The Effects of Exercise Training on Glucose Homeostasis and Muscle Metabolism in Type 1 Diabetic Female Mice
Abstract
1. Introduction
2. Results
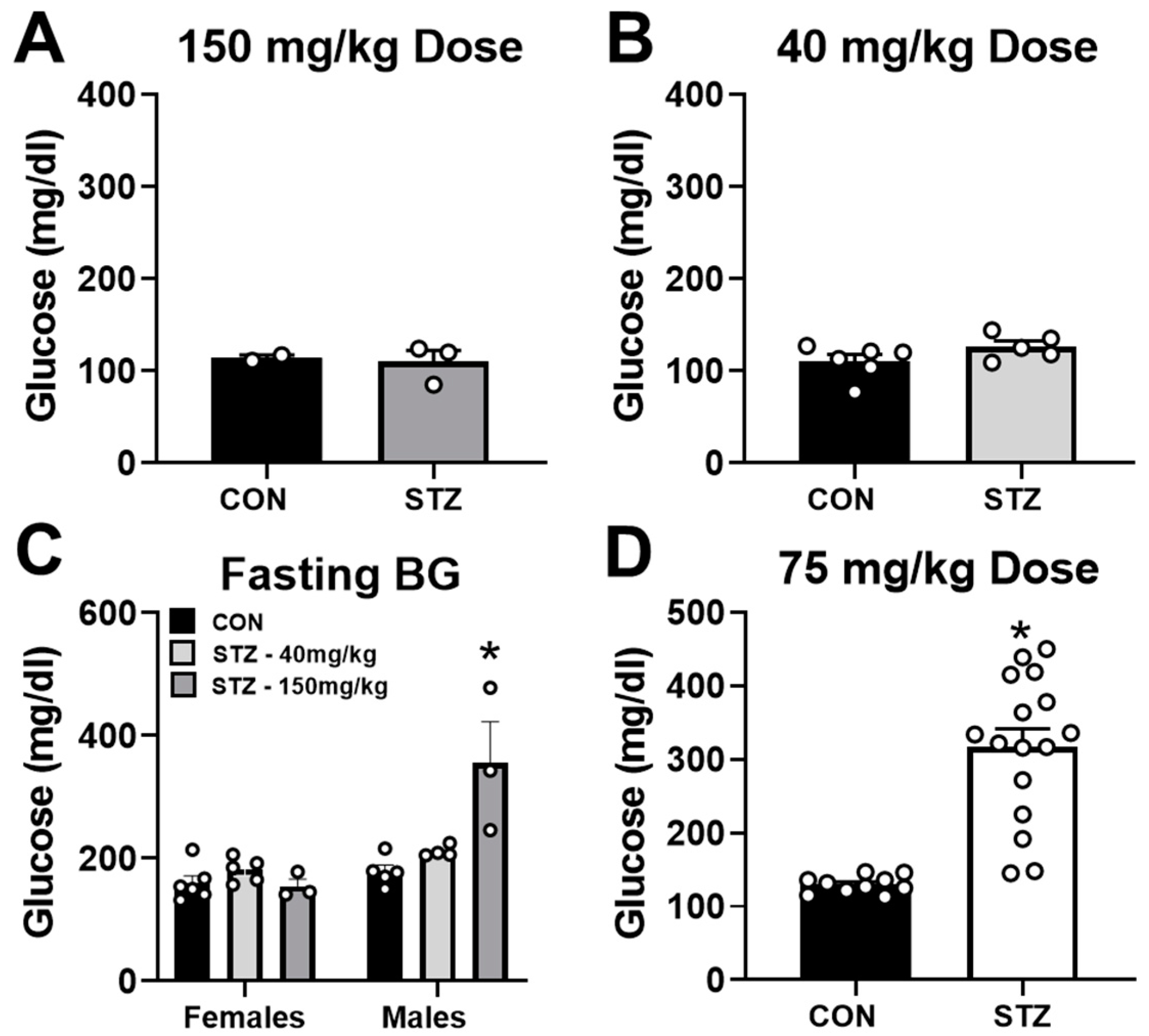
2.1. Streptozotocin Dose of 75 mg/kg Induces T1D in Female Mice
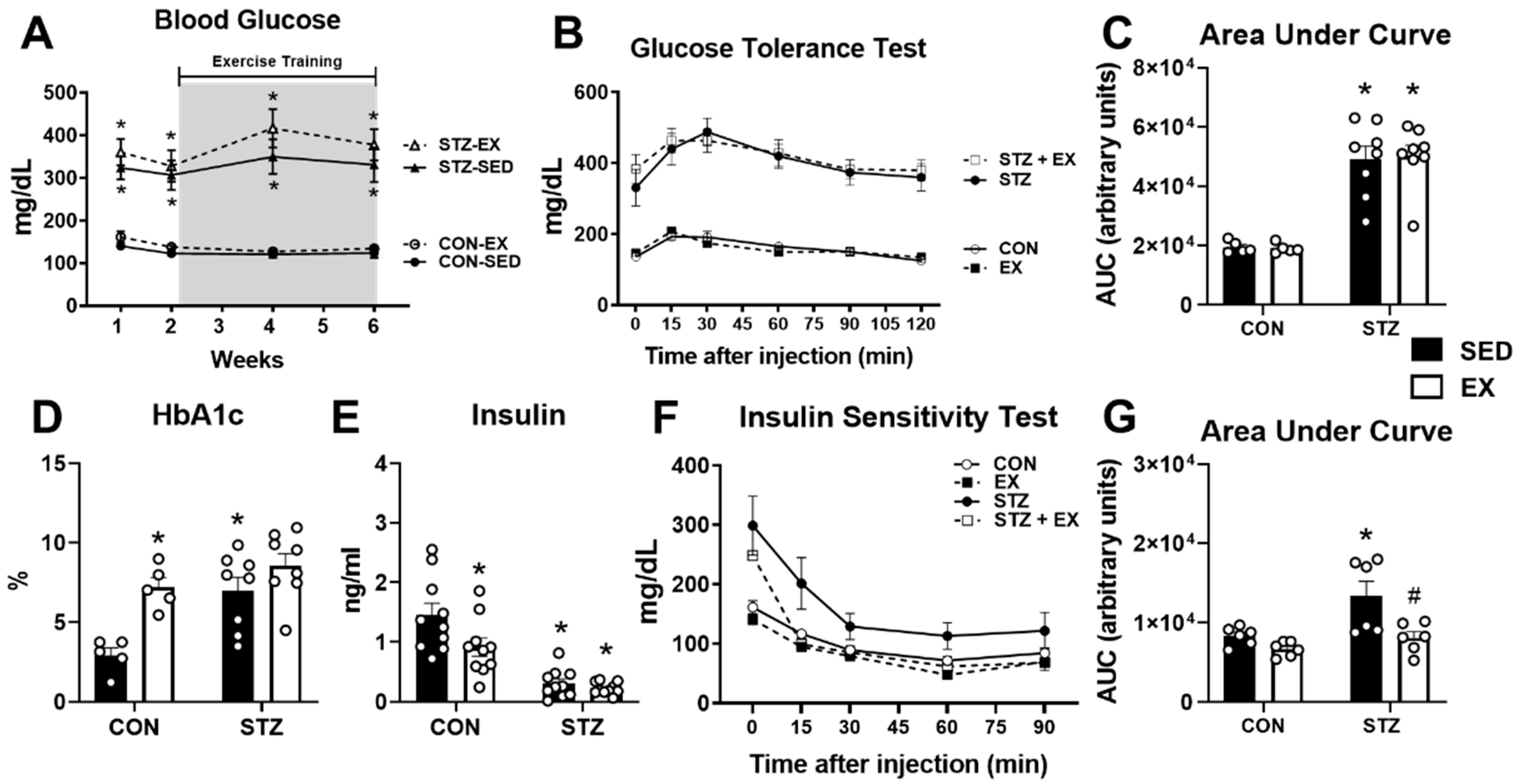
2.2. Exercise Training Does Not Improve Glucose Homeostasis in T1D Female Mice
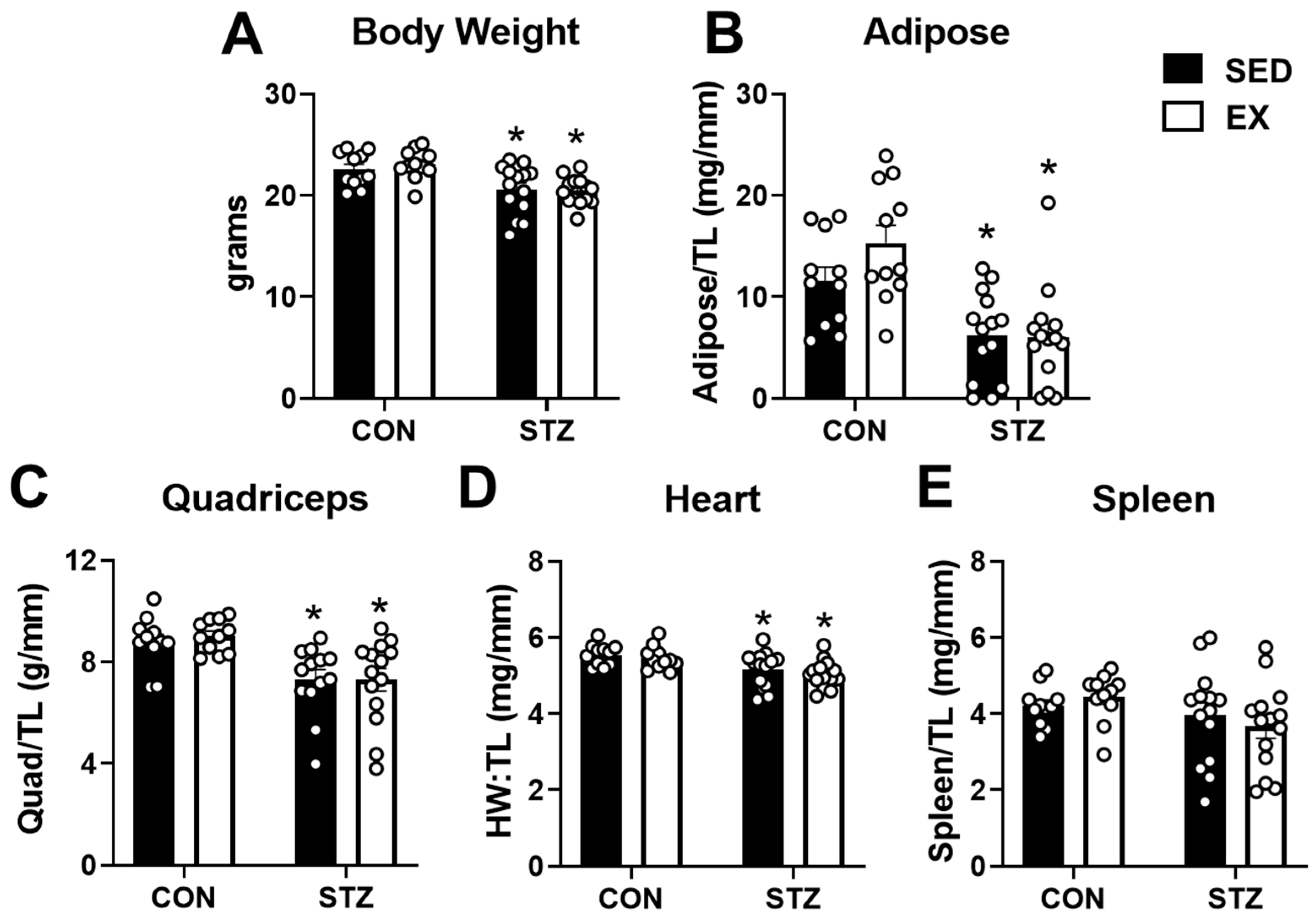
2.3. Exercise Training Does Not Protect against the Negative Effects of T1D
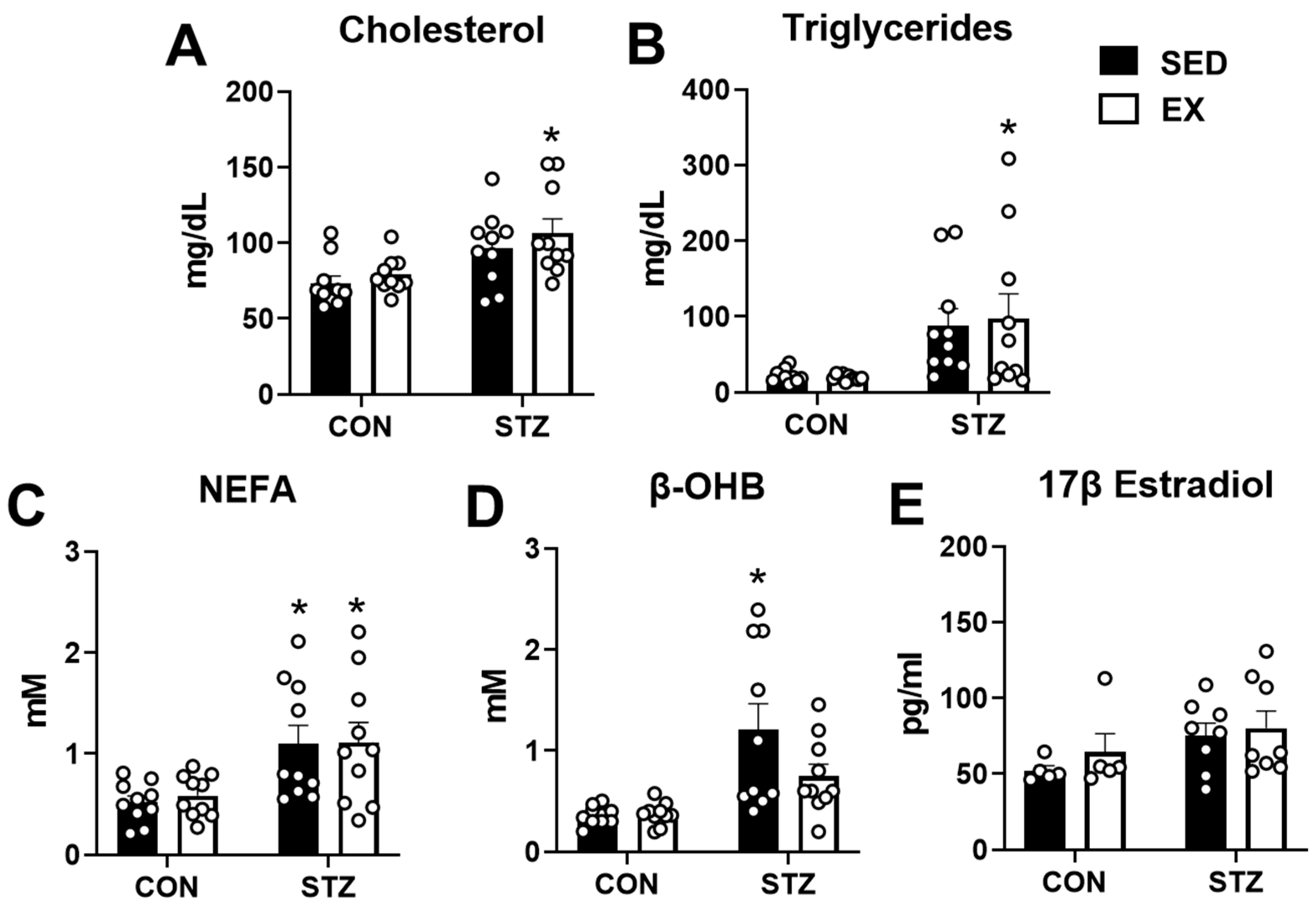
2.4. Exercise Training Does Not Prevent Dyslipidemia in T1D
2.5. Exercise Training Prevents Depletion of Triacylglycerol Content in T1D Skeletal Muscle
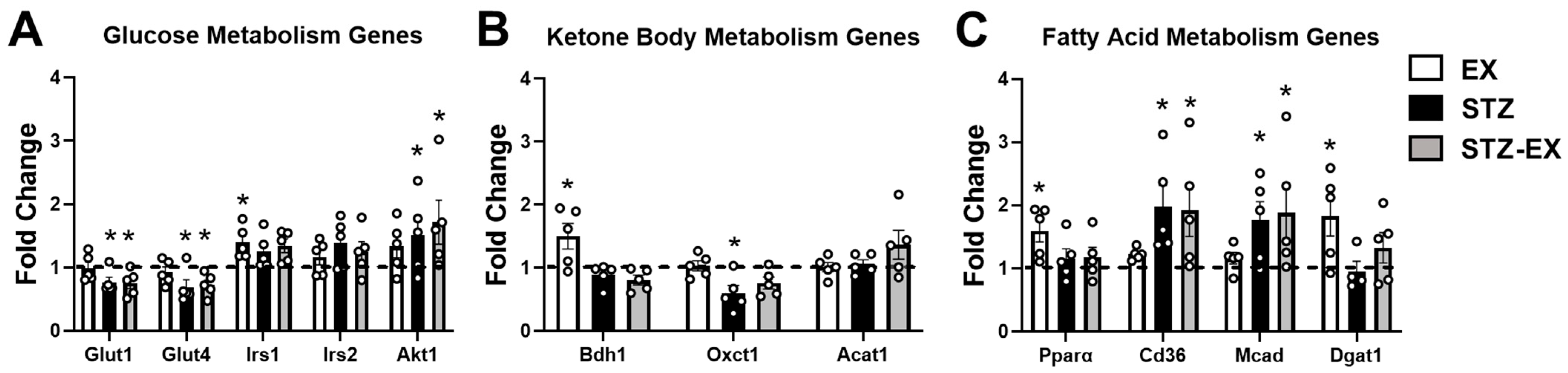
2.6. Exercise Training Does Not Prevent Altered Gene Expression in Female T1D Mice
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Streptozotocin Injections
4.3. Blood Glucose Assessment
4.4. Exercise Training Protocol
4.5. Glucose Tolerance Test
4.6. Insulin Sensitivity Tests
4.7. Tissue Harvest
4.8. Serum Analysis
4.9. Tissue Analysis
4.10. Gene Expression Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Peters, S.A.; Mishra, G.D.; Woodward, M. Risk of All-Cause Mortality and Vascular Events in Women Versus Men with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Lancet Diabetes Endocrinol. 2015, 3, 198–206. [Google Scholar] [CrossRef]
- Manicardi, V.; Russo, G.; Napoli, A.; Torlone, E.; Volsi, P.L.; Giorda, C.B.; Musacchio, N.; Nicolucci, A.; Suraci, C.; Lucisano, G.; et al. Gender-Disparities in Adults with Type 1 Diabetes: More Than a Quality of Care Issue. A Cross-Sectional Observational Study from the Amd Annals Initiative. PLoS ONE 2016, 11, e0162960. [Google Scholar] [CrossRef] [PubMed]
- Millstein, R.J.; Pyle, L.L.; Bergman, B.C.; Eckel, R.H.; Maahs, D.M.; Rewers, M.J.; Schauer, I.E.; Snell-Bergeon, J.K. Sex-Specific Differences in Insulin Resistance in Type 1 Diabetes: The Cacti Cohort. J. Diabetes Complicat. 2018, 32, 418–423. [Google Scholar] [CrossRef]
