Gene Dose-Dependent and Additive Effects of ABCG2 rs2231142 and SLC2A9 rs3733591 Genetic Polymorphisms on Serum Uric Acid Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. DNA Extraction, Polymerase Chain Reaction, and Pyrosequencing
2.3. Measurement of Uric Acid
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Primer | Sequences | Size, bp | PCR Tm, °C | |
|---|---|---|---|---|
| ABCG2 rs2231137 | Forward | 5′-GCTCATTGCCACACATTT-3′ | 188 | 60 |
| Reverse | B 5′-GAAGCCATTGGTGTTTCC-3′ | |||
| Sequencing | 5′-ATGTCGAAGTTTTTATCC-3′ | |||
| ABCG2 rs2231142 | Forward | B 5′-ATGTTGTGATGGGCACTCTGAC-3′ | 210 | 60 |
| Reverse | 5′- TATCCACACAGGGAAAGTCCTACT-3′ | |||
| Sequencing | 5′-GAAGAGCTGCTGAGAACT-3′ | |||
| ABCG2 rs72552713 | Forward | 5′-GTCTTAGCTGCAAGGAAAGAT-3′ | 166 | 60 |
| Reverse | B 5′-CCAAAGCACTTACCCATATAGA-3′ | |||
| Sequencing | 5′-AATGTAATTCAGGTTAYGTG-3′ | |||
| SLC2A9 rs734553 | Forward | B 5′-ACCCCATGATCTGATTATT-3′ | 176 | 60 |
| Reverse | 5′-ACCCACCCTCATGATTTA-3′ | |||
| Sequencing | 5′-GGCTGACTGATTAGATCC-3′ | |||
| SLC2A9 rs16890979 | Forward | 5′-GCATTAGACATGATGGACACTC-3′ | 223 | 60 |
| Reverse | B 5′-AGGCCATGGTGACAATCA-3′ | |||
| Sequencing | 5′-TTCTTGGGTAAAGCAGAC-3′ | |||
| SLC2A9 rs3733591 | Forward | 5′-GCATTAGACATGATGGACACTC-3′ | 223 | 60 |
| Reverse | B 5′-AGGCCATGGTGACAATCA-3′ | |||
| Sequencing | 5′-GGAGGTCCTGGCTGAGAG-3′ |
| Haplotypes | ABCG2 rs2231142 | SLC2A9 rs3733591 | Frequencies |
|---|---|---|---|
| H1 | C | A | 0.5427 |
| H2 | C | G | 0.1993 |
| H3 | A | A | 0.1773 |
| H4 | A | G | 0.0807 |
References
- Fathallah-Shaykh, S.A.; Cramer, M.T. Uric acid and the kidney. Pediatr. Nephrol. 2014, 29, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, L.; Malaguarnera, M. Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int. J. Med. Sci. 2007, 4, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.A.; Puig, J.G.; Mateos, F.A.; Jimenez, M.L.; Kim, M.; Simmonds, H. Inherited superactivity of phosphoribosylpyrophosphate synthetase: Association of uric acid overproduction and sensorineural deafness. Am. J. Med. 1988, 85, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Losman, M.J.; Rosenberg, A.L.; Mehlman, I.; Levinson, D.J.; Holmes, E.W. Phosphoribosylpyrophosphate synthetase superactivity: A study of five patients with catalytic defects in the enzyme. Arthritis Rheum. 1986, 29, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, W. Abnormal serum uric acid levels in children. J. Pediatr. 1996, 128, 731–741. [Google Scholar] [CrossRef]
