A Targeted and an Untargeted Metabolomics Approach to the Volatile Aroma Profile of Young ‘Maraština’ Wines
Abstract
:1. Introduction
2. Methods and Materials
2.1. Chemicals and Reagents
2.2. Vineyard Parcel Characteristics
2.3. Wine Samples
2.4. Climate Data
2.5. Solid-Phase Extraction for GC-MS/MS and GC×GC/TOF-MS Analysis
2.6. GC-MS/MS Analysis
2.7. GC×GC/TOF-MS Analysis
2.8. Data Analysis
3. Results and Discussion
3.1. GC-MS/MS Analysis
3.2. GC×GC/TOF-MS
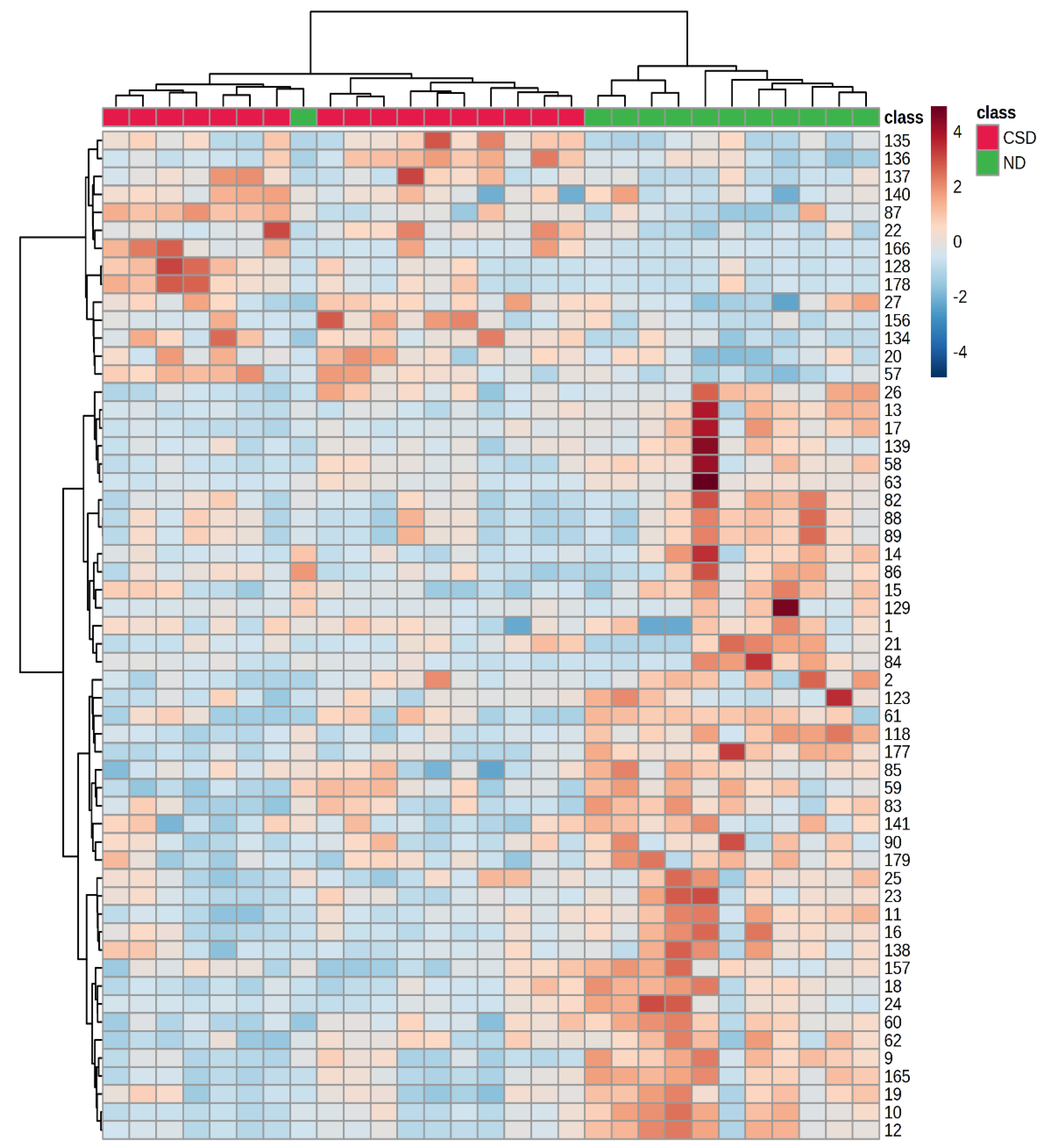
3.3. Multivariate Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Styger, G.; Prior, B.; Bauer, F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145. [Google Scholar] [CrossRef]
- Beckner Whitener, M.E.; Carlin, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; Du Toit, M.; Vrhovsek, U. Early fermentation volatile metabolite profile of non-Saccharomyces yeasts in red and white grape must: A targeted approach. LWT—Food Sci. Technol. 2015, 64, 412–422. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Milanović, V.; Cardinali, F.; Ferrocino, I.; Boban, A.; Franciosa, I.; Gajdoš Kljusurić, J.; Mucalo, A.; Osimani, A.; Aquilanti, L.; Garofalo, C.; et al. Croatian white grape variety Maraština: First taste of its indigenous mycrobiota. Food Res. Int. 2022, 162, 111917. [Google Scholar] [CrossRef]
- Petronilho, S.; Coimbra, M.A.; Rocha, S.M. A critical review on extraction techniques and gas chromatography based determination of grapevine derived sesquiterpenic compounds. Anal. Chim. Acta 2014, 846, 8–35. [Google Scholar] [CrossRef]
- Xue, L.; Xu, J.; Feng, C.; Lu, D.; Zhou, Z. Optimal normalization method for GC-MS/MS based large-scale targeted metabolomics. J. Anal. Chem. 2022, 77, 361–368. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Lotti, C.; Masuero, D.; Carlin, S.; Weingart, G.; Mattivi, F. Quantitative metabolic profiling of grape, apple, and raspberry volatile compounds (VOCs) using a GC/MS/MS method. J. Chromatogr. B Biomed. Appl. 2014, 966, 132–139. [Google Scholar] [CrossRef]
- Weldegergis, B.T.; de Villiers, A.; McNeish, C.; Seethapathy, S.; Mostafa, A.; Górecki, T.; Crouch, A.M. Characterisation of volatile components of Pinotage wines using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GCxGC–TOF-MS). Food Chem. 2011, 129, 188–199. [Google Scholar] [CrossRef]
- Welke, J.E.; Manfroi, V.; Zanus, M.; Lazarotto, M.; Alcaraz Zini, C. Characterization of the volatile profile of Brazilian Merlot wines through comprehensive two-dimensional gas chromatography time-of-flight mass spectrometric detection. J. Chromatogr. A 2012, 1226, 124–139. [Google Scholar] [CrossRef] [Green Version]
- Górecki, O.; Panić, N.; Oldridge, N. Recent advances in comprehensive two-dimensional gas chromatography (GC×GC). J. Liq. Chromatogr. Rel. Technol. 2006, 29, 1077–1104. [Google Scholar] [CrossRef]
- Mucalo, A.; Lukšić, K.; Budić-Leto, I.; Zdunić, G. Cluster Thinning Improves Aroma Complexity of White Maraština (Vitis vinifera L.) Wines Compared to Defoliation under Mediterranean Climate. Appl. Sci. 2022, 12, 7327. [Google Scholar] [CrossRef]
- Budić-Leto, I.; Humar, I.; Gajdoš Kljusurić, J.; Zdunić, G.; Zlatić, E. Free and bound volatile aroma compounds of ’Maraština’ grapes as influenced by dehydration techniques. Appl. Sci. 2020, 10, 8928. [Google Scholar] [CrossRef]
- Lukić, I.; Radeka, S.; Budić-Leto, I.; Bubola, M.; Vrhovsek, U. Targeted UPLC-QqQ-MS/MS profiling of phenolic compounds for differentiation of monovarietal wines and corroboration of particular varietal typicity concepts. Food Chem. 2019, 1, 300. [Google Scholar] [CrossRef] [PubMed]
- Gajdoš Kljusurić, J.; Boban, A.; Mucalo, A.; Budić-Leto, I. Novel application of NIR spectroscopy for non-destructive determination of ‘Maraština’ wine parameters. Foods 2022, 11, 1172. [Google Scholar] [CrossRef]
- Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_03_32_641.html (accessed on 6 December 2022).
