Abstract
Natural bioactive compounds are proposed as alternatives in mitigating obesity-associated skeletal muscle dysfunction. The objective of this study was to test the hypothesis that the combination of geranylgeraniol (GGOH) and green tea polyphenols (GTPs) can alleviate high-fat-diet (HFD)-induced muscle atrophy and alter gut microbiome composition. Male C57BL/6J mice fed an HFD were assigned to four groups (12 mice each) in a 2 (no GGOH vs. 400 mg GGOH/kg diet) × 2 (no GTPs vs. 0.5% weight/volume GTPs in water) factorial design. After 14 weeks of diet intervention, skeletal muscle and cecal samples were collected and examined. Compared to the control groups, the group that consumed a combination of GGOH and GTPs (GG + GTPs) had significantly decreased body and fat mass but increased skeletal muscle mass normalized by body weight and cross-sectional area. In soleus muscle, the GG + GTP diet increased citrate synthase activity but decreased lipid peroxidation. Gut microbiome beta-diversity analysis revealed a significant difference in the microbiome composition between diet groups. At the species level, the GG + GTP diet decreased the relative abundance of Dorea longicatena, Sporobacter termitidis, and Clostridium methylpentosum, and increased that of Akkermansia muciniphila and Subdoligranulum variabile. These results suggest that the addition of GGOH and GTPs to an HFD alleviates skeletal muscle atrophy, which is associated with changes in the gut microbiome composition.
1. Introduction
Skeletal muscle is the most important organ for whole-body glucose homeostasis [1] and is responsible for approximately 80 to 90% of insulin-stimulated whole-body glucose uptake and disposal under normal conditions [2,3]. The gut microbiome is a complex community of microbes inhabiting our bodies, and in this context, the cecum, and plays a vital role in our health. Growing evidence suggests that crosstalk between gut microbiota and skeletal muscle regulates systemic low-grade inflammation, oxidative stress, mitochondrial function, and the development of metabolic diseases [4]. Disturbances of gut microbiota have also been shown to decrease skeletal muscle mass and function [4,5]. Further studies suggested that gut microbiota modulates muscle glycogen, a key energetic substrate for prolonged exercise, thus affecting glycogen’s muscle availability and function in mice [6].
In recent years, the use of bioactive compounds has become an alternative approach to preventing and alleviating obesity-associated skeletal muscle dysfunction. Among these bioactive compounds, geranylgeraniol (GGOH) and green tea polyphenols (GTPs) are good candidates. GGOH, which is found in fruits, vegetables, and grains [7], modifies testosterone production [8] and suppresses pro-inflammatory production (monocyte chemoattractant protein-1 and interleukin-6 in adipose tissue) [9]. However, the effects of GGOH on skeletal muscle properties in the development of obesity have not been investigated. Besides GGOH, GTPs and their bioactive compounds exhibit strong antioxidant and antiobesity properties, which can regulate gut microbiota composition [10]. Furthermore, previous studies have reported the beneficial effects of green tea on obesity-induced skeletal muscle disorders [11,12,13]. For example, green tea extracts mitigate HFD-induced muscle atrophy in senescent-accelerated mouse prone-8 mice [13]. GTP treatment ameliorates metabolic abnormalities and insulin resistance by enhancing insulin signaling in the skeletal muscle of Zucker fatty rats [12]. However, the effects of GGOH and GTPs on skeletal muscle-associated parameters along with gut microbiota have not been analyzed.
In the present study, we investigated the benefits of two dietary bioactive components, GGOH and GTPs, individually and in combination, on skeletal muscle properties and the gut microbiome in HFD-fed mice. We hypothesized that the combination of GGOH and GTPs would alleviate HFD-induced muscle atrophy and alter the gut microbiome composition. Findings from this study will advance our understanding of these bioactive compounds and their effects on skeletal muscle biology in the progression of obesity in humans.
2. Materials and Methods
2.1. Animals and Treatments
Five-week-old male C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Following a 5-day acclimation period on standard chow, all mice were fed an HFD (60% of calories from fat, catalog number: D12492, Research Diets, Inc., New Brunswick, NJ, USA) and randomly assigned to four experimental groups (n = 12/group, group-housed with 3 mice per cage): Control (HFD only), GG (HFD supplemented with GGOH in 400 mg/kg diet), GTPs (HFD supplemented with 0.5% weight/volume GTPs in water), and GG + GTPs group (HFD supplemented with a combination of GGOH and GTPs). This study design yielded a 2 × 2 factorial design (2 (no GGOH vs. 400 mg GGOH/kg diet) × 2 (no GTPs vs. GTPs)). The diet composition of the HFD was previously reported [14,15]. GGOH (GG-Gold™, extracted from annatto, 85% purity with the remaining 15% consisting of fatty acids, plant terpenoids, sterols, and waxes) was provided by American River Nutrition (Hadley, MA, USA). GTPs (decaffeinated with a purity of 98.5%, consisting of 65.3% epigallocatechin gallate, 19.08% epicatechin-3-gallate, 9.87% epicatechin, 4.14% epigallocatechin, and 1.54% catechin, Zhejiang Yixin Pharmaceutical Co., Ltd., Zhejiang, China) were dissolved in distilled water and prepared fresh daily [16,17]. Mice were housed under a controlled temperature of 21 ± 2 °C with a 12 h light–dark cycle. Mice were fed and watered ad libitum, and body weight, food intake, and water consumption were recorded weekly. The Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee approved all handling, conditions, and experiments performed.
2.2. Sample Collection
After 14 weeks, mice were fasted for four hours and euthanized with isoflurane. Soleus and gastrocnemius muscles were harvested, weighed, snap-frozen in liquid nitrogen, and stored at −80 °C for later analyses. White adipose tissue (i.e., epididymal fat) was collected and weighed. The cecal samples were collected from the cecum and frozen at −80 °C for later microbiome analyses.