- Monaco, C.M.F.; Bellissimo, C.A.; Hughes, M.C.; Ramos, S.V.; Laham, R.; Perry, C.G.R.; Hawke, T.J. Sexual Dimorphism in Human Skeletal Muscle Mitochondrial Bioenergetics in Response to Type 1 Diabetes. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E44–E51. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.M.F.; Tarnopolsky, M.A.; Dial, A.G.; Nederveen, J.P.; Rebalka, I.A.; Nguyen, M.; Turner, L.V.; Perry, C.G.R.; Ljubicic, V.; Hawke, T.J. Normal to Enhanced Intrinsic Mitochondrial Respiration in Skeletal Muscle of Middle- to Older-Aged Women and Men with Uncomplicated Type 1 Diabetes. Diabetologia 2021, 64, 2517–2533. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.T.; Bieuzen, F.; Bleakley, C.M. Where Are All the Female Participants in Sports and Exercise Medicine Research? Eur. J. Sport. Sci. 2014, 14, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Zucker, I. Sex Bias in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Tian, R. Glucose Transporters in Cardiac Metabolism and Hypertrophy. Compr. Physiol. 2015, 6, 331–351. [Google Scholar] [CrossRef]
- Zisman, A.; Peroni, O.D.; Abel, E.D.; Michael, M.D.; Mauvais-Jarvis, F.; Lowell, B.B.; Wojtaszewski, J.F.; Hirshman, M.F.; Virkamaki, A.; Goodyear, L.J.; et al. Targeted Disruption of the Glucose Transporter 4 Selectively in Muscle Causes Insulin Resistance and Glucose Intolerance. Nat. Med. 2000, 6, 924–928. [Google Scholar] [CrossRef]
- Bryant, N.J.; Govers, R.; James, D.E. Regulated Transport of the Glucose Transporter Glut4. Nat. Rev. Mol. Cell Biol. 2002, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Kurth-Kraczek, E.J.; Hirshman, M.F.; Goodyear, L.J.; Winder, W.W. 5′ Amp-Activated Protein Kinase Activation Causes Glut4 Translocation in Skeletal Muscle. Diabetes 1999, 48, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, Glut4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Teupe, B.; Bergis, K. Epidemiological Evidence for “Double Diabetes”. Lancet 1991, 337, 361–362. [Google Scholar] [CrossRef]
- Cleland, S.J.; Fisher, B.M.; Colhoun, H.M.; Sattar, N.; Petrie, J.R. Insulin Resistance in Type 1 Diabetes: What Is ‘Double Diabetes’ and What Are the Risks? Diabetologia 2013, 56, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.A.; Durstine, J.L.; Roberts, C.K.; Barnard, R.J. Impact of Diet and Exercise on Lipid Management in the Modern Era. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Ostman, C.; Jewiss, D.; King, N.; Smart, N.A. Clinical Outcomes to Exercise Training in Type 1 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2018, 139, 380–391. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.K.; da Silva, S.; Serafini, E.; de Souza, D.R.; Farias, H.R.; Silveira, G.d.; Silveira, P.C.; de Souza, C.T.; Portela, L.V.; Muller, A.P. Prior Exercise Training Prevent Hyperglycemia in Stz Mice by Increasing Hepatic Glycogen and Mitochondrial Function on Skeletal Muscle. J. Cell Biochem. 2017, 118, 678–685. [Google Scholar] [CrossRef]
- Dotzert, M.S.; McDonald, M.W.; Murray, M.R.; Nickels, J.Z.; Noble, E.G.; Melling, C.W.J. Effect of Combined Exercise Versus Aerobic-Only Training on Skeletal Muscle Lipid Metabolism in a Rodent Model of Type 1 Diabetes. Can. J. Diabetes 2018, 42, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Dotzert, M.S.; Murray, M.R.; McDonald, M.W.; Olver, T.D.; Velenosi, T.J.; Hennop, A.; Noble, E.G.; Urquhart, B.L.; Melling, C.W. Metabolomic Response of Skeletal Muscle to Aerobic Exercise Training in Insulin Resistant Type 1 Diabetic Rats. Sci. Rep. 2016, 6, 26379. [Google Scholar] [CrossRef]
- Hall, K.E.; McDonald, M.W.; Grise, K.N.; Campos, O.A.; Noble, E.G.; Melling, C.W. The Role of Resistance and Aerobic Exercise Training on Insulin Sensitivity Measures in Stz-Induced Type 1 Diabetic Rodents. Metabolism 2013, 62, 1485–1494. [Google Scholar] [CrossRef]
- Huang, H.H.; Farmer, K.; Windscheffel, J.; Yost, K.; Power, M.; Wright, D.E.; Stehno-Bittel, L. Exercise Increases Insulin Content and Basal Secretion in Pancreatic Islets in Type 1 Diabetic Mice. Exp. Diabetes Res. 2011, 2011, 481427. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.R.; Seo, D.Y.; Park, S.H.; Kwak, H.B.; Kim, M.; Ko, K.S.; Rhee, B.D.; Han, J. Aerobic Exercise Training Decreases Cereblon and Increases Ampk Signaling in the Skeletal Muscle of Stz-Induced Diabetic Rats. Biochem. Biophys. Res. Commun. 2018, 501, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Day, S.; Wu, W.; Mason, R.; Rochon, P.A. Measuring the Data Gap: Inclusion of Sex and Gender Reporting in Diabetes Research. Res. Integr. Peer Rev. 2019, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Yardley, J.E.; Brockman, N.K.; Bracken, R.M. Could Age, Sex and Physical Fitness Affect Blood Glucose Responses to Exercise in Type 1 Diabetes? Front. Endocrinol. 