L-Arginine and Cardioactive Arginine Derivatives as Substrates and Inhibitors of Human and Mouse NaCT/Nact
Abstract
1. Introduction
2. Results
2.1. L-Arginine and Arginine Derivatives as Substrates of Human and Mouse NaCT/Nact
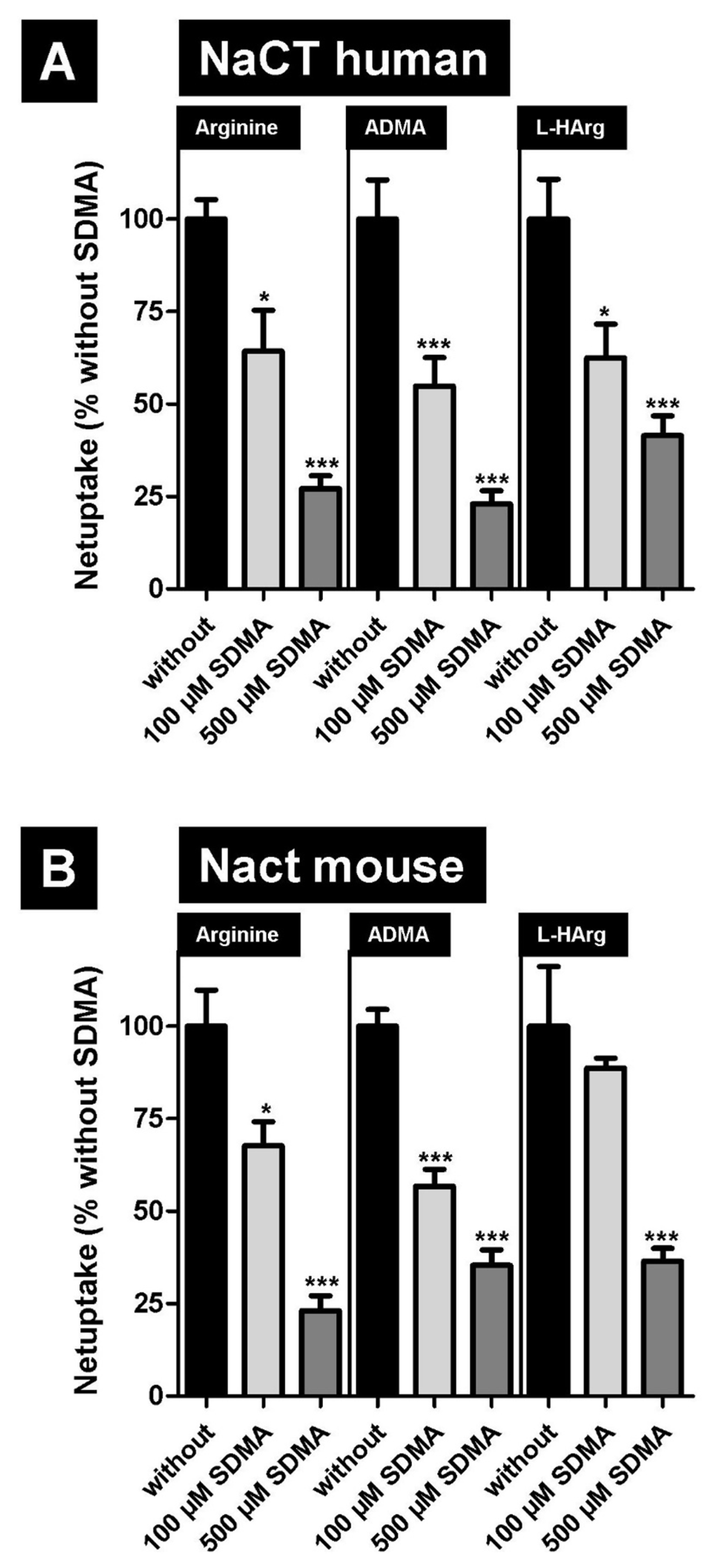
2.2. Inhibition of NaCT/Nact-Mediated Transport of L-Arginine and Arginine Derivatives by Symmetric Dimethylarginine (SDMA)
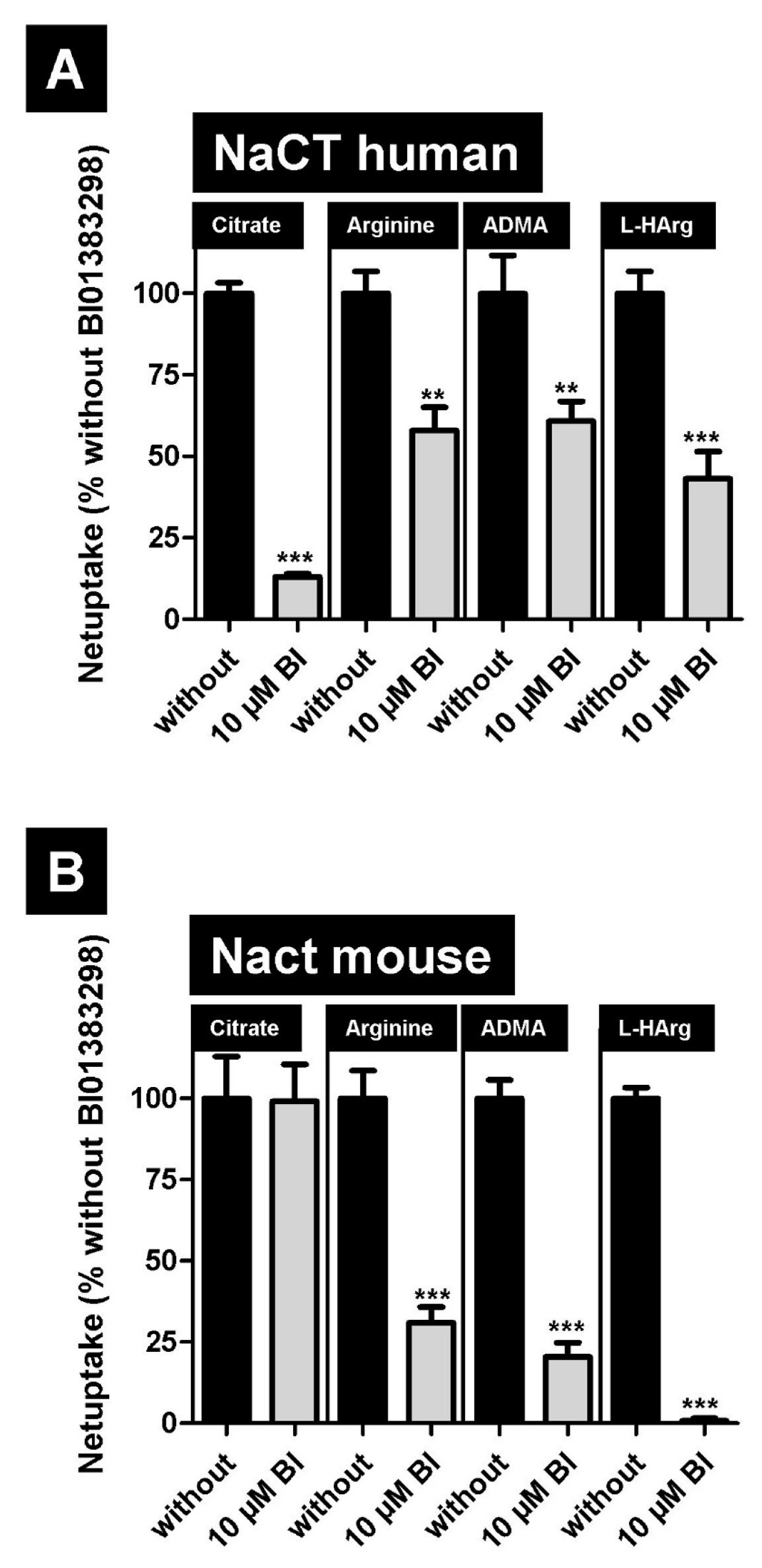
2.3. Inhibition of NaCT-/Nact-Mediated Transport of L-Arginine and Arginine Derivatives by BI01383298
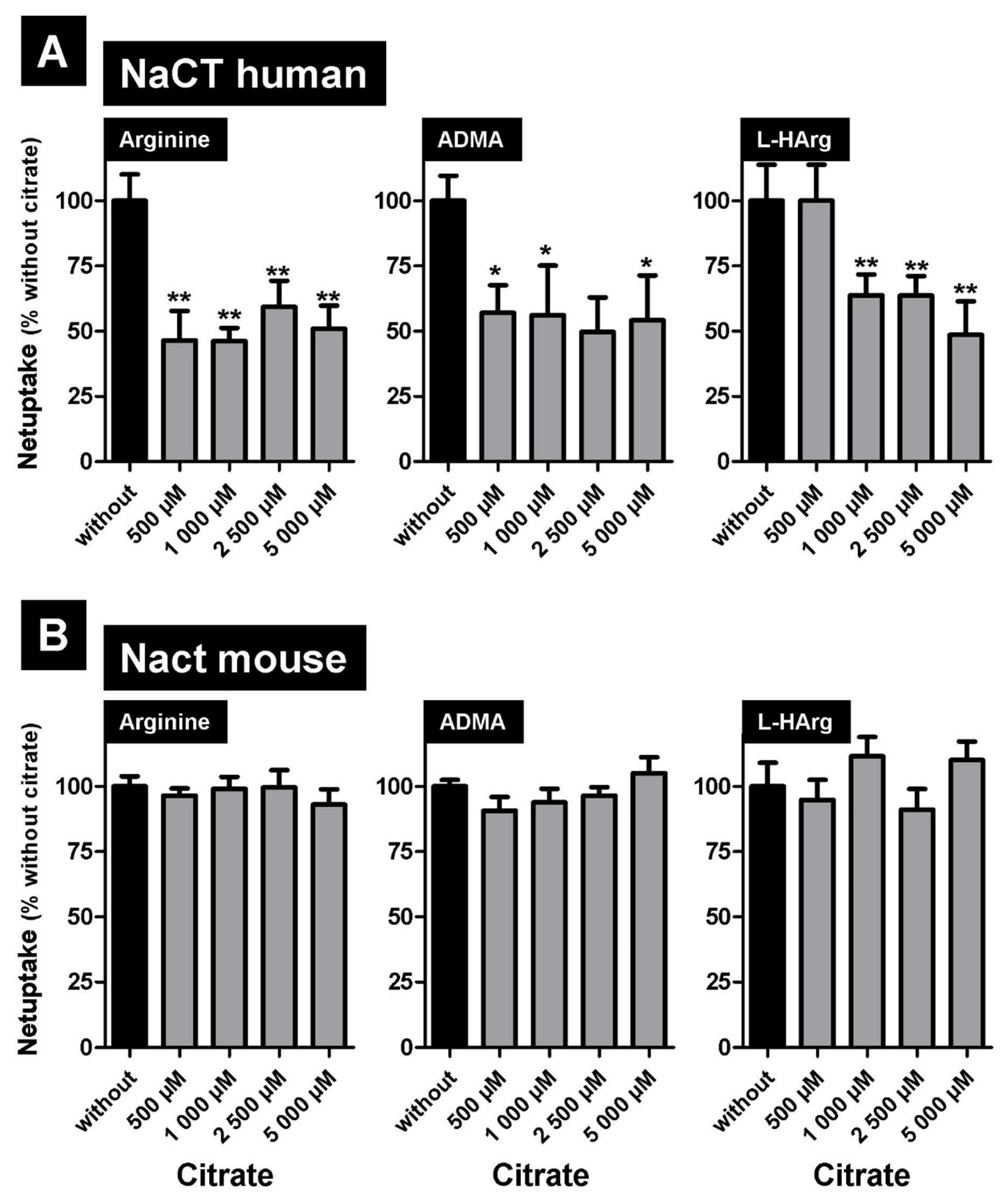
2.4. Inhibition of NaCT/Nact-Mediated Transport of L-Arginine and Arginine Derivatives by the Prototypic Substrate Citrate
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Transport and Inhibition Studies
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hediger, M.A.; Romero, M.F.; Peng, J.B.; Rolfs, A.; Takanaga, H.; Bruford, E.A. The ABCs of solute carriers: Physiological, pathological and therapeutic implications of human membrane transport proteins. Pflugers Arch. 2004, 447, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Allikmets, R.; Gerrard, B.; Hutchinson, A.; Dean, M. Characterization of the human ABC superfamily: Isolation and mapping of 21 new genes using the expressed sequence tags database. Hum. Mol. Genet. 1996, 5, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Keppler, D. Multidrug resistance proteins (MRPs, ABCCs): Importance for pathophysiology and drug therapy. In Drug Transporters; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 299–323. [Google Scholar] [CrossRef]
- Pajor, A.M. Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflugers Arch. 2014, 466, 119–130. [Google Scholar] [CrossRef]
- Inoue, K.; Zhuang, L.; Maddox, D.M.; Smith, S.B.; Ganapathy, V. Structure, function, and expression pattern of a novel sodium-coupled citrate transporter (NaCT) cloned from mammalian brain. J. Biol. Chem. 2002, 277, 39469–39476. [Google Scholar] [CrossRef]
- Zwart, R.; Peeva, P.M.; Rong, J.X.; Sher, E. Electrophysiological characterization of human and mouse sodium-dependent citrate transporters (NaCT/SLC13A5) reveal species differences with respect to substrate sensitivity and cation dependence. J. Pharmacol. Exp. Ther. 2015, 355, 247–254. [Google Scholar] [CrossRef]
