Risks of Oral Anticoagulants: Interactions with Drugs and Medicinal Plants
Abstract
1. Introduction
2. The Literature Search
3. Interactions Involving Oral Anticoagulants
3.1. Summary
3.2. Interactions with Drugs and Oral Anticoagulants
3.3. Interactions with Drugs and Wafarin, and Their Effects on INR
3.4. Possible Interactions Between Medicinal Plants and Warfarin
3.5. Possible Interactions Between Medicinal Plants and DOACs
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| ADRs | Adverse drug reactions |
| AUC | Area under the curve |
| BCRP | Breast cancer resistance protein |
| CBD | Cannabidiol |
| CISCS-UBI | Health Sciences Research Centre, University of Beira Interior |
| CMF | Cyclophosphamide, methotrexate, fluorouracil |
| CYP | Cytochrome P450 |
| CYP1A2 | Cytochrome P450 1A2 |
| CYP2C8 | Cytochrome P450 2C8 |
| CYP2C9 | Cytochrome P450 2C9 |
| CYP2C19 | Cytochrome P450 2C19 |
| CYP2D6 | Cytochrome P450 2D6 |
| CYP3A4 | Cytochrome P450 3A4 |
| DOACs | Direct oral anticoagulants |
| FEDER | Fundo Europeu de Desenvolvimento Regional |
| INR | International Normalized Ratio |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| P-gp | P-glycoprotein |
| PPIs | Proton pump inhibitors |
| SNRIs | Serotonin and norepinephrine reuptake inhibitors |
| SSRIs | Selective serotonin reuptake inhibitors |
| VKAs | Vitamin K antagonists |
| VKORC1 | Vitamin K epoxide reductase complex subunit 1 |
References
- Oliveira, R.P.; Jesus, A. Interações Medicamentosas Potenciais em Farmácia Comunitária-Estudo Exploratório. Acta Farm. Port. 2022, 11, 12–27. [Google Scholar]
- Revista De Fitoterapia. Published 2009. Available online: https://www.fitoterapia.net/php/descargar_documento.php?id=4523&doc_r=sn&num_volumen=22&secc_volumen=5959 (accessed on 11 July 2024).
- OIPM—Observatório De Interações Planta-Medicamento. Available online: http://www.oipm.uc.pt/interacoes/ (accessed on 11 July 2024).
- Ordem Dos Farmacêuticos Podemos Minimizar O Risco De Interações Medicamentosas? Published 2021. Available online: https://www.ordemfarmaceuticos.pt/pt/artigos/podemos-minimizar-o-risco-de-interacoes-medicamentosas/ (accessed on 13 July 2024).
- Jandu, J.S.; Mohanaselvan, A.; Dahal, R.; Bista, S. Strategies to Reduce Polypharmacy in Older Adults. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK574550/ (accessed on 7 July 2025).
- Ansari, J. Drug interaction and pharmacist. J. Young Pharm. 2010, 2, 326–331. [Google Scholar] [CrossRef]
- Guirguis, L.M. Mixed methods evaluation: Pharmacists’ experiences and beliefs toward an interactive communication approach to patient interactions. Patient Educ. Couns. 2011, 83, 432–442. [Google Scholar] [CrossRef]
- The ESC Handbook on Cardiovascular Pharmacotherapy; Oxford University Press: Oxford, UK, 2019. [CrossRef]
- Delafuente, J.C. Understanding and preventing drug interactions in elderly patients. Crit. Rev. Oncol. Hematol. 2003, 48, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Frisullo, G.; Profice, P.; Brunetti, V.; Scala, I.; Bellavia, S.; Broccolini, A.; Caliandro, P.; Iorio, R.D.; Morosetti, R.; Pilato, F.; et al. Prospective observational study of safety of early treatment with edoxaban in patients with ischemic stroke and atrial fibrillation (SATES study). Brain Sci. 2021, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Lima, N. Warfarin: An evidence-based review of food and drug interactions. Port. J. Gen. Pract. 2008, 24, 475–482. [Google Scholar] [CrossRef]
- Afonso, A.; Marques, G.; Gonçalves, A.; Barroso, P.; Gonzalez, A.; Rodrigues, H.; Ferreira, M.J. Antithrombotic Therapy: Current and Under Development. Angiol. Vasc. Surg. 2016, 12, 170–179. [Google Scholar] [CrossRef]
- Furtado, C.; Silva, A. Anticoagulants 2000–2013. Published Online 2014. Available online: https://www.infarmed.pt/documents/15786/17838/Relatorio_Anticoagulantes_05012015.pdf/c8c00f82-0541-4bcf-9eb2-80db9f569f99 (accessed on 30 September 2024).