- Seegmiller, J.E.; Rosenbloom, F.M.; Kelley, W.N. Enzyme Defect Associated with a Sex-Linked Human Neurological Disorder and Excessive Purine Synthesis. Science 1967, 155, 1682–1684. [Google Scholar] [CrossRef] [PubMed]
- Page, T.; Bakay, B.; Nissinen, E.; Nyhan, W.L. Hypoxanthine--guanine phosphoribosyltransferase variants: Correlation of clinical phenotype with enzyme activity. J. Inherit. Metab. Dis. 1981, 4, 203–206. [Google Scholar] [CrossRef]
- Sculley, D.G.; Dawson, P.A.; Emmerson, B.T.; Gordon, R.B. A review of the molecular basis of hypoxanthine-guanine phosphoribosyltransferase (HPRT) deficiency. Hum. Genet. 1992, 90, 195–207. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Johnson, R.J. Uric Acid: The Oxidant-Antioxidant Paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [Green Version]
- Kanellis, J.; Kang, D.-H. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin. Nephrol. 2005, 25, 39–42. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.-S.; Pae, C.-U.; Yoon, S.-J.; Jang, W.-Y.; Lee, N.J.; Kim, J.-J.; Lee, S.-J.; Lee, C.; Paik, I.-H.; Lee, C.-U. Decreased plasma antioxidants in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2006, 21, 344–348. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.L.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Serum uric acid levels and the risk of Parkinson disease. Ann. Neurol. 2005, 58, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Döring, A.; Gieger, C.; Mehta, D.; Gohlke, H.; Prokisch, H.; Coassin, S.; Fischer, G.; Henke, K.; Klopp, N.; Kronenberg, F.; et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat. Genet. 2008, 40, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.; Shafiu, M.; Mu, W.; Johnson, R.J. SLC2A9--a fructose transporter identified as a novel uric acid transporter. Nephrol. Dial. Transplant. 2008, 23, 2746–2749. [Google Scholar] [CrossRef] [PubMed]
- Itahana, Y.; Han, R.; Barbier, S.; Lei, Z.; Rozen, S.; Itahana, K. The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense. Oncogene 2014, 34, 1799–1810. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, A.; Nakanishi, T.; Fujita, T.; Tamai, I. Extra-Renal Elimination of Uric Acid via Intestinal Efflux Transporter BCRP/ABCG2. PLoS ONE 2012, 7, e30456. [Google Scholar] [CrossRef] [Green Version]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate–anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef]
- Anzai, N.; Ichida, K.; Jutabha, P.; Kimura, T.; Babu, E.; Jin, C.J.; Srivastava, S.; Kitamura, K.; Hisatome, I.; Endou, H.; et al. Plasma Urate Level Is Directly Regulated by a Voltage-driven Urate Efflux Transporter URATv1 (SLC2A9) in Humans. J. Biol. Chem. 2008, 283, 26834–26838. [Google Scholar] [CrossRef] [Green Version]