- Neretti, N.; Wang, P.Y.; Brodsky, A.S.; Nyguyen, H.H.; White, K.P.; Rogina, B.; Helfand, S.L. Long-lived Indy induces reduced mitochondrial reactive oxygen species production and oxidative damage. Proc. Natl. Acad. Sci. USA 2009, 106, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Karadeniz, Z.; Fischer-Rosinsky, A.; Willmes, D.M.; Spranger, J.; Birkenfeld, A.L. Knockdown of Indy/CeNac2 extends Caenorhabditis elegans life span by inducing AMPK/aak-2. Aging 2015, 7, 553–567. [Google Scholar] [CrossRef][Green Version]
- Wang, P.Y.; Neretti, N.; Whitaker, R.; Hosier, S.; Chang, C.; Lu, D.; Rogina, B.; Helfand, S.L. Long-lived Indy and calorie restriction interact to extend life span. Proc. Natl. Acad. Sci. USA 2009, 106, 9262–9267. [Google Scholar] [CrossRef]
- Birkenfeld, A.L.; Lee, H.Y.; Guebre-Egziabher, F.; Alves, T.C.; Jurczak, M.J.; Jornayvaz, F.R.; Zhang, D.Y.; Hsiao, J.J.; Martin-Montalvo, A.; Fischer-Rosinsky, A.; et al. Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metabolism 2011, 14, 184–195. [Google Scholar] [CrossRef]
- Thevenon, J.; Milh, M.; Feillet, F.; St-Onge, J.; Duffourd, Y.; Juge, C.; Roubertie, A.; Heron, D.; Mignot, C.; Raffo, E.; et al. Mutations in SLC13A5 cause autosomal-recessive epileptic encephalopathy with seizure onset in the first days of life. Am. J. Hum. Genet. 2014, 95, 113–120. [Google Scholar] [CrossRef]
- Hardies, K.; de Kovel, C.G.; Weckhuysen, S.; Asselbergh, B.; Geuens, T.; Deconinck, T.; Azmi, A.; May, P.; Brilstra, E.; Becker, F.; et al. Recessive mutations in SLC13A5 result in a loss of citrate transport and cause neonatal epilepsy, developmental delay and teeth hypoplasia. Brain 2015, 138, 3238–3250. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.; Porter, B.E.; Colas, C.; Schlessinger, A.; Pajor, A.M. Mutations in the Na(+)/citrate cotransporter NaCT (SLC13A5) in pediatric patients with epilepsy and developmental delay. Mol. Med. 2016, 22, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Selch, S.; Chafai, A.; Sticht, H.; Birkenfeld, A.L.; Fromm, M.F.; König, J. Analysis of naturally occurring mutations in the human uptake transporter NaCT important for bone and brain development and energy metabolism. Sci. Rep. 2018, 8, 11330. [Google Scholar] [CrossRef]
