Fibromyalgia and Depression in Women: An 1H-NMR Metabolomic Study
Abstract
:1. Introduction
2. Results
2.1. Clinical and Autoimmune Analysis
2.2. Psychological Test Results
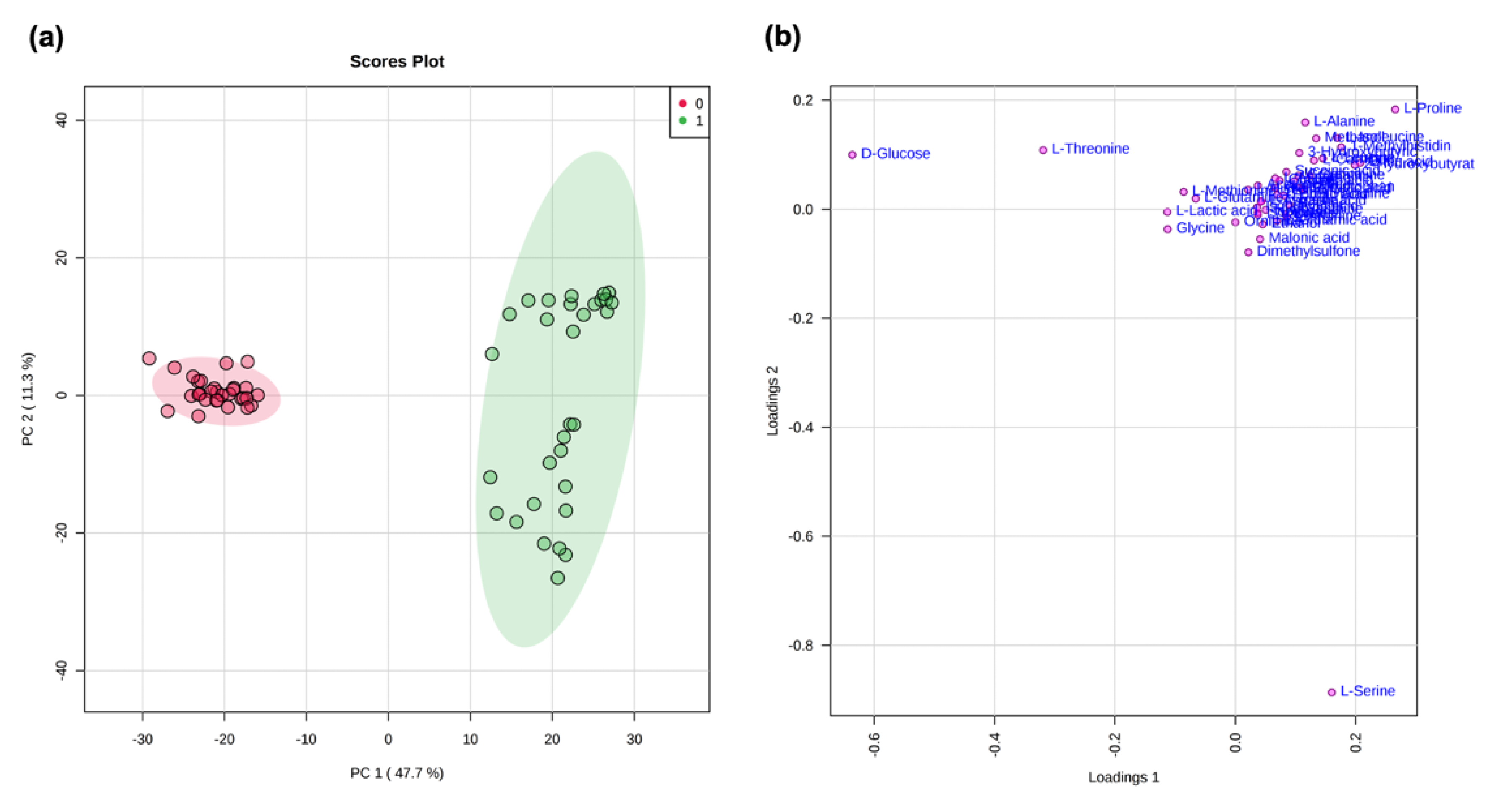
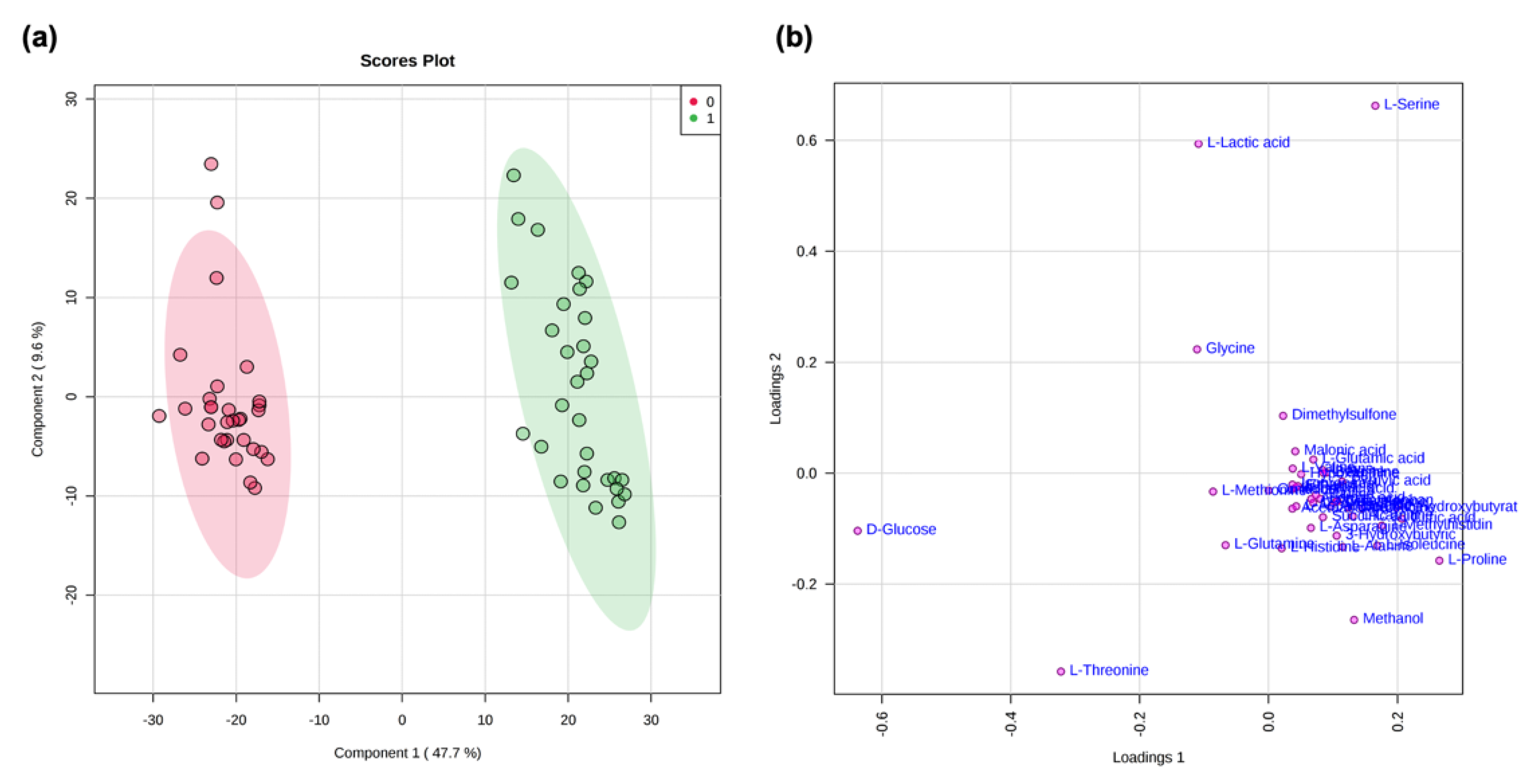
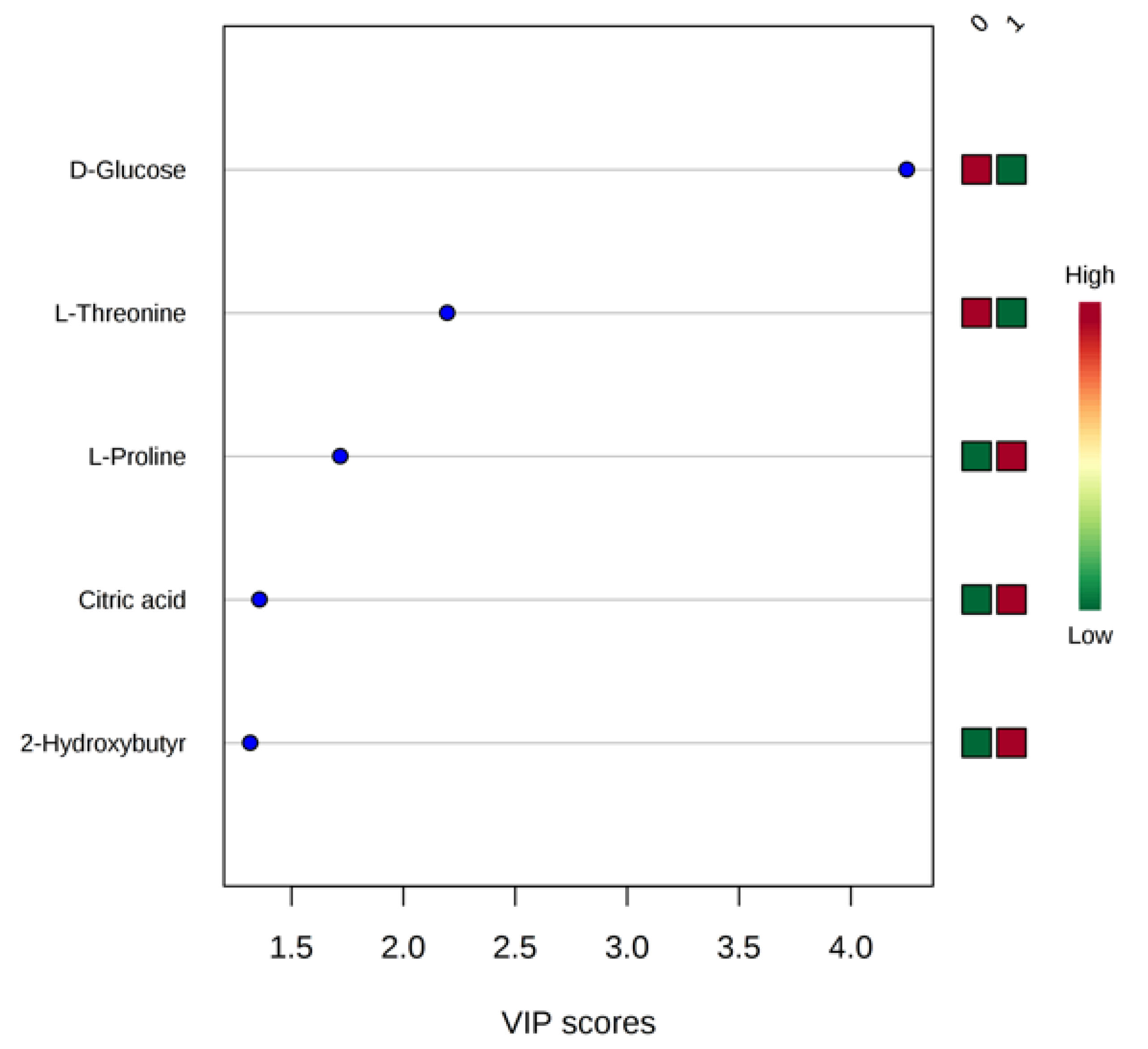
2.3. Statistical Analysis
Statistical Analysis of NMR Data
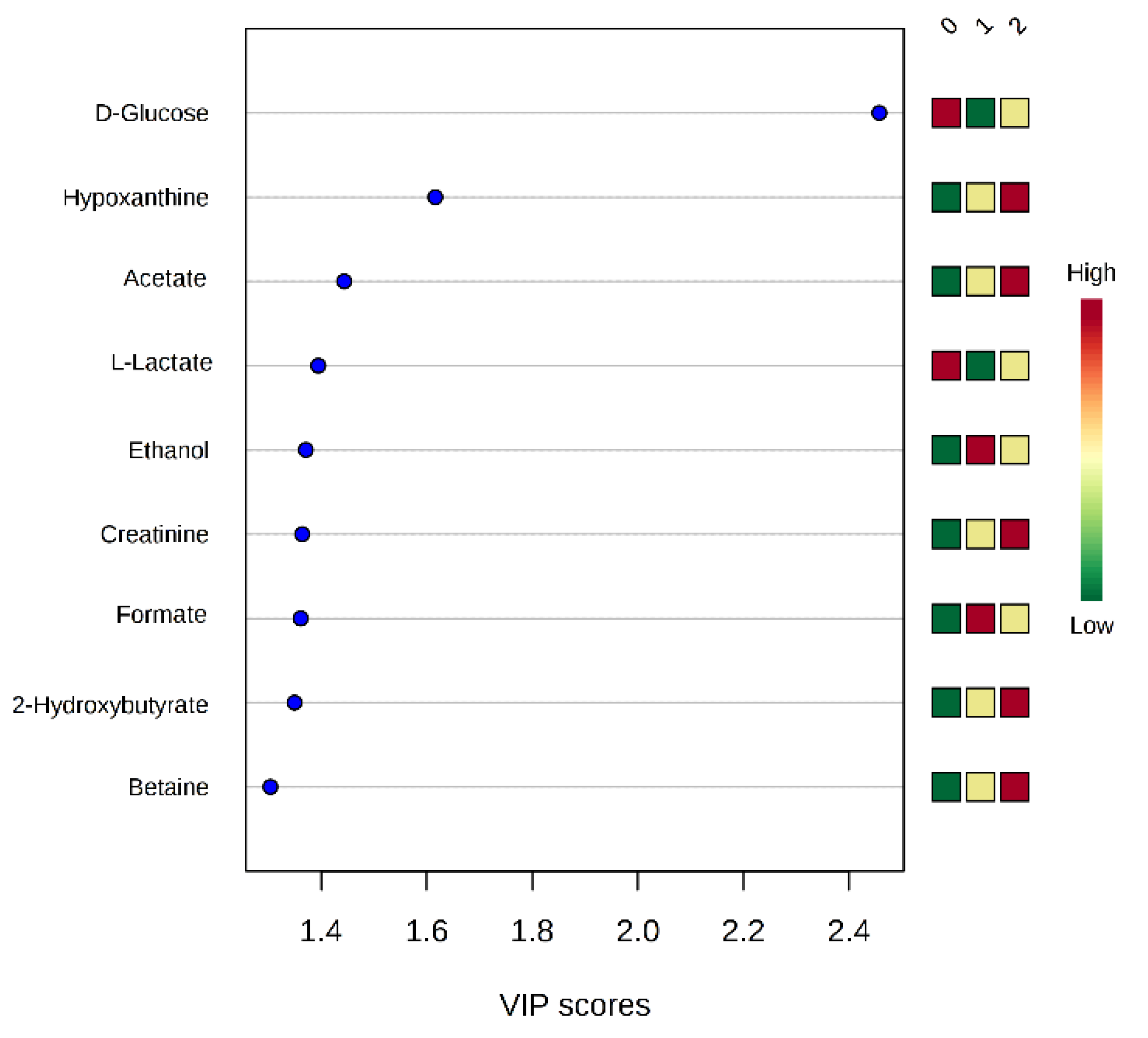
2.4. NMR Data and Psychological Tests
3. Discussion
4. Materials and Methods
4.1. Partecipants and Study Design
4.2. Autoimmune Parameter Analysis
4.3. Psychological Test: Hamilton Anxiety Test (HAM-A) and Hamilton Anxiety Depression (HAM-D)
4.4. Sample Pretreatment for NMR Analysis
4.5. NMR Data Acquisition
4.6. NMR Data Processing
4.7. Multivariate Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staud, R.; Rodriguez, M.E. Mechanisms of Disease: Pain in fibromyalgia syndrome. Nat. Clin. Pract. Rheumatol. 2006, 2, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L.; Greene, L.; Ali, A.; Faridi, Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med. Hypotheses 2007, 69, 517–525. [Google Scholar] [CrossRef]
- Alonso-Blanco, M.C.; Fernández-De-Las-Peñas, C.; de la Llave, A.I.; Zarco-Moreno, P.; Galán-Del-Río, F.; Svensson, P. Characteristics of referred muscle pain to the head from active trigger points in women with myofascial temporomandibular pain and fibromyalgia syndrome. J. Headache Pain 2012, 13, 625–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahan, F.; Nanji, K.; Qidwai, W.; Qasim, R. Fibromyalgia syndrome: An overview of pathophysiology, diagnosis and management. Oman Med. J. 2012, 27, 192–195. [Google Scholar] [CrossRef]
- Malatji, B.G.; Meyer, H.; Mason, S.; Engelke, U.F.; Wevers, R.A.; Van Reenen, M.; Reinecke, C.J. A diagnostic biomarker profile for fibromyalgia syndrome based on an NMR metabolomics study of selected patients and controls. BMC Neurol. 2017, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Menzies, V.; Starkweather, A.; Yao, Y.; Thacker, L.R.; Garrett, T.J.; Swift--Scanlan, T.; Kelly, D.L.; Patel, P.; Lyon, D.E. Metabolomic differentials in women with and without fibromyalgia. Clin. Transl. Sci. 2020, 13, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forseth, K.O.; Gran, J.T.; Husby, G. A population study of the incidence of fibromyalgia among women aged 26–55 yr. Rheumatology 1997, 36, 1318–1323. [Google Scholar] [CrossRef] [Green Version]
- Baggio, G.; Corsini, A.; Floreani, A.; Giannini, S.; Zagonel, V. Gender medicine: A task for the third millennium. Clin. Chem. Lab. Med. 2013, 51, 713–727. [Google Scholar] [CrossRef]
- Yunus, M.B. The role of gender in fibromyalgia syndrome. Curr. Rheumatol. Rep. 2001, 3, 128–134. [Google Scholar] [CrossRef]