2018, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Konhilas, J.P.; Maass, A.H.; Luckey, S.W.; Stauffer, B.L.; Olson, E.N.; Leinwand, L.A. Sex Modifies Exercise and Cardiac Adaptation in Mice. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2768-76. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, L.E.; Rowe, P.; O’Neill, C.C.; DeWitt, E.A.; Kolwicz, S.C., Jr. Sex Differences in Endurance Exercise Capacity and Skeletal Muscle Lipid Metabolism in Mice. Physiol. Rep. 2022, 10, e15174. [Google Scholar] [CrossRef]
- Saadane, A.; Lessieur, E.M.; Du, Y.; Liu, H.; Kern, T.S. Successful Induction of Diabetes in Mice Demonstrates No Gender Difference in Development of Early Diabetic Retinopathy. PLoS ONE 2020, 15, e0238727. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Chicco, A.J.; Jew, K.N.; Johnson, M.S.; Lynch, J.M.; Watson, P.A.; Moore, R.L. Cardioprotection Afforded by Chronic Exercise Is Mediated by the Sarcolemmal, and Not the Mitochondrial, Isoform of the Katp Channel in the Rat. J. Physiol. 2005, 569, 913–924. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex Differences in Lipid and Lipoprotein Metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex Differences in Metabolic Regulation and Diabetes Susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]
- Kolb, H. Mouse Models of Insulin Dependent Diabetes: Low-Dose Streptozocin-Induced Diabetes and Nonobese Diabetic (Nod) Mice. Diabetes Metab. Rev. 1987, 3, 751–778. [Google Scholar] [CrossRef]
- Le May, C.; Chu, K.; Hu, M.; Ortega, C.S.; Simpson, E.R.; Korach, K.S.; Tsai, M.J.; Mauvais-Jarvis, F. Estrogens Protect Pancreatic Beta-Cells from Apoptosis and Prevent Insulin-Deficient Diabetes Mellitus in Mice. Proc. Natl. Acad. Sci. USA 2006, 103, 9232–9237. [Google Scholar] [CrossRef]
- Luo, J.; Sobkiw, C.L.; Hirshman, M.F.; Logsdon, M.N.; Li, T.Q.; Goodyear, L.J.; Cantley, L.C. Loss of Class Ia Pi3k Signaling in Muscle Leads to Impaired Muscle Growth, Insulin Response, and Hyperlipidemia. Cell Metab. 2006, 3, 355–366. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.T.; Lee, K.Y.; Klaus, K.; Softic, S.; Krumpoch, M.T.; Fentz, J.; Stanford, K.I.; Robinson, M.M.; Cai, W.; Kleinridders, A.; et al. Insulin and Igf-1 Receptors Regulate Foxo-Mediated Signaling in Muscle Proteostasis. J. Clin. Investig. 