2.3. Skeletal Muscle Analyses
Gastrocnemius muscles (n = 3 per treatment group) were fixed with 10% phosphate-buffered formalin and stained with hematoxylin–eosin to visualize tissue architecture and measure the cross-sectional area (CSA) described previously [11]. Briefly, the CSA was measured by tracing the outer perimeter of the cell with image J (NIH) over 2000 fibers per group.
All other experiments were performed using soleus muscles, which contain a high percentage of myosin heavy-chain I isoforms (MyHCs I) and are classified as Type I fibers since Type I fibers negatively correlate with obesity and metabolic disorders [18,19]. Citrate synthase (CS) activity (CS0720; Sigma-Aldrich, St. Louis, MO, USA), cytochrome c oxidase (COX) activity (CYTOCOX1; Sigma-Aldrich, St. Louis, MO, USA)), malondialdehyde (MDA) levels (#10009055, Cayman Chemical, Ann Arbor, MI, USA), and the production of hydrogen peroxide (H2O2) were determined using an Amplex red hydrogen peroxide H2O2 assay kit (A22188: Invitrogen, Waltham, MA, USA) following the respective manufacturers’ instructions.
The expression of the skeletal muscle MyHC gene was measured by quantitative real-time polymerase chain reaction (RT-qPCR) as described previously [20]. Primers for MyHC-I, IIa, and IIx were listed previously [21]. The common reference genes including Gapdh, Hprt1, Actb, 18S, and Tbp were evaluated using M-value, and reference gene expression stability was determined using Bio-Rad CFX Manager 3.2. (Biorad, Hercules, CA, USA). Tbp was the most stable among groups, so mRNA levels were normalized to Tbp using the cycle threshold (ΔΔCT), and relative fold changes were reported compared to the Control. The primers of Tbp were forward 5′-GCCTTCCACCTTATGCTCAG-3′ reverse 5′-GTTGTTGCTGCTGCTGTTG-3′.
2.4. Microbiome Profiling
This was performed as previously described [22]. Briefly, stool samples were collected from mice cecum, and then microbial DNA was isolated using MoBio PowerFecal® DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA). The V3–V4 regions of the bacterial 16S rRNA gene were amplified using the bacterial universal primer sets 341F and 805R [23]. DNA was quantified; then, libraries were prepared using Nextera XT index kit v2 (Illumina, San Diego, CA, USA) following the manufacturer’s instructions. The libraries were then sequenced on a MiSeq sequencer (Illumina Inc., San Diego, CA, USA) using a 600-cycle v3 sequencing kit at the Center for Biotechnology and Genomics, Texas Tech University, Lubbock, TX, USA. Raw sequencing data have been deposited under BioProject accessions PRJNA551310 (for the HFD group), PRJNA637806 (for the GG group), PRJNA589473 (for the GTP group), and PRJNA843790 (for the GG + GTP group) in the National Center for Biotechnology Information (NCBI) BioProject database.
2.5. Statistical Analysis
2.5.1. Physiological Data Analysis
The data are presented as a mean ± standard error of the mean (SEM). SigmaStat software version 14.0 (Systat Software, Inc., San Jose, CA, USA) was used for data analysis of these parameters by the two-way analysis of variance (ANOVA) test, followed by the post hoc Fisher’s Least Significant Difference (LSD) test. A significance level of p-value < 0.05 applies to all statistical tests. The figures were made using GraphPad Prism software version 9.0 (GraphPad, San Diego, CA, USA).
2.5.2. Microbiome Data Analysis
16S rRNA gene sequencing data were analyzed using QIIME 2 [24]. Briefly, reads were filtered, denoised, and merged. Then, DADA2 plugin (within QIIME 2) was used to determine the exact amplicon sequence variants (ASVs). For taxonomy assignment, Greengenes database version 13.8 was used. For redundancy analysis, we used Calypso software, which generated the redundancy analysis (RDA) plot and measured the statistical significance of the diet effect on microbial community composition [25]. We used the nonparametric Kruskal–Wallis test followed by Dunn’s test for multiple comparisons. Results were regarded as significant when the p-value < 0.05.
3. Results
3.1. Final Body Weight and Fat Pad Weight
We previously showed that mice fed an HFD had significantly increased body weight and fat pad weight, impaired glucose tolerance, and reduced insulin sensitivity compared to mice fed a low-fat diet [14,26]. In addition, we reported an alteration in gut microbiome composition in the HFD group compared to the low-fat-diet group [15,26]. Thus, in this study, we tested bioactive compounds in the mice fed an HFD. The initial body weight was not different across treatment groups. As shown in Figure 1A, starting at 12 weeks of intervention, mice fed a combination of GGOH and GTPs (GG + GTPs) showed a significantly lower body weight than the control group. At 14 weeks, the GG + GTPs group also showed a lower body weight compared to the group administered GGOH (GG), resulting in the order of control group = GG group > GTP group > GG + GTPs group. The overall food intake was similar among groups, except for week 11, where food intake was lower in all treatment groups compared to the control group (Figure 1B). The water intake was significantly lower with GTP administration (GTPs and GG + GTPs) in weeks 9 and 11 (Figure 1C). Two-way ANOVA factorial analysis indicated that the individual and combination of GGOH and GTP supplementation lowered white adipose tissue (WAT) weight (Figure 1D). We did not observe an interaction between GGOH and GTP supplementation on WAT weight (Figure 1D).
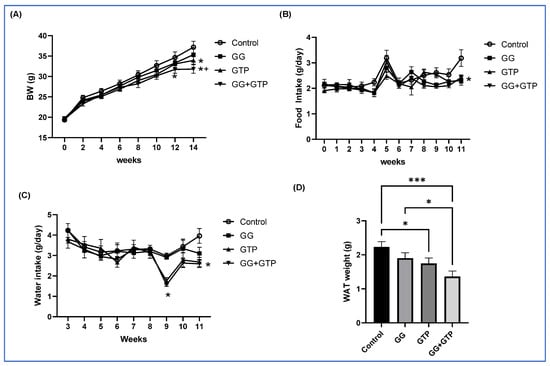
Figure 1.