- Downes, J. Cause of illness among males and females. Milbank Mem. Fund Q. 1950, 28, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadabhoy, D.; Crofford, L.J.; Spaeth, M.; Russell, I.J.; Clauw, D.J. Biology and therapy of fibromyalgia. Evidence-based biomarkers for fibromyalgia syndrome. Arthritis Res. Ther. 2008, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Bänsch, M.; Berg, P.A. Clinical relevance of antibodies against serotonin and gangliosides in patients with primary fibromyalgia syndrome. Psychoneuroendocrinology 1992, 17, 593–598. [Google Scholar] [CrossRef]
- Okifuji, A.; Turk, D.C.; Sherman, J.J. Evaluation of the relationship between depression and fibromyalgia syndrome: Why 438 aren’t all patients depressed? J. Rheumatol. 2000, 27, 212–219. [Google Scholar]
- Ethod, N. Somatization and depression in fibromyalgia syndrome. Am. J. Psychiatry 1988, 145, 950–954. [Google Scholar]
- Burckhardt, C.S.; O’Reilly, C.A.; Wiens, A.N.; Clark, S.R.; Campbell, S.M.; Bennett, R.M. Assessing depression in fibromyalgia patients. Arthritis Rheum. 1994, 7, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Maurer, D.M.; Raymond, T.J.; Davis, B.N. Depression: Screening and diagnosis. Am. Fam. Physician 2018, 98, 508–515. [Google Scholar]
- Robins, L.N.; Helzer, J.; Croughan, J.; Ratcliff, K. The NIMH Diagnostic Interview Schedule: Its History, Characteristics and 444 Validity; Washington University School of Medicine: St. Louis, WA, USA, 1980. [Google Scholar]
- Williams, J.B.W. A structured interview guide for the hamilton depression rating scale. Arch. Gen. Psychiatry 1988, 45, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, G.; Pagano, I.; Grimaldi, M.; Marino, C.; Molettieri, P.; Santoro, A.; Stillitano, I.; Romano, R.; Montoro, P.; D’Ursi, A.M.; et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: A nuclear magnetic resonance-based metabolomic Study. J. Proteome Res. 2021, 20, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, M.; Palisi, A.; Marino, C.; Montoro, P.; Capasso, A.; Novi, S.; Tecce, M.F.; D’Ursi, A.M. NMR-based metabolomic profile of hypercholesterolemic human sera: Relationship with in vitro gene expression? PLoS ONE 2020, 15, e0231506. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, M.; Marino, C.; Buonocore, M.; Santoro, A.; Sommella, E.; Merciai, F.; Salviati, E.; De Rosa, A.; Nuzzo, T.; Errico, F.; et al. Prenatal and early postnatal cerebrald-aspartate depletion influencesl-amino acid pathways, bioenergetic processes, and developmental brain metabolism. J. Proteome Res. 2021, 20, 727–739. [Google Scholar] [CrossRef]
