Polyphenol-Rich Leaf of Annona squamosa Stimulates Insulin Release from BRIN-BD11 Cells and Isolated Mouse Islets, Reduces (CH2O)n Digestion and Absorption, and Improves Glucose Tolerance and GLP-1 (7-36) Levels in High-Fat-Fed Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Plant Extracts
2.2. In Vitro Insulin-Releasing Studies
2.3. Membrane Potential and Intracellular Calcium Concentration ([Ca2+]i)
2.4. Glycation of Insulin
2.5. Cellular Glucose Uptake
2.6. In Vitro DPP-IV Enzyme Activity
2.7. Starch Digestion
2.8. In Vitro Glucose Diffusion
2.9. Animals
2.10. Oral Glucose Tolerance
2.11. In Vivo DPP-IV Enzyme Activity
2.12. Residual Gut Sucrose Content
2.13. Intestinal Glucose Absorption
2.14. Gastrointestinal Motility
2.15. Feeding Test
2.16. Crude Extract Purification
2.17. Mass Spectrometry Analysis
2.18. Statistical Analysis
3. Results
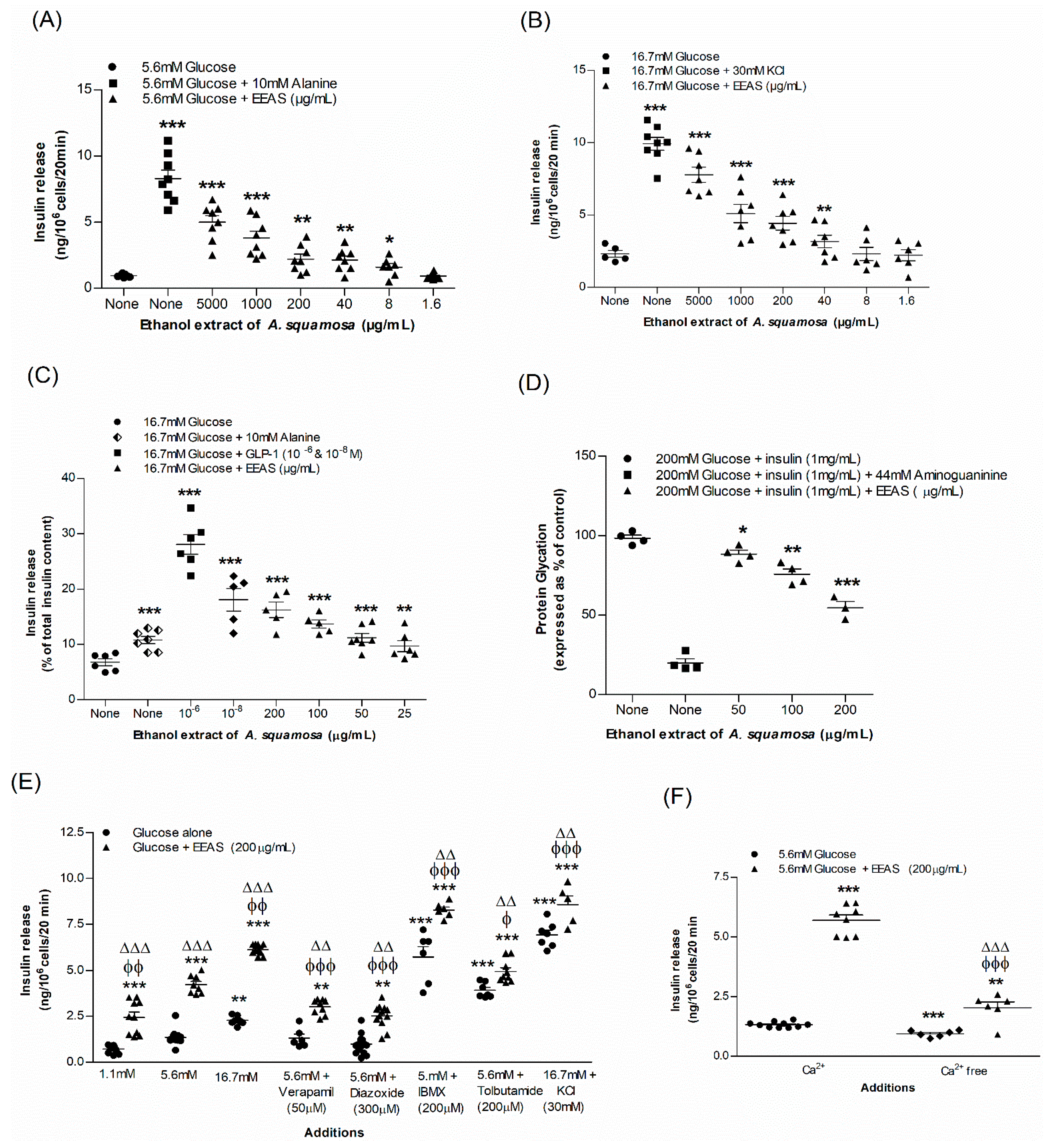
3.1. EEAS and Insulin Release from BRIN-BD11 Cells
3.2. EEAS and Glycation of Insulin
3.3. EEAS and Membrane Depolarisation and [Ca2+]i
3.4. EEAS and Glucose Uptake and Insulin Action
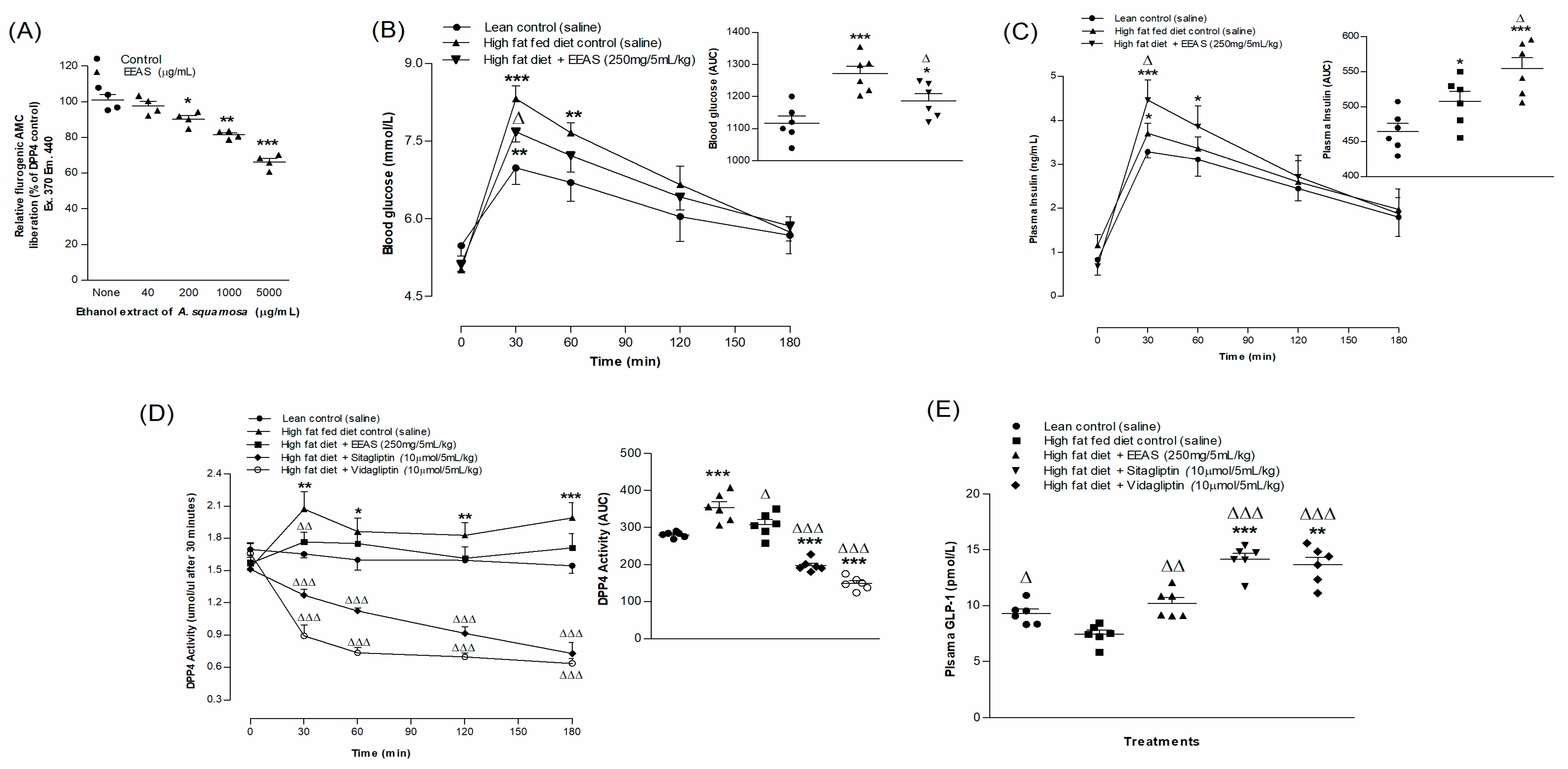
3.5. EEAS and Starch Digestion
3.6. EEAS and Glucose Diffusion In Vitro
3.7. EEAS and In Vitro DPP-IV Enzymatic Activity
3.8. EEAS and Oral Glucose Tolerance and Plasma Insulin, DPP-IV and Active GLP-1 (7-36) Levels
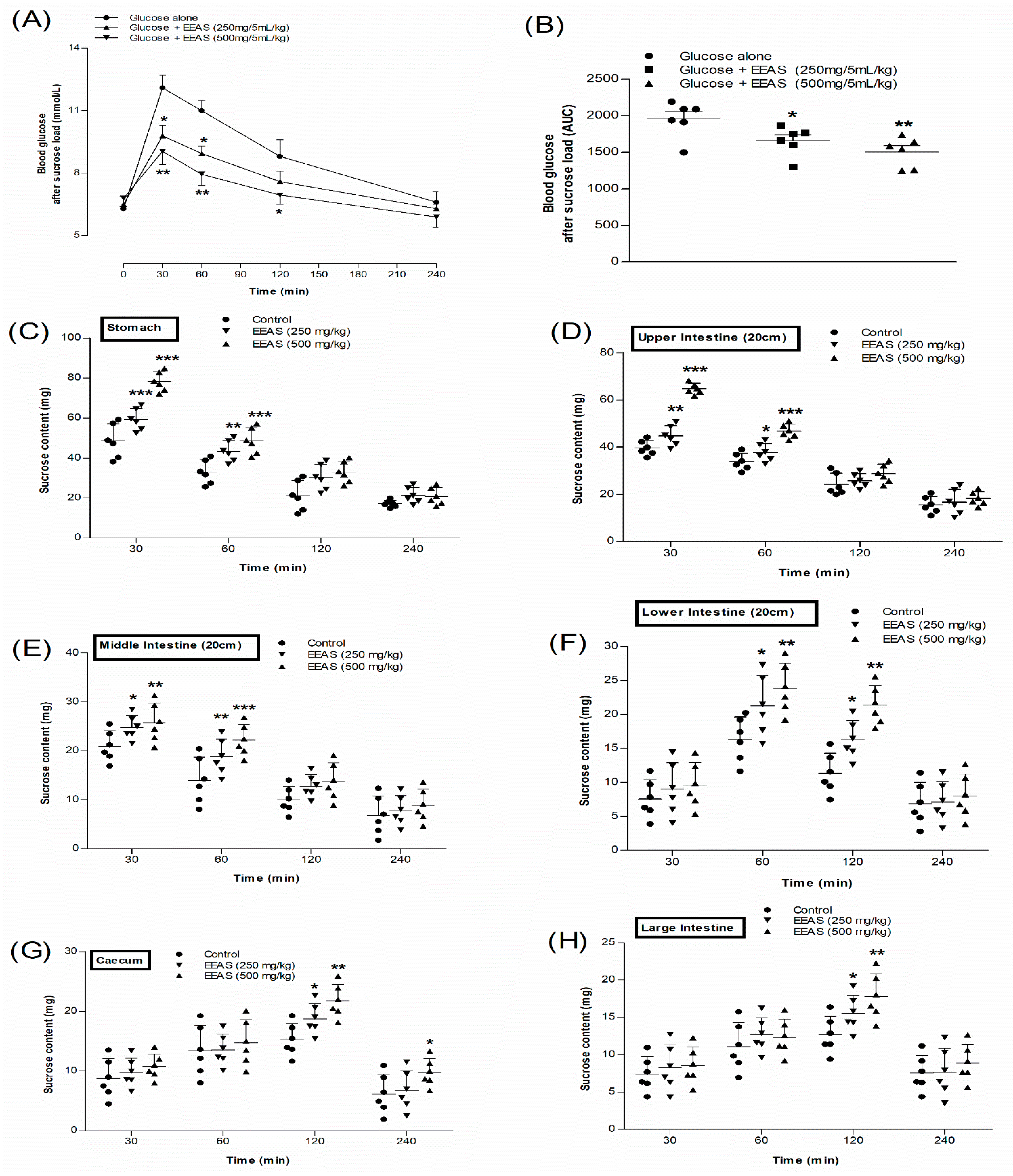
3.9. EEAS and Blood Glucose after Sucrose Load
3.10. EEAS and Residual Gut Sucrose Content
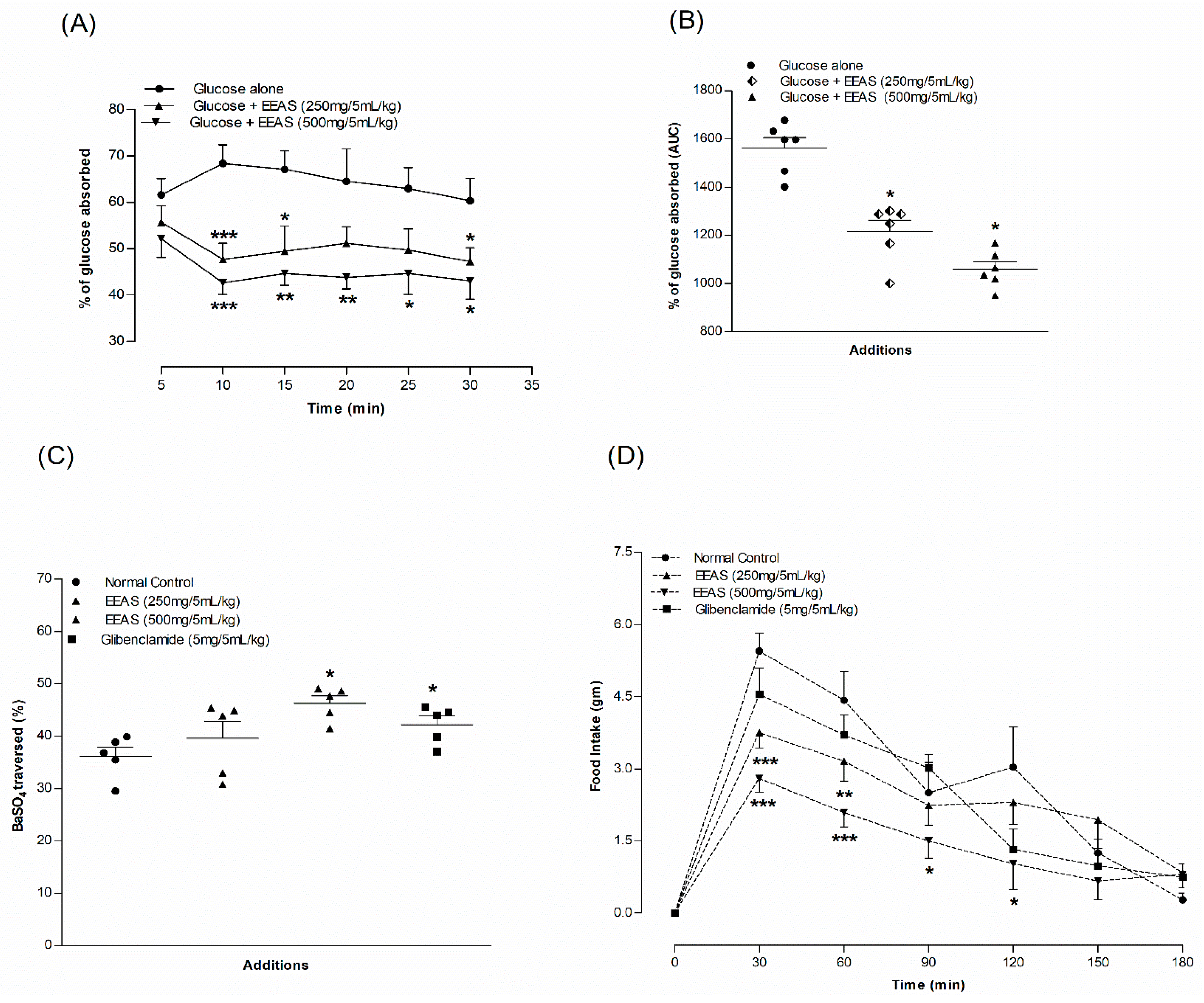
3.11. EEAS and Intestinal Gut Perfusion In Situ
3.12. EEAS and Gut Motility
3.13. EEAS and Feeding Test
3.14. Extract Purification and Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-NBDG | 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose |
| AGE | Advanced glycation end products |
| AWERB | Animal Welfare and Ethical Review Board |
| BaSO4 | Barium sulphate |
| cAMP | Cyclic adenosine monophosphate |
| CVD | Cardiovascular diseases |
| DPP-IV | Dipeptidyl peptidase IV |
| EEAS | Ethanol extract of Annona squamosa |
| FLIPR | Fluorometric imaging plate reader |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-1R | Glucagon-like peptide-1 receptor |
| GLUT4 | Glucose transporter type 4 |
| HFF | High-fat-fed |
| IBMX | 3-isobutyl-1-methylxanthine |
| IDF | International Diabetes Federation |
| KCl | Potassium chloride |
| LC-ESI-MS | Liquid chromatography–electrospray ionisation mass spectrometry |
| PI3 | Phosphatidylinositol |