2016, 126, 3433–3446. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; de Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive Loss of Skeletal Muscle Mass in Older Adults with Type 2 Diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Accelerated Loss of Skeletal Muscle Strength in Older Adults with Type 2 Diabetes: The Health, Aging, and Body Composition Study. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.J. Signalling Pathways That Mediate Skeletal Muscle Hypertrophy and Atrophy. Nat. Cell Biol. 2003, 5, 87–90. [Google Scholar] [CrossRef]
- Bassel-Duby, R.; Olson, E.N. Signaling Pathways in Skeletal Muscle Remodeling. Annu. Rev. Biochem. 2006, 75, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Dekel, Y.; Glucksam, Y.; Elron-Gross, I.; Margalit, R. Insights into Modeling Streptozotocin-Induced Diabetes in Icr Mice. Lab. Anim. 2009, 38, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.C.; Khurana, M.; Ryan, E.A.; Finegood, D.T. Gender Differences in the Metabolic Response to Graded Numbers of Transplanted Islets of Langerhans. Endocrinology 1994, 135, 2681–2687. [Google Scholar] [CrossRef]
- Cortright, R.N.; Collins, H.L.; Chandler, M.P.; Lemon, P.W.; DiCarlo, S.E. Diabetes Reduces Growth and Body Composition More in Male Than in Female Rats. Physiol. Behav. 1996, 60, 1233–1238. [Google Scholar] [CrossRef]
- Harvey, K.L.; Holcomb, L.E.; Kolwicz, S.C., Jr. Ketogenic Diets and Exercise Performance. Nutrients 2019, 11, 2296. [Google Scholar] [CrossRef]
- Goldberg, I.J. Clinical Review 124: Diabetic Dyslipidemia: Causes and Consequences. J. Clin. Endocrinol. Metab. 2001, 86, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H.; Hordern, M.D.; Miller, T.; Chyun, D.A.; Bertoni, A.G.; Blumenthal, R.S.; Philippides, G.; Rocchini, A. Exercise Training for Type 2 Diabetes Mellitus: Impact on Cardiovascular Risk: A Scientific Statement from the American Heart Association. Circulation 2009, 119, 3244–3262. [Google Scholar] [CrossRef] [PubMed]
- Verges, B. Dyslipidemia in Type 1 Diabetes: Amaskeddanger. Trends Endocrinol. Metab. 2020, 31, 422–434. [Google Scholar] [CrossRef]