- Grimaldi, M.; Palisi, A.; Rossi, G.; Stillitano, I.; Faiella, F.; Montoro, P.; Rodriquez, M.; Palladino, R.; D’Ursi, A.M.; Romano, R. Saliva of patients affected by salivary gland tumour: An NMR metabolomics analysis. J. Pharm. Biomed. Anal. 2018, 160, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Palisi, A.; Grimaldi, M.; Sabatini, P.; Montoro, P.; Scrima, M.; Rodriquez, M.; D’Ursi, A.M. A serum nuclear magnetic resonance-based metabolomic signature of antiphospholipid syndrome. J. Pharm. Biomed. Anal. 2017, 133, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.; Bundy, J.; Holmes, E.; Lindon, J.; Nicholson, J. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Malatji, B.G.; Mason, S.; Mienie, J.; Wevers, R.A.; Meyer, H.; Van Reenen, M.; Reinecke, C.J. The GC–MS metabolomics signature in patients with fibromyalgia syndrome directs to dysbiosis as an aspect contributing factor of FMS pathophysiology. Metabolomics 2019, 15, 54. [Google Scholar] [CrossRef]
- Heymann, R.E.; Paiva, E.S.; Martinez, J.E.; Helfenstein, M.; Rezende, M.C.; Provenza, J.R.; Ranzolin, A.; De Assis, M.R.; Feldman, D.P.; Ribeiro, L.S.; et al. New guidelines for the diagnosis of fibromyalgia. Rev. Bras. Reum. 2017, 57, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.S.; Proietti, F.A. Interrelations between fibromyalgia, thyroid autoantibodies, and depression. J. Rheumatol. 2004, 31, 2036. [Google Scholar]
- Jacobsen, S.; HØYer--Madsen, M.; Danneskiold--SamsØE, B.; Wiik, A. Screening for autoantibodies in patients with 474 primary fibromyalgia syndrome and a matched control group. APMIS 1990, 98, 655–658. [Google Scholar] [CrossRef]
- Hamilton, M. The hamilton rating scale for depression. In Assessment of Depression; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1986; pp. 143–152. [Google Scholar]
- Thompson, E. Hamilton Rating Scale for Anxiety (HAM-A). Occup. Med. 2015, 65, 601. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Mandal, R.; Sinelnikov, I.V.; Broadhurst, D.; Wishart, D.S. MetaboAnalyst 2.0--a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012, 40, W127–W133. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.1–14.10.91. [Google Scholar] [CrossRef] [PubMed]
- Hajian-Tilaki, K.O.; Hanley, J.A.; Joseph, L.; Collet, J.-P. A Comparison of parametric and nonparametric approaches to ROC analysis of quantitative diagnostic tests. Med. Decis. Mak. 1997, 17, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) curve analysis for medical diagnostic test evaluation. Casp. J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S.; Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, S.; Bono, H.; Ogata, H.; Fujibuchi, W.; Nishioka, T.; Sato, K.; Kanehisa, M. Organizing and computing metabolic pathway data in terms of binary relations. Pac. Symp. Biocomput. Pac. Symp. Biocomput. 1997, 175–186. [Google Scholar]
- Aguilera, M.; Paz, C.; Compañ, V.; Medina, J.C.; Feixas, G. Cognitive rigidity in patients with depression and fibromyalgia. Int. J. Clin. Health Psychol. 2019, 19, 160–164. [Google Scholar] [CrossRef]
