Abstract
Diclofenac, an aryl-acetic acid derivative from the non-steroidal anti-inflammatory drug class, is the subject of multiple non-clinical and clinical studies regarding its usefulness in treating some dermatologic pathologies with an inflammatory, auto-immune, or proliferative component. Diclofenac is now approved for the topical treatment of actinic keratoses (AK), pre-malignant entities that have the risk of transformation into skin carcinomas. The hypothesis that diclofenac increases granular layer development in the mice tail model, having an anti-psoriatic effect, was demonstrated in a previous study in which 1% and 2% diclofenac ointment was evaluated. The aim of the present study was to perform experimental research on the topical effect of diclofenac in the mice tail model, by testing 4% and 8% diclofenac ointment, which is presented in the first part of the manuscript. In the second part of the manuscript, we also aimed to conduct a literature review regarding topical diclofenac uses in specific dermatological entities by evaluating the articles published in PubMed and Scopus databases during 2014–2025. The studies regarding the efficacy of topical diclofenac in dermatological diseases such as AK and field cancerization, actinic cheilitis, basal cell carcinoma, Bowen disease, Darier disease, seborrheic keratoses, and porokeratosis, were analyzed. The results of the experimental work showed a significant effect of 4% and 8% diclofenac ointment on orthokeratosis degree when compared to the negative control groups. Diclofenac in the concentration of 4% and 8% significantly increased the orthokeratosis degree compared to the negative control with untreated mice (p = 0.006 and p = 0.011, respectively, using the Kruskal–Wallis test) and to the negative control with vehicle (p = 0.006 and p = 0.011, respectively, using the Kruskal–Wallis test). The mean epidermal thickness was increased for the diclofenac groups, but not significantly when compared to the control groups. The results are concordant with our previous experiment, emphasizing the need for future clinical trials on the use of topical diclofenac in psoriasis.
1. Introduction
Diclofenac is an aryl-acetic acid derivative that inhibits cyclo-oxygenases (COX) and consequently prostaglandin (PG) synthesis, and has been approved since 1973 and occurs in various pharmaceutical forms, with systemic or topical administration. Diclofenac is indicated mainly for its anti-inflammatory and analgesic properties. Although diclofenac is considered a non-specific COX inhibitor, in vitro studies showed that diclofenac and celecoxib, a COX-2 selective inhibitor, act similarly on COX, when diclofenac is administered in a daily dosage of under 150 milligrams [1]. By this mechanism, diclofenac inhibits PG, prostacyclin, and thromboxane synthesis and decreases inflammation. Diclofenac has good transdermal absorption, in various concentrations, from 1% to 2.32%, being used for its anti-inflammatory properties. Diclofenac is classified as having low solubility and high membrane permeability. It is well absorbed when administered by the oral route and concentrates in inflamed tissue, where it dissociates from proteins due to the acidic environment and diffuses into intercellular space, having an excellent therapeutic effect. In the synovial liquid, diclofenac accumulates and remains for a longer time than in the bloodstream. When metabolized, the diclofenac metabolites are excreted renally and biliary, entering the entero-hepatic circuit. Diclofenac is poorly tolerated when internally administrated, as it irritates the gastric mucosa and has hepatic, renal, and cardiovascular toxicity [2,3,4]. The effect on the gastric mucosa is due to the reduction in PG synthesis, followed by a decreased secretion of mucosal protectors such as mucus and bicarbonate [5]. The gastrointestinal adverse effects are dependent on the systemic circulatory levels of diclofenac [6].