- Satoh, M.; Mioh, H.; Shiotsu, Y.; Ogawa, Y.; Tamaoki, T. Mouse Bone Marrow Stromal Cell Line Mc3t3-G2/Pa6 with Hematopoietic-Supporting Activity Expresses High Levels of Stem Cell Antigen Sca-1. Exp. Hematol. 1997, 25, 972–979. [Google Scholar] [PubMed]
- Fischer, Y.; Thomas, J.; Sevilla, L.; Munoz, P.; Becker, C.; Holman, G.; Kozka, I.J.; Palacin, M.; Testar, X.; Kammermeier, H.; et al. Insulin-Induced Recruitment of Glucose Transporter 4 (Glut4) and Glut1 in Isolated Rat Cardiac Myocytes. Evidence of the Existence of Different Intracellular Glut4 Vesicle Populations. J. Biol. Chem. 1997, 272, 7085–7092. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Kayano, T.; Buse, J.B.; Edwards, Y.; Pilch, P.F.; Bell, G.I.; Seino, S. Cloning and Characterization of the Major Insulin-Responsive Glucose Transporter Expressed in Human Skeletal Muscle and Other Insulin-Responsive Tissues. J. Biol. Chem. 1989, 264, 7776–7779. [Google Scholar] [CrossRef]
- Kainulainen, H.; Breiner, M.; Schurmann, A.; Marttinen, A.; Virjo, A.; Joost, H.G. In Vivo Glucose Uptake and Glucose Transporter Proteins Glut1 and Glut4 in Heart and Various Types of Skeletal Muscle from Streptozotocin-Diabetic Rats. Biochim. Biophys. Acta 1994, 1225, 275–282. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of Hba1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef]
- Dholakia, U.; Bandyopadhyay, S.; Hod, E.A.; Prestia, K.A. Determination of Rbc Survival in C57bl/6 and C57bl/6-Tg(Ubc-Gfp) Mice. Comp. Med. 2015, 65, 196–201. [Google Scholar]
- van der Kooij, M.A.; Jene, T.; Treccani, G.; Miederer, I.; Hasch, A.; Voelxen, N.; Walenta, S.; Muller, M.B. Chronic Social Stress-Induced Hyperglycemia in Mice Couples Individual Stress Susceptibility to Impaired Spatial Memory. Proc. Natl. Acad. Sci. USA 2018, 115, E10187–E10196. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.J.; Kim, S.K.; Massett, M.P. Differences in Exercise Capacity and Responses to Training in 24 Inbred Mouse Strains. Front. Physiol. 2017, 8, 974. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.A.; McNally, L.A.; Riggs, D.W.; Conklin, D.J.; Bhatnagar, A.; Hill, B.G. Fvb/Nj Mice Are a Useful Model for Examining Cardiac Adaptations to Treadmill Exercise. Front. Physiol. 2016, 7, 636. [Google Scholar] [CrossRef] [PubMed]