| RP-HPLC | Reverse-phase high-performance liquid chromatography |
| SGLT-2 | Sodium–glucose cotransporter-2 |
| STZ | Streptozotocin |
| T2DM | Type 2 diabetes mellitus |
| UARC | University Ayurvedic Research Center |
References
- Bastaki, S. Diabetes mellitus and its treatment. Int. J. Diabetes Metab. 2005, 13, 111–134. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Distelmaier, K.; Lanza, I.R.; Irving, B.A.; Robinson, M.M.; Konopka, A.R.; Shulman, G.I.; Nair, K.S. Mechanism by Which Caloric Restriction Improves Insulin Sensitivity in Sedentary Obese Adults. Diabetes 2016, 65, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R.A. General aspects of diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Dyson, P.A.; Kelly, T.; Deakin, T.; Duncan, A.; Frost, G.; Harrison, Z.; Khatri, D.; Kunka, D.; McArdle, P.; Mellor, D.; et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet. Med. 2011, 28, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Azam, S.; Seidel, V.; Abdel-Wahab, Y.H.A. In vitro and in vivo antihyperglycemic activity of the ethanol extract of Heritiera fomes bark and characterization of pharmacologically active phytomolecules. J. Pharm. Pharmacol. 2022, 74, 415–425. [Google Scholar] [CrossRef]
- Bastin, M.; Andreelli, F. Dual GIP–GLP1-receptor agonists in the treatment of type 2 diabetes: A short review on emerging data and therapeutic potential. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Azam, S.; Hannan, J.M.A.; Flatt, P.R.; Wahab, Y.H.A. Anti-hyperglycaemic activity of H. rosa-sinensis leaves is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of insulin secretion. J. Ethnopharmacol. 2020, 253, 112647. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Evaluation of the antidiabetic and Insulin Releasing Effects of A. squamosa, Including Isolation and Characterization of Active Phytochemicals. Plants 2020, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Anti-hyperglycaemic and insulin-releasing effects of Camellia sinensis leaves and isolation and characterisation of active compounds. Brit. J. Nutr. 2021, 126, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Identification of Multiple Pancreatic and Extra-Pancreatic Pathways Underlying the Glucose-Lowering Actions of Acacia arabica Bark in Type-2 Diabetes and Isolation of Active Phytoconstituents. Plants 2021, 10, 1190. [Google Scholar] [CrossRef]