- Singh, G.; Kaul, S. Anxiety and depression are common in fibromyalgia patients and correlate with symptom severity score. Indian J. Rheumatol. 2018, 13, 168. [Google Scholar] [CrossRef]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef]
- Carrozzino, D.; Patierno, C.; Fava, G.A.; Guidi, J. The hamilton rating scales for depression: A critical review of clinimetric properties of different versions. Psychother. Psychosom. 2020, 89, 133–150. [Google Scholar] [CrossRef]
- Arout, C.A.; Sofuoglu, M.; Bastian, L.A.; Rosenheck, R.A. Gender differences in the prevalence of fibromyalgia and in concomitant medical and psychiatric disorders: A national veterans health administration study. J. Women’s Health 2018, 27, 1035–1044. [Google Scholar] [CrossRef]
- Eisinger, J.; Plantamura, A.; Ayavou, T. Glycolysis abnormalities in fibromyalgia. J. Am. Coll. Nutr. 1994, 13, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Barker, K.L.; Warren, J.C. Estrogen control of carbohydrate metabolism in the rat uterus: Pathways of glucose metabolism. Endocrinology 1966, 78, 1205–1212. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clegg, D.J. Minireview: The year in review of estrogen regulation of metabolism. Mol. Endocrinol. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.-J.; Qin, Z.; Wang, P.-Y.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef]
- Proia, P.; Amato, A.; Contrò, V.; Monaco, A.L.; Brusa, J.; Brighina, F.; Messina, G. Relevance of lactate level detection in migrane and fibromyalgia. Eur. J. Transl. Myol. 2019, 29, 8202. [Google Scholar] [CrossRef] [Green Version]
- McIver, K.L.; Evans, C.; Kraus, R.M.; Ispas, L.; Sciotti, V.M.; Hickner, R. NO-mediated alterations in skeletal muscle nutritive blood flow and lactate metabolism in fibromyalgia. Pain 2006, 120, 161–169. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Meredith, D. The SLC16 gene family?from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch. Eur. J. Physiol. 2004, 447, 619–628. [Google Scholar] [CrossRef]
- Neeck, G.; Crofford, L.J. Neuroendocrine perturbations in fibromyalgia and chronic fatigue syndrome. Rheum. Dis. Clin. N. Am. 2000, 26, 989–1002. [Google Scholar] [CrossRef]
- Walter, B.; Vaitl, D.; Frank, R. Affective distress in fibromyalgia syndrome is associated with pain severity. Z. Rheumatol. 1998, 57, S101–S104. [Google Scholar] [CrossRef]
- Brown, A.E.; Jones, D.E.; Walker, M.; Newton, J.L. Abnormalities of AMPK activation and glucose uptake in cultured skeletal muscle cells from individuals with chronic fatigue syndrome. PLoS ONE 2015, 10, e0122982. [Google Scholar] [CrossRef] [PubMed]
- Steardo, L., Jr.; Fabrazzo, M.; Sampogna, G.; Monteleone, A.M.; D’Agostino, G.; Monteleone, P.; Maj, M. Impaired glucose 527 metabolism in bipolar patients and response to mood stabilizer treatments. J. Affect. Disord. 2019, 245, 174–179. [Google Scholar] [CrossRef]