- Jaramillo-Martinez, V.; Urbatsch, I.L.; Ganapathy, V. Functional Distinction between Human and Mouse Sodium-Coupled Citrate Transporters and Its Biologic Significance: An Attempt for Structural Basis Using a Homology Modeling Approach. Chem. Rev. 2021, 121, 5359–5377. [Google Scholar] [CrossRef]
- Von Loeffelholz, C.; Lieske, S.; Neuschäfer-Rube, F.; Willmes, D.M.; Raschzok, N.; Sauer, I.M.; König, J.; Fromm, M.; Horn, P.; Chatzigeorgiou, A.; et al. The human longevity gene homolog INDY and Interleukin-6 interact in hepatic lipid metabolism. Hepatology 2017, 66, 616–630. [Google Scholar] [CrossRef]
- Higuchi, K.; Kopel, J.J.; Sivaprakasam, S.; Jaramillo-Martinez, V.; Sutton, R.B.; Urbatsch, I.L.; Ganapathy, V. Functional analysis of a species-specific inhibitor selective for human Na+-coupled citrate transporter (NaCT/SLC13A5/mINDY). Biochem. J. 2020, 477, 4149–4165. [Google Scholar] [CrossRef]
- Atzler, D.; Schwedhelm, E.; Choe, C.U. L-homoarginine and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 83–88. [Google Scholar] [CrossRef]
- Atzler, D.; Gore, M.O.; Ayers, C.R.; Choe, C.U.; Böger, R.H.; de Lemos, J.A.; McGuire, D.K.; Schwedhelm, E. Homoarginine and cardiovascular outcome in the population-based Dallas Heart Study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Sonntag, S.R.; Lieb, W.; Maas, R. Asymmetric and symmetric dimethylarginine as risk markers for total mortality and cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. PLoS ONE 2016, 11, e0165811. [Google Scholar] [CrossRef]
- Taghikhani, E.; Maas, R.; Fromm, M.F.; König, J. The renal transport protein OATP4C1 mediates uptake of the uremic toxin asymmetric dimethylarginine (ADMA) and efflux of cardioprotective L-homoarginine. PLoS ONE 2019, 14, e0213747. [Google Scholar] [CrossRef]
- Banjarnahor, S.; Rodionov, R.N.; König, J.; Maas, R. Transport of L-arginine related cardiovascular risk markers. J. Clin. Med. 2020, 9, 3975. [Google Scholar] [CrossRef] [PubMed]