- Carlin, S.; Lotti, C.; Correggi, L.; Mattivi, F.; Arapitsas, P.; Vrhovsek, U. Measurement of the effect of accelerated aging on the aromatic compounds of Gewürztraminer and Teroldego wines, using an SPE-GC-MS/MS protocol. Metabolites 2022, 12, 180. [Google Scholar] [CrossRef]
- Šuklje, K.; Carlin, S.; Stanstrup, J.; Antalick, G.; Blackman, J.W.; Meeks, C.; Deloire, A.; Schmidtke, L.M.; Vrhovsek, U. Unravelling wine volatile evolution during shiraz grape ripening by untargeted HS-SPME-GCxGC-TOFMS. Food Chem. 2019, 277, 753–765. [Google Scholar] [CrossRef]
- Carlin, S.; Vrhovsek, U.; Franceschi, P.; Lotti, C.; Bontempo, L.; Camin, F.; Toubiana, D.; Zottele, F.; Toller, G.; Fait, A. Regional features of northern Italian sparkling wines, identified using solid-phase micro extraction and comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. Food Chem. 2016, 208, 68–80. [Google Scholar] [CrossRef]
- MetaboAnalayst 5.0. Available online: https://www.metaboanalyst.ca/ (accessed on 5 December 2022).
- Van Leeuwen, C.; Barbe, J.C.; Darriet, P.; Geffroy, O.; Gomès, E.; Guillaumie, S.; Helwi, P.; Laboyrie, J.; Lytra, G.; Le Menn, N. Recent advancements in understanding the terroir effect on aromas in grapes and wines. Oeno One 2020, 54, 985–1006. [Google Scholar] [CrossRef]
- Šuklje, K.; Lisjak, K.; Česnik, H.B.; Janes, L.; Toit, W.D.; Coetzee, Z.; Vanzo, A.; Deloire, A. Classification of grape berries according to diameter and total soluble solids to study the effect of light and temperature on methoxypyrazine, glutathione, and hydroxycinnamate evolution during ripening of Sauvignon blanc (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 9454–9461. [Google Scholar] [CrossRef]
- Dziadas, M.; Jeleń, H.H. Analysis of terpenic compounds in white wines using SPE-SPME-GC/MS approach. Anal. Chim. Acta 2010, 677, 43–49. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. The Chemistry of Wine. In Handbook of Enology, 2nd ed.; John Wiley & Sons: Chichester, UK, 2006; pp. 205–230. [Google Scholar]
- Strauss, C.R.; Wilson, B.; Gooley, P.R.; Williams, P.J. Biogeneration of Aromas: Role of Monoterpenic Compounds in Grape and Wine Flavor, 1st ed.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 1986; p. 222. [Google Scholar]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J. Agric. Food Chem. 2007, 55, 6674–6684. [Google Scholar] [CrossRef] [PubMed]
- Luzzini, G.; Slaghenaufi, D.; Pasetto, F.; Ugliano, M. Influence of grape composition and origin, yeast strain and spontaneous fermentation on aroma profile of Corvina and Corvinone wines. LWT 2021, 143, 111120. [Google Scholar] [CrossRef]
- Ong, P.K.C.; Acree, T.E. Similarities in the aroma chemistry of Gewürztraminer variety wines and lychee (Litchi chinesis sonn.) fruit. J. Agric. Food Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef]
- Chigo-Hernandez, M.M.; DuBois, A.; Tomasino, E. Aroma perception of rose oxide, linalool and α-terpineol combinations in Gewürztraminer wine. Fermentation 2022, 8, 30. [Google Scholar] [CrossRef]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Katarína, F.; Katarína, M.; Katarína, D.; Ivan, Š.; Fedor, M. Influence of yeast strain on aromatic profile of Gewürztraminer wine. LWT-Food Sci. Technol. 2014, 59, 256–262. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M. Carotenoid breakdown products-The norisoprenoids-In wine aroma. Arch. Biochem. Biophys. 2009, 483, 236–245. [Google Scholar] [CrossRef]
- Francis, L.; Newton, J. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Loyd, N.D.R.; Capone, D.L.; Ugliano, M.; Taylor, D.K.; Skouroumounis, G.K.; Sefton, M.A. Formation of damascenone under both commercial and model fermentation conditions. J. Agric. Food Chem. 2011, 59, 1338–1343. [Google Scholar] [CrossRef]
- Winterhalter, P.; Gök, R. TDN and β-Damascenone: Two Important Carotenoid Metabolites in Wine, 1st ed.; ACS Publications: Washington, DC, USA, 2013; pp. 125–137. [Google Scholar]
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J.C. Study of sensory interactions among red wine fruity esters in a model solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache roséwines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Pérez, D.; Assof, M.; Bolcato, E.; Sari, S.; Fanzone, M. Combined effect of temperature and ammonium addition on fermentation profile and volatile aroma composition of Torrontés Riojano wines. LWT—Food Sci. Technol. 2018, 87, 488–497. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Assessment of spontaneous fermentation and non-Saccharomyces sequential fermentation in Verdicchio wine at winery scale. Beverages 2022, 8, 49. [Google Scholar] [CrossRef]
- Tufariello, M.; Capone, S.; Siciliano, P. Volatile components of Negroamaro red wines produced in Apulian Salento area. Food Chem. 2012, 132, 2155–2164. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [Green Version]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods 2021, 10, 51. [Google Scholar] [CrossRef]
- Jeromel, A.; Korenika, A.-M.J.; Tomaz, I. An Influence of Different Yeast Species on Wine Aroma Composition, 1st ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 171–285. [Google Scholar]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avram, V.; Floare, C.G.; Hosu, A.; Cimpoiu, C.; Măruţoiu, C.; Moldovan, Z. Characterization of Romanian wines by gas chromatography–mass spectrometry. Anal. Lett. 2015, 48, 1099–1116. [Google Scholar] [CrossRef]