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H.A. Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.; Manjula, P.; Paari, N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013, 2, 196–200. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- Berglund, L.M.; Lyssenko, V.; Ladenvall, C.; Kotova, O.; Edsfeldt, A.; Pilgaard, K.; Alkayyali, S.; Brøns, C.; Forsblom, C.; Jonsson, A.; et al. Glucose-Dependent Insulinotropic Polypeptide Stimulates Osteopontin Expression in the Vasculature via Endothelin-1 and CREB. Diabetes 2015, 65, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Flatt, P.R. Dipeptidyl Peptidase IV (DPP IV) and Related Molecules in Type 2 Diabetes. Front. Biosci. 2008, 13, 3648. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Sinha, A.; Perween, Z. Important medicinal plants with their medicinal uses from Jharkhand State. Int. J. Res. Eng. Sci. Manag. 2020, 3, 532–542. [Google Scholar]
- Padmanabhan, P.; Paliyath, G. Annonaceous Fruits. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 169–173. [Google Scholar] [CrossRef]
- Bhat, R.; Paliyath, G. Fruits of Tropical Climates: Dietary Importance and Health Benefits. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 144–149. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Chakraverty, R. The pharmacological properties of Annona squamosa Linn: A Review. Int. J. Pharm. Eng. 2016, 4, 692–699. [Google Scholar]
- Pandey, N.; Barve, D. Phytochemical and pharmacological review on Annona squamosa Linn. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 1404–1412. [Google Scholar]
- Kaleem, M.; Asif, M.; Ahmed, Q.; Bano, B. Antidiabetic and antioxidant activity of Annona squamosa extract in streptozotocin-induced diabetic rats. Singap. Med. J. 2006, 47, 670. [Google Scholar]
- Hannan, J.M.A.; Ansari, P.; Azam, S.; Flatt, P.R.; Wahab, Y.H.A. Effects of Spirulina platensis on insulin secretion, dipeptidyl peptidase IV activity and both carbohydrate digestion and absorption indicate potential as an adjunctive therapy for diabetes. Brit. J. Nutr. 2020, 124, 1021–1034. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Insulinotropic and antidiabetic properties of Eucalyptus citriodora leaves and isolation of bioactive phytomolecules. J. Pharm. Pharmacol. 2021, 73, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Insulin secretory and antidiabetic actions of Heritiera fomes bark together with isolation of active phytomolecules. PLoS ONE 2022, 17, e0264632. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.M.; Flatt, P.R.; Duffy, G.; Abdel-Wahab, Y.H.A. The Effects of Traditional Antidiabetic Plants on in Vitro Glucose Diffusion. Nutr. Res. 2003, 23, 413–424. [Google Scholar] [CrossRef]