Diclofenac is available in multiple pharmaceutical forms for oral, rectal, and injectable administrations, as well as for topical application. There are several topical pharmaceutical forms available for diclofenac, such as cream, ointment, gel, foam, cutaneous solution, and TTS (transdermal therapeutic patches, e.g., patches). Since diclofenac has a good absorption rate into the dermis, but also in the muscular tissue, it is often indicated as topical therapy for long-term usage. In localized inflammatory conditions, topical treatment is often indicated, as it reduces the risk of systemic adverse reactions, and therefore patient adherence increases. In topical application of a substance, there are multiple factors that can influence bioavailability, like the thickness of the stratum corneum, epidermal hydration, and dermal vascularization degree, but also the modality by which the substance is applied, because, as with an occlusive dressing, the absorption is better. Newer transdermal delivery systems can have a significant influence on the absorption of diclofenac when topically administered [7].
In dermatology, diclofenac 3% gel is approved for the topical treatment of AK, which are considered precancerous because of their potential of developing into skin carcinomas, especially squamous cell carcinomas (SCCs) [8].
All skin cells represent a potential site for PG synthesis. COX-1 is constitutive while COX-2 is synthesized through the influence of growth factors, cytokines, or tumoral factors. The proliferative roles of COX-2 are expressed by PG that bind to specific receptors for PGE, PGF, and PGI. Nuclear factor kB (NF-kB) is involved in a variety of inflammatory and immunological processes of differentiation, proliferation, and apoptosis. NF-kB regulates COX-2 activity. COX-2 is also implicated in melanocytic and non-melanocytic tumoral development. Recent studies showed high levels of NF-kB and COX-2 in chronic psoriatic plaques. Thus, we underline the necessity of researching a new potential target for psoriasis treatment by inhibiting COX-2 [9].
The aim of the present study was to perform experimental research on the topical effect of diclofenac in the mice tail model, by testing 4% and 8% diclofenac ointment. We also conducted a literature review regarding topical diclofenac uses in specific dermatological entities by evaluating the articles published in PubMed and Scopus databases during 2014–2025, using “diclofenac”, “topical”, and “dermatology” as keywords.
2. Materials and Methods
2.1. Part I: Experimental Study
2.1.1. Animals and Substances
For each group, 6 male Albino mice were used. The substances used (diclofenac sodium, white soft paraffin) were provided by a pure substance provider (Fagron, Bucharest, Romania).
The animal study protocol was approved by the Ethics Committee of “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania (13310/27 May 2021) for studies involving animals.
2.1.2. Experimental Design
The mice tail model for psoriasis was used in order to evaluate the possible anti-psoriatic effect of 4% and 8% diclofenac ointment. The same experimental design as in our previous published work was used [10]. In this experiment, male Albino mice weighing 20–30 g were individually housed with ad libitum access to water and food for the entire duration of the experiment and in constant environmental conditions (humidity, temperature, 12 h light/dark cycle). We used four groups of animals: two negative control groups (untreated mice group, n = 6, and negative control with white soft paraffin as vehicle, n = 6) and two test groups (diclofenac 4% group, n = 6, and diclofenac 8% group, n = 6). For each group, the substance was topically applied in the proximal part of the tail, daily, under occlusion for 120 min, after which the ointment was washed with warm water. The same procedure was repeated once daily, 5 days per week, for two consecutive weeks. At the end of the experiment, the mice were sacrificed after general anesthesia according to the ethical guidelines for lab animal research, and the tails were fixed in 10% neutral-buffered formalin. After dehydration in graded ethanol, and clarification in butanol and infiltration with paraffin, 4 μm sections were obtained and stained with Mayer hematoxylin and eosin [10].
The sections were analyzed morphometrically using a Zeiss optical microscope (Carl Zeiss AG, Jena, Germany) and the Zen Blue program (Carl Zeiss AG, Jena, Germany), for the length of the continuous granular layer developed within the scale length, measured between two hair follicles, and for the epidermal thickness calculated from the inferior part of the epidermis to the stratum corneum. The main parameters obtained were orthokeratosis degree, relative drug efficacy, and mean epidermal thickness, as in our previous experiment [10].
- The orthokeratosis degree was calculated according to the following formula:
- G/S × 100
- G = the granular layer length developed within the scale.
- S = the scale length measured between two hair follicles.