- Kis, E.; Nagy, T.; Jani, M.; Molnar, E.; Janossy, J.; Ujhellyi, O.; Nemet, K.; Heredi-Szabo, K.; Krajcsi, P. Leflunomide and its metabolite A771726 are high affinity substrates of BCRP: Implications for drug resistance. Ann. Rheum. Dis. 2008, 68, 1201–1207. [Google Scholar] [CrossRef]
- Otero, J.A.; Miguel, V.; González-Lobato, L.; Villalba, R.G.; Espín, J.C.; Prieto, J.G.; Merino, G.; Álvarez, A.I. Effect of bovine ABCG2 polymorphism Y581S SNP on secretion into milk of enterolactone, riboflavin and uric acid. Animal 2016, 10, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Auberson, M.; Stadelmann, S.; Stoudmann, C.; Seuwen, K.; Koesters, R.; Thorens, B.; Bonny, O. SLC2A9 (GLUT9) mediates urate reabsorption in the mouse kidney. Pflugers. Arch. 2018, 470, 1739–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolz, M.; Johnson, T.; Sanna, S.; Teumer, A.; Vitart, V.; Perola, M.; Mangino, M.; Albrecht, E.; Wallace, C.; Farrall, M.; et al. Meta-Analysis of 28,141 Individuals Identifies Common Variants within Five New Loci That Influence Uric Acid Concentrations. PLoS Genet. 2009, 5, e1000504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, H.-P.; Chen, C.-J.; Tovosia, S.; Ko, A.M.-S.; Lee, C.-H.; Ou, T.-T.; Lin, G.-T.; Chang, S.-J.; Chiang, S.-L.; Chiang, H.-C.; et al. Associations of a non-synonymous variant in SLC2A9 with gouty arthritis and uric acid levels in Han Chinese subjects and Solomon Islanders. Ann. Rheum. Dis. 2010, 69, 887–890. [Google Scholar] [CrossRef]
- Meng, Q.; Yue, J.; Shang, M.; Shan, Q.; Qi, J.; Mao, Z.; Li, J.; Zhang, F.; Wang, B.; Zhao, T.; et al. Correlation of GLUT9 Polymorphisms with Gout Risk. Medicine 2015, 94, e1742. [Google Scholar] [CrossRef]
- Lukkunaprasit, T.; Rattanasiri, S.; Turongkaravee, S.; Suvannang, N.; Ingsathit, A.; Attia, J.; Thakkinstian, A. The association between genetic polymorphisms in ABCG2 and SLC2A9 and urate: An updated systematic review and meta-analysis. BMC Med. Genet. 2020, 21, 210. [Google Scholar] [CrossRef]
- Tu, H.-P.; Ko, A.M.-S.; Chiang, S.-L.; Lee, S.-S.; Lai, H.-M.; Chung, C.-M.; Huang, C.-M.; Lee, C.-H.; Kuo, T.-M.; Hsieh, M.-J.; et al. Joint Effects of Alcohol Consumption and ABCG2 Q141K on Chronic Tophaceous Gout Risk. J. Rheumatol. 2014, 41, 749–758. [Google Scholar] [CrossRef]
- Takei, R.; Cadzow, M.; Markie, D.; Bixley, M.; Phipps-Green, A.; Major, T.J.; Li, C.; Choi, H.K.; Li, Z.; Hu, H.; et al. Trans-ancestral dissection of urate- and gout-associated major loci SLC2A9 and ABCG2 reveals primate-specific regulatory effects. J. Hum. Genet. 2021, 66, 161–169. [Google Scholar] [CrossRef]