- Ansari, P.; Hannon-Fletcher, M.P.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Effects of 22 Traditional Anti-Diabetic Medicinal Plants on DPP-IV Enzyme Activity and Glucose Homeostasis in High-Fat Fed Obese Diabetic Rats. Biosci. Rep. 2021, 41, BSR20203824. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ali, L.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Antihyperglycaemic activity of Asparagus racemosus roots is partly mediated by inhibition of carbohydrate digestion and absorption, and enhancement of cellular insulin action. Brit. J. Nutr. 2012, 107, 1316–1323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hannan, J.M.A.; Ansari, P.; Haque, A.; Sanju, A.; Huzaifa, A.; Rahman, A.; Ghosh, A.; Azam, S. Nigella Sativa Stimulates Insulin Secretion from Isolated Rat Islets and Inhibits the Digestion and Absorption of (CH2O)N in the Gut. Biosci. Rep. 2019, 39, BSR20190723. [Google Scholar] [CrossRef] [PubMed]
- Hannan, J.M.A.; Marenah, L.; Ali, L.; Rokeya, B.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Insulin secretory actions of extracts of Asparagus racemosus root in perfused pancreas, isolated islets and clonal pancreatic β-cells. J. Endocrinol. 2007, 192, 159–168. [Google Scholar] [CrossRef]
- Azad, S.; Ansari, P.; Azam, S.; Hossain, S.; Shahid, M.I.-B.; Hasan, M.; Hannan, J.M.A. Anti-Hyperglycaemic Activity of Moringa Oleifera Is Partly Mediated by Carbohydrase Inhibition and Glucose-Fibre Binding. Biosci. Rep. 2017, 37, bsr20170059. [Google Scholar] [CrossRef] [PubMed]
- Hannan, J.M.A.; Ali, L.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Aqueous extracts of husks of Plantago ovata reduce hyperglycaemia in type 1 and type 2 diabetes by inhibition of intestinal glucose absorption. Brit. J. Nutr. 2006, 96, 131–137. [Google Scholar] [CrossRef]
- Amin, M.N. In-Vivo Evaluation of Anti-Onciceptive, Anti-Inflammatory, Antipyretic, Hypoxia and Gastro-Intestinal Potentials of SwasKas Chintamani Ras. Discov. Med. 2019, 6, 1. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kesari, A.N.; Diwakar, S.; Tyagi, A.; Tandon, V.; Chandra, R.; Watal, G. In vivo evaluation of anti-oxidant and anti-lipidimic potential of Annona squamosa aqueous extract in Type 2 diabetic models. J. Ethnopharmacol. 2008, 118, 21–25. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Rajendran, K.; Kumar, C.D.; Bodla, R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin–nicotinamide type 2 diabetic rats. J. Ethnopharmacol. 2004, 91, 171–175. [Google Scholar] [CrossRef]