- Schmidt, H.D.; Shelton, R.C.; Duman, R.S. Functional Biomarkers of Depression: Diagnosis, Treatment, and Pathophysiology. Neuropsychopharmacology 2011, 36, 2375–2394. [Google Scholar] [CrossRef]
- Głombik, K.; Detka, J.; Góralska, J.; Kurek, A.; Solnica, B.; Budziszewska, B. Brain metabolic alterations in rats showing depression-like and obesity phenotypes. Neurotox. Res. 2019, 37, 406–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerozissis, K.J.E.J.O.P. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur. J. Pharmacol. 2008, 533–585, 38–49. [Google Scholar] [CrossRef]
- Duarte, A.I.; Moreira, P.I.; Oliveira, C.R. Insulin in central nervous system: More than just a peripheral hormone. J. Aging Res. 2012, 2012, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, M.; Kazemi, M.H.; Yoosefi, A.; Ghasemi, A.; Paragomi, P.; Amini, H.; Afzali, M.H. Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res. 2014, 215, 355–361. [Google Scholar] [CrossRef]
- Ruggiero, V.; Era, B.; Cacace, E.; Molin, L.; Corda, M.; Fais, A.; Utzeri, S. A preliminary study on serum proteomics in fibromyalgia syndrome. Clin. Chem. Lab. Med. 2014, 52, 207. [Google Scholar] [CrossRef] [Green Version]
- Fais, A.; Cacace, E.; Corda, M.; Era, B.; Peri, M.; Utzeri, S.; Ruggiero, V. Purine metabolites in fibromyalgia syndrome. Clin. Biochem. 2013, 46, 37–39. [Google Scholar] [CrossRef]
- Talotta, R.; Bazzichi, L.; Di Franco, M.; Casale, R.; Batticciotto, A.; Gerardi, M.C.; Sarzi-Puttini, P. One year in review 2017: Fibromyalgia. Clin. Exp. Rheumatol. 2017, 105, 6–12. [Google Scholar]
- Haroon, E.; Miller, A.H. Inflammation Effects on Brain Glutamate in Depression: Mechanistic Considerations and Treatment Implications. Curr. Top. Behav. Neurosci. 2016, 31, 173–198. [Google Scholar] [CrossRef]
- Altamura, C.; Maes, M.; Dai, J.; Meltzer, H. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur. Neuropsychopharmacol. 1995, 5, 71–75. [Google Scholar] [CrossRef]
- Bellato, E.; Marini, E.; Castoldi, F.; Barbasetti, N.; Mattei, L.; Bonasia, D.E.; Blonna, D. Fibromyalgia Syndrome: Etiology, pathogenesis, diagnosis, and treatment. Pain Res. Treat. 2012, 2012, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Afari, N.; Mostoufi, S.; Noonan, C.; Poeschla, B.; Succop, A.; Chopko, L.; Strachan, E. C-reactive protein and pain sensitivity: Findings from female twins. Ann. Behav. Med. 2011, 42, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Feinberg, T.; Sambamoorthi, U.; Lilly, C.; Innes, K.K. Potential Mediators between Fibromyalgia and C-Reactive protein: Results from a Large U.S. Community Survey. BMC Musculoskelet. Disord. 2017, 18, 294. [Google Scholar] [CrossRef] [Green Version]