- Correia, A.; Costa, I.; Caldeira, S. Oral Anticoagulants—Usage and Expenditure. Published 2019. Available online: https://www.infarmed.pt/documents/15786/2713015/Anticoagulantes+Orais+-+Utilização+e+Despesa/01c3bd05-9002-43e8-ac54-eec12791ff90 (accessed on 30 September 2024).
- Figueiredo, I.V.; Lavrador, M.; Freitas, A.M.; Madureira, B.; Campos, C.M.; Gonçalves, V.; Machado, F.; Fortuna, J.; Feio, J.; Castel-Branco, M.; et al. Atualizações em Coagulação: Os Anticoagulantes Orais Não Antagonistas da Vitamina K (NOACs). Rev. Port. Farmacoter. 2018, 10, 9–19. [Google Scholar][Green Version]
- Fernandes, M.; Manuel, M.; Martinez, J. Direct Oral Anticoagulants. Boletim CIM. Published 2018. Available online: https://www.ordemfarmaceuticos.pt/fotos/publicacoes/boletimcim_out_dez_2018_online_15881267745c51cf9600571.pdf (accessed on 16 July 2024).
- Vazquez, S.R. Drug-drug interactions in an era of multiple anticoagulants: A focus on clinically relevant drug interactions. Blood 2018, 132, 2230–2239. [Google Scholar] [CrossRef]
- Di Minno, A.; Frigerio, B.; Spadarella, G.; Ravani, A.; Sansaro, D.; Amato, M.; Kitzmiller, J.P.; Pepi, M.; Tremoli, E.; Baldassarre, D. Old and new oral anticoagulants: Food, herbal medicines and drug interactions. Blood Rev. 2017, 31, 193–203. [Google Scholar] [CrossRef]
- Mekaj, Y.H.; Mekaj, A.Y.; Duci, S.B.; Miftari, E.I. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk Manag. 2015, 11, 967–977. [Google Scholar] [CrossRef]
- Leite, P.M.; Martins, M.A.P.; Carvalho, M.d.G.; Castilho, R.O. Mechanisms and interactions in concomitant use of herbs and warfarin therapy: An updated review. Biomed. Pharmacother. 2021, 143, 112103. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.S.; Lee, S.W.H. Warfarin and food, herbal or dietary supplement interactions: A systematic review. Br. J. Clin. Pharmacol. 2021, 87, 352–374. [Google Scholar] [CrossRef] [PubMed]
- Grześk, G.; Rogowicz, D.; Wołowiec, Ł.; Ratajczak, A.; Gilewski, W.; Chudzińska, M.; Sinkiewicz, A.; Banach, J. The clinical significance of drug-food interactions of direct oral anticoagulants. Int. J. Mol. Sci. 2021, 22, 8531. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fuentes, A.; Rivera-Caravaca, J.M.; López-Gálvez, R.; Marín, F.; Roldán, V. Non-vitamin K Antagonist Oral Anticoagulants and Drug-Food Interactions: Implications for Clinical Practice and Potential Role of Probiotics and Prebiotics. Front. Cardiovasc. Med. 2022, 8, 787235. [Google Scholar] [CrossRef]
- Ge, B.; Zhang, Z.; Zuo, Z. Updates on the clinical evidenced herb-warfarin interactions. Evid. Based Complement. Altern. Med. 2014, 2014, 957362. [Google Scholar] [CrossRef]
- Sikorska, J.; Uprichard, J. Direct oral anticoagulants: A quick guide. Eur. Cardiol. Rev. 2017, 12, 40. [Google Scholar] [CrossRef]
- Wang, M.; Zeraatkar, D.; Obeda, M.; Lee, M.; Garcia, C.; Nguyen, L.; Agarwal, A.; Al-Shalabi, F.; Benipal, H.; Ahmad, A.; et al. Drug-drug interactions with warfarin: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2021, 87, 4051–4100. [Google Scholar] [CrossRef]