- Sharma, A.; Chand, T.; Khardiya, M.; Yadav, K.C.; Mangal, R.; Sharma, A. Antidiabetic and antihyperlipidemic activity of Annona squamosa fruit peel in streptozotocin induced diabetic rats. Int. J. Toxicol. Pharmacol. 2013, 5, 15–21. [Google Scholar]
- Hannan, J.M.A.; Ali, L.; Rokeya, B.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Soluble Dietary Fibre Fraction OfTrigonella Foenum-Graecum(Fenugreek) Seed Improves Glucose Homeostasis in Animal Models of Type 1 and Type 2 Diabetes by Delaying Carbohydrate Digestion and Absorption, and Enhancing Insulin Action. Brit. J. Nutr. 2007, 97, 514–521. [Google Scholar] [CrossRef]
- Attiq, A.; Jalil, J.; Husain, K. Annonaceae: Breaking the wall of inflammation. Front. Pharmacol. 2017, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Billington, C.K.; Ojo, O.O.; Penn, R.B.; Ito, S. CAMP Regulation of Airway Smooth Muscle Function. Pulm. Pharmacol. Ther. 2013, 26, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chiang, S.-H.; Saltiel, A.R. Insulin signaling and the regulation of glucose transport. Mol. Med. 2004, 10, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.K.R.; Zierath, J.R. Insulin Signaling and Glucose Transport in Insulin Resistant Human Skeletal Muscle. Cell Biochem. Biophys. 2007, 48, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Lee, L.-C.; Hu, K.-Y.; Tsai, W.-J.; Huang, C.; Tsay, H.-J.; Liu, H.-K. The application of post-translational modification oriented serum proteomics to assess experimental diabetes with complications. PLoS ONE 2018, 13, e0206509. [Google Scholar] [CrossRef] [PubMed]
- Flatt, P.R.; Abdel-Wahab, Y.H.A.; Boyd, A.C.; Barnett, C.R.; O’Harte, F.P. Pancreatic B-cell dysfunction and glucose toxicity in non-insulin-dependent diabetes. Proc. Nutr. Soc. 1997, 56, 243–262. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J. Advanced Glycation End Products (AGE) and Diabetes: Cause, Effect, or Both? Curr. Diabetes Rep. 2013, 14, 453. [Google Scholar] [CrossRef]
- Abdel-Wahab, Y.H.A.; Marenah, L.; Flatt, P.R.; Conlon, J.M. Insulin releasing properties of the temporin family of antimicrobial peptides. Protein Pept. Lett. 2007, 14, 702–707. [Google Scholar] [CrossRef]