- Shariatpanaahi, M.V.; Moshtaaghi, M.; Shahbaazi, S.H.; Abadi, A.; Shariatpanaahi, Z.V. The relationship between depression and serum ferritin level. Eur. J. Clin. Nutr. 2006, 61, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Ortancil, O.; Sanli, A.; Eryuksel, R.; Basaran, A.; Ankarali, H. Association between serum ferritin level and fibromyalgia syndrome. Eur. J. Clin. Nutr. 2010, 64, 308–312. [Google Scholar] [CrossRef]
- Okan, S.; Türk, A.Ç.; Şıvgın, H.; Özsoy, F.; Okan, F. Association of ferritin levels with depression, anxiety, sleep quality, and physical functioning in patients with fibromyalgia syndrome: A cross-sectional study. Croat. Med. J. 2019, 60, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Arnold, L.M.; Hudson, J.I.; Keck, P.E., Jr.; Auchenbach, M.B.; Javaras, K.N.; Hess, E.V. Comorbidity of fibromyalgia and 562 psychiatric disorders. J. Clin. Psychiatry 2006, 11, 333–338. [Google Scholar]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdrich, S.; Hawrelak, J.A.; Myers, S.P.; Harnett, J.E. Determining the association between fibromyalgia, the gut microbiome and its biomarkers: A systematic review. BMC Musculoskelet. Disord. 2020, 21, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.; Gyamfi, A.; Cong, X.S. Altered composition of gut microbiota in depression: A systematic review. Front. Psychiatry 2020, 11, 541–569. [Google Scholar] [CrossRef]
- Voigt, J.; Krause, C.; Rohwäder, E.; Saschenbrecker, S.; Hahn, M.; Danckwardt, M.; Feirer, C.; Ens, K.; Fechner, K.; Barth, E.; et al. Automated indirect immunofluorescence evaluation of antinuclear autoantibodies on HEp-2 Cells. Clin. Dev. Immunol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Krause, C.; Ens, K.; Fechner, K.; Voigt, J.; Fraune, J.; Rohwäder, E.; Hahn, M.; Danckwardt, M.; Feirer, C.; Barth, E.; et al. EUROPattern Suite technology for computer-aided immunofluorescence microscopy in autoantibody diagnostics. Lupus 2015, 24, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Radice, A.; Sinico, R.A.; Damoiseaux, J.; Seaman, A.; Buckmelter, K.; Vizjak, A.; Buchner, C.; Binder, W.L.; 576 Fritzler, M.J. Performance evaluation of a novel chemiluminescence assay for detection of anti-GBM antibodies: An 577 international multicenter study. Nephrol. Dial. Transplant. 2012, 27, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Bentow, C.; Serra, J.; Fritzler, M.J. Detection of autoantibodies using chemiluminescence technologies. Immunopharmacol. Immunotoxicol. 2015, 38, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shovman, O.; Gilburd, B.; Chayat, C.; Amital, H.; Langevitz, P.; Watad, A.; Guy, A.; Perez, D.; Azoulay, D.; Blank, M.; et al. Prevalence of anti-DFS70 antibodies in patients with and without systemic autoimmune rheumatic diseases. Clin. Exp. Rheumatol. 2017, 36, 121–126. [Google Scholar]