Effects of GGOH and GTPs on BW, food intake, water intake, and WAT weight in male mice fed an HFD. (A) Body weight, (B) food intake, and (C) water intake over time. (D) Final WAT weight: two-way ANOVA found no interaction effects (A–D). BW, body weight; Control, HFD only; GG, HFD supplemented with GGOH in 400 mg/kg diet; GTPs, HFD supplemented with 0.5% weight/volume GTPs in water; GG + GTPs, HFD supplemented with a combination of GGOH and GTP; WAT, white adipose tissue; *, p < 0.05 compared to the control; +, p < 0.05 compared to GG for (A–C); *, p < 0.05; ***, p < 0.001 by Fisher’s Least Significant Difference (LSD) test post hoc analysis (D).
3.2. Muscle Mass and Cross-Sectional Area
The absolute muscle weights (soleus and gastrocnemius muscles) were not changed with treatment (Figure 2A,B). However, both the GG and GG + GTPs groups had significantly increased relative soleus muscle weight (i.e., soleus muscle weight normalized to body weight) (Figure 2C). Only the GTP group, and not the GG group, had an increased relative gastrocnemius weight (i.e., gastrocnemius muscle normalized to body weight) (Figure 2D). However, the CSA of gastrocnemius muscles was significantly different, resulting in the order of GG + GTP group = GTP group > GG group > control group (Figure 2E). We did not observe the interaction between GGOH and GTP supplementation on both soleus and gastrocnemius weights of animals (Figure 2C,D), but significant interaction effects on the CSA were seen (Figure 2E). Representative images are shown in Figure 2F.
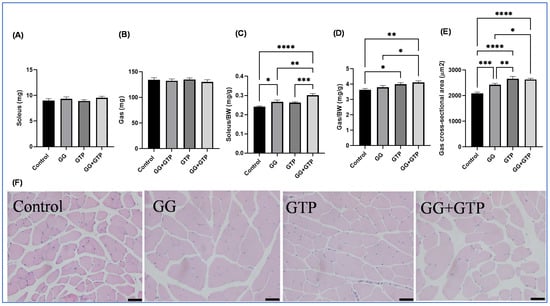
Figure 2.
Effects of GGOH and GTPs on muscle morphology. (A) Soleus weight, (B) gastrocnemius weight, (C) soleus weight normalized by BW. (D) Gastrocnemius weight normalized by BW. (E) Gastrocnemius muscle CSA. (F) Representative images of control, GG, GTPs, and GG + GTPs gastrocnemius muscle sections stained with hematoxylin and eosin. The scale bar = 100 μm. Over 2000 fibers per group were counted. BW, body weight; Control, HFD only; GG, HFD supplemented with GGOH in 400 mg/kg diet; GTPs, HFD supplemented with 0.5% weight/volume GTPs in water; GG + GTPs, HFD supplemented with a combination of GGOH and GTPs; *, p < 0.05; **, p < 0.01; ***, p < 0.001, and ****, p < 0.0001 by Fisher’s Least Significant Difference (LSD) test post hoc analysis.
3.3. Mitochondrial Enzyme Activity and Oxidative Stress of Skeletal Muscle
Here, we showed the effects of GGOH and GTP supplementation on mitochondrial enzyme activity and oxidative stress of soleus muscle (Figure 3). The combination of GGOH and GTPs (GG + GTPs) significantly increased CS activity (Figure 3A). Only the GTP group, not the GG group, had significantly increased COX activity. There was no interaction between GGOH and GTP supplementation in CS and COX activity (Figure 3A,B). We measured hydrogen peroxide (H2O2) production and thiobarbituric-acid-reactive substances (TBARSs) as indirect oxidative stress markers (Figure 3C,D). There was a significant interaction between GGOH and GTP supplementation in both markers (p < 0.05). The GTP group had suppressed H2O2 production as well as TBARS levels. The GG group had decreased TBARS levels, and the control group had the highest H2O2 and TBARS levels among all groups (Figure 3C,D).
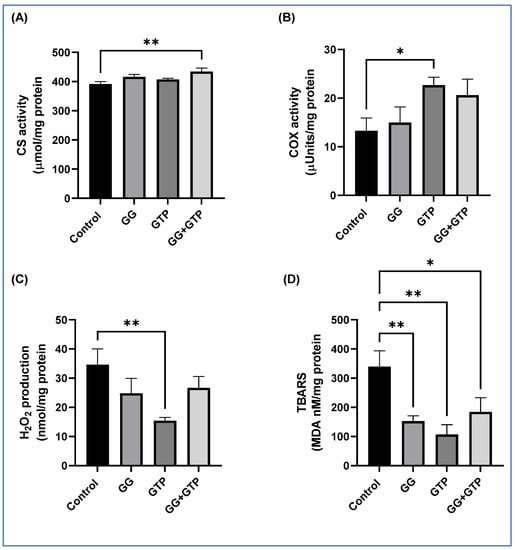
Figure 3.
Effect of GGOH and GTPs on (A) CS activity, (B) COX activity, (C) H2O2 production, and (D) TBARS level of the soleus muscle. Values are mean (n = 6/group) with the standard error of the mean (SEM). CS, citrate synthase; COX, cytochrome c oxidase; Control, HFD only; GG, HFD supplemented with GGOH in 400 mg/kg diet; GTPs, HFD supplemented with 0.5% weight/volume GTPs in water; GG + GTPs, HFD supplemented with a combination of GGOH and GTPs; H2O2, hydrogen peroxidase; TBARSs, thiobarbituric-acid-reactive substances; MDA, malondialdehyde. *, p < 0.05; **, p < 0.01 by Fisher’s Least Significant Difference (LSD) test post hoc analysis.