- Vranckx, P.; Valgimigli, M.; Heidbuchel, H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythmia Electrophysiol. Rev. 2018, 7, 55. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Vilares, C.; Pereira, C.; Martins, M. Anticoagulantes Orais—Não Antagonistas da vitamina K (NOACs). 2017; 1–56. [Google Scholar]
- Infarmed, I.P. Summary of Product Characteristics-Varfine. Published 2023. Available online: https://extranet.infarmed.pt/INFOMED-fo/detalhes-medicamento.xhtml (accessed on 15 July 2024).
- European Medicines Agency. Summary of Product Characteristics-Lixiana. Published 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/lixiana-epar-product-information_pt.pdf (accessed on 16 July 2024).
- Drug Interaction Checker-For Drugs, Food, and Alcohol. Published 2024. Available online: https://www.drugs.com/drug_interactions.html (accessed on 17 July 2024).
- Mendell, J.; Zahir, H.; Matsushima, N.; Noveck, R.; Lee, F.; Chen, S.; Zhang, G.; Shi, M. Drug-drug interaction studies of cardiovascular drugs involving p-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor xa inhibitor. Am. J. Cardiovasc. Drugs 2013, 13, 331–342. [Google Scholar] [CrossRef]
- Mar, P.L.; Gopinathannair, R.; Gengler, B.E.; Chung, M.K.; Perez, A.; Dukes, J.; Ezekowitz, M.D.; Lakkireddy, D.; Lip, G.Y.H.; Miletello, M.; et al. Drug Interactions Affecting Oral Anticoagulant Use. Circ. Arrhythmia Electrophysiol. 2022, 15, e007956. [Google Scholar] [CrossRef] [PubMed]
- Kvasnicka, T.; Malikova, I.; Zenahlikova, Z.; Kettnerova, K.; Brzezkova, R.; Zima, T.; Ulrych, J.; Briza, J.; Netuka, I.; Kvasnicka, J. Rivaroxaban-Metabolism, Pharmacologic Properties and Drug Interactions. Curr. Drug Metab. 2017, 18, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Mueck, W.; Stampfuss, J.; Kubitza, D.; Becka, M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin. Pharmacokinet. 2014, 53, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Corsini, A.; Ferri, N.; Proietti, M.; Boriani, G. Edoxaban and the Issue of Drug-Drug Interactions: From Pharmacology to Clinical Practice. Drugs 2020, 80, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Colombo, E.; Tenconi, M.; Baldessin, L.; Corsini, A. Drug-Drug Interactions of Direct Oral Anticoagulants (DOACs): From Pharmacological to Clinical Practice. Pharmaceutics 2022, 14, 1120. [Google Scholar] [CrossRef]
- Undas, A.; Brummel-Ziedins, K.E.; Mann, K.G. Anticoagulant effects of statins and their clinical implications. Thromb. Haemost. 2013, 111, 392–400. [Google Scholar] [CrossRef]
- Byon, W.; Garonzik, S.; Boyd, R.A.; Frost, C.E. Apixaban: A Clinical Pharmacokinetic and Pharmacodynamic Review. Clin. Pharmacokinet. 2019, 58, 1265–1279. [Google Scholar] [CrossRef]
- European Medicines Agency. Summary of Product Characteristics-Pradaxa. Published 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/pradaxa-epar-product-information_pt.pdf (accessed on 16 July 2024).