- Maier, W.; Buller, R.; Philipp, M.; Heuser, I. The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 1988, 14, 61–68. [Google Scholar] [CrossRef]
- Bruss, G.S.; Gruenberg, A.M.; Goldstein, R.D.; Barber, J. Hamilton anxiety rating scale interview guide: Joint interview and test-retest methods for interrater reliability. Psychiatry Res. 1994, 53, 191–202. [Google Scholar] [CrossRef]
- Fava, G.A.; Kellner, R.; Munari, F.; Pavan, L. The hamilton depression rating scale in normals and depressives. Acta Psychiatr. Scand. 1982, 66, 26–32. [Google Scholar] [CrossRef]
- Van, Q.N.; Chmurny, G.N.; Veenstra, T.D. The depletion of protein signals in metabonomics analysis with the WET–CPMG pulse sequence. Biochem. Biophys. Res. Commun. 2003, 301, 952–959. [Google Scholar] [CrossRef]
- Mo, H.; Raftery, D. Pre-SAT180, a simple and effective method for residual water suppression. J. Magn. Reson. 2008, 190, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranibar, N.; Borys, M.; Mackin, N.A.; Ly, V.; Abu-Absi, N.; Abu-Absi, S.; Niemitz, M.; Schilling, B.; Li, Z.J.; Brock, B.; et al. NMR-based metabolomics of mammalian cell and tissue cultures. J. Biomol. NMR 2011, 49, 195–206. [Google Scholar] [CrossRef]
- Jupin, M.; Michiels, P.; Girard, F.; Spraul, M.; Wijmenga, S. NMR metabolomics profiling of blood plasma mimics shows that medium- and long-chain fatty acids differently release metabolites from human serum albumin. J. Magn. Reson. 2014, 239, 34–43. [Google Scholar] [CrossRef]
- Weljie, A.M.; Newton, J.; Mercier, P.; Carlson, E.; Slupsky, C.M. Targeted Profiling: Quantitative analysis of1H NMR metabolomics data. Anal. Chem. 2006, 78, 4430–4442. [Google Scholar] [CrossRef] [PubMed]
| VIP Score | 1 | 2 | p-Value | |
|---|---|---|---|---|
| Ferritin | 2.60 | + | - | 0.0054 |
| C-reactive protein | 1.35 | - | + | 0.0134 |
| Creatine kinase | 1.00 | + | - | 0.0471 |
| Pathway Name | Pathway Source | Hits | Raw p | FDR |
|---|---|---|---|---|
| Alanine, aspartate, and glutamate metabolism | Metaboanalyst 4.0 | 8 | 4.09 × 10−18 | 1.60 × 10−16 |
| Purine metabolism | Metaboanalyst 4.0 | 2 | 1.42 × 10−12 | 2.04 × 10−11 |
| Glyoxylate and dicarboxylate metabolism | Metaboanalyst 4.0 | 8 | 1.76 × 10−12 | 2.04 × 10−11 |
| Aminoacyl-tRNA biosynthesis | Metaboanalyst 4.0 | 19 | 2.09 × 10−12 | 2.04 × 10−11 |
| Glycine, serine, and threonine metabolism | Metaboanalyst 4.0 | 7 | 1.20 × 10−8 | 9.36 × 10−8 |
| Glycolysis/gluconeogenesis | Metaboanalyst 4.0 | 3 | 8.12 × 10−8 | 3.52 × 10−7 |
| Arginine and proline metabolism | Metaboanalyst 4.0 | 6 | 6.76 × 10−7 | 2.46 × 10−6 |
| Arginine biosynthesis | Metaboanalyst 4.0 | 5 | 6.93 × 10−7 | 2.46 × 10−6 |
| Pyruvate metabolism | Metaboanalyst 4.0 | 3 | 3.45 × 10−6 | 1.12 × 10−5 |
| Cysteine and methionine metabolism | Metaboanalyst 4.0 | 3 | 4.70 × 10−6 | 1.41 × 10−5 |