- Liu, J.; Yang, W.; Li, Y.; Wei, Z.; Dan, X. ABCG2 rs2231142 variant in hyperuricemia is modified by SLC2A9 and SLC22A12 polymorphisms and cardiovascular risk factors in an elderly community-dwelling population. BMC Med. Genet. 2020, 21, 54. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.J.; Ahn, J.; Hwang, J.; Han, S.W.; Lee, K.N.; Kim, J.B.; Lee, S.; Na, J.O.; Lim, H.E.; Kim, J.W.; et al. Relationship between uric acid and blood pressure in different age groups. Clin. Hypertens. 2015, 21, 14. [Google Scholar] [CrossRef]
- Zand, S.; Shafiee, A.; Boroumand, M.; Jalali, A.; Nozari, Y. Serum Uric Acid Is Not an Independent Risk Factor for Premature Coronary Artery Disease. Cardiorenal Med. 2013, 3, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.W.J.; Ford, E.S.; Gao, X.; Choi, H.K. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: The third national health and nutrition examination survey. Arthritis Rheum. 2007, 59, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.J. The increase in serum uric acid concentration caused by diuretics might be beneficial in heart failure. Eur. J. Heart Fail. 2005, 7, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Ene-Stroescu, D.; Gorbien, M.J. Gouty arthritis. A primer on late-onset gout. Geriatrics 2005, 60, 24–31. [Google Scholar] [PubMed]
- Mikuls, T.R.; Farrar, J.T.; Bilker, W.B.; Fernandes, S.; Schumacher, H.R., Jr.; Saag, K.G. Gout epidemiology: Results from the UK General Practice Research Database, 1990-1999. Ann. Rheum. Dis. 2005, 64, 267–272. [Google Scholar] [CrossRef]
- Lawrence, R.C.; Helmick, C.G.; Arnett, F.C.; Deyo, R.A.; Felson, D.T.; Giannini, E.H.; Heyse, S.P.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998, 41, 778–799. [Google Scholar] [CrossRef]
- Wallace, K.L.; Riedel, A.A.; Joseph-Ridge, N.; Wortmann, R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J. Rheumatol. 2004, 31, 1582–1588. [Google Scholar]
- Lin, X.; Wang, X.; Li, X.; Song, L.; Meng, Z.; Yang, Q.; Zhang, W.; Gao, Y.; Yang, Z.; Cai, H.; et al. Gender- and Age-Specific Differences in the Association of Hyperuricemia and Hypertension: A Cross-Sectional Study. Int. J. Endocrinol. 2019, 2019, 7545137. [Google Scholar] [CrossRef] [Green Version]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-A.; Joo, H.-J.; Park, J.-Y. ABCG2 polymorphisms, 34G>A and 421C>A in a Korean population: Analysis and a comprehensive comparison with other populations. J. Clin. Pharm. Ther. 2010, 35, 705–712. [Google Scholar] [CrossRef]
