | 570 | 724 | 839 | 1018 | 1170 | 59.1 |
| C6 | 760 | 906 | 983 | 1146 | 1297 | 88.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakoumaki, F.; Kyrkou, C.; Fotiou, M.; Dimitropoulou, A.; Biliaderis, C.G.; Athanasiadis, A.P.; Menexes, G.; Michaelidou, A.-M. Framework of Methodology to Assess the Link between A Posteriori Dietary Patterns and Nutritional Adequacy: Application to Pregnancy. Metabolites 2022, 12, 395. https://doi.org/10.3390/metabo12050395
Tsakoumaki F, Kyrkou C, Fotiou M, Dimitropoulou A, Biliaderis CG, Athanasiadis AP, Menexes G, Michaelidou A-M. Framework of Methodology to Assess the Link between A Posteriori Dietary Patterns and Nutritional Adequacy: Application to Pregnancy. Metabolites. 2022; 12(5):395. https://doi.org/10.3390/metabo12050395
Chicago/Turabian StyleTsakoumaki, Foteini, Charikleia Kyrkou, Maria Fotiou, Aristea Dimitropoulou, Costas G. Biliaderis, Apostolos P. Athanasiadis, Georgios Menexes, and Alexandra-Maria Michaelidou. 2022. "Framework of Methodology to Assess the Link between A Posteriori Dietary Patterns and Nutritional Adequacy: Application to Pregnancy" Metabolites 12, no. 5: 395. https://doi.org/10.3390/metabo12050395
APA StyleTsakoumaki, F., Kyrkou, C., Fotiou, M., Dimitropoulou, A., Biliaderis, C. G., Athanasiadis, A. P., Menexes, G., & Michaelidou, A.-M. (2022). Framework of Methodology to Assess the Link between A Posteriori Dietary Patterns and Nutritional Adequacy: Application to Pregnancy. Metabolites, 12(5), 395. https://doi.org/10.3390/metabo12050395