- Ten consecutive scales were analyzed for each animal, 60 scales per group. Dimensions were calculated in micrometers.
- The relative drug efficacy was calculated according to:
- (OKtest − OKcontrol)/(100 − OKcontrol) × 100.
- OKtest = mean orthokeratosis values obtained for the test substances.
- OKcontrol = mean values of orthokeratosis obtained for negative control with the ointment base.
- Mean epidermal thickness was calculated after measuring the epidermal thickness from the inferior part of the epidermis to the stratum corneum (5 measurements for each scale, 10 scales per animal, 300 measurements per group).
2.1.3. Statistical Analysis
The statistical analysis was performed with SPSS (IBM, USA) version 25 using the Kruskal–Wallis non-parametric test, with statistical significance set at p ≤ 0.05, because the groups were not uniformly distributed following the Gauss curve.
2.1.4. Experimental Results
The experimental results regarding the main parameters evaluated in the experiment are shown in Table 1. The orthokeratosis degree was in the following order: diclofenac 4% > diclofenac 8% > negative control with vehicle > untreated mice (see Table 1).

Table 1.
Main parameters evaluated in the experiment.
The mean epidermal thickness was in the following order: negative control with vehicle > diclofenac 4% >diclofenac 8% > untreated mice (see Table 1).
The relative drug efficacy was 66.16% for diclofenac 4% and 66.12% for diclofenac 8%. (see Table 1).
In Table 2 and Table 3, the statistical comparisons made between two groups regarding orthokeratosis degree and mean epidermal thickness can be observed, in order to evaluate the model validity using the negative control groups, but also the statistical significance between diclofenac 4% and 8%.

Table 2.
Orthokeratosis degree (Kruskal–Wallis test).

Table 3.
Mean epidermal thickness (Kruskal–Wallis test).
The relative drug efficacy derives from the orthokeratosis degree parameter and shows the percentual intensity of the orthokeratosis effect of diclofenac related to the maximum intensity of this effect. This parameter allows for the comparison with other substances in various experimental settings. In Figure 1, the relative drug efficacy for diclofenac and tretinoin in the mice tail model can be observed. Diclofenac 4% and 8% had higher relative drug efficacy than tretinoin 0.05%, diclofenac 1%, and diclofenac 2% tested in our previous experiment [10].
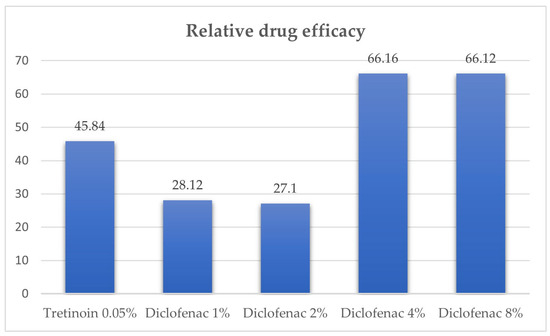
Figure 1.
Relative drug efficacy for diclofenac and tretinoin in mice tail model.
Taking into account the results obtained in this research for diclofenac 4% and 8%, and in our previous research for diclofenac 1% and 2% [10], in inducing granular layer development and normalization of the granular layer in the tail model for psoriasis, we further performed a literature review regarding topical diclofenac use in dermatology between 2014 and 2025.
2.2. Part II: Literature Review Regarding the Use of Topical Diclofenac in Dermatological Diseases (2014–2025)
2.2.1. Method
The literature search was conducted in PubMed and Scopus databases using the following keywords “diclofenac” AND “topical” AND “dermatology” and related terms. We further performed the search inside the references of the articles initially selected.
The data extracted from each article refer to diclofenac indication, dose concentration, and mechanism of action implied.
The PubMed database search revealed 90 articles, of which 39 were excluded, and 51 articles remained of interest. The Scopus database search revealed another 48 articles, of which 27 articles were excluded, and 21 articles remained of interest. A total of 14 articles were found in both databases, from which one article was excluded and 13 articles were of interest. After removing duplicates, 59 articles were included. In Figure 2 the article selection process can be observed.