- Cruz, M.P.; Valente, I.M.; Gonçalves, L.M.; Rodrigues, J.A.; Barros, A.A. Application of gas-diffusion microextraction to the analysis of free and bound acetaldehyde in wines by HPLC–UV and characterization of the extracted compounds by MS/MS detection. Anal Bioanal. Chem. 2012, 403, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Oliemans Punter & Partners: Utrecht, The Netherlands, 2011; p. 485. [Google Scholar]
- Zhang, X.; Kontoudakis, N.; Blackman, J.; Šuklje, K.; Antalick, G.; Clark, A.C. Determination of 13 volatile aldehyde compounds in wine by GC-QQQ-MS: P-benzoquinone to dissociate hydrogen sulfite addition products. Food Anal. Methods 2019, 12, 1285–1297. [Google Scholar] [CrossRef]
- Kourist, R.; Hilterhaus, L. Microorganisms in Biorefineries, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 275–301. [Google Scholar]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-vine grape drying effect on volatile compounds and aromatic series in must from Pedro Ximénez grape variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef]
- Ferreira, V.; Culleré, L.; López, R.; Cacho, J. Determination of important odor-active aldehydes of wine through gas chromatography-mass spectrometry of their O-(2,3,4,5,6-pentafluorobenzyl) oximes formed directly in the solid phase extraction cartridge used for selective isolation. J. Chromatogr. A 2004, 1028, 339–345. [Google Scholar] [CrossRef]
- Allamy, L.; Darriet, P.; Pons, A. Molecular interpretation of dried-fruit aromas in Merlot and Cabernet Sauvignon musts and young wines: Impact of over-ripening. Food Chem. 2018, 266, 245–253. [Google Scholar] [CrossRef]
- Lukić, I.; Carlin, S.; Vrhovsek, U. Comprehensive 2D gas chromatography with TOF-MS detection confirms the matchless discriminatory power of monoterpenic compounds and provides in-depth volatile profile information for highly efficient white wine varietal differentiation. Foods 2020, 9, 1787. [Google Scholar] [CrossRef]
- Qian, M.C.; Fang, Y.; Shellie, K. Volatile composition of Merlot wine from different vine water status. J. Agric. Food Chem. 2009, 57, 7459–7463. [Google Scholar] [CrossRef]
- Moreira, N.; Guedes de Pinho, P.; Santos, C.; Vasconcelos, I. Volatile sulphur compounds composition of monovarietal white wines. Food Chem. 2010, 123, 1198–1203. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Cabrera-Valido, H.M.; Pérez-Trujillo, J.P.; Cacho, J. Bound aroma compounds of Gual and Listán blanco grape varieties and their influence in the elaborated wines. Food Chem. 2011, 127, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Cacho, J.; Ferreira, V. Isolation and identification of odorants generated in wine during its oxidation: A gas chromatography–olfactometric study. Eur. Food Res. Technol. 2000, 211, 105–110. [Google Scholar] [CrossRef]

| No. | Compound | tR (min:s) | LOQ (µg/L) | F-Ratio | Concentration (µg/L) | S | |
|---|---|---|---|---|---|---|---|
| ND | CSD | ||||||
| 1 | cis-Rose oxide | 07:38.3 | 0.036 | 15.954 | 0.07 ± 0.03 | 0.04 ± 0.01 | * |
| 2 | trans-Rose oxide | 07:46.0 | 0.014 | 13.046 | 0.02 ± 0.01 | 0.01 ± 0.01 | * |
| 3 | cis-Linalool oxide | 08:29.9 | 0.114 | 10.211 | 0.27 ± 0.07 | 0.34 ± 0.06 | * |
| 4 | trans-Linalool oxide | 08:18.2 | 0.082 | 4.889 | 0.46 ± 0.11 | 0.54 ± 0.10 | * |
| 5 | trans-Terpin | 11:23.9 | 0.100 | 2.490 | 0.30 ± 0.14 | 0.23 ± 0.10 | ns |
| 6 | 1,8-Cineole | 06:24.8 | 0.050 | 2.292 | 0.09 ± 0.04 | 0.07 ± 0.01 | ns |
| 7 | α-Terpineol | 09:51.2 | 0.100 | 1.634 | 1.71 ± 0.43 | 1.50 ± 0.45 | ns |
| 8 | Eugenol | 11:38.8 | 0.150 | 1.626 | 0.17 ± 0.07 | 0.22 ± 0.12 | ns |
| 9 | Geraniol | 10:28.8 | 0.250 | 1.062 | 5.23 ± 1.14 | 4.74 ± 1.35 | ns |
| 10 | Terpinen-4-ol | 09:21.5 | 0.075 | 0.475 | 0.13 ± 0.09 | 0.22 ± 0.44 | ns |
| 11 | β-Ionone | 10:55.2 | 0.050 | 0.340 | 0.07 ± 0.01 | 0.06 ± 0.03 | ns |
| 12 | β-Citronellol | 10:07.7 | 1.000 | 0.233 | 10.01 ± 3.79 | 9.45 ± 2.57 | ns |
| 13 | Linalool | 08:55.3 | 0.100 | 0.153 | 6.88 ± 1.23 | 6.63 ± 1.95 | ns |
| 14 | Safranal | 09:38.6 | 0.100 | 0.009 | 0.12 ± 0.06 | 0.12 ± 0.05 | ns |
| ∑ Terpenic compounds | 25.51 ± 3.84 | 24.18 ± 5.16 | ns | ||||
| 15 | β-Damascenone | 10:27.8 | 0.100 | 38.577 | 1.89 ± 0.64 | 0.84 ± 0.27 | * |
| 16 | TDN | 10:09.0 | 0.050 | 16.381 | 0.68 ± 0.16 | 0.45 ± 0.15 | * |
| 17 | Vitispirani (mix of isomers) | 08:56.5 | 0.500 | 4.149 | 0.64 ± 0.31 | 0.39 ± 0.35 | ns |
| ∑ C13-norisoprenoids | 3.21 ± 0.76 | 1.67 ± 0.51 | * | ||||
| 18 | Ethyl caprylate | 08:12.4 | 1.000 | 33.678 | 221.09 ± 68.24 | 176.73 ± 65.92 | ns |
| 19 | Diethyl succinate | 09:42.4 | 0.250 | 19.764 | 399.80 ± 195.07 | 170.80 ± 82.73 | * |
| 20 | Ethyl valerate | 05:37.9 | 0.050 | 4.846 | 1.22 ± 0.37 | 1.52 ± 0.35 | * |
| 21 | Ethyl laurate | 10:29.2 | 0.075 | 3.699 | 28.64 ± 46.24 | 7.47 ± 7.29 | ns |
| 22 | Ethyl heptanoate | 07:27.1 | 0.050 | 3.170 | 1.03 ± 0.24 | 1.42 ± 0.75 | ns |
| 23 | Ethyl caprate | 09:32.9 | 0.050 | 3.170 | 73.77 ± 73.89 | 45.76 ± 24.78 | ns |
| 24 | Ethyl isovalerate | 04:53.6 | 0.100 | 3.147 | 11.06 ± 6.23 | 8.02 ± 3.15 | ns |
| 25 | Ethyl 2-methylbutyrate | 04:42.5 | 0.050 | 3.097 | 7.55 ± 4.18 | 5.39 ± 2.56 | ns |
| 26 | Ethyl leucate | 08:55.9 | 0.250 | 2.878 | 36.78 ± 11.11 | 14.24 ± 9.94 | * |