- Das, S.; Das, S.; De, B. In Vitro Inhibition of Key Enzymes Related to Diabetes by the Aqueous Extracts of Some Fruits of West Bengal, India. Curr. Res. Nutr. Food Sci. 2012, 8, 19–24. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kesari, A.N.; Watal, G.; Murthy, P.S.; Chandra, R.; Maithal, K.; Tandon, V. Hypoglycemic and Antidiabetic Effect of Aqueous Extract of Leaves of Annona squamosa (L.) in Experimental Animal. Curr. Sci. 2005, 88, 8. [Google Scholar]
- Ding, Y.; Zhang, Z.; Dai, X.; Jiang, Y.; Bao, L.; Li, Y.; Li, Y. Grape Seed Proanthocyanidins Ameliorate Pancreatic Beta-Cell Dysfunction and Death in Low-Dose Streptozotocin- and High-Carbohydrate/High-Fat Diet-Induced Diabetic Rats Partially by Regulating Endoplasmic Reticulum Stress. Nutr. Metab. 2013, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int. J. Clin. Pract. 2006, 60, 1454–1470. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, A.; Yasutake, Y.; Gao, J.; Matsuda, M.; Takahashi, I.; Takeuchi, H.; Hirata, M. Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS ONE 2013, 8, e57375. [Google Scholar] [CrossRef]
- Zander, M.; Madsbad, S.; Madsen, J.L.; Holst, J.J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: A parallel-group study. Lancet 2002, 359, 824–830. [Google Scholar] [CrossRef]
- Deacon, C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.-T.; Yin, Y.-C.; Xing, S.; Li, W.-N.; Fu, X.-Q. Hypoglycemic effect and mechanism of isoquercitrin as an inhibitor of dipeptidyl peptidase-4 in type 2 diabetic mice. RSC Adv. 2018, 8, 14967–14974. [Google Scholar] [CrossRef]
- Kashiwada, M.; Nakaishi, S.; Usuda, A.; Miyahara, Y.; Katsumoto, K.; Katsura, K.; Terakado, I.; Jindo, M.; Nakajima, S.; Ogawa, S.; et al. Analysis of Anti-Obesity and Anti-Diabetic Effects of Acacia Bark-Derived Proanthocyanidins in Type 2 Diabetes Model KKAy Mice. J. Nat. Med. 2021, 75, 893–906. [Google Scholar] [CrossRef]
- Gupta, A.; Jacobson, G.A.; Burgess, J.R.; Jelinek, H.F.; Nichols, D.S.; Narkowicz, C.K.; Al-Aubaidy, H.A. Citrus Bioflavonoids Dipeptidyl Peptidase-4 Inhibition Compared with Gliptin Antidiabetic Medications. Biochem. Biophys. Res. Commun. 2018, 503, 21–25. [Google Scholar] [CrossRef]
- Mathijs, I.; Da Cunha, D.A.; Himpe, E.; Ladriere, L.; Chellan, N.; Roux, C.R.; Joubert, E.; Muller, C.; Cnop, M.; Louw, J.; et al. Phenylpropenoic acid glucoside augments pancreatic beta cell mass in high-fat diet-fed mice and protects beta cells from ER stress-induced apoptosis. Mol. Nutr. Food Res. 2014, 58, 1980–1990. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Moneim, A.A.; Yazid, I.A.; Mahmoud, A.M. Antihyperglycemic, Antihyperlipidemic and Antioxidant Effects and the Probable Mechanisms of Action of Ruta Graveolens Infusion and Rutin in Nicotinamide-Streptozotocin-Induced Diabetic Rats. Diabetol. Croat. 2010, 39, 15–35. [Google Scholar]