- Kim, K.-A.; Joo, H.-J.; Park, J.-Y. Effect of ABCG2 genotypes on the pharmacokinetics of A771726, an active metabolite of prodrug leflunomide, and association of A771726 exposure with serum uric acid level. Eur. J. Clin. Pharmacol. 2010, 67, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Song, W.-G.; Lee, H.-M.; Joo, H.-J.; Park, J.-Y. Effect of P2Y1 and P2Y12 genetic polymorphisms on the ADP-induced platelet aggregation in a Korean population. Thromb. Res. 2013, 132, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-T.; Wu, S.; Su, C.-W.; Teng, M.-S.; Hsu, L.-A.; Ko, Y.-L. Association of ABCG2 rs2231142-A allele and serum uric acid levels in male and obese individuals in a Han Taiwanese population. J. Formos. Med. Assoc. 2017, 116, 18–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.P.; Barry, P.E.; Dawber, T.R.; McNamara, P.M. Epidemiology of gout and hyperuricemia. A long-term population study. Am. J. Med. 1967, 42, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Campion, E.W.; Glynn, R.J.; DeLabry, L.O. Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. Am. J. Med. 1987, 82, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Cheng, J.; Huangfu, N.; Zhao, R.; Xu, Z.; Zhang, F.; Zheng, W.; Zhang, D. Hyperuricemia and Cardiovascular Disease. Curr. Pharm. Des. 2019, 25, 700–709. [Google Scholar] [CrossRef]
- Yip, K.; Cohen, R.E.; Pillinger, M.H. Asymptomatic hyperuricemia: Is it really asymptomatic? Curr. Opin. Rheumatol. 2020, 32, 71–79. [Google Scholar] [CrossRef]
- Álvarez-Lario, B.; Alonso-Valdivielso, J.L. Hyperuricemia and gout; the role of diet. Nutr. Hosp. 2014, 29, 760–770. [Google Scholar] [CrossRef]
- Petreski, T.; Ekart, R.; Hojs, R.; Bevc, S. Hyperuricemia, the heart, and the kidneys—To treat or not to treat? Ren. Fail. 2020, 42, 978–986. [Google Scholar] [CrossRef]
- Li, C.; Hsieh, M.-C.; Chang, S.-J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013, 25, 210–216. [Google Scholar] [CrossRef]
- De Becker, B.; Borghi, C.; Burnier, M.; van de Borne, P. Uric acid and hypertension: A focused review and practical recom-mendations. J. Hypertens. 2019, 37, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Liu, M.; Kim, M.J.; Park, S. The haplotype of SLC2A9_rs3733591, PKD2_rs2725220 and ABCG2_rs2231142 increases the hyperuricaemia risk and alcohol, chicken and processed meat intakes and smoking interact with its risk. Int. J. Food Sci. Nutr. 2021, 72, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Ieiri, I.; Hirota, T.; Takane, H.; Maegawa, S.; Kigawa, J.; Suzuki, H.; Nanba, E.; Oshimura, M.; Terakawa, N.; et al. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab. Dispos. 