| 27 | Butyl acetate | 04:55.8 | 0.150 | 1.549 | 0.59 ± 0.38 | 0.76 ± 0.38 | ns |
| 28 | Ethyl caproate | 06:36.0 | 0.050 | 1.532 | 178.75 ± 23.78 | 161.34 ± 44.5 | ns |
| 29 | Isoamyl acetate | 05:29.6 | 0.250 | 1.166 | 491.93 ± 309.43 | 579.53 ± 126.75 | ns |
| 30 | Ethyl phenylacetate | 10:16.3 | 0.050 | 1.067 | 4.73 ± 1.46 | 4.13 ± 1.62 | ns |
| 31 | Isobutyl acetate | 04:13.2 | 0.500 | 0.501 | 11.24 ± 6.80 | 12.49 ± 2.70 | ns |
| 32 | Hexyl acetate | 06:56.6 | 0.075 | 0.127 | 1.93 ± 2.62 | 2.24 ± 2.04 | ns |
| 33 | Phenylethyl acetate | 10:24.5 | 0.075 | 0.051 | 113.91 ± 30.45 | 110.87 ± 39.36 | ns |
| 34 | Ethyl butyrate | 04:30.0 | 0.100 | 0.001 | 67.10 ± 16.39 | 66.89 ± 15.94 | ns |
| ∑ Esters | 1651.12 ± 437.54 | 1369.58 ± 291.48 | * | ||||
| 35 | Benzyl alcohol | 10:37.4 | 0.150 | 4.216 | 11.06 ± 3.39 | 16.07 ± 7.94 | * |
| 36 | cis-3-Hexen-1-ol | 07:49.3 | 0.014 | 1.664 | 52.28 ± 32.29 | 40.97 ± 15.4 | ns |
| 37 | trans-3-Hexen-1-ol | 07:39.6 | 0.050 | 0.590 | 28.76 ± 18.30 | 24.57 ± 11.70 | ns |
| 38 | 1-Hexanol | 07:34.8 | 0.075 | 0.005 | 301.42 ± 75.5 | 303.70 ± 98.49 | ns |
| ∑ Alcohols | 393.53 ± 117.99 | 385.30 ± 115.34 | ns | ||||
| 39 | Geranic acid | 12:13.3 | 5.000 | 8.813 | 4.32 ± 4.56 | 8.22 ± 2.65 | * |
| 40 | Octanoic acid | 11:14.2 | 50.000 | 5.916 | 2453.81 ± 420.15 | 2083.9 ± 400.08 | * |
| 41 | Decanoic acid | 11:57.5 | 50.000 | 4.083 | 967.72 ± 337.98 | 766.39 ± 209.3 | ns |
| 42 | Nonanoic acid | 11:35.3 | 10.000 | 1.833 | 18.18 ± 4.73 | 20.18 ± 3.36 | ns |
| 43 | Valeric acid | 09:59.8 | 5.000 | 0.903 | 41.03 ± 9.07 | 43.7 ± 6.34 | ns |
| ∑ Acids | 3480.74 ± 669.79 | 2914.16 ± 575.91 | * | ||||
| 44 | 4-Vinylguaiacol | 11:44.5 | 5.000 | 5.536 | 155.17 ± 107.71 | 271.54 ± 146.66 | * |
| 45 | 4-Ethyl phenol | 11:37.7 | 0.050 | 4.260 | 0.08 ± 0.05 | 0.13 ± 0.06 | * |
| 46 | Guaiacol | 10:34.0 | 0.100 | 0.501 | 0.07 ± 0.07 | 0.09 ± 0.05 | ns |
| 47 | 4-Ethyl guaiacol | 11:10.9 | 0.075 | 0.304 | 0.09 ± 0.03 | 0.10 ± 0.03 | ns |
| ∑ Phenols | 155.41 ± 107.82 | 271.85 ± 146.72 | * | ||||
| 48 | Phenylacetaldehyde | 09:35.0 | 1.000 | 5.401 | 39.96 ± 6.79 | 31.85 ± 10.70 | * |
| 49 | Benzaldehyde | 08:51.4 | 0.150 | 1.056 | 0.21 ± 0.26 | 0.31 ± 0.27 | ns |
| ∑ Aldehydes | 40.16 ± 6.89 | 32.15 ± 10.78 | * | ||||
| 50 | 2-Aminoacetophenone | 11:52.7 | 0.050 | 1.017 | 0.21 ± 0.06 | 0.24 ± 0.08 | ns |
| 51 | Zingerone | 14:34.6 | 0.050 | 0.610 | 2.92 ± 1.81 | 3.42 ± 1.65 | ns |
| ∑ Ketones | 3.13 ± 1.79 | 3.66 ± 1.66 | ns | ||||
| 52 | γ-nonalactone | 11:14.2 | 0.150 | 0.989 | 2.85 ± 2.11 | 2.27 ± 1.12 | ns |
| 53 | γ-octalactone | 10:50.6 | 0.100 | 0.568 | 1.90 ± 1.42 | 2.46 ± 2.28 | ns |
| 54 | γ-decalactone | 11:50.1 | 0.100 | 0.529 | 0.75 ± 0.15 | 0.80 ± 0.25 | ns |
| 55 | δ-decalactone | 11:38.0 | 0.150 | 0.009 | 8.61 ± 2.29 | 8.69 ± 2.26 | ns |
| ∑ Lactones | 14.11 ± 3.70 | 14.22 ± 3.86 | ns | ||||
| 56 | Benzothiazole | 11:00.6 | 0.500 | 2.937 | 1.04 ± 0.76 | 0.73 ± 0.08 | ns |
| ∑ Indole | 1.04 ± 0.76 | 0.73 ± 0.08 | ns | ||||
| No. | Compound | m/z | 1 tR (min:s) | 2 tR (min:s) | LRIexp | LRIlit | ND | CSD | F | S | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | % | Area | % | |||||||||
| 1 | 8-Hydroxylinalool | 101 | 30:42.0 | 00:01.2 | 2300 | 2294 | 6426 | 0.01 | 5214 | 0.01 | 5.608 | * |
| 2 | Hotrienol | 71 | 18:48.0 | 00:01.3 | 1604 | 1605 | 4887 | 0.00 | 2844 | 0.00 | 5.067 | * |
| 3 | 2,3-Dihydrofarnesol | 69 | 30:07.0 | 00:01.6 | 2273 | 2265 | 18,585 | 0.02 | 15,003 | 0.02 | 1.936 | ns |
| 4 | β-Citronellol | 69 | 21:50.0 | 00:01.4 | 1757 | 1762 | 20,982 | 0.02 | 18,151 | 0.02 | 1.571 | ns |
| 5 | Linalool | 93 | 17:31.0 | 00:01.4 | 1547 | 1544 | 15,457 | 0.01 | 14,273 | 0.02 | 0.674 | ns |
| 6 | Geraniol | 69 | 23:14.0 | 00:01.4 | 1844 | 1839 | 18,645 | 0.02 | 17,447 | 0.02 | 0.477 | ns |
| 7 | trans-Farnesol | 69 | 31:24.0 | 00:01.6 | 2350 | 2355 | 12,911 | 0.01 | 14,418 | 0.02 | 0.233 | ns |
| ∑ Terpenic compounds | 97,893 | 0.09 | 87,349 | 0.10 | 1.057 | ns | ||||||
| 8 | 3-Oxo-α-ionol | 108 | 35:15.0 | 00:01.4 | 2641 | - | 27,266 | 0.03 | 31,116 | 0.04 | 0.921 | ns |
| ∑ C13-norisoprenoids | 27,266 | 0.03 | 31,116 | 0.04 | 1.808 | ns | ||||||
| 9 | Ethyl 2-hydroxy-4-methylvalerate | 69 | 17:31.0 | 00:01.3 | 1547 | 1547 | 188,279 | 0.18 | 74,936 | 0.09 | 33.650 | * |
| 10 | Ethyl isopentyl succinate | 101 | 24:17.0 | 00:01.7 | 1900 | 1897 | 52,727 | 0.05 | 20,056 | 0.02 | 23.051 | * |
| 11 | Isoamyl lactate | 45 | 18:06.0 | 00:01.3 | 1583 | 1583 | 136,300 | 0.13 | 75,713 | 0.09 | 22.459 | * |
| 12 | Diethyl butanedioate | 101 | 20:12.0 | 00:01.5 | 1686 | 1679 | 5,015,783 | 4.84 | 1,878,508 | 2.19 | 21.671 | * |
| 13 | Decyl 2,2-dimethylpropanoate | 70 | 23:14.0 | 00:01.3 | 1844 | - | 13,321 | 0.01 | 6138 | 0.01 | 14.324 | * |
| 14 | Ethyl 3-hydroxypropionate | 73 | 18:34.0 | 00:01.1 | 1597 | - | 185,916 | 0.18 | 83,103 | 0.10 | 14.318 | * |
| 15 | Ethyl 3-formylpropionate | 85 | 28:01.0 | 00:01.2 | 2145 | - | 24,799 | 0.02 | 13,960 | 0.02 | 13.896 | * |
| 16 | Diethyl 2-hydroxypentanedioate | 85 | 28:36.0 | 00:01.3 | 2143 | - | 418,513 | 0.40 | 209,095 | 0.24 | 12.786 | * |
| 17 | Ethyl 2-phenylethyl oxalate | 104 | 31:03.0 | 00:01.4 | 2337 | - | 7245 | 0.01 | 2795 | 0.00 | 12.337 | * |
| 18 | Diethyl 2-methylbutanedioate | 115 | 31:17.0 | 00:01.1 | 2346 | - | 6585 | 0.01 | 3881 | 0.00 | 12.317 | * |