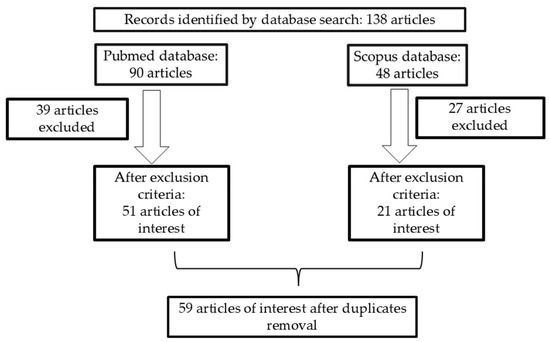
Figure 2.
Article selection process.
Exclusion criteria: articles that do not provide data about diclofenac topical treatment, articles that are not centered on topical diclofenac treatment results following dermatologic indication, and articles that are not written in English [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72].
2.2.2. Actinic Keratosis, Actinic Cheilitis, and Field Cancerization
The majority of articles evaluated diclofenac topical treatment of AK and field cancerization. The term field cancerization requires for diagnosis at least two of the following signs: atrophy, “sandpaper” texture, telangiectasia, and hyperpigmentation disorders [73]. For topical treatment of field cancerization, various substances can be used, e.g., diclofenac, imiquimod, and 5-fluorouracil (5-FU), and also surgical excision, cryotherapy, curettage, and photodynamic therapy (PDT).
In a synopsis of the 18 evidence-based recommendations for the treatment of AK detailed in the Guidelines of Care for the Management of Actinic Keratosis, diclofenac is conditionally approved to be used in the treatment of AK, with low quality of evidence. Also, the black box warning for cardiovascular and gastrointestinal adverse effects was underlined, as with other oral and topical medications in the non-steroidal anti-inflammatory drug class [74]. Strong recommendations were made for the use of UV protection, cryosurgery, topical imiquimod, and topical 5-FU, whereas conditional recommendations were for the use of PDT and topical diclofenac individually or in combination therapy [74].
Diclofenac sodium 3% in hyaluronic acid 2.5% gel as field-directed treatment is labeled as moderately effective, with evidence level 1 in immune-competent patients and level 3 in immune-suppressed patients [42,75].
According to an expert opinion report by Dréno et al. [76], diclofenac gel is well tolerated but less effective than 5-FU or imiquimod. Its use is recommended for non-hyperkeratotic or superficial AK. It was also noted that the maximum therapeutic effect is seen 30 days after treatment cessation.
Diclofenac 3% in sodium hyaluronate is less effective than other treatments of single and multiple AK and field cancerization treatments (grade recommendation A and level of evidence 1) according to the Oxford Centre for Evidence-Based Medicine, European Association of Dermato-Oncology, European Dermatology Forum, European Academy of Dermatology and Venereology, and Union of Medical Specialists (Union Européenne des Médecins Spécialistes) [77].
The Polish dermatological society recommends that the use of topical diclofenac 3% gel should be reserved for mild AK and can be combined before or after with other treatment modalities (cryosurgery or photodynamic therapy) [78].
Topical treatment with ingenol mebutate has been shown to have superior efficacy when compared to topical diclofenac sodium hyaluronate according to an expert opinion by Stockfleth et al. [79].
A review conducted by Bernal Masferrer et al. [80] that evaluated the topical treatment of AK and cancerization field revealed some studies regarding diclofenac’s mechanism of action, effectiveness, and side effects. The mechanisms of action implicated are a decrease in inflammatory markers (COX-2, CD3, CD8), and a reduction in apoptosis markers (p53, p21) and angiogenesis markers (CD31) [81]. The AK cure rate was 36.9 ± 9.5% at 3 months and 45% at 6 months follow-up. The noted adverse events were irritation, oedema, pain, and pruritus, similar to other topical treatments.