- Massett, M.P.; Berk, B.C. Strain-Dependent Differences in Responses to Exercise Training in Inbred and Hybrid Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1006–R1013. [Google Scholar] [CrossRef] [PubMed]
- Billat, V.L.; Mouisel, E.; Roblot, N.; Melki, J. Inter-and Intrastrain Variation in Mouse Critical Running Speed. J. Appl. Physiol. 2005, 98, 1258–1263. [Google Scholar] [CrossRef]
- Brown, D.A.; Johnson, M.S.; Armstrong, C.J.; Lynch, J.M.; Caruso, N.M.; Ehlers, L.B.; Fleshner, M.; Spencer, R.L.; Moore, R.L. Short-Term Treadmill Running in the Rat: What Kind of Stressor Is It? J. Appl. Physiol. 2007, 103, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Yechoor, V.K.; Patti, M.E.; Saccone, R.; Kahn, C.R. Coordinated Patterns of Gene Expression for Substrate and Energy Metabolism in Skeletal Muscle of Diabetic Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 10587–10592. [Google Scholar] [CrossRef] [PubMed]
- Brahma, M.K.; Ha, C.M.; Pepin, M.E.; Mia, S.; Sun, Z.; Chatham, J.C.; Habegger, K.M.; Abel, E.D.; Paterson, A.J.; Young, M.E.; et al. Increased Glucose Availability Attenuates Myocardial Ketone Body Utilization. J. Am. Heart Assoc. 2020, 9, e013039. [Google Scholar] [CrossRef]
- Svensson, K.; Albert, V.; Cardel, B.; Salatino, S.; Handschin, C. Skeletal Muscle Pgc-1alpha Modulates Systemic Ketone Body Homeostasis and Ameliorates Diabetic Hyperketonemia in Mice. FASEB J. 2016, 30, 1976–1986. [Google Scholar] [CrossRef]
- Dobrzyn, P.; Pyrkowska, A.; Jazurek, M.; Szymanski, K.; Langfort, J.; Dobrzyn, A. Endurance Training-Induced Accumulation of Muscle Triglycerides Is Coupled to Upregulation of Stearoyl-Coa Desaturase 1. J. Appl. Physiol. 2010, 109, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Priya, G.; Kalra, S. A Review of Insulin Resistance in Type 1 Diabetes: Is There a Place for Adjunctive Metformin? Diabetes Ther. 2018, 9, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Kaul, K.; Apostolopoulou, M.; Roden, M. Insulin Resistance in Type 1 Diabetes Mellitus. Metabolism 2015, 64, 1629–1639. [Google Scholar] [CrossRef]
- DiMenna, F.J.; Arad, A.D. The Acute Vs. Chronic Effect of Exercise on Insulin Sensitivity: Nothing Lasts Forever. Cardiovasc. Endocrinol. Metab. 2021, 10, 149–161. [Google Scholar] [CrossRef]