| 19 | Ethyl hydrogen succinate | 128 | 31:45.0 | 00:01.1 | 2363 | 2368 | 6,682,871 | 6.44 | 5,000,549 | 5.84 | 11.107 | * |
| 20 | Ethyl pyruvate | 43 | 11:27.0 | 00:01.2 | 1268 | 1267 | 114,024 | 0.11 | 181,394 | 0.21 | 9.854 | * |
| 21 | Ethyl 2-acetamido-4-methylpentanoate | 128 | 27:47.0 | 00:01.4 | 2117 | - | 7613 | 0.01 | 3598 | 0.00 | 9.168 | * |
| 22 | Methyl 4-hydroxybutanoate | 74 | 21:50.0 | 00:01.1 | 1757 | - | 7964 | 0.01 | 16,086 | 0.02 | 8.420 | * |
| 23 | Diethyl malate | 117 | 26:37.0 | 00:01.3 | 2031 | 2041 | 211,154 | 0.20 | 128,189 | 0.15 | 7.498 | * |
| 24 | Methyl ethyl succinate | 115 | 19:23.0 | 00:01.4 | 1641 | 1632 | 23,799 | 0.02 | 10,165 | 0.01 | 7.464 | * |
| 25 | Ethyl 2-hydroxypropanoate | 45 | 13:05.0 | 00:01.1 | 1344 | 1353 | 2,411,977 | 2.33 | 1,963,777 | 2.29 | 5.328 | * |
| 26 | 2-Phenylethyl propionate | 104 | 24:03.0 | 00:01.7 | 1892 | - | 7125 | 0.01 | 5276 | 0.01 | 4.853 | * |
| 27 | Methyl 2-methyl-3-oxobutanoate | 88 | 26:02.0 | 00:01.2 | 2000 | - | 7922 | 0.01 | 10,553 | 0.01 | 4.573 | * |
| 28 | Ethyl linoleate | 105 | 34:47.0 | 00:02.2 | 2517 | - | 38,773 | 0.04 | 22,431 | 0.03 | 4.163 | ns |
| 29 | Ethyl laurate | 88 | 23:21.0 | 00:02.2 | 1844 | 1846 | 127,797 | 0.12 | 27,741 | 0.03 | 3.102 | ns |
| 30 | Ethyl 2-phenylacetate | 91 | 22:18.0 | 00:01.6 | 1793 | 1786 | 83,794 | 0.08 | 65,586 | 0.08 | 2.936 | ns |
| 31 | Ethyl octanoate | 88 | 15:11.0 | 00:02.0 | 1440 | 1440 | 1,534,067 | 1.48 | 1,216,408 | 1.42 | 2.934 | ns |
| 32 | Ethyl heptanoate | 88 | 12:58.0 | 00:01.9 | 1322 | 1327 | 8473 | 0.01 | 11,930 | 0.01 | 2.919 | ns |
| 33 | α-Terpinyl acetate | 59 | 20:33.0 | 00:01.5 | 1696 | 1693 | 13,056 | 0.01 | 10,422 | 0.01 | 2.915 | ns |
| 34 | Ethyl undecenoate | 152 | 38:24.0 | 00:01.2 | 2883 | - | 8752 | 0.01 | 4496 | 0.01 | 2.913 | ns |
| 35 | Ethyl pentadecanoate | 88 | 30:00.0 | 00:02.3 | 2268 | 2161 | 75,987 | 0.07 | 41,633 | 0.05 | 2.587 | ns |
| 36 | Ethyl vanillate | 151 | 35:15.0 | 00:01.3 | 2641 | 2653 | 4552 | 0.00 | 7188 | 0.01 | 2.452 | ns |
| 37 | Ethyl dec-9-enoate | 88 | 20:26.0 | 00:02.0 | 1693 | 1703 | 67,674 | 0.07 | 42,785 | 0.05 | 2.026 | ns |
| 38 | Ethyl decanoate | 88 | 19:30.0 | 00:02.1 | 1645 | 1642 | 330,113 | 0.32 | 201,810 | 0.24 | 1.944 | ns |
| 39 | Ethyl 4-hydroxybutanoate | 87 | 22:32.0 | 00:01.2 | 1800 | 1796 | 6,613,274 | 6.38 | 7,833,802 | 9.14 | 1.694 | ns |
| 40 | Ethyl acetaminoacetate | 72 | 28:22.0 | 00:01.2 | 2155 | - | 25,266 | 0.02 | 21,895 | 0.03 | 1.583 | ns |
| 41 | Ethyl 3-cyclohexylpropanoate | 88 | 31:10.0 | 00:01.4 | 2341 | - | 7943 | 0.01 | 6381 | 0.01 | 1.527 | ns |
| 42 | Ethyl 3-hydroxyoctanoate | 117 | 24:03.0 | 00:01.4 | 1892 | 1892 | 29,452 | 0.03 | 24,961 | 0.03 | 1.226 | ns |
| 43 | Methyl 2,3-dihydroxybenzoate | 136 | 30:00.0 | 00:01.2 | 2268 | - | 11,774 | 0.01 | 6125 | 0.01 | 1.179 | ns |
| 44 | Ethyl 2-(4-hydroxyphenyl)acetate | 180 | 38:52.0 | 00:01.2 | 2904 | - | 6564 | 0.01 | 7742 | 0.01 | 0.953 | ns |
| 45 | Ethyl 3-hydroxybutanoate | 71 | 16:56.0 | 00:01.2 | 1510 | 1505 | 53,677 | 0.05 | 44,603 | 0.05 | 0.890 | ns |
| 46 | Ethyl 3-hydroxyhexanoate | 71 | 20:12.0 | 00:01.3 | 1686 | 1690 | 9462 | 0.01 | 8390 | 0.01 | 0.559 | ns |
| 47 | N-Acetyl-L-valine ethyl ester | 72 | 26:23.0 | 00:01.4 | 2019 | - | 17,871 | 0.02 | 14,638 | 0.02 | 0.366 | ns |
| 48 | Methyl pyruvate | 43 | 21:57.0 | 00:01.4 | 1761 | 1217 | 122,105 | 0.12 | 137,910 | 0.16 | 0.199 | ns |
| 49 | Ethyl hexanoate | 88 | 10:38.0 | 00:01.8 | 1232 | 1238 | 810,509 | 0.78 | 862,228 | 1.01 | 0.155 | ns |
| 50 | Ethyl 4-acetoxybutanoate | 87 | 21:01.0 | 00:01.5 | 1732 | - | 30,519 | 0.03 | 32,622 | 0.04 | 0.110 | ns |
| 51 | 2-Phenylethyl acetate | 104 | 22:53.0 | 00:01.6 | 1811 | 1811 | 1,474,492 | 1.42 | 1,411,052 | 1.65 | 0.084 | ns |
| 52 | 2-Methylbutyl acetate | 43 | 08:04.0 | 00:01.6 | 1131 | 1128 | 3,851,619 | 3.71 | 4,093,266 | 4.78 | 0.072 | ns |
| 53 | Ethyl 4-hydroxybenzoate | 121 | 40:09.0 | 00:01.2 | 2996 | - | 13,799 | 0.01 | 14,950 | 0.02 | 0.060 | ns |
| 54 | Ethyl 2-phenylethyl dimethylmalonate | 104 | 37:35.0 | 00:01.4 | 2811 | - | 12,272 | 0.01 | 11,503 | 0.01 | 0.059 | ns |
| 55 | Diisoproply phthalate | 149 | 36:04.0 | 00:01.8 | 2702 | - | 7430 | 0.01 | 7370 | 0.01 | 0.001 | ns |
| 56 | Hexyl acetate | 43 | 11:34.0 | 00:01.7 | 1270 | 1275 | 46,249 | 0.04 | 45,864 | 0.05 | 0.000 | ns |
| ∑ Esters | 30,961,232 | 29.85 | 25,925,505 | 30.26 | 6.558 | * | ||||||
| 57 | 2,4,7,9-Tetramethyl-5-decyne-4,7-diol | 109 | 27:26.0 | 00:01.3 | 2106 | - | 2419 | 0.00 | 4759 | 0.01 | 16.214 | * |
| 58 | 3-Methylpentan-1-ol | 56 | 12:44.0 | 00:01.1 | 1316 | 1340 | 1,029,814 | 0.99 | 510,705 | 0.60 | 8.382 | * |
| 59 | 2,7-Dimethyloctane-4,5-diol | 69 | 21:22.0 | 00:01.1 | 1743 | - | 56,054 | 0.05 | 37,359 | 0.04 | 7.861 | * |
| 60 | 2-(4-Methoxyphenyl) ethanol | 121 | 31:03.0 | 00:01.3 | 2337 | 2335 | 54,485 | 0.05 | 41,628 | 0.05 | 7.723 | * |
| 61 | 2-Phenylethanol | 45 | 24:24.0 | 00:01.2 | 1904 | 1909 | 9,329,193 | 8.99 | 4,663,562 | 5.44 | 7.509 | * |
| 62 | Nonan-2-ol | 45 | 16:56.0 | 00:01.4 | 1510 | 1528 | 60,114 | 0.06 | 44,710 | 0.05 | 7.000 | * |
| 63 | 4-Methylpentan-1-ol | 56 | 12:23.0 | 00:01.1 | 1306 | 1301 | 138,557 | 0.13 | 57,418 | 0.07 | 5.757 | * |