- Ramos, S.; Martins, S.O. Inovação Na Terapêutica Anticoagulante. Rev. Port. Farmacoter. 2013, 5, 30–43. [Google Scholar]
- European Medicines Agency. Summary of Product Characteristics-Xarelto. Published 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/xarelto-epar-product-information_pt.pdf (accessed on 16 July 2024).
- European Medicines Agency. Summary of Product Characteristics-Eliquis. Published 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_pt.pdf (accessed on 16 July 2024).
- Shikdar, S.; Vashisht, R.; Bhattacharya, P.T. International Normalized Ratio (INR). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507707/ (accessed on 23 October 2024).
- Campos, M.d.G.; Costa, M.L.; Falcão, A. Intervenção Farmacêutica na Determinação de Interações Planta-Medicamento. Boletim CIM. Published 2012. Available online: https://www.ordemfarmaceuticos.pt/fotos/publicacoes/bc.103_importancia_da_farmacogenomica_medicamentos_orfaos_int.farm._na_determinacao_de_inter._planta_medicamento_16324747125a12ef2ad9b11.pdf (accessed on 6 July 2024).
- Gamboa, A.; Severiano, A.; Martins, A.S.; Mousinho, C.; Bragança, F.; Hergy, F.; Pedro, M.; Silva, M.; Catalão, P.; Duarte, S. Boletim De Farmacovigilância Volume 24; Número 11. Published Online 2020. Available online: https://www.infarmed.pt/documents/15786/3591914/Boletim+de+Farmacovigilância%2C+Volume+24%2C+no11%2C+novembro+de+2020/ab8e3344-835e-34a3-420d-174cfc835e19 (accessed on 17 July 2024).
- OIPM—Observatório de Interações Planta-Medicamento. Available online: https://oipm.uc.pt/ (accessed on 18 July 2024).
- Izzo, A.A.; Hoon-Kim, S.; Radhakrishnan, R.; Williamson, E.M. A Critical Approach to Evaluating Clinical Efficacy, Adverse Events and Drug Interactions of Herbal Remedies. Phyther. Res. 2016, 30, 691–700. [Google Scholar] [CrossRef]
- Gamboa, A.; Severiano, A.; Martins, A.S.; Mousinho, C.; Bragança, F.; Hergy, F.; Pedro, M.; Silva, M.; Catalão, P.; Duarte, S. Boletim De Farmacovigilância Volume 24; Número 3. Published 2020. Available online: https://www.infarmed.pt/documents/15786/3591914/Boletim+de+Farmacovigilância%2C+Volume+24%2C+no3%2C+março+de+202/62d0a9c0-a579-8b7b-ec9e-c1ea975c412a?version=1.3 (accessed on 18 July 2024).
- Abdul, M.I.M.; Jiang, X.; Williams, K.M.; Day, R.O.; Roufogalis, B.D.; Liauw, W.S.; Xu, H.; Matthias, A.; Lehmann, R.P.; McLachlan, A.J. Pharmacokinetic and pharmacodynamic interactions of echinacea and policosanol with warfarin in healthy subjects. Br. J. Clin. Pharmacol. 2010, 69, 508–515. [Google Scholar] [CrossRef]
- Li, A.; Li, M.K.; Crowther, M.; Vazquez, S.R. Drug-drug interactions with direct oral anticoagulants associated with adverse events in the real world: A systematic review. Thromb. Res. 2020, 194, 240–245. [Google Scholar] [CrossRef]
- Milić, N.; Miloševíc, N.; Goloeòrbin Kon, S.; Bozǐć, T.; Abenavoli, L.; Borrelli, F. Warfarin interactions with medicinal herbs. Nat. Prod. Commun. 2014, 9, 1934578X1400900835. [Google Scholar] [CrossRef]
- Nutescu, E.; Chuatrisorn, I.; Hellenbart, E. Drug and dietary interactions of warfarin and novel oral anticoagulants: An update. J. Thromb. Thrombolysis 2011, 31, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y.K. Interaction between warfarin and danshen (Salvia miltiorrhiza). Ann. Pharmacother. 2001, 35, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Karatoprak, G.S. Horse Chestnut. Nonvitam. Nonmineral Nutr. Suppl. 2019, 295–299. [Google Scholar] [CrossRef]
- Bilberry. LiverTox Clin. Res. Inf. Drug-Induced Liver Inj. Published Online 20 February 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548250/ (accessed on 30 September 2024).