- Müller, M.; Canfora, E.E.; Blaak, E.E. Gastrointestinal Transit Time, Glucose Homeostasis and Metabolic Health: Modulation by Dietary Fibers. Nutrients 2018, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Mujahid, M.; Singh, P.K.; Farooqui, S.; Singh, K.; Parveen, S.; Arif, M. Annona squamosa linn. (Custard apple): An aromatic medicinal plant fruit with immense nutraceutical and therapeutic potentials (Review). Int. J. Pharm. Sci. Res. 2018, 9, 1745–1759. [Google Scholar]
- Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Sasi, M.; Maheshwari, C.; Singh, S.; Dhumal, S.; et al. Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Biological Activities. Biomolecules 2021, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Barhate, C.; Biyani, S.; Kulkarni, S.; Nagarsenker, M. Quantitative Analysis of Flavonoids in Annona squamosa Leaf Extracts and Its Pellet Formulation by Validated High-Performance Thin-Layer Chromatographic Technique. J. Planar Chromatogr. Mod. TLC 2011, 24, 306–311. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Yang, Y.-L.; Chen, M.; Chang, F.-R.; Chen, S.-L.; Wu, S.-H.; Wu, Y.-C. Mono-Tetrahydrofuran Annonaceous Acetogenins from Annona Squamosa as Cytotoxic Agents and Calcium Ion Chelators. J. Nat. Prod. 2008, 71, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chai, W.-M.; Yang, Q.; Wang, R.; Peng, Y. Novel Insights into the Inhibitory Effect and Mechanism of Proanthocyanidins from Pyracantha fortuneana Fruit on α-Glucosidase. J. Food Sci. 2017, 82, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative Evaluation of Quercetin, Isoquercetin and Rutin as Inhibitors of Alpha-Glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.; Zohari, F.; Sadeghi, H. Antioxidant and Protective Effects of Major Flavonoids from Teulimacrium polium on β-Cell Destruction in a Model of Streptozotocin-Induced Diabetes. Planta Med. 2009, 75, 1418–1420. [Google Scholar] [CrossRef]
- Yokozawa, T.; Cho, E.J.; Park, C.H.; Kim, J.H. Protective Effect of Proanthocyanidin against Diabetic Oxidative Stress. Evid.-Based Complement. Altern. Med. 2011, 2012, e623879. [Google Scholar] [CrossRef] [PubMed]