| 64 | 4-Hexen-3-ol | 71 | 28:08.0 | 00:01.2 | 2148 | - | 31,532 | 0.03 | 27,392 | 0.03 | 4.185 | ns |
| 65 | cis-4-Hydroxymethyl-2-methyl-1,3-dioxolane | 103 | 20:05.0 | 00:01.1 | 1682 | - | 40,436 | 0.04 | 112,028 | 0.13 | 3.949 | ns |
| 66 | 3-heptyn-2-ol | 43 | 24:10.0 | 00:01.1 | 1896 | - | 140,268 | 0.14 | 193,684 | 0.23 | 3.516 | ns |
| 67 | 3-Ethyl-4-methyl-1-pentanol | 69 | 16:42.0 | 00:01.3 | 1503 | 1507 | 3100 | 0.00 | 15,929 | 0.02 | 2.985 | ns |
| 68 | trans-4-hydroxymethyl-2-methyl-1,3-dioxolane | 103 | 18:55.0 | 00:01.1 | 1607 | 35,623 | 0.03 | 116,338 | 0.14 | 2.303 | ns | |
| 69 | 3-Hexen-1-ol | 67 | 14:01.0 | 00:01.2 | 1386 | 1380 | 173,450 | 0.17 | 117,245 | 0.14 | 2.180 | ns |
| 70 | 3-Ethoxy-1-propanol | 59 | 13:47.0 | 00:01.1 | 1363 | 1377 | 44,867 | 0.04 | 24,178 | 0.03 | 2.092 | ns |
| 71 | 2,6-Dimethyl-7-octen-2,6-diol | 71 | 25:27.0 | 00:01.2 | 1959 | 1964 | 7915 | 0.01 | 7044 | 0.01 | 1.734 | ns |
| 72 | Heptan-1-ol | 70 | 15:39.0 | 00:01.3 | 1453 | 1456 | 2,585,574 | 2.49 | 2,431,741 | 2.84 | 1.035 | ns |
| 73 | Phenoxyethanol | 94 | 28:15.0 | 00:01.2 | 2151 | 2142 | 10,304 | 0.01 | 12,013 | 0.01 | 0.683 | ns |
| 74 | 1-Butanol | 56 | 08:25.0 | 00:01.1 | 1140 | 1146 | 140,783 | 0.14 | 154,607 | 0.18 | 0.429 | ns |
| 75 | Isoamyl alcohol | 55 | 10:10.0 | 00:04.9 | 1221 | 1230 | 445,386 | 0.43 | 395,568 | 0.46 | 0.357 | ns |
| 76 | Pentan-1-ol | 42 | 10:59.0 | 00:01.1 | 1241 | 1244 | 43,391 | 0.04 | 37,469 | 0.04 | 0.219 | ns |
| 77 | 2-Methyl-3-butene-1,2-diol | 71 | 23:49.0 | 00:02.2 | 1863 | - | 17,499 | 0.02 | 16,048 | 0.02 | 0.175 | ns |
| 78 | Butane-1,3-diol | 45 | 18:06.0 | 00:01.0 | 1583 | 1576 | 1,564,242 | 1.51 | 1,473,432 | 1.72 | 0.109 | ns |
| 79 | (2S,3S)-Butane-2,3-diol | 45 | 17:24.0 | 00:01.0 | 1543 | 1545 | 5,140,609 | 4.96 | 5,339,535 | 6.23 | 0.084 | ns |
| 80 | (3,4,5-Trimethoxyphenyl) methanol | 198 | 38:31.0 | 00:01.4 | 2889 | - | 9875 | 0.01 | 10,396 | 0.01 | 0.020 | ns |
| 81 | 4-Methyl-5-thiazoleethanol | 113 | 30:42.0 | 00:01.2 | 2300 | 2311 | 17,739 | 0.02 | 18,441 | 0.02 | 0.019 | ns |
| ∑ Alcohols | 21,123,230 | 20.37 | 15,863,228 | 18.52 | 7.556 | * | ||||||
| 82 | Hexanoic acid | 60 | 23:21.0 | 00:01.1 | 1848 | 1854 | 7,588,282 | 7.32 | 5,184,808 | 6.05 | 10.652 | * |
| 83 | 2-Oxopentanedioic acid | 101 | 37:35.0 | 00:01.1 | 2811 | - | 177,608 | 0.17 | 121,733 | 0.14 | 9.957 | * |
| 84 | Dodecanoic acid | 60 | 33:16.0 | 00:01.2 | 2489 | - | 62,061 | 0.06 | 24,576 | 0.03 | 9.084 | * |
| 85 | Isovaleric acid | 60 | 20:05.0 | 00:01.0 | 1682 | 1680 | 7,103,710 | 6.85 | 5,799,112 | 6.77 | 8.570 | * |
| 86 | Butanoic acid | 60 | 19:16.0 | 00:01.0 | 1638 | 1637 | 1,073,005 | 1.03 | 828,020 | 0.97 | 7.530 | * |
| 87 | Acetic acid | 60 | 15:39.0 | 00:01.0 | 1453 | 1465 | 499,185 | 0.48 | 741,473 | 0.87 | 7.121 | * |
| 88 | Caprylic acid | 60 | 26:58.0 | 00:01.1 | 2050 | 2046 | 6,104,658 | 5.89 | 4,619,455 | 5.39 | 6.266 | * |
| 89 | Octanoic acid | 60 | 27:12.0 | 00:01.1 | 2098 | 2096 | 6,104,658 | 5.89 | 4,619,455 | 5.39 | 6.266 | * |
| 90 | Succinic acid | 56 | 30:49.0 | 00:01.0 | 2304 | - | 375,686 | 0.36 | 211,649 | 0.25 | 4.411 | * |
| 91 | Butanedioic acid | 56 | 37:00.0 | 00:00.9 | 2782 | - | 308,343 | 0.30 | 147,377 | 0.17 | 4.215 | ns |
| 92 | Dec-9-enoic acid | 69 | 31:10.0 | 00:01.1 | 2341 | 2341 | 279,052 | 0.27 | 184,202 | 0.22 | 3.734 | ns |
| 93 | Decanoic acid | 60 | 30:14.0 | 00:01.2 | 2279 | 2275 | 1,334,398 | 1.29 | 965,662 | 1.13 | 3.674 | ns |
| 94 | 4-Methyl-2-oxovaleric acid | 57 | 15:25.0 | 00:01.3 | 1447 | - | 66,486 | 0.06 | 19,862 | 0.02 | 3.495 | ns |
| 95 | 5-Oxotetrahydrofuran-2-carboxylic acid | 103 | 38:31.0 | 00:01.1 | 2889 | - | 80,224 | 0.08 | 60,831 | 0.07 | 3.393 | ns |
| 96 | 2-Methylbutanoic acid | 74 | 20:05.0 | 00:01.1 | 1682 | 1674 | 5,108,137 | 4.93 | 4,341,982 | 5.07 | 2.652 | ns |
| 97 | 3-Hexenoic acid | 68 | 25:13.0 | 00:01.0 | 1952 | - | 6614 | 0.01 | 3529 | 0.00 | 2.602 | ns |
| 98 | Malic acid | 71 | 37:28.0 | 00:01.0 | 2806 | 277,579 | 0.27 | 215,559 | 0.25 | 2.528 | ns | |
| 99 | 2-Hydroxy-4-methylpentanoic acid | 76 | 33:44.0 | 00:01.0 | 2592 | - | 75,528 | 0.07 | 37,324 | 0.04 | 1.723 | ns |
| 100 | Hexadecanoic acid | 60 | 38:45.0 | 00:01.4 | 2900 | 2900 | 77,788 | 0.07 | 60,127 | 0.07 | 1.506 | ns |
| 101 | 5-Hexenoic acid | 60 | 24:24.0 | 00:01.0 | 1904 | 1900 | 53,357 | 0.05 | 34,362 | 0.04 | 0.869 | ns |
| 102 | 4-Methoxy-4-oxobutanoic acid | 101 | 31:17.0 | 00:01.0 | 2346 | - | 40,500 | 0.04 | 33,313 | 0.04 | 0.776 | ns |
| 103 | o-Anisic acid | 105 | 37:07.0 | 00:01.2 | 2792 | - | 8878 | 0.01 | 6783 | 0.01 | 0.748 | ns |
| 104 | Heptanoic acid | 60 | 25:13.0 | 00:01.1 | 1952 | 1960 | 46,171 | 0.04 | 52,421 | 0.06 | 0.716 | ns |
| 105 | Homovanillic acid | 137 | 40:02.0 | 00:01.4 | 2992 | 3099 | 6456 | 0.01 | 5866 | 0.01 | 0.648 | ns |
| 106 | Pyruvic acid | 85 | 32:20.0 | 00:01.0 | 2411 | - | 7576 | 0.01 | 6720 | 0.01 | 0.574 | ns |
| 107 | trans-3-Hexenoic acid | 68 | 24:59.0 | 00:01.0 | 1923 | 1915 | 4188 | 0.00 | 3508 | 0.00 | 0.445 | ns |
| 108 | 2-(4-Hexyl-2,5-dioxofuran-3-yl)acetic acid | 126 | 27:40.0 | 00:01.6 | 2113 | 2110 | 23,221 | 0.02 | 16,851 | 0.02 | 0.437 | ns |
| 109 | 2-Propenoic acid | 45 | 19:30.0 | 00:01.0 | 1645 | - | 22,808 | 0.02 | 33,948 | 0.04 | 0.414 | ns |
| 110 | Benzeneacetic acid | 91 | 34:19.0 | 00:01.1 | 2572 | 2565 | 489,308 | 0.47 | 537,374 | 0.63 | 0.368 | ns |