3.4. Gene Expression of Skeletal Muscle Motor Protein
The impact of GGOH and GTP supplementation on MyHC isoforms of the soleus muscles was determined (Figure 4A–C). There was no effect on the expression of MyHC-I (Figure 4A). There was a significant difference between the GTP group and the GG + GTP group, but no interaction was found in mRNA expression of MyHC-IIa (Figure 4B). The GG group had significantly decreased mRNA expression of MyHC-IIx compared to the control group, while the GG + GTP group had significantly increased levels compared to the GG group with significant interaction effects (Figure 4C).
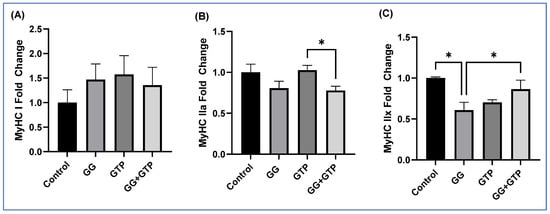
Figure 4.
The effects of GGOH and GTP supplementation on myosin heavy-chain isoform mRNA gene expression in soleus muscle. (A) MyHC-I, (B) MyHC IIa, (C) MyHC IIx. n = 4 control; n = 5 GG, GTPs, and GG + GTPs, respectively. Control, HFD only; GG, HFD supplemented with GGOH in 400 mg/kg diet; GTPs, HFD supplemented with 0.5% weight/volume GTPs in water; GG + GTPs, HFD supplemented with a combination of GGOH and GTPs; MyHC, myosin heavy chain; *, p < 0.05 by Fisher’s Least Significant Difference (LSD) test post hoc analysis.
3.5. Gut Microbiome Diversity and Composition
Microbiome alpha diversity (i.e., microbiome richness and evenness) did not differ with different supplementations. Beta diversity (i.e., a measure of the similarity or dissimilarity of microbiome communities) was examined using redundancy analysis, which revealed a significant difference in the gut microbiome composition based on diet supplementation (Figure 5A). The most abundant phyla were Verrucomicrobia and Firmicutes among all treatment groups, while Bacteroidetes, Actinobacteria, and Proteobacteria were detected at a much lower abundance (Figure 5B). While the relative abundance of Firmicutes decreased in the GG + GTP group, the relative abundance of Verrucomicrobia increased in the GG + GTP group. Such an effect on Bacteroidetes and Proteobacteria was observed with the same pattern in the GG + GTP group (Figure 5B).
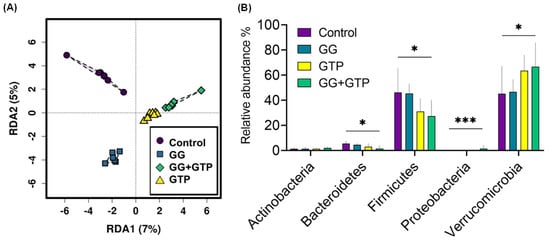
Figure 5.
Microbiome composition overview of different diet groups. (A) Beta-diversity multivariate analysis using redundancy analysis (RDA). (B) Relative abundance differences in major phyla among diet groups. Control, HFD only; GG, HFD supplemented with GGOH in 400 mg/kg diet; GTPs, HFD supplemented with 0.5% weight/volume GTPs in water; GG + GTPs, HFD supplemented with a combination of GGOH and GTPs; * p < 0.05 and *** p < 0.001. Kruskal–Wallis test was performed followed by Dunn’s test.
From the microbiome analysis of mice ceca, we observed changes in the abundance of several species (Figure 6). While the individual groups of GG or GTPs affected the relative abundance of a few species, a stronger and statistically significant effect was observed with the combined treatment (the GG + GTP group) and in some cases with the GTP group only. The GTP group decreased the abundance of Clostridium methylpentosum and Dorea longicatena. Compared to the control group, the effect of GG + GTPs increased the relative abundance of Akkermansia muciniphila (A. muciniphila) and Subdoligranulum variabile (S. variabile) and decreased the abundance of Sporobacter termitidis (Figure 6).
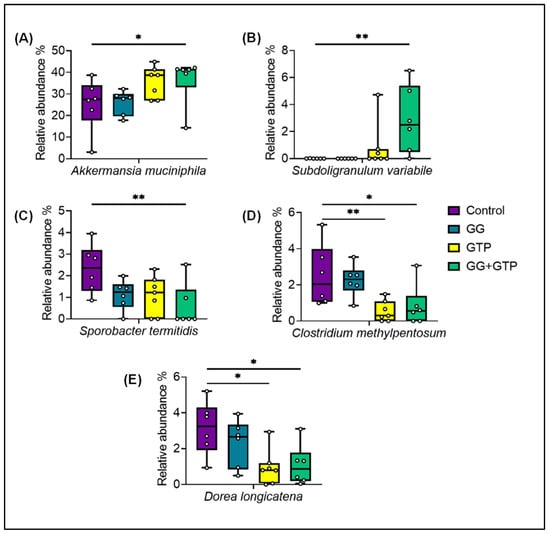
Figure 6.
Amplicon sequence variants (ASVs) with a statistically significant differential abundance of (A) Akkermansia muciniphila, (B) Subdoligranulum variabile, (C) Sporobacter termitidi, (D) Clostridium methylpentosum, and (E) Dorea longicatena among diet groups. Control, HFD only; GG, HFD supplemented with GGOH in 400 mg/kg diet; GTPs, HFD supplemented with 0.5% weight/volume GTPs in water; GG + GTPs, HFD supplemented with a combination of GGOH and GTPs. * p < 0.05 and ** p < 0.01. Kruskal–Wallis test was performed followed by Dunn’s test.
4. Discussion
Accumulating evidence suggests that skeletal muscle atrophy associated with metabolic diseases is highly linked to oxidative stress, mitochondrial dysfunction, and dysbiosis (i.e., imbalanced gut microbiota) [4,27,28,29]. Supporting our hypothesis, mice fed an HFD supplemented with GGOH and GTPs for 14 weeks had independent benefits, while the combination of GGOH and GTPs (GG + GTP group) rather than individual supplementation had much greater effects on the muscle mass, fat mass, oxidative stress markers, and gut microbiome composition. This is the first study showing that supplementation of GGOH and GTPs for 14 weeks in mice fed an HFD could alter skeletal muscle properties and gut microbiome composition.