- Ivy, J.L.; Young, J.C.; Mclane, J.A.; Fell, R.D.; Holloszy, J.O. Exercise Training and Glucose Uptake by Skeletal Muscle in Rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 1393–1396. [Google Scholar] [CrossRef]
- Janez, A.; Guja, C.; Mitrakou, A.; Lalic, N.; Tankova, T.; Czupryniak, L.; Tabak, A.G.; Prazny, M.; Martinka, E.; Smircic-Duvnjak, L. Insulin Therapy in Adults with Type 1 Diabetes Mellitus: A Narrative Review. Diabetes Ther. 2020, 11, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Simsek, M.; Naziroglu, M.; Erdinc, A. Moderate Exercise with a Dietary Vitamin C and E Combination Protects against Streptozotocin-Induced Oxidative Damage to the Kidney and Lens in Pregnant Rats. Exp. Clin. Endocrinol. Diabetes 2005, 113, 53–59. [Google Scholar] [CrossRef]
- Wu, K.K.; Huan, Y. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2008, 70, 5–47. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, C.C.; Locke, E.J.; Sipf, D.A.; Thompson, J.H.; Drebushenko, E.K.; Berger, N.S.; Segich, B.S.; Kolwicz, S.C., Jr. The Effects of Exercise Training on Glucose Homeostasis and Muscle Metabolism in Type 1 Diabetic Female Mice. Metabolites 2022, 12, 948. https://doi.org/10.3390/metabo12100948
O’Neill CC, Locke EJ, Sipf DA, Thompson JH, Drebushenko EK, Berger NS, Segich BS, Kolwicz SC Jr. The Effects of Exercise Training on Glucose Homeostasis and Muscle Metabolism in Type 1 Diabetic Female Mice. Metabolites. 2022; 12(10):948. https://doi.org/10.3390/metabo12100948
Chicago/Turabian StyleO’Neill, Caitlin C., Erica J. Locke, Darren A. Sipf, Jack H. Thompson, Erin K. Drebushenko, Nathan S. Berger, Brooke S. Segich, and Stephen C. Kolwicz, Jr. 2022. "The Effects of Exercise Training on Glucose Homeostasis and Muscle Metabolism in Type 1 Diabetic Female Mice" Metabolites 12, no. 10: 948. https://doi.org/10.3390/metabo12100948
APA StyleO’Neill, C. C., Locke, E. J., Sipf, D. A., Thompson, J. H., Drebushenko, E. K., Berger, N. S., Segich, B. S., & Kolwicz, S. C., Jr. (2022). The Effects of Exercise Training on Glucose Homeostasis and Muscle Metabolism in Type 1 Diabetic Female Mice. Metabolites, 12(10), 948. https://doi.org/10.3390/metabo12100948