| 111 | Propionic acid | 74 | 17:24.0 | 00:01.0 | 1543 | 1547 | 58,564 | 0.06 | 56,625 | 0.07 | 0.137 | ns |
| 112 | Benzoic acid | 105 | 32:34.0 | 00:01.1 | 2423 | 2423 | 26,705 | 0.03 | 25,854 | 0.03 | 0.104 | ns |
| 113 | Isobutyric acid | 73 | 18:06.0 | 00:01.0 | 1583 | 1581 | 539,147 | 0.52 | 559,134 | 0.65 | 0.027 | ns |
| 114 | trans-2-Hexenoic acid | 73 | 25:27.0 | 00:01.0 | 1959 | 1969 | 9305 | 0.01 | 9517 | 0.01 | 0.027 | ns |
| 115 | 7-Octenoic acid | 57 | 27:54.0 | 00:01.1 | 2120 | - | 14,525 | 0.01 | 14,436 | 0.02 | 0.010 | ns |
| 116 | Pentanoic acid | 60 | 21:22.0 | 00:01.0 | 1743 | 1744 | 76,463 | 0.07 | 77,245 | 0.09 | 0.008 | ns |
| 117 | Nonanoic acid | 60 | 28:36.0 | 00:01.1 | 2162 | 2165 | 12,275 | 0.01 | 12,228 | 0.01 | 0.001 | ns |
| ∑ Acids | 38,142,449 | 36.78 | 29,672,925 | 34.64 | 17.718 | * | ||||||
| 118 | 2-Ethoxy-6-(methoxymethyl)phenol | 137 | 31:17.0 | 00:01.4 | 2346 | - | 6209 | 0.01 | 2609 | 0.00 | 46.882 | * |
| 119 | 4-Vinylguaiacol | 135 | 29:11.0 | 00:01.3 | 2200 | 2203 | 287,108 | 0.28 | 483,388 | 0.56 | 3.766 | ns |
| 120 | 2,4-Ditert-butylphenol | 191 | 30:49.0 | 00:01.3 | 2304 | 2316 | 8225 | 0.01 | 6636 | 0.01 | 1.416 | ns |
| 121 | 2,6-Ditert-butyl-4-methylphenol | 205 | 24:31.0 | 00:02.0 | 1908 | 1906 | 85,342 | 0.08 | 91,286 | 0.11 | 1.015 | ns |
| 122 | Phenol | 94 | 26:09.0 | 00:01.1 | 2000 | 2008 | 15,548 | 0.01 | 16,802 | 0.02 | 0.864 | ns |
| ∑ Phenols | 402,432 | 0.39 | 600,722 | 0.70 | 3.701 | ns | ||||||
| 123 | 4-Hydroxybenzaldehyde | 121 | 39:48.0 | 00:01.2 | 2960 | 2958 | 140,741 | 0.14 | 97,154 | 0.11 | 4.282 | * |
| 124 | Benzaldehyde | 106 | 17:10.0 | 00:01.4 | 1536 | 1534 | 6935 | 0.01 | 8158 | 0.01 | 3.959 | ns |
| 125 | 6,6-Trimethyl-1-cyclohexene-1-propenal | 163 | 24:59.0 | 00:01.6 | 1923 | 3759 | 0.00 | 4395 | 0.01 | 1.300 | ns | |
| 126 | Benzeneacetaldehyde | 91 | 19:37.0 | 00:01.4 | 1648 | 1648 | 52,327 | 0.05 | 56,856 | 0.07 | 0.159 | ns |
| 127 | Hydroxy methyl furfural | 97 | 33:30.0 | 00:01.1 | 2500 | - | 22,283 | 0.02 | 22,031 | 0.03 | 0.027 | ns |
| ∑ Aldehydes | 226,044 | 0.22 | 188,594 | 0.22 | 2.768 | ns | ||||||
| 128 | 2-Methyl-4-phenyl-3-pentanone | 105 | 25:27.0 | 00:02.0 | 1959 | - | 18,102 | 0.02 | 104,172 | 0.12 | 8.945 | * |
| 129 | 3-Hydroxy-2-butanone | 45 | 11:48.0 | 00:01.1 | 1276 | 1280 | 59,058 | 0.06 | 21,029 | 0.02 | 4.706 | * |
| 130 | 1-Phenylethanone | 105 | 19:44.0 | 00:01.4 | 1652 | 1656 | 18,532 | 0.02 | 21,882 | 0.03 | 3.013 | ns |
| 131 | 4,5-Dimethyl-1,3-dioxol-2-one | 114 | 27:54.0 | 00:01.1 | 2120 | - | 22,813 | 0.02 | 24,528 | 0.03 | 0.452 | ns |
| 132 | Acetovanillone | 151 | 35:29.0 | 00:01.2 | 2650 | 2651 | 93,840 | 0.09 | 85,845 | 0.10 | 0.162 | ns |
| 133 | Zingerone | 137 | 37:21.0 | 00:01.3 | 2800 | 2790 | 7157 | 0.01 | 6754 | 0.01 | 0.083 | ns |
| ∑ Ketones | 219,503 | 0.21 | 264,211 | 0.31 | 0.484 | ns | ||||||
| 134 | 2-Benzofuran-1(3H)-one | 105 | 31:38.0 | 00:01.4 | 2359 | 2356 | 4335 | 0.00 | 6958 | 0.01 | 18.421 | * |
| 135 | δ-Valerolactone | 42 | 22:46.0 | 00:01.3 | 1808 | - | 28,596 | 0.03 | 63,074 | 0.07 | 11.676 | * |
| 136 | DL Mevalolactone | 71 | 34:12.0 | 00:01.1 | 2566 | - | 36,733 | 0.04 | 54,671 | 0.06 | 10.112 | * |
| 137 | 2,3-Dihydro-1-benzofuran | 120 | 31:59.0 | 00:01.1 | 2371 | 2389 | 574,035 | 0.55 | 1,642,628 | 1.92 | 7.665 | * |
| 138 | 5-(Hydroxymethyl)dihydrofuran-2(3H)-one | 85 | 35:50.0 | 00:01.1 | 2664 | - | 3,008,921 | 2.90 | 2,223,477 | 2.60 | 5.411 | * |
| 139 | δ-Octalactone | 99 | 25:34.0 | 00:01.5 | 1963 | 1965 | 29,764 | 0.03 | 21,141 | 0.02 | 4.794 | * |
| 140 | 3-Hydroxy-4,4-dimethyldihydrofuran-2(3H)-one | 128 | 26:30.0 | 00:01.1 | 2025 | - | 2356 | 0.00 | 2954 | 0.00 | 4.547 | * |
| 141 | 4-Hydroxy-2-ethyl-5-methyl-3(2H)-furanone | 56 | 27:26.0 | 00:01.1 | 2106 | 22,976 | 0.02 | 16,587 | 0.02 | 4.410 | * | |
| 142 | 4-(1-Hydroxyethyl)-γ-butanolactone | 86 | 31:03.0 | 00:01.2 | 2337 | 2328 | 51,285 | 0.05 | 39,634 | 0.05 | 2.961 | ns |
| 143 | 5-Ethoxydihydro-2(3H)-furanone | 85 | 21:08.0 | 00:01.4 | 1735 | 1728 | 96,235 | 0.09 | 67,581 | 0.08 | 2.558 | ns |
| 144 | δ-Hexanolactone | 42 | 22:25.0 | 00:01.4 | 1796 | 1792 | 53,111 | 0.05 | 28,094 | 0.03 | 2.431 | ns |
| 145 | α-Amino-γ-butyrolactone | 57 | 28:43.0 | 00:01.1 | 2165 | - | 15,428 | 0.01 | 17,230 | 0.02 | 2.156 | ns |
| 146 | cis-4-Hydroxy-3-methylundecanoic acid lactone | 99 | 25:41.0 | 00:01.6 | 1967 | - | 22,731 | 0.02 | 17,913 | 0.02 | 1.168 | ns |
| 147 | γ-Hexalactone | 85 | 20:47.0 | 00:01.4 | 1704 | 1703 | 12,815 | 0.01 | 14,975 | 0.02 | 0.815 | ns |
| 148 | 3-Hydroxy-4,4-dimethyldihydrofuran-2(3H)-one | 71 | 26:37.0 | 00:01.1 | 2031 | 2034 | 108,610 | 0.10 | 115,306 | 0.13 | 0.672 | ns |
| 149 | δ-Decalactone | 99 | 29:11.0 | 00:01.6 | 2200 | 2192 | 26,224 | 0.03 | 24,117 | 0.03 | 0.420 | ns |
| 150 | 5-(Hydroxy[methoxy(5-oxotetrahydro-2-furanyl)methoxy]methyl)dihydro-2(3H)-furanone | 85 | 31:24.0 | 00:01.2 | 2360 | - | 30,839 | 0.03 | 25,851 | 0.03 | 0.411 | ns |
| 151 | γ-Octalactone | 85 | 24:38.0 | 00:01.5 | 1911 | 1916 | 40,055 | 0.04 | 46,792 | 0.05 | 0.210 | ns |
| 152 | γ-Nonalactone | 85 | 28:22.0 | 00:01.6 | 2155 | 7610 | 0.01 | 7212 | 0.01 | 0.165 | ns | |
| 153 | 3,4-Dihydroxy-5-methyl-dihydrofuran-2-one | 60 | 38:59.0 | 00:01.1 | 2908 | - | 20,306 | 0.02 | 21,262 | 0.02 | 0.056 | ns |