The absolute weights were not altered, which agrees with previous studies [18,30]. Absolute skeletal muscle mass can be attributed to extracellular matrix content and intramuscular adipose tissue, which significantly increases with obesity [31]. We believe the cross-sectional data in addition to relative muscle mass demonstrate the positive effects of GG and GTPs on skeletal muscle mass. The observations that GGOH increased skeletal muscle weight are corroborated by previous work. For example, a low dose of GGOH increases the CSA of muscle by suppressing atrogin-1 expression in denervation-induced muscle atrophy [32]. GGOH administration has also been shown to upregulate testosterone synthesis in testis-derived cells [33], which can promote muscle hypertrophy by suppressing the expression of atrogin-1 and MuRF-1 [34,35]. The increased muscle mass by GTP supplementation shown in this study is supported by previous studies. For example, GTP extract protects against endoplasmic reticulum stress, oxidative stress, and protein degradation in HFD-induced muscle atrophy [36]. The administration of epigallocatechin-3-gallate (EGCG), the most abundant catechin in GTPs, to mice significantly increased muscle fiber size after muscle damage by induction of myogenic markers, including myogenin and muscle creatine kinase [37]. GTPs mitigate HFD-induced muscle atrophy in senescence-accelerated mice [13].
Interestingly, the GG + GTP group had much larger increases in muscle mass than the GG group despite previous studies showing that GGOH can upregulate testosterone synthesis [33]. The dose of GGOH used in this study may not be sufficient to activate hypertrophic pathways or inhibit the atrophic pathways [32], but may regulate inflammation [38]. It is accepted that crosstalk between adipose tissue and skeletal muscle determines muscle quality [39]. Inflammatory cytokines and hormones released by adipose tissue in the states of both obesity and metabolic diseases negatively affect muscle mass [40,41]. In contrast, myokines induced by skeletal muscle through exercise training decrease leptin and inflammatory cytokines in circulation, which contributes to decreased adipose tissue size [42]. We previously reported that GTPs increased the fat-free mass and decreased fat mass in HFD-induced obese rats [16,43]. Based on previous studies, we can speculate that the antioxidant and anti-inflammatory effects of GTPs [44] with GGOH administration may result in the highest muscle mass (i.e., GG + GTPs group), which may then contribute to the WAT mass.
Obesity is also associated with reduced skeletal muscle oxidative capacity [45,46] and mitochondrial dysfunction is a quite common pathology in metabolic disorders [28]. CS activity, a biomarker for mitochondrial density in skeletal muscle [47], was increased, and the level of TABARS, a lipid peroxidation maker, was decreased when used in a combination (i.e., GG + GTP group) in agreement with a previous study on dystrophic (i.e., mdx) mice [48]. The increased COX activity and decreased H2O2 production by GTP supplementation seen in our study also agree with a previous study in human cultured neurons and astrocytes treated with EGCG [49] and obese mice treated with GTPs [11]. Therefore, our results suggest that GTPs and GGOH can protect from obesity-associated oxidative stress, but mitochondrial functional assessment warrants future study.
Skeletal muscles are heterogeneous and composed of different fiber types based on myosin heavy-chain (MyHC) expression (i.e., Type I, IIa, and IIx) [50]. Type I fibers are more insulin-sensitive and have a higher oxidative capacity than Type II fibers [51], and thus, are more susceptible to metabolic diseases [19,52,53]. A decreased number of Type I fibers was shown in subjects with insulin resistance [52] and obesity [19]. Although our results indicate that fiber-type alterations can occur with the use of bioactive compounds in an obesogenic environment, the functional changes due to fiber-type alteration warrant further study. In addition, most skeletal muscles are hybrid, expressing mixed fibers (i.e., I/IIa, IIa/IIx, etc.). Therefore, a study on single-fiber proteomics instead of whole muscle lysates would likely provide muscle pathophysiology [54].
The species A. muciniphila is well-known for its beneficial properties against metabolic syndrome and has been validated in studies using rodents and humans [55]. A. muciniphila is more abundant in physically active women than in either sedentary people [56] or healthy subjects [57]. Improved glucose homeostasis by metformin treatment, the most used antidiabetic drug, in diet-induced obese mice was highly correlated with increased A. muciniphila [58,59]. Supplementation with A. muciniphila improved metabolic parameters in overweight/obese insulin-resistant humans [60]. Concord grape polyphenols [61] and epigallocatechin gallate from green tea increased A. muciniphila with improved metabolic outcomes. We did not observe any changes in the gut microbiome in the GG-treated group. Like muscle data, the dose we used in this study may not be sufficient to alter gut microbiome composition. Previously, we showed a 800 mg/kg diet of GGOH (vs. 400 mg/kg diet in this study) increased the relative abundance of Butyricicoccus pullicaecorum and decreased the abundance of Dorea longicatena compared to the control group. Thus, it is imperative to investigate the dose–response test. S. variabile, another butylate-producing species, was increased with GTPs in high-fat-diet-induced insulin-resistant mice [22]. An increased abundance of S. variabile was highly correlated with HDL cholesterol levels and negatively correlated with fat mass and insulin resistance in humans [62]. Previous studies suggested that butyrate-producing bacterial species, such as A. muciniphila and S. variabile, regulate skeletal muscle metabolism and function [63]; thus, the validation of causality warrants future study.
Interestingly, the combination of GGOH and GTPs (GG + GTP group) produced much greater effects on skeletal muscle, WAT, and gut microbiome composition than individual supplementation (GG group or GTP group). Sishi et al. [29] reported that diet-induced obesity increased oxidative stress and low-grade inflammation, which activate atrophic signaling pathways and apoptosis in skeletal muscle. GGOH may act as an antioxidant, as shown in lower TBARS levels in our study, or an anti-inflammatory, as previously shown in lipopolysaccharide-induced inflammatory responses [38]. Thus, a low dose of GGOH could act synergistically in the presence of GTPs. Supporting our speculation, previous studies show that GTPs can act as pro-oxidants with the coadministration of tocotrienol, another antioxidant [11,64]. Thus, further investigation is needed to establish a dose relationship, particularly when supplementations are coadministered.