- Neske, A.; Ruiz Hidalgo, J.; Cabedo, N.; Cortes, D. Acetogenins from Annonaceae Family. Their Potential Biological Applications. Phytochemistry 2020, 174, 112332. [Google Scholar] [CrossRef]
- Lima, L.A.R.S.; Pimenta, L.P.S.; Boaventura, M.A.D. Acetogenins from Annona Cornifolia and Their Antioxidant Ca pacity. Food Chem. 2010, 122, 1129–1138. [Google Scholar] [CrossRef]


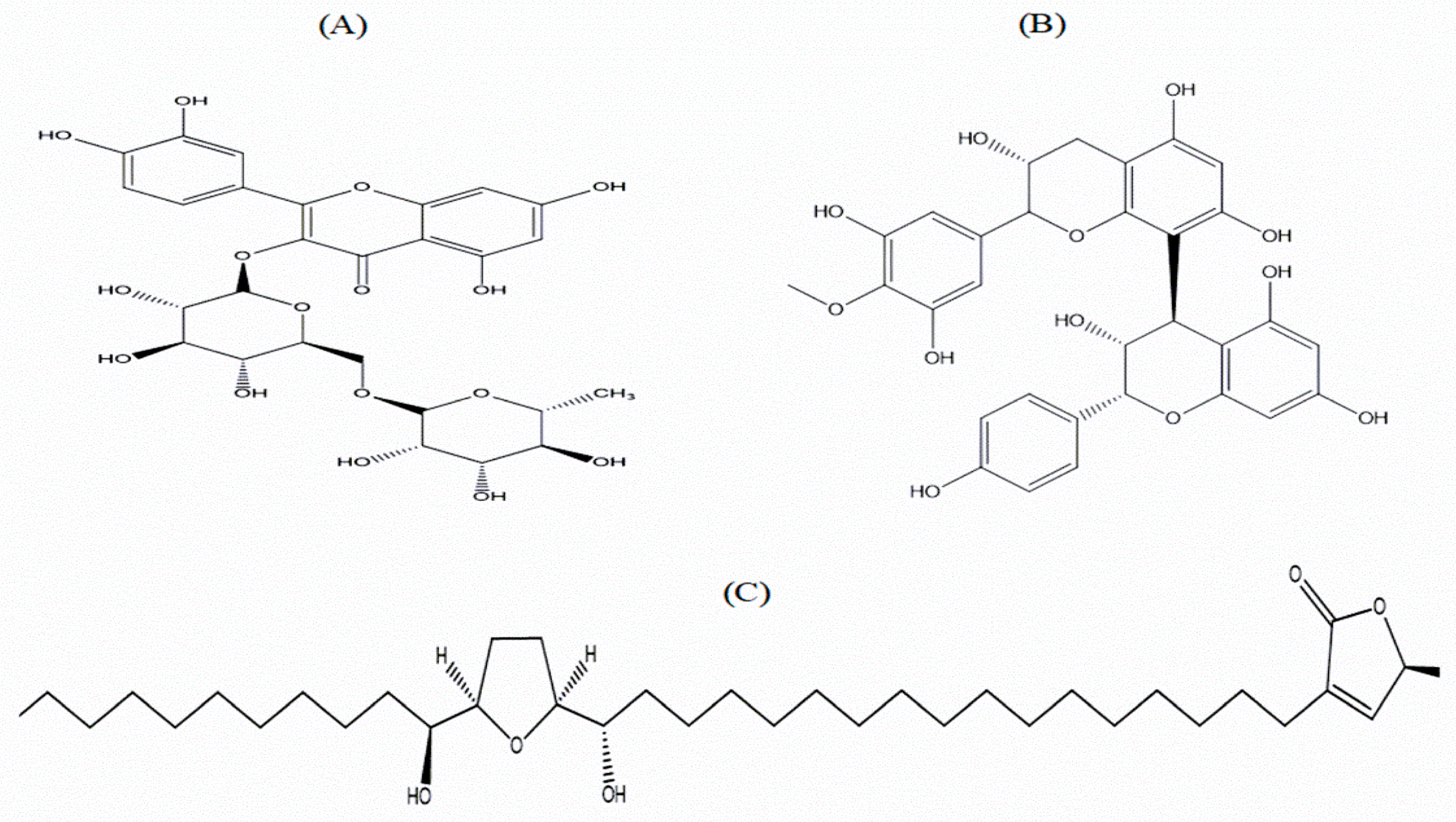
| Peak Samples | Retention Time (Min) | Theoretical Molecular Weight (Da) | Found Molecular Weight (Da) | Predicted Compounds |
|---|---|---|---|---|
| P1 | 5 | - | - | Not determined |
| P2 | 10.3 | - | - | Not determined |
| P3 | 11.8 | - | - | Not determined |
| P4 | 13 | - | - | Not determined |
| P5 | 14 | - | 741.4 | Unknown |
| P6 | 15 | 610.5 | 609.3 | Rutin |
| P7 | 15.6 | 592.9 | 592.3 | Squafosacin G |
| P8 | 16 | 592.5 | 592.0 | Proanthocyanidin |
| P9 | 16.5 | - | - | Not determined |
| P10 | 20 | - | - | Not determined |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, P.; Hannan, J.M.A.; Seidel, V.; Abdel-Wahab, Y.H.A. Polyphenol-Rich Leaf of Annona squamosa Stimulates Insulin Release from BRIN-BD11 Cells and Isolated Mouse Islets, Reduces (CH2O)n Digestion and Absorption, and Improves Glucose Tolerance and GLP-1 (7-36) Levels in High-Fat-Fed Rats. Metabolites 2022, 12, 995. https://doi.org/10.3390/metabo12100995
Ansari P, Hannan JMA, Seidel V, Abdel-Wahab YHA. Polyphenol-Rich Leaf of Annona squamosa Stimulates Insulin Release from BRIN-BD11 Cells and Isolated Mouse Islets, Reduces (CH2O)n Digestion and Absorption, and Improves Glucose Tolerance and GLP-1 (7-36) Levels in High-Fat-Fed Rats. Metabolites. 2022; 12(10):995. https://doi.org/10.3390/metabo12100995
Chicago/Turabian StyleAnsari, Prawej, J.M.A. Hannan, Veronique Seidel, and Yasser H.A. Abdel-Wahab. 2022. "Polyphenol-Rich Leaf of Annona squamosa Stimulates Insulin Release from BRIN-BD11 Cells and Isolated Mouse Islets, Reduces (CH2O)n Digestion and Absorption, and Improves Glucose Tolerance and GLP-1 (7-36) Levels in High-Fat-Fed Rats" Metabolites 12, no. 10: 995. https://doi.org/10.3390/metabo12100995
APA StyleAnsari, P., Hannan, J. M. A., Seidel, V., & Abdel-Wahab, Y. H. A. (2022). Polyphenol-Rich Leaf of Annona squamosa Stimulates Insulin Release from BRIN-BD11 Cells and Isolated Mouse Islets, Reduces (CH2O)n Digestion and Absorption, and Improves Glucose Tolerance and GLP-1 (7-36) Levels in High-Fat-Fed Rats. Metabolites, 12(10), 995. https://doi.org/10.3390/metabo12100995