2004, 33, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Jia, J.; Sun, Z.; Liu, C.; Li, Z.; Xiao, Y.; Yu, J.; Du, F.; Shi, Y.; Sun, J.; et al. Polymorphism of FGD4 and myelosuppression in patients with esophageal squamous cell carcinoma. Futur. Oncol. 2021, 17, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- Urano, W.; Taniguchi, A.; Anzai, N.; Inoue, E.; Sekita, C.; Endou, H.; Kamatani, N.; Yamanaka, H. Association between GLUT9 and gout in Japanese men. Ann. Rheum. Dis. 2010, 69, 932–933. [Google Scholar] [CrossRef] [PubMed]
- Hollis-Moffatt, J.E.; Gow, P.J.; Harrison, A.A.; Highton, J.; Jones, P.B.; Stamp, L.K.; Dalbeth, N.; Merriman, T.R. The SLC2A9 nonsynonymous Arg265His variant and gout: Evidence for a population-specific effect on severity. Arthritis Res. Ther. 2011, 13, R85. [Google Scholar] [CrossRef] [Green Version]
- McArdle, P.F.; Parsa, A.; Chang, Y.-P.C.; Weir, M.R.; O’Connell, J.R.; Mitchell, B.; Shuldiner, A. Association of a common nonsynonymous variant in GLUT9 with serum uric acid levels in old order amish. Arthritis Rheum. 2008, 58, 2874–2881. [Google Scholar] [CrossRef] [Green Version]
- Lim, E.; Miyamura, J.; Chen, J.J. Racial/Ethnic-Specific Reference Intervals for Common Laboratory Tests: A Comparison among Asians, Blacks, Hispanics, and White. Hawaii J. Med. Public Health 2015, 74, 302–310. [Google Scholar]
- Singh, J.A. Racial and Gender Disparities Among Patients with Gout. Curr. Rheumatol. Rep. 2013, 15, 307. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, L.; Xie, R.; Dai, W.; Gao, C.; Shen, P.; Huang, X.; Zhang, F.; Yang, X.; Ji, G. Association of Serum Uric Acid with Body Mass Index: A Cross-Sectional Study from Jiangsu Province, China. Iran. J. Public Health 2014, 43, 1503–1509. [Google Scholar]
- Palmer, T.M.; Nordestgaard, B.G.; Benn, M.; Tybjaerg-Hansen, A.; Smith, G.D.; Lawlor, D.A.; Timpson, N.J. Association of plasma uric acid with ischaemic heart disease and blood pressure: Mendelian randomisation analysis of two large cohorts. Bmj 2013, 347, f4262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsa, A.; Brown, E.; Weir, M.R.; Fink, J.C.; Shuldiner, A.R.; Mitchell, B.D.; McArdle, P.F. Genotype-based changes in serum uric acid affect blood pressure. Kidney Int. 2012, 81, 502–507. [Google Scholar] [CrossRef] [PubMed]
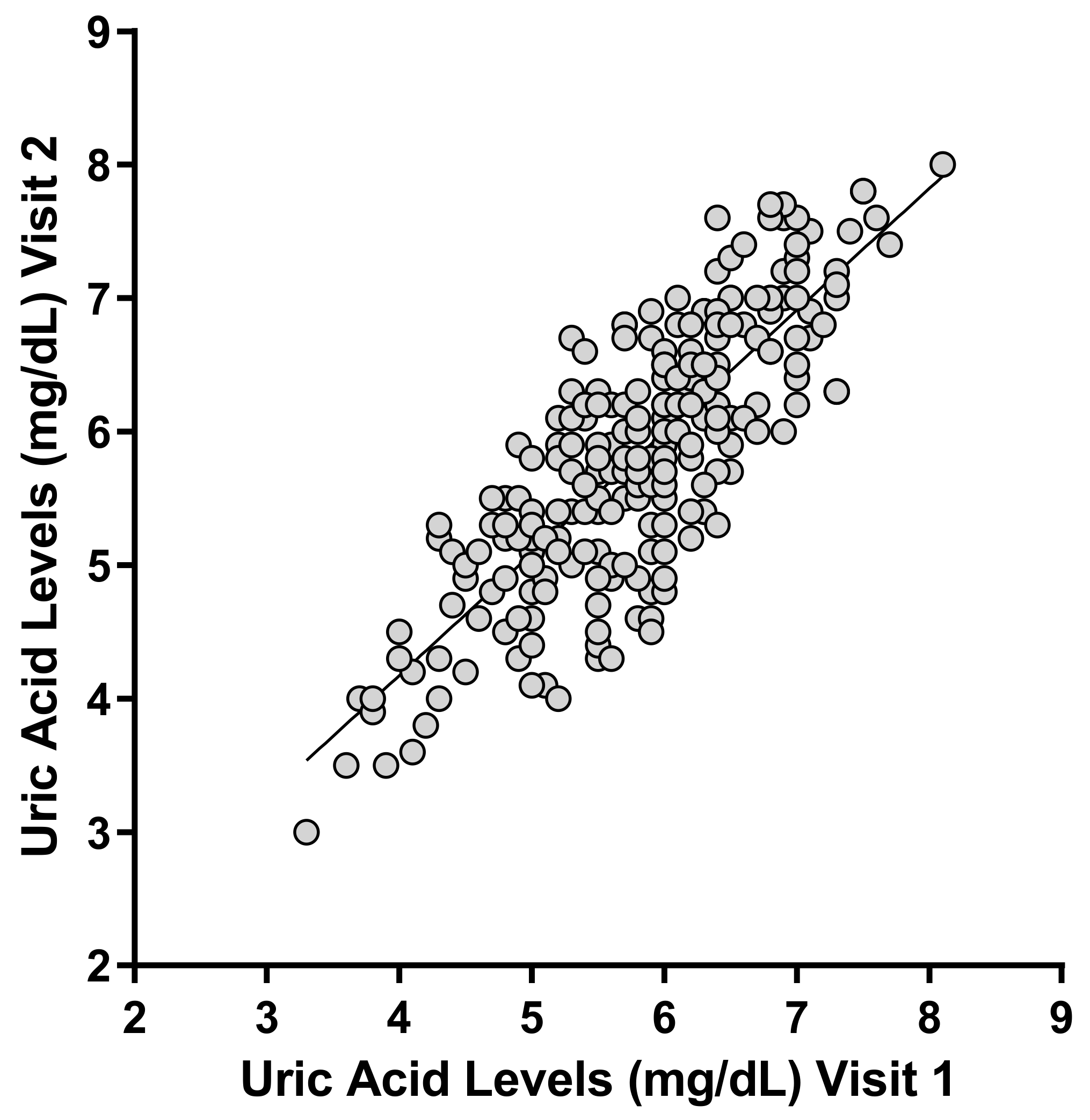

| Wild Type | Heterozygous Mutant | Homozygous Mutant | p Value | ||
|---|---|---|---|---|---|
| ABCG2 rs2231137 | GG (n = 167) | GA (n = 70) | AA (n = 13) | ||
| Weight (kg) | 69.3 ± 6.7 | 69.4 ± 6.7 | 67.8 ± 6.0 | 0.714 | |
| Height (cm) | 175.4 ± 4.9 | 174.5 ± 4.9 | 174.7 ± 3.4 | 0.354 | |