- Karimpour-Reihan, S.; Firuzei, E.; Khosravi, M.; Abbaszade, M. Coagulation Disorder following Red Clover (Trifolium pratense) Misuse: A Case Report. Adv. J. Emerg. Med. 2018, 2, e20. [Google Scholar] [CrossRef]
- Grymonprez, M.; Petrovic, M.; De Backer, T.L.; Steurbaut, S.; Lahousse, L. The Impact of Polypharmacy on the Effectiveness and Safety of Non-vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Thromb. Haemost. 2023, 124, 135–148. [Google Scholar] [CrossRef]
- Tomaszuk-Kazberuk, A.; Koziński, M.; Domienik-Karłowicz, J.; Jaguszewski, M.; Darocha, S.; Wybraniec, M.; Dobrowolski, P.; Kupczyńska, K.; Michalski, B.; Wańha, W.; et al. Pharmacotherapy of atrial fibrillatioin COVID-19 patients. Cardiol. J. 2021, 28, 758–766. [Google Scholar] [CrossRef]
| Mechanism | Warfarin | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|---|---|
| Antiarrhythmics | ||||||
| Amiodarone | Moderate inhibitor of P-glycoprotein and CYP3A4 inhibitor | 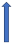 +12% to 60% AUC ~10–40%) Cmax (SmPC) | 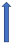 +12% to 60% (SmPc) | 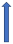 Minimal effect (SmPC) | 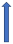 (SmPC) |  +40% |
| Diltiazem | CYP3A4 inhibitor and weak P-glycoprotein inhibitor | 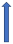 ~10% to 20% AUC | No effect (SmPc) | No effect |  | 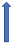 39.8% AUC |
| Propafenone | CYP3A4 inhibitor | 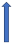 ~10% to 20% AUC (SmPC) |  |  | 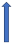 | 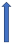 |
| Dronedarone | CYP3A4 and P-glycoprotein inhibitor | 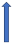 | 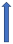 +70% to 100% | Moderate effects. Combination to be avoided | With caution | 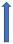 +85% (dose reduction to 30 mg once a daily by label) (SmPC) |
| Propranolol | CYP1A2 inhibitor | 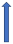 | No relevant interaction | No relevant interaction | No relevant interaction | No relevant interaction |
| Quinidine | P-glycoprotein inhibitor and CYP2D6 inhibitor | 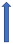 ~30% to 53% AUC Cmax ~15% a 25% | 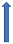 +53% (SmPc) | Unknown increase extension |  | 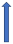 +77% (No dose reduction required by label) |
| Verapamil | Weak CYP3A4 inhibitor and P-glycoprotein inhibitor | 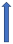 +12% to +180% AUC | 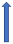 +12% to 180% (if taken simultaneously) (SmPC) | 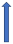 +40% (probably not relevant) | 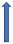 (SmPC) | 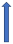 +53 (no dose reduction required by label) (SmPC) |
| Other Cardiovascular Drugs | ||||||
| Atorvastatin | P-glycoprotein inhibitor and CYP3A4 competition | 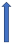 3–5% AUC (SmPC) | No relevant interaction | No relevant interaction | With caution | 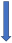 Decreased the 24-h concentration for edoxaban by 25.7% |
| Ticagrelor | P-glycoprotein inhibitor and CYP3A4 inhibitor |  No significant change | 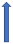 +24% to 65% (give loading dose 2 h after dabigatran) (SmPC) | With caution | With caution | With caution |
| Antibiotics | ||||||