| 154 | Ƴ-Butyrolactone | 68 | 22:32.0 | 00:01.1 | 1800 | - | 3,075,844 | 2.97 | 3,137,551 | 3.66 | 0.014 | ns |
| 155 | γ-Heptalactone | 85 | 22:39.0 | 00:01.4 | 1804 | 1796 | 9714 | 0.01 | 9998 | 0.01 | 0.012 | ns |
| ∑ Lactones and Furanoids | 7,278,522 | 7.02 | 7,605,007 | 8.88 | 0.347 | ns | ||||||
| 156 | Ethyl 3-methylthiopropanoate | 74 | 18:06.0 | 00:01.5 | 1583 | 1580 | 15,294 | 0.01 | 38,729 | 0.05 | 6.358 | * |
| 157 | 3-(Methylthio)propionic acid | 61 | 30:35.0 | 00:01.0 | 2295 | 2298 | 119,593 | 0.12 | 72,314 | 0.08 | 6.004 | * |
| 158 | 2-Methyldihydrothiophen-3(2H)-one | 60 | 17:17.0 | 00:01.5 | 1540 | - | 822,873 | 0.79 | 550,872 | 0.64 | 3.146 | ns |
| 159 | 3-(Ethylthio)propanol | 61 | 22:04.0 | 00:01.2 | 1785 | 1802 | 30,717 | 0.03 | 40,517 | 0.05 | 2.897 | ns |
| 160 | N-acetylmethionine ethyl ester | 99 | 35:50.0 | 00:01.4 | 2664 | - | 14,010 | 0.01 | 15,774 | 0.02 | 0.919 | ns |
| 161 | 3-Methylthiopropyl acetate | 61 | 19:16.0 | 00:01.5 | 1638 | 1633 | 6213 | 0.01 | 7236 | 0.01 | 0.669 | ns |
| 162 | S-(3-Hydroxypropyl) ethanethioate | 74 | 25:48.0 | 00:01.2 | 1971 | - | 76,117 | 0.07 | 86,477 | 0.10 | 0.660 | ns |
| 163 | 5-Acetyldihydrofuran-2(3H)-one | 85 | 27:05.0 | 00:01.2 | 2092 | 2096 | 23,593 | 0.02 | 22,169 | 0.03 | 0.388 | ns |
| 164 | 3-Methylmercapto-1-propanol | 106 | 20:54.0 | 00:01.2 | 1707 | 1715 | 1,677,408 | 1.62 | 1,720,142 | 2.01 | 0.035 | ns |
| ∑ Sulfur-containing compounds | 2,785,819 | 2.69 | 2,554,230 | 2.98 | 0.601 | ns | ||||||
| 165 | 2-Ethylbutan-1-amine | 101 | 35:08.0 | 00:01.1 | 2636 | - | 192,419 | 0.19 | 98,372 | 0.11 | 26.914 | * |
| 166 | N-Phenethylacetamide | 104 | 34:33.0 | 00:01.3 | 2583 | 2590 | 10,957 | 0.01 | 72,921 | 0.09 | 8.741 | * |
| 167 | 3-Methylpiperazine-2,5-dione | 85 | 34:05.0 | 00:01.0 | 2560 | - | 5863 | 0.01 | 4539 | 0.01 | 5.000 | ns |
| 168 | Benzothiazole | 135 | 25:20.0 | 00:01.5 | 1956 | 1959 | 8013 | 0.01 | 4187 | 0.00 | 3.423 | ns |
| 169 | N,N-Dibutylformamide | 72 | 21:57.0 | 00:01.7 | 1761 | 1767 | 9573 | 0.01 | 12,233 | 0.01 | 1.776 | ns |
| 170 | N-Acetylcysteamine | 60 | 15:18.0 | 00:01.3 | 1443 | - | 16,226 | 0.02 | 30,705 | 0.04 | 1.708 | ns |
| 171 | 1H-indole | 117 | 32:48.0 | 00:01.2 | 2434 | 2435 | 9900 | 0.01 | 34,798 | 0.04 | 1.101 | ns |
| 172 | N-(3-Methylbutyl)acetamide | 72 | 23:35.0 | 00:01.2 | 1856 | 1855 | 3509 | 0.00 | 7045 | 0.01 | 1.042 | ns |
| 173 | 1H-Isoindole-1,3(2H)-dione | 120 | 39:13.0 | 00:01.3 | 2940 | - | 137,672 | 0.13 | 122,523 | 0.14 | 0.982 | ns |
| 174 | 3-Ethyl-4-methyl-1H-pyrrole-2,5-dione | 139 | 30:14.0 | 00:01.2 | 2279 | 2260 | 20,059 | 0.02 | 22,456 | 0.03 | 0.453 | ns |
| 175 | 2-(Oxan-4-yl)ethanamine | 85 | 28:22.0 | 00:01.1 | 2155 | - | 10,950 | 0.01 | 10,492 | 0.01 | 0.138 | ns |
| 176 | 2-Propoxyethylamine | 68 | 36:39.0 | 00:00.9 | 2730 | - | 3229 | 0.00 | 3729 | 0.00 | 0.037 | ns |
| ∑ Nitrogen-containing compounds | 428,370 | 0.41 | 424,000 | 0.49 | 0.003 | ns | ||||||
| 177 | 4-Hydroxy-6-pentyltetrahydro-2H-pyran-2-one | 102 | 37:28.0 | 00:01.5 | 2806 | - | 6418 | 0.01 | 2634 | 0.00 | 14.628 | * |
| 178 | 1,1-Di(2-methyl butoxy)ethane | 71 | 12:16.0 | 00:02.5 | 1303 | - | 169,495 | 0.16 | 688,162 | 0.80 | 7.905 | * |
| 179 | Succinic acid anhydride | 56 | 35:01.0 | 00:01.0 | 2631 | - | 450,941 | 0.43 | 285,403 | 0.33 | 7.558 | * |
| 180 | 2-Methyl-2-propyl-1,3-dioxolane | 87 | 35:43.0 | 00:01.3 | 2659 | - | 4732 | 0.00 | 6170 | 0.01 | 1.151 | ns |
| 181 | Isothiocyanatocyclohexane | 141 | 20:12.0 | 00:02.0 | 1686 | 1670 | 17,086 | 0.02 | 17,710 | 0.02 | 1.141 | ns |
| 182 | 1,4-Dioxanyl hydroperoxide | 115 | 20:05.0 | 00:01.7 | 1682 | - | 7069 | 0.01 | 9086 | 0.01 | 0.946 | ns |
| 183 | 2,3-Diphenylbutane | 105 | 18:55.0 | 00:01.6 | 1607 | - | 3817 | 0.00 | 4115 | 0.00 | 0.157 | ns |
| 184 | 2-Methyl-2H-pyran-3,4,5 (6H)-trione | 142 | 30:35.0 | 00:01.1 | 2296 | - | 2767 | 0.00 | 2992 | 0.00 | 0.137 | ns |
| 185 | Methylsuccinic anhydride | 68 | 23:07.0 | 00:01.1 | 1841 | 1855 | 4237 | 0.00 | 5186 | 0.01 | 0.086 | ns |
| 186 | 4,5-Dimethyl-2-pentadecyl-1,3-dioxolane | 101 | 33:37.0 | 00:01.1 | 2500 | 52,080 | 0.05 | 50,362 | 0.06 | 0.045 | ns | |
| 187 | Ethoxy-1-pentoxyethane | 73 | 07:43.0 | 00:02.0 | 1103 | 1104 | 1,298,503 | 1.25 | 1,373,564 | 1.60 | 0.043 | ns |
| 188 | trans-7-tetradecene | 83 | 15:25.0 | 00:02.8 | 1447 | 1435 | 8217 | 0.01 | 8055 | 0.01 | 0.034 | ns |
| ∑ Other compounds | 2,025,363 | 1.95 | 2,453,438 | 2.86 | 0.775 | ns | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boban, A.; Vrhovsek, U.; Carlin, S.; Mucalo, A.; Budić-Leto, I. A Targeted and an Untargeted Metabolomics Approach to the Volatile Aroma Profile of Young ‘Maraština’ Wines. Metabolites 2022, 12, 1295. https://doi.org/10.3390/metabo12121295
Boban A, Vrhovsek U, Carlin S, Mucalo A, Budić-Leto I. A Targeted and an Untargeted Metabolomics Approach to the Volatile Aroma Profile of Young ‘Maraština’ Wines. Metabolites. 2022; 12(12):1295. https://doi.org/10.3390/metabo12121295
Chicago/Turabian StyleBoban, Ana, Urska Vrhovsek, Silvia Carlin, Ana Mucalo, and Irena Budić-Leto. 2022. "A Targeted and an Untargeted Metabolomics Approach to the Volatile Aroma Profile of Young ‘Maraština’ Wines" Metabolites 12, no. 12: 1295. https://doi.org/10.3390/metabo12121295