- Dizikes, G.J.; Grody, W.W.; Kern, R.M.; Cederbaum, S.D. Isolation of human liver arginase cDNA and demonstration of nonhomology between the two human arginase genes. Biochem. Biophys. Res. Commun. 1986, 141, 53–59. [Google Scholar] [CrossRef]
- Schlune, A.; Vom Dahl, S.; Haussinger, D.; Ensenauer, R.; Mayatepek, E. Hyperargininemia due to arginase I deficiency: The original patients and their natural history, and a review of the literature. Amino Acids 2015, 47, 1751–1762. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Wu, G. Homoarginine, arginine, and relatives: Analysis, metabolism, transport, physiology, and pathology. Amino Acids 2015, 47, 1697–1702. [Google Scholar] [CrossRef]
- Deignan, J.L.; De Deyn, P.P.; Cederbaum, S.D.; Fuchshuber, A.; Roth, B.; Gsell, W.; Marescau, B. Guanidino compound levels in blood, cerebrospinal fluid, and post-mortem brain material of patients with argininemia. Mol. Genet. Metab. 2010, 100 (Suppl. 1), S31–S36. [Google Scholar] [CrossRef]
- Hiramatsu, M. A role for guanidino compounds in the brain. Mol. Cell. Biochem. 2003, 244, 57–62. [Google Scholar] [CrossRef]
- Bernstein, H.G.; Jager, K.; Dobrowolny, H.; Steiner, J.; Keilhoff, G.; Bogerts, B.; Laube, G. Possible sources and functions of L-homoarginine in the brain: Review of the literature and own findings. Amino Acids 2015, 47, 1729–1740. [Google Scholar] [CrossRef]
- Uchiyama, T.; Fujita, T.; Gukasyan, H.J.; Kim, K.J.; Borok, Z.; Crandall, E.D.; Lee, V.H. Functional characterization and cloning of amino acid transporter B(0,+) (ATB(0,+)) in primary cultured rat pneumocytes. J. Cell. Physiol. 2008, 214, 645–654. [Google Scholar] [CrossRef]
- Closs, E.I.; Graf, P.; Habermeier, A.; Cunningham, J.M.; Förstermann, U. Human cationic amino acid transporters hCAT-1, hCAT-2A, and hCAT-2B: Three related carriers with distinct transport properties. Biochemistry 1997, 36, 6462–6468. [Google Scholar] [CrossRef]