| Clarithromycin | Strong CYP3A4 inhibitor and P-glycoprotein inhibitor | 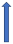 +19% a to 40% AUC +15% a 30% Cmax | 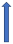 +19% AUC +15% Cmax (SmPC) | 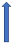 +50% AUC +40% Cmax | 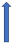 +60% AUC + 30% Cmax (SmPC) |  +53% AUCa +27% Cmax (SmPC) |
| Erythromycin | Strong CYP3A4 inhibitor and P-glycoprotein inhibitor |  30% AUC +30% Cmax 15% (SmPC) | +19% AUC + 15% Cmax (SmPC) | 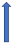 +30% AUC +30% Cmax (SmPC) | 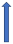 (SmPC) | 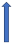 +85% AUC + 68% Cmax (dose reduction to 30 mg once daily by label) (SmPC) |
| Rifampicin | CYP3A4, P-glycoprotein/BCRP and CYP2C9 inducer | 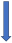 −67% AUC −22% to 35% Cmax (SmPC) | 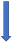 −66% AUC −67% Cmax (SmPC) | 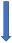 −50% AUC −22% Cmax (SmPC) | 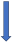 −54% AUC −42% Cmax (SmPC) |  −35% AUC (but with compensatory increase of active metabolite) (SmPC) |
| Isoniazid | CYP2C9 inhibitor | 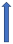 | No relevant interaction | No relevant interaction | No relevant interaction | No relevant interaction |
| Metronidazole | CYP1A2 and CYP2C9 inhibitor | 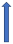 +40% AUC +20% to 50% Cmax (SmPC) | No relevant interaction | No relevant interaction | No relevant interaction | No relevant interaction |
| Quinolones | Strong CYP1A2 inhibitor (Ciprofloxacin) | 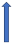 | No relevant interaction | No relevant interaction | No relevant interaction | No relevant interaction |
| Sulfamethoxazole + Trimethoprim | Inhibitor CYP3A4 and CYP2C9 | 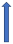 | No relevant interaction | No relevant interaction | No relevant interaction | No relevant interaction |
| Antivirals | ||||||
| Protease inhibitors (e.g., ritonavir) | CYP3A4 inhibitor and P-glycoprotein/BCRP inhibition or induction |  +53% AUC + 55% Cmax (SmPC) | Variable decrease or increase (SmPC) | 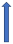 +153% AUC + 55% Cmax (SmPC) | 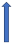 | 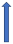 Elevated risk of bleeding. |
| Antifungals | ||||||
| Fluconazole | Moderate CYP3A4 and CYP2C9 inhibitor | 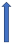 +42% AUC + 30% Cmax (SmPC) | No relevant interaction | 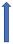 +42% AUC + 30% Cmax (SmPC) |  ~+40% AUC | No relevant interaction |
| Voriconazole | Strong CYP3A4 inhibitor | 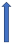 +80% to 100% AUC + 60% to 80% Cmax (SmPC) | No data | Combination to be avoided (SmPC) | Combination to be avoided (SmPC) | No data |
| Ketoconazole | Strong CYP3A4 inhibitor and P-glycoprotein/BCRP competition | 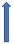 +140% to 150% AUC + 100% to 160% Cmax (SmPC) | 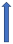 +140% to 150% AUC | 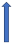 | 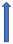 +100% AUC + 64% Cmax | 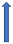 +160% AUC + 72% Cmax (SmPC) |
| Itraconazole | Strong CYP3A4 inhibitor and P-glycoprotein/BCRP competition | 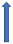 +70% to 100% AUC + 50% to 70% Cmax (SmPC) | 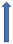 | 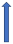 | 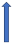 | 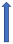 |