There are some limitations to consider: (1) The CSA was measured on gastrocnemius, while the other experiments were performed on the soleus muscle. Obesity and its associated diseases result in decreased oxidative Type I fibers, which have a higher glucose handling and oxidative capacity, with a proportional increase in fast-twitch fibers, such as IIx fibers, which are glycolytic [51]. Thus, we investigated the biochemical properties using soleus muscles. However, the mass of soleus muscle was only 7–12 mg in our studied mice, so it was not feasible to perform all experiments with soleus muscle. (2) We noted that there was a peak in food intake around the fifth week and a sharp decline in the amount of drinking water consumed around the ninth week. Such changes were consistent among all animals studied, regardless of group, with the same trend during the same period. Since this study had a 14-week feeding period, changes in food intake and water consumption in the middle of the feeding experiment should not have affected the research questions we wished to address. (3) Finally, it would be informative if we could make correlations between skeletal muscle size with the changes in gut microbiome species. However, the sample size was not sufficient to determine a correlation.
In conclusion, we demonstrated that geranylgeraniol and green tea polyphenols mitigate the negative effects of a high-fat diet on adipose tissue, skeletal muscle atrophy, and the gut microbiome.
Author Contributions
Conceptualization, C.-L.S. and E.C.; methodology, C.-L.S., M.M.E., A.H. and E.C.; Validation, K.G., H.E.J., A.U.J., M.M.E., C.-L.S. and E.C.; Formal analysis, C.-L.S., M.M.E. and E.C.; investigation, C.-L.S., M.M.E., K.G., H.E.J., A.U.J. and E.C. Resources, C.-L.S. and E.C.; data curation, M.M.E.; writing—original draft preparation, C.-L.S., M.M.E. and E.C.; writing—review and editing, C.-L.S., M.M.E., K.G., H.E.J., A.U.J., A.H. and E.C.; visualization, C.-L.S., M.M.E. and E.C; supervision, C.-L.S. and E.C.; project administration, C.-L.S. and E.C.; funding acquisition, C.-L.S. and E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by American River Nutrition, LLC., Hadley, MA.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of Texas Tech University Health Sciences Center (#15001, 03/26/2015).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
H.E.J. was supported by the Undergraduate Project fund from the Texas Tech University Center for Active Learning and Undergraduate Engagement. A.U.J. was supported by the Texas Tech University Honors College through the Undergraduate Research Scholar program. M.M.E. was supported by the doctoral dissertation competition fellowship from the graduate school at Texas Tech University. E.C. was supported by the University of Texas in San Antonio, Office of the Vice President for Research, Economic Development, and Knowledge Enterprise. We thank Michael D. Tomison for the animal care of the study, Pratibha Kottapalli and Kameswara Rao Kottapalli for microbiome profiling, and Salvatore N. Campise and Antonio Bollinger for quantification of the cross-sectional area of gastrocnemius muscles.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
CS, citrate synthase activity; COX, cytochrome c oxidase activity; CSA, cross-sectional area; H2O2, hydrogen peroxide; HFD, high-fat diet; GG, HFD supplemented with GGOH in 400 mg/kg diet; GGOH, geranylgeraniol; GTPs, green tea polyphenols as well as the experimental group of HFD supplemented with 0.5% vol/wt GT in water; GG + GTPs, HFD supplemented with a combination of GGOH and GTPs; MDA, malondialdehyde; TBARSs, thiobarbituric-acid-reactive substances.
References
- Petersen, K.F.; Dufour, S.; Savage, D.B.; Bilz, S.; Solomon, G.; Yonemitsu, S.; Cline, G.W.; Befroy, D.; Zemany, L.; Kahn, B.B.; et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 12587–12594. [Google Scholar] [CrossRef] [PubMed]
- De Fronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Gizard, F.; Fernandez, A.; De Vadder, F. Interactions between gut microbiota and skeletal muscle. Nutr. Metab. Insights 2020, 13. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef] [PubMed]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rue, O.; Mariadassou, M.; et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.K.; Zabot, G.L.; Grazielle, N.-N.; Nogueira, G.C.; Meireles, A.M.A. Process Engineering Applying Supercritical Technology for Obtaining Functional and Therapeutic Products. In Advances in Biotechnology for Food Industry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 327–358. [Google Scholar]
- Ho, H.J.; Shirakawa, H.; Giriwono, P.E.; Ito, A.; Komai, M. A novel function of geranylgeraniol in regulating testosterone production. Biosci. Biotechnol. Biochem. 2018, 82, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Okin, D.; Medzhitov, R. The Effect of Sustained Inflammation on Hepatic Mevalonate Pathway Results in Hyperglycemia. Cell 2016, 165, 343–356. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Chung, E.; Campise, S.N.; Joiner, H.E.; Tomison, M.D.; Kaur, G.; Dufour, J.M.; Cole, L.; Ramalingam, L.; Moustaid-Moussa, N.; Shen, C.L. Effect of annatto-extracted tocotrienols and green tea polyphenols on glucose homeostasis and skeletal muscle metabolism in obese male mice. J. Nutr. Biochem. 2019, 67, 36–43. [Google Scholar] [CrossRef]