| Age (years) | 24.5 ± 2.2 | 24.7 ± 2.4 | 24.6 ± 2.4 | 0.838 | |
| BMI (kg/m2) | 22.6 ± 2.4 | 22.8 ± 2.6 | 22.2 ± 2.3 | 0.609 | |
| ABCG2 rs2231142 | CC (n = 138) | CA (n = 95) | AA (n = 17) | ||
| Weight (kg) | 68.5 ± 6.4 | 70.1 ± 6.5 | 70.7 ± 8.8 | 0.139 | |
| Height (cm) | 175.0 ± 4.6 | 175.2 ± 5.1 | 175.9 ± 4.8 | 0.728 | |
| Age (years) | 24.5 ± 2.3 | 24.7 ± 2.4 | 24.7 ± 1.8 | 0.724 | |
| BMI (kg/m2) | 22.4 ± 2.4 | 22.9 ± 2.5 | 22.9 ± 2.9 | 0.354 | |
| ABCG2 rs72552713 | CC (n = 241) | CT (n = 9) | |||
| Weight (kg) | 69.2 ± 6.6 | 72.2 ± 8.6 | 0.174 | ||
| Height (cm) | 175.0 ± 4.8 | 177.6 ± 3.3 | 0.121 | ||
| Age (years) | 24.6 ± 2.3 | 24.4 ± 2.3 | 0.868 | ||
| BMI (kg/m2) | 22.6 ± 2.4 | 22.9 ± 3.0 | 0.690 | ||
| SLC2A9 rs734553 | TT (n = 240) | TG (n = 10) | |||
| Weight (kg) | 69.2 ± 6.6 | 71.3 ± 7.9 | 0.323 | ||
| Height (cm) | 175.1 ± 4.8 | 174.8 ± 5.3 | 0.831 | ||
| Age (years) | 24.5 ± 2.3 | 25.4 ± 2.2 | 0.237 | ||
| BMI (kg/m2) | 22.6 ± 2.4 | 23.4 ± 3.2 | 0.300 | ||
| SLC2A9 rs16890979 | GG (n = 241) | GT (n = 9) | |||
| Weight (kg) | 69.2 ± 6.6 | 70.0 ± 9.7 | 0.722 | ||
| Height (cm) | 175.1 ± 4.8 | 174.8 ± 5.5 | 0.829 | ||
| Age (years) | 24.5 ± 2.3 | 25.7 ± 2.1 | 0.139 | ||
| BMI (kg/m2) | 22.6 ± 2.4 | 23.0 ± 3.9 | 0.605 | ||
| SLC2A9 rs3733591 | AA (n = 126) | AG (n = 108) | GG (n = 16) | ||
| Weight (kg) | 68.9 ± 6.1 | 70.1 ± 7.4 | 66.3 ± 5.1 | 0.071 | |
| Height (cm) | 174.8 ± 4.9 | 175.4 ± 4.8 | 175.6 ± 4.8 | 0.613 | |
| Age (years) | 24.5 ± 2.4 | 24.7 ± 2.3 | 24.1 ± 1.2 | 0.512 | |
| BMI (kg/m2) | 22.6 ± 2.4 | 22.8 ± 2.7 | 21.5 ± 1.6 | 0.147 |
| Genotype Frequencies | Allele Frequencies | χ2 | p Value | ||||
|---|---|---|---|---|---|---|---|
| ABCG2 rs2231137 | GG | GA | AA | G | A | ||
| n | 167 | 70 | 13 | ||||
| Frequencies | 0.668 | 0.280 | 0.052 | 0.808 | 0.192 | 1.792 | 0.181 |
| ABCG2 rs2231142 | CC | CA | AA | C | A | ||
| n | 138 | 95 | 17 | ||||
| Frequencies | 0.552 | 0.380 | 0.068 | 0.742 | 0.258 | 0.0022 | 0.963 |
| ABCG2 rs72552713 | CC | CT | TT | C | T | ||
| n | 241 | 9 | 0 | ||||
| Frequencies | 0.964 | 0.036 | 0 | 0.982 | 0.018 | 2.248 | 0.134 |
| SLC2A9 rs734553 | TT | TG | GG | T | G | ||
| n | 240 | 10 | 0 | ||||
| Frequencies | 0.96 | 0.04 | 0 | 0.980 | 0.020 | 1.666 | 0.197 |
| SLC2A9 rs16890979 | GG | GA | AA | G | A | ||
| n | 241 | 9 | 0 | ||||
| Frequencies | 0.964 | 0.036 | 0 | 0.982 | 0.018 | 2.248 | 0.134 |
| SLC2A9 rs3733591 | AA | AG | GG | A | G | ||
| n | 126 | 108 | 16 | ||||
| Frequencies | 0.504 | 0.432 | 0.064 | 0.720 | 0.280 | 0.946 | 0.331 |
| Genotypes | Alleles | n | Mean ± SD | 95% CI |