| Posaconazole | Strong CYP3A4 inhibitor and P-glycoprotein | 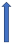 (SmPC) | 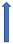 With caution | 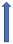 Combination to be avoided | 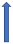 Combination to be avoided | 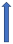 With caution |
| Others | ||||||
| Carbamazepine | Strong CYP3A4 and P-glycoprotein inducer | 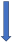 +66% to 75% AUC + 50% to 60% Cmax (SmPC) | 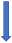 (SmPC) |  (SmPC) | 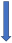 (SmPC) |  (SmPC) |
| Phenytoin | Strong CYP3A4 inducer and P-glycoprotein competition | 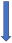 +20% to 40% AUC + 20% to 30% Cmax (SmPC) |  (SmPC) | 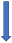 (SmPC) | 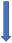 (SmPC) | 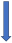 (SmPC) |
| Naproxen | P-glycoprotein competition. Pharmacodynamic interaction (increased bleeding time) | 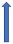 (SmPC) | 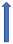 Increase the risk of bleeding. | No significant difference in AUC (SmPC) |  +55% AUC + 61% Cmax (SmPC) | No significant effect on AUC (SmPC) |
| H2 Receptor Antagonists, PPIs, Antacids (Aluminum Hydroxide + Magnesium Hydroxide) | Gastrointestinal absorption |  | Minimal effect | No relevant interaction | No relevant interaction | No relevant interaction |
| SSRIs, SNRIs | Pharmacodynamic effect on platelets |  | With caution (SmPC) | With caution (SmPC) | With caution (SmPC) | With caution(SmPC) |
| Increase in INR | Decrease in INR |
|---|---|
| Citalopram, Disulfiram, Entacapone, Phenytoin, Fluoxetine, Fluvoxamine, Paracetamol, Allopurinol, Celecoxib, Indomethacin, Methylprednisolone, Piroxicam, Tramadol, Cimetidine, Omeprazole, Orlistat, CMF, Ifosfamide, Levonorgestrel, Tamoxifen, Acetylsalicylic acid, Amiodarone, Dronedarone, Atorvastatin, Quinidine, Clofibrate, Diltiazem, Fenofibrate, Glucagon, Lovastatin, Propafenone, Propranolol, Simvastatin, Fluconazole, Miconazole, Lysine acetylsalicylate, Acetylsalicylic acid, NSAIDs, Clopidogrel, Dipyridamole, SSRIs, Ticlopidine, Thyroxine, Erythromycin, Isoniazid, Levofloxacin, Metronidazole, Sulfamethoxazole | Barbiturates (phenobarbital, thiopental), Carbamazepine, Chlordiazepoxide, Propofol, Azathioprine, Mesalazine, Sulfasalazine, Cyclosporine, Mercaptopurine, Metamizole, Raloxifene, Cholestyramine, Telmisartan, Rifampicin, Phenobarbital, Ritonavir, Saquinavir, Estrogens, Dicloxacillin, Griseofulvin |
| Medicinal Plant | Interaction Mechanism | Interaction Outcome |
|---|---|---|
| Danshen (Salvia milthiorriza) | Anticoagulant and vasodilator properties that potentiate warfarin’s anticoagulant effect. | Increased anticoagulant effect. |
| Don quai (Angelica sinensis) | Contains natural coumarin derivatives as well as antithrombotic and antiarrhythmic properties that potentiate warfarin’s effect. | Increased anticoagulant effect. |
| Ginseng (Panax quinquefolius) | Exact mechanism unknown but thought to induce warfarin metabolism due to ginsenoside’s steroid effects. | Decreased anticoagulant effect. |
| Goji berries (Lycium barbarum) | CYP2C9 inhibition. | Increased anticoagulant effect. |
| St. John’s Wort (Hypericum perforatum) | Induction of CYP1A2, CYP2C9, and CYP3A4. | Decreased anticoagulant effect. |