- Cheng, J.; Tan, Y.; Zhou, J.; Xiao, L.; Johnson, M.; Qu, X. Green tea polyphenols ameliorate metabolic abnormalities and insulin resistance by enhancing insulin signalling in skeletal muscle of Zucker fatty rats. Clin. Sci. 2020, 134, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Ishino, M.; Kitazawa, H.; Yoto, A.; Shimba, Y.; Mochizuki, Y.; Unno, K.; Meguro, S.; Tokimitsu, I.; Miura, S. Green tea extracts ameliorate high-fat diet-induced muscle atrophy in senescence-accelerated mouse prone-8 mice. PLoS ONE 2018, 13, e0195753. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; Ramalingam, L.; Menikdiwela, K.; Scoggin, S.; Shen, C.L.; Tomison, M.D.; Kaur, G.; Dufour, J.M.; Chung, E.; Kalupahana, N.S.; et al. Effects of delta-tocotrienol on obesity-related adipocyte hypertrophy, inflammation and hepatic steatosis in high-fat-fed mice. J. Nutr. Biochem. 2017, 48, 128–137. [Google Scholar] [CrossRef]
- Chung, E.; Elmassry, M.M.; Kottapalli, P.; Kottapalli, K.R.; Kaur, G.; Dufour, J.M.; Wright, K.; Ramalingam, L.; Moustaid-Moussa, N.; Wang, R.; et al. Metabolic benefits of annatto-extracted tocotrienol on glucose homeostasis, inflammation, and gut microbiome. Nutr. Res. 2020, 77, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Han, J.; Wang, S.; Chung, E.; Chyu, M.C.; Cao, J.J. Green tea supplementation benefits body composition and improves bone properties in obese female rats fed with high-fat diet and caloric restricted diet. Nutr. Res. 2015, 35, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Brackee, G.; Song, X.; Tomison, M.D.; Finckbone, V.; Mitchell, K.T.; Tang, L.; Chyu, M.C.; Dunn, D.M.; Wang, J.S. Safety Evaluation of Green Tea Polyphenols Consumption in Middle-aged Ovariectomized Rat Model. J. Food Sci. 2017, 82, 2192–2205. [Google Scholar] [CrossRef]
- Denies, M.S.; Johnson, J.; Maliphol, A.B.; Bruno, M.; Kim, A.; Rizvi, A.; Rustici, K.; Medler, S. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol. Rep. 2014, 2, e00204. [Google Scholar] [CrossRef]
- Tanner, C.J.; Barakat, H.A.; Dohm, G.L.; Pories, W.J.; MacDonald, K.G.; Cunningham, P.R.; Swanson, M.S.; Houmard, J.A. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1191–E1196. [Google Scholar] [CrossRef]
- Chung, E.; Joiner, H.E.; Skelton, T.; Looten, K.D.; Manczak, M.; Reddy, P.H. Maternal exercise upregulates mitochondrial gene expression and increases enzyme activity of fetal mouse hearts. Physiol. Rep. 2017, 5, e13184. [Google Scholar] [CrossRef]
- Vogel, J.; Figueiredo de Rezende, F.; Rohrbach, S.; Zhang, M.; Schroder, K. Nox4 Is Dispensable for Exercise Induced Muscle Fibre Switch. PLoS ONE 2015, 10, e0130769. [Google Scholar] [CrossRef]
- Elmassry, M.M.; Chung, E.; Cao, J.J.; Hamood, A.N.; Shen, C.L. Osteoprotective effect of green tea polyphenols and annatto-extracted tocotrienol in obese mice is associated with enhanced microbiome vitamin K2 biosynthetic pathways. J. Nutr. Biochem. 2020, 86, 108492. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.-J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome–environment interactions. Bioinformatics 2016, 33, 782–783. [Google Scholar] [CrossRef]
- Chung, E.; Elmassry, M.M.; Cao, J.J.; Kaur, G.; Dufour, J.M.; Hamood, A.N.; Shen, C.-L. Beneficial effect of dietary geranylgeraniol on glucose homeostasis and bone microstructure in obese mice is associated with suppression of proinflammation and modification of gut microbiome. Nutr. Res. 2021, 93, 27–37. [Google Scholar] [CrossRef]
- Rodriguez, J.; Delzenne, N.M. Modulation of the gut microbiota-adipose tissue-muscle interactions by prebiotics. J. Endocrinol. 2021, 249, R1–R23. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Sishi, B.; Loos, B.; Ellis, B.; Smith, W.; du Toit, E.F.; Engelbrecht, A.M. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp. Physiol. 2011, 96, 179–193. [Google Scholar] [CrossRef]
- Sousa, L.G.O.; Marshall, A.G.; Norman, J.E.; Fuqua, J.D.; Lira, V.A.; Rutledge, J.C.; Bodine, S.C. The effects of diet composition and chronic obesity on muscle growth and function. J. Appl. Physiol. 2021, 130, 124–138. [Google Scholar] [CrossRef]
- Tam, C.S.; Covington, J.D.; Bajpeyi, S.; Tchoukalova, Y.; Burk, D.; Johannsen, D.L.; Zingaretti, C.M.; Cinti, S.; Ravussin, E. Weight gain reveals dramatic increases in skeletal muscle extracellular matrix remodeling. J. Clin. Endocrinol. Metab. 2014, 99, 1749–1757. [Google Scholar] [CrossRef]
- Miyawaki, A.; Rojasawasthien, T.; Hitomi, S.; Aoki, Y.; Urata, M.; Inoue, A.; Matsubara, T.; Morikawa, K.; Habu, M.; Tominaga, K.; et al. Oral Administration of Geranylgeraniol Rescues Denervation-induced Muscle Atrophy via Suppression of Atrogin-1. In Vivo 2020, 34, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.J.; Shirakawa, H.; Yoshida, R.; Ito, A.; Maeda, M.; Goto, T.; Komai, M. Geranylgeraniol enhances testosterone production via the cAMP/protein kinase A pathway in testis-derived I-10 tumor cells. Biosci. Biotechnol. Biochem. 2016, 80, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Hourde, C.; Jagerschmidt, C.; Clement-Lacroix, P.; Vignaud, A.; Ammann, P.; Butler-Browne, G.S.; Ferry, A. Androgen replacement therapy improves function in male rat muscles independently of hypertrophy and activation of the Akt/mTOR pathway. Acta Physiol. 2009, 195, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Pires-Oliveira, M.; Maragno, A.L.; Parreiras-e-Silva, L.T.; Chiavegatti, T.; Gomes, M.D.; Godinho, R.O. Testosterone represses ubiquitin ligases atrogin-1 and Murf-1 expression in an androgen-sensitive rat skeletal muscle in vivo. J. Appl. Physiol. 2010, 108, 266–273. [Google Scholar] [CrossRef]