| D-glutamine and D-glutamate metabolism | Metaboanalyst 4.0 | 2 | 2.63 × 10−5 | 6.84 × 10−5 |
| Nitrogen metabolism | Metaboanalyst 4.0 | 2 | 2.63 × 10−5 | 6.84 × 10−5 |
| Citrate cycle (TCA cycle) | Metaboanalyst 4.0 | 3 | 4.15 × 10−5 | 1.01 × 10−4 |
| Porphyrin and chlorophyll metabolism | Metaboanalyst 4.0 | 2 | 5.56 × 10−4 | 1.14 × 10−2 |
| Glutathione metabolism | Metaboanalyst 4.0 | 3 | 7.58 × 10−3 | 1.48 × 10−3 |
| Propanoate metabolism | Metaboanalyst 4.0 | 2 | 1.80 × 10−3 | 3.53 × 10−2 |
| Valine, leucine, and isoleucine biosynthesis | Metaboanalyst 4.0 | 4 | 2.36 × 10−1 | 4.19 × 10−1 |
| Tyrosine metabolism | Metaboanalyst 4.0 | 3 | 0.00010325 | 0.00017 |
| Defective SLC16A1 causes symptomatic deficiency in lactate transport (SDLT) | Reactome | 2 | 0.005466565 | 0.37265 |
| Creatine metabolism | Reactome | 3 | 0.007243495 | 0.37265 |
| Proton-coupled monocarboxylate transport | Reactome | 2 | 0.007763673 | 0.37265 |
| Transport of bile salts and organic acids, metal ions, and amine compounds | Reactome | 5 | 0.037453248 | 0.50662 |
| Organic cation/anion/zwitterion transport | Reactome | 3 | 0.038381951 | 0.50662 |
| SLC-mediated transmembrane transport | Reactome | 8 | 0.042023926 | 0.50662 |
| Organic anion transporters | Reactome | 2 | 0.042882355 | 0.50662 |
| Fibromyalgic Group (N = 31) | Control Group (N = 31) | |
|---|---|---|
| Sex (male/female) | 0/31 | 0/31 |
| Age (mean ± SD, years) | 42.8 ± 14.04 | 50.0 ± 9.90 |
| Number of participants psychological tests | 19/31 | 19/31 |
| ANA positive | 19/31 | 0/31 |
| ENA positive | 0/31 | 0/31 |
| ACPA positive | 2/31 | 0/31 |
| HAM-A > 17 and HAM-D < 21 | 12/19 | 0/19 |
| HAM-A > 17 and HAM-D > 21 | 7/19 | 0/19 |
| HAM-A < 17 and HAM-D < 21 | 0/19 | 19/19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, C.; Grimaldi, M.; Sabatini, P.; Amato, P.; Pallavicino, A.; Ricciardelli, C.; D’Ursi, A.M. Fibromyalgia and Depression in Women: An 1H-NMR Metabolomic Study. Metabolites 2021, 11, 429. https://doi.org/10.3390/metabo11070429
Marino C, Grimaldi M, Sabatini P, Amato P, Pallavicino A, Ricciardelli C, D’Ursi AM. Fibromyalgia and Depression in Women: An 1H-NMR Metabolomic Study. Metabolites. 2021; 11(7):429. https://doi.org/10.3390/metabo11070429
Chicago/Turabian StyleMarino, Carmen, Manuela Grimaldi, Paola Sabatini, Patrizia Amato, Arianna Pallavicino, Carmen Ricciardelli, and Anna Maria D’Ursi. 2021. "Fibromyalgia and Depression in Women: An 1H-NMR Metabolomic Study" Metabolites 11, no. 7: 429. https://doi.org/10.3390/metabo11070429
APA StyleMarino, C., Grimaldi, M., Sabatini, P., Amato, P., Pallavicino, A., Ricciardelli, C., & D’Ursi, A. M. (2021). Fibromyalgia and Depression in Women: An 1H-NMR Metabolomic Study. Metabolites, 11(7), 429. https://doi.org/10.3390/metabo11070429