| Green Tea (Camellia sinensis) | Antagonizes warfarin’s effect due to vitamin K content. | Decreased anticoagulant effect. High doses of green tea reduce INR. |
| Ginkgo (Ginkgo biloba) | Platelet aggregation inhibition. | Increased anticoagulant effect. |
| Chamomile (Matricaria recutita) | In vitro inhibition of CYP3A4 and CYP1A2 activity. | Increased anticoagulant effect. |
| Ginger (Zingiber officinale) | Anticoagulant properties that potentiate warfarin’s effect. | Increased anticoagulant effect. |
| Cannabis (Cannabis sativa) | CYP2C9 inhibition. | Increased anticoagulant effect. |
| Gotu Kola (Centella asiatica) | In vitro inhibition of CYP2C9. | Increased anticoagulant effect. |
| Licorice (Glycyrrhiza glabra) | Inhibition of CYP3A4. | Increased anticoagulant effect. |
| Milk Thistle (Silybum marianum) | Inhibition of CYP2C9. | Increased anticoagulant effect. |
| Myrrh (Commiphora myrrha) | Liver enzyme inducer. | Decreased anticoagulant effect. |
| Soy (Glycine max) | May increase warfarin’s effect due to in vitro inhibition of CYP3A4 and CYP2C9. May also reduce its effect due to vitamin K content. | Decreased or increased anticoagulant effect. |
| Sweet Clover (Melilotus officinalis) | Contains coumarin that is converted to dicoumarol which causes a reduction in levels of coagulation factors II, VII, IX, and X. | Increased anticoagulant effect. |
| Turmeric (Curcuma longa) | Curcumin potentiates the effects of warfarin. | Increased anticoagulant effect. |
| Aloe Vera (Aloe barbadensis) | Decreased intestinal absorption of warfarin and/or increased renal elimination. | Decreased anticoagulant effect. |
| Garlic (Allium sativum) | Inhibition of platelet aggregation. | Increased anticoagulant effect. |
| Cranberry (Vaccinium macrocarpon) | Contains anthocyanins that are metabolized by CYP2C9 and CYP3A4. | Increased anticoagulant effect. |
| Devil’s Claw (Harpagophytum procumbens) | Inhibits in vitro activity of CYP2C8, CYP2C9, CYP2C19, and CYP3A4. | Increased anticoagulant effect. |
| Bilberry (Vaccinium myrtillus) | Antiplatelet properties that can increase warfarin’s anticoagulant effect. | Increased anticoagulant effect. |
| Horse Chestnut (Aesculus hippocastanum) | Synergistic effect with warfarin. | Increased anticoagulant effect. |
| Echinacea (Echinacea purpurea) | Accelerates the breakdown of warfarin, reducing its effectiveness. | Decreased anticoagulant effect. |
| Saw Palmetto (Serenoa repens) | Inhibits some cytochrome P450 isoenzymes, including CYP2D6,CYP2C9, and CYP3A4. | Increased anticoagulant effect. |
| Red Clover (Trifolium pratense) | Inhibits CYP2C9 and contained coumarins. | Increased anticoagulant effect. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.S.; Monteiro, C.; Duarte, A.P. Risks of Oral Anticoagulants: Interactions with Drugs and Medicinal Plants. Sci. Pharm. 2025, 93, 35. https://doi.org/10.3390/scipharm93030035
Martins AS, Monteiro C, Duarte AP. Risks of Oral Anticoagulants: Interactions with Drugs and Medicinal Plants. Scientia Pharmaceutica. 2025; 93(3):35. https://doi.org/10.3390/scipharm93030035
Chicago/Turabian StyleMartins, Ana Sofia, Cristina Monteiro, and Ana Paula Duarte. 2025. "Risks of Oral Anticoagulants: Interactions with Drugs and Medicinal Plants" Scientia Pharmaceutica 93, no. 3: 35. https://doi.org/10.3390/scipharm93030035
APA StyleMartins, A. S., Monteiro, C., & Duarte, A. P. (2025). Risks of Oral Anticoagulants: Interactions with Drugs and Medicinal Plants. Scientia Pharmaceutica, 93(3), 35. https://doi.org/10.3390/scipharm93030035