- Rodriguez, J.; Gilson, H.; Jamart, C.; Naslain, D.; Pierre, N.; Deldicque, L.; Francaux, M. Pomegranate and green tea extracts protect against ER stress induced by a high-fat diet in skeletal muscle of mice. Eur. J. Nutr. 2015, 54, 377–389. [Google Scholar] [CrossRef]
- Kim, A.R.; Kim, K.M.; Byun, M.R.; Hwang, J.H.; Park, J.I.; Oh, H.T.; Kim, H.K.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. Catechins activate muscle stem cells by Myf5 induction and stimulate muscle regeneration. Biochem. Biophys. Res. Commun. 2017, 489, 142–148. [Google Scholar] [CrossRef]
- Giriwono, P.E.; Shirakawa, H.I.; Ohsaki, Y.; Sato, S.; Aoyama, Y.; Ho, H.J.; Goto, T.; Komai, M. Geranylgeraniol Suppresses the Expression of IRAK1 and TRAF6 to Inhibit NFκB Activation in Lipopolysaccharide-Induced Inflammatory Responses in Human Macrophage-Like Cells. Int. J. Mol. Sci. 2019, 20, 2320. [Google Scholar] [CrossRef]
- Rahemi, H.; Nigam, N.; Wakeling, J.M. The effect of intramuscular fat on skeletal muscle mechanics: Implications for the elderly and obese. J. R. Soc. Interface 2015, 12, 20150365. [Google Scholar] [CrossRef]
- Argiles, J.M.; Lopez-Soriano, J.; Almendro, V.; Busquets, S.; Lopez-Soriano, F.J. Cross-talk between skeletal muscle and adipose tissue: A link with obesity? Med. Res. Rev. 2005, 25, 49–65. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Rouault, C.; Rodriguez-Cuenca, S.; Albert, V.; Edom-Vovard, F.; Vidal-Puig, A.; Clement, K.; Butler-Browne, G.S.; Lacasa, D. Human Adipocytes Induce Inflammation and Atrophy in Muscle Cells During Obesity. Diabetes 2015, 64, 3121–3134. [Google Scholar] [CrossRef]
- Stanford, K.I.; Goodyear, L.J. Muscle-Adipose Tissue Cross Talk. Cold Spring Harb. Perspect. Med. 2018, 8, a029801. [Google Scholar] [CrossRef]
- Shen, C.L.; Cao, J.J.; Dagda, R.Y.; Chanjaplammootil, S.; Lu, C.; Chyu, M.C.; Gao, W.; Wang, J.S.; Yeh, J.K. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet-induced obese rats. Nutr. Res. 2012, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Yeh, J.K.; Cao, J.J.; Tatum, O.L.; Dagda, R.Y.; Wang, J.S. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation-induced bone loss model. J. Nutr. Biochem. 2010, 21, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Coen, P.M.; Hames, K.C.; Leachman, E.M.; DeLany, J.P.; Ritov, V.B.; Menshikova, E.V.; Dube, J.J.; Stefanovic-Racic, M.; Toledo, F.G.; Goodpaster, B.H. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity 2013, 21, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef] [PubMed]
- Vigelsø, A.; Andersen, N.B.; Dela, F. The relationship between skeletal muscle mitochondrial citrate synthase activity and whole body oxygen uptake adaptations in response to exercise training. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 84–101. [Google Scholar]
- Call, J.A.; Voelker, K.A.; Wolff, A.V.; McMillan, R.P.; Evans, N.P.; Hulver, M.W.; Talmadge, R.J.; Grange, R.W. Endurance capacity in maturing mdx mice is markedly enhanced by combined voluntary wheel running and green tea extract. J. Appl. Physiol. 2008, 105, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Gonzalez, G.; Pichaud, N.; Ballard, J.W.; Bessede, A.; Marcal, H.; Guillemin, G.J. Epigallocatechin-3-gallate induces oxidative phosphorylation by activating cytochrome c oxidase in human cultured neurons and astrocytes. Oncotarget 2016, 7, 7426–7440. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Albers, P.H.; Pedersen, A.J.; Birk, J.B.; Kristensen, D.E.; Vind, B.F.; Baba, O.; Nohr, J.; Hojlund, K.; Wojtaszewski, J.F. Human muscle fiber type-specific insulin signaling: Impact of obesity and type 2 diabetes. Diabetes 2015, 64, 485–497. [Google Scholar] [CrossRef]
- Stuart, C.A.; McCurry, M.P.; Marino, A.; South, M.A.; Howell, M.E.; Layne, A.S.; Ramsey, M.W.; Stone, M.H. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J. Clin. Endocrinol. Metab. 2013, 98, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, A.; Patel, K. Skeletal muscle fiber plasticity in response to selected envrionmental and physiological stimuli. Histol. Histopathol. 2009, 24, 611–629. [Google Scholar] [PubMed]
- Murgia, M.; Nogara, L.; Baraldo, M.; Reggiani, C.; Mann, M.; Schiaffino, S. Protein profile of fiber types in human skeletal muscle: A single-fiber proteomics study. Skelet. Muscle 2021, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Uchiyama, K.; Takagi, T. A next-generation beneficial microbe: Akkermansia muciniphila. J. Clin. Biochem. Nutr. 2018, 63, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velasquez-Mejia, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated with Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Van Hul, M.; Le Roy, T.; Prifti, E.; Dao, M.C.; Paquot, A.; Zucker, J.D.; Delzenne, N.M.; Muccioli, G.; Clement, K.; Cani, P.D. From correlation to causality: The case of Subdoligranulum. Gut Microbes 2020, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).