- Strobel, J.; Müller, F.; Zolk, O.; Endreß, B.; König, J.; Fromm, M.F.; Maas, R. Transport of asymmetric dimethylarginine (ADMA) by cationic amino acid transporter 2 (CAT2), organic cation transport 2 (OCT2) and multidrug and toxin extrusion protein 1 (MATE1). Amino Acids 2013, 45, 898–1002. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Teerlink, T.; Siroen, M.P.; van Lambalgen, A.A.; Rauwerda, J.A.; van Leeuwen, P.A. The liver is an important organ in the metabolism of asymmetrical dimethylarginine (ADMA). Clin. Nutr. 2003, 22, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Siroen, M.P.; van der Sijp, J.R.; Teerlink, T.; van Schaik, C.; Nijveldt, R.J.; van Leeuwen, P.A. The human liver clears both asymmetric and symmetric dimethylarginine. Hepatology 2005, 41, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, R.P.; Dalton, R.N.; Davies, N.A.; Hodges, S.J.; Turner, C.; Williams, R.; Jalan, R. Inflammation is an important determinant of levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA) in acute liver failure. Liver Transpl. 2007, 13, 400–405. [Google Scholar] [CrossRef]
- Pilz, S.; Putz-Bankuti, C.; Meinitzer, A.; März, W.; Kienreich, K.; Stojakovic, T.; Pieber, T.R.; Stauber, R.E. Association of homoarginine and methylarginines with liver dysfunction and mortality in chronic liver disease. Amino Acids 2015, 47, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, R.P.; Malaki, M.; Davies, N.A.; Hodges, S.J.; Dalton, R.N.; Turner, C.; Sen, S.; Williams, R.; Leiper, J.; Vallance, P.; et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology 2007, 45, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Willmes, D.M.; Daniels, M.; Kurzbach, A.; Lieske, S.; Bechmann, N.; Schumann, T.; Henke, C.; El-Agroudy, N.N.; Da Costa Goncalves, A.C.; Peitzsch, M.; et al. The longevity gene mIndy (I’m Not Dead, Yet) affects blood pressure through sympathoadrenal mechanisms. JCI Insight 2021, 6, e136083. [Google Scholar] [CrossRef] [PubMed]
- Bieck, P.R.; Leibowitz, M.; Lachno, D.R.; Ledent, E.; Padich, R.; Jhee, S. Dihydroxyphenylglycol as a biomarker of norepinephrine transporter inhibition by atomoxetine: Human model to assess central and peripheral effects of dosing. J. Clin. Psychopharmacol. 2016, 36, 675–683. [Google Scholar] [CrossRef]