|---|---|---|---|---|
| ABCG2 rs2231137 | GG | 167 | 5.55 ± 0.88 | (5.66–5.93) |
| GA | 70 | 5.88 ± 0.84 | (5.68–6.08) | |
| AA | 13 | 5.80 ± 0.88 | (5.02–6.09) | |
| p value | 0.448 | |||
| ABCG2 rs2231142 | CC | 138 | 5.51 ± 0.83 | (5.37–5.65) |
| CA | 95 | 6.10 ± 0.74 | (5.94–6.25) | |
| AA | 17 | 6.57 ± 0.74 | (6.19–6.96) | |
| p value | < 0.001 | |||
| ABCG2 rs72552713 | CC | 241 | 5.79 ± 0.87 | (5.68–5.90) |
| CT | 9 | 6.25 ± 0.53 | (5.84–6.65) | |
| p value | 0.118 | |||
| SLC2A9 rs734553 | TT | 240 | 5.80 ± 0.87 | (5.69–5.91) |
| TG | 10 | 6.07 ± 0.74 | (5.54–6.60) | |
| p value | 0.328 | |||
| SLC2A9 rs16890979 | GG | 241 | 5.80 ± 0.87 | (5.69–5.91) |
| GA | 9 | 6.02 ± 0.79 | (5.41–6.63) | |
| p value | 0.449 | |||
| SLC2A9 rs3733591 | AA | 126 | 5.42 ± 0.80 | (5.28–5.56) |
| AG | 108 | 6.12 ± 0.75 | (5.98–6.26) | |
| GG | 16 | 6.74 ± 0.48 | (6.48–7.00) | |
| p value | < 0.001 |
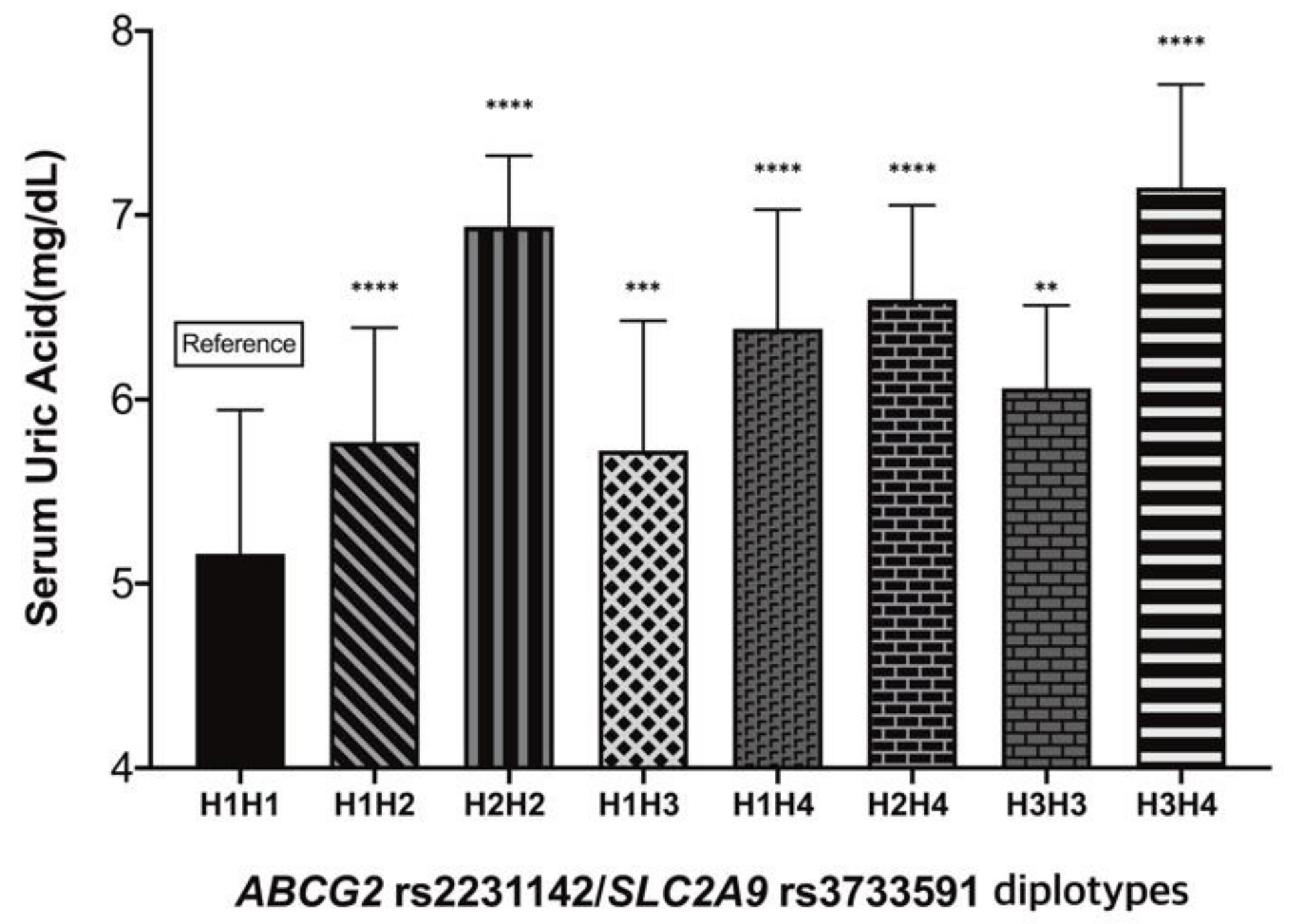
| Diplotypes | Number | Frequency (%) | Mean ± SD | 95% CI |
|---|---|---|---|---|
| H1H1 | 74 | 29.6 | 5.16 ± 0.78 | 5.00–5.32 |
| H1H2 | 56 | 22.4 | 5.77 ± 0.62 | 5.62–5.92 |
| H1H3 | 43 | 17.2 | 5.72 ± 0.70 | 5.53–5.92 |
| H1H4 | 44 | 17.6 | 6.38 ± 0.65 | 6.21–6.56 |
| H2H2 | 8 | 3.2 | 6.54 ± 0.51 | 6.66–7.22 |
| H2H4 | 8 | 3.2 | 6.54 ± 0.51 | 6.18–6.91 |
| H3H3 | 9 | 3.6 | 6.06 ± 0.45 | 5.76–6.36 |
| H3H4 | 8 | 3.2 | 7.15 ± 0.56 | 6.75–7.56 |
| H4H4 | 0 | 0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-W.; Noh, J.-H.; Kim, J.-M.; Lee, H.-Y.; Kim, K.-A.; Park, J.-Y. Gene Dose-Dependent and Additive Effects of ABCG2 rs2231142 and SLC2A9 rs3733591 Genetic Polymorphisms on Serum Uric Acid Levels. Metabolites 2022, 12, 1192. https://doi.org/10.3390/metabo12121192
Park J-W, Noh J-H, Kim J-M, Lee H-Y, Kim K-A, Park J-Y. Gene Dose-Dependent and Additive Effects of ABCG2 rs2231142 and SLC2A9 rs3733591 Genetic Polymorphisms on Serum Uric Acid Levels. Metabolites. 2022; 12(12):1192. https://doi.org/10.3390/metabo12121192
Chicago/Turabian StylePark, Jin-Woo, Ji-Hyeon Noh, Jong-Min Kim, Hwa-Young Lee, Kyoung-Ah Kim, and Ji-Young Park. 2022. "Gene Dose-Dependent and Additive Effects of ABCG2 rs2231142 and SLC2A9 rs3733591 Genetic Polymorphisms on Serum Uric Acid Levels" Metabolites 12, no. 12: 1192. https://doi.org/10.3390/metabo12121192
APA StylePark, J.-W., Noh, J.-H., Kim, J.-M., Lee, H.-Y., Kim, K.-A., & Park, J.-Y. (2022). Gene Dose-Dependent and Additive Effects of ABCG2 rs2231142 and SLC2A9 rs3733591 Genetic Polymorphisms on Serum Uric Acid Levels. Metabolites, 12(12), 1192. https://doi.org/10.3390/metabo12121192