- Terheggen, H.G.; Lowenthal, A.; Lavinha, F.; Colombo, J.P. Familial hyperargininaemia. Arch. Dis. Child. 1975, 50, 57–62. [Google Scholar] [CrossRef][Green Version]
- Deignan, J.L.; Marescau, B.; Livesay, J.C.; Iyer, R.K.; De Deyn, P.P.; Cederbaum, S.D.; Grody, W.W. Increased plasma and tissue guanidino compounds in a mouse model of hyperargininemia. Mol. Genet. Metab. 2008, 93, 172–178. [Google Scholar] [CrossRef]
- May, M.; Kayacelebi, A.A.; Batkai, S.; Jordan, J.; Tsikas, D.; Engeli, S. Plasma and tissue homoarginine concentrations in healthy and obese humans. Amino Acids 2015, 47, 1847–1852. [Google Scholar] [CrossRef]
- Mancusso, R.; Gregorio, G.G.; Liu, Q.; Wang, D.N. Structure and mechanism of a bacterial sodium-dependent dicarboxylate transporter. Nature 2012, 491, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Sauer, D.B.; Song, J.; Wang, B.; Hilton, J.K.; Karpowich, N.K.; Mindell, J.A.; Rice, W.J.; Wang, D.N. Structure and inhibition mechanism of the human citrate transporter NaCT. Nature 2021, 591, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; König, J.; Buchholz, J.K.; Spring, H.; Leier, I.; Keppler, D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol. Pharmacol. 1999, 55, 929–937. [Google Scholar] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surrer, D.B.; Fromm, M.F.; Maas, R.; König, J. L-Arginine and Cardioactive Arginine Derivatives as Substrates and Inhibitors of Human and Mouse NaCT/Nact. Metabolites 2022, 12, 273. https://doi.org/10.3390/metabo12040273
Surrer DB, Fromm MF, Maas R, König J. L-Arginine and Cardioactive Arginine Derivatives as Substrates and Inhibitors of Human and Mouse NaCT/Nact. Metabolites. 2022; 12(4):273. https://doi.org/10.3390/metabo12040273
Chicago/Turabian StyleSurrer, Daniela B., Martin F. Fromm, Renke Maas, and Jörg König. 2022. "L-Arginine and Cardioactive Arginine Derivatives as Substrates and Inhibitors of Human and Mouse NaCT/Nact" Metabolites 12, no. 4: 273. https://doi.org/10.3390/metabo12040273
APA StyleSurrer, D. B., Fromm, M. F., Maas, R., & König, J. (2022). L-Arginine and Cardioactive Arginine Derivatives as Substrates and Inhibitors of Human and Mouse NaCT/Nact. Metabolites, 12(4), 273. https://doi.org/10.3390/metabo12040273