A systematic review conducted by Patel et al. [82] evaluated 20 original randomized control trials regarding field cancerization for topical treatment. Topical diclofenac sodium 3% received a clinical recommendation grade B, after topical 0.5% (5-FU) salicylic acid association and 0.5% 5-FU.
An updated review performed by Aggarwal et al. [83] also evaluated topical treatments of field cancerization. The study showed that photodynamic therapy (PDT) with either aminolevulinic acid (ALA) or methyl aminolevulinate (MAL), as well as topical treatments with 5-FU, diclofenac gel, piroxicam, imiquimod, and ingenol mebutate, had higher efficacy than vehicle treatments. The combination of 5-FU and salicylic acid (5-FU–SA) was the most effective but also caused the most frequent adverse reactions. Tirbanibulin had a favorable safety profile related to imiquimod, 5-FU, and diclofenac. Ingenol mebutate is no longer recommended for the treatment of AKs because of the increased risk of cutaneous SCC development.
In a review by Arcuri et al. [84] regarding pharmacological agents used in the prevention and treatment of AK, multiple clinical and preclinical studies with diclofenac were reviewed. Diclofenac sodium 3% in 2.5% hyaluronan gel was applied twice daily for 30–90 days. The optimal duration of treatment was 90 days, with no difference in outcomes regarding clinical and histological response when compared to a longer duration of 6 months. The most frequent adverse reactions were erythema, skin dryness, and pruritus. A 2019 study evaluated the effect on pig ears of the combination of diclofenac with a group of naturally occurring compounds with antiproliferative effects, with promising results [84,85].
In a review by Collins et al. [86], diclofenac 3% gel applied twice daily for 16 weeks led to a 41% clearance of AK in organ transplant patients.
In a review by Dodds et al. [87] regarding treatment of AK, diclofenac produced a complete resolution in 50% of cases when topically applied for 3 months, with good tolerance and minimal adverse reactions (contact dermatitis, xerosis). Treatment compliance was lower when the treatment was longer. Diclofenac had a higher rate of complete response after cryotherapy (64% vs. 32%, respectively).
In a review by Micali et al. [88], diclofenac is considered to have lower overall efficacy in treating AK, but is well tolerated, being an alternative therapy for patients who are not willing to endure the side effects associated with 5-FU or imiquimod. Another advantage of diclofenac gel is represented by its pregnancy category B classification, which allows for safer use compared to other topical medications in women of childbearing age with AKs.
A review by Piaserico et al. [89] compared the efficacy of PDT combined with topical treatment (diclofenac, imiquimod, adapalene, 5-FU, calcitriol), systemic treatment (acitretin, methotrexate, and polypodium leucotomos) and mechanical–physical treatment for AK. It was concluded that the comparison should be made after more than four weeks of topically applying diclofenac, two times per day. Pretreatment with imiquimod, adapalene, 5-FU, and calcipotriol showed greater effectiveness for AK treatment than topical treatment in monotherapy but evidence regarding diclofenac was poor.
Two studies with topical diclofenac: a randomized, parallel-group clinical trial with 28 patients by Segatto et al. [90] and a single-center, open-label, prospective, randomized controlled clinical trial of 200 patients by Zane et al. (2014) [91] were assessed in a review of patient satisfaction regarding topical treatment for AK by Khanna et al. [92]). In the first study, patients either applied 3% sodium diclofenac gel twice daily for 12 weeks or 5% 5-FU cream twice daily for 4 weeks. Patient satisfaction was similar in both groups, with no significant difference. A total of 54% of patients treated with 5-FU reported all lesions to be healed in comparison to 20% of patients treated with diclofenac sodium. Diclofenac was better tolerated, with higher satisfaction regarding adverse events (93.3% versus 38.4%, p = 0.008). In the second study, a comparison was drawn between treatment with diclofenac 3% plus hyaluronic acid gel (DHA) and MAL-PDT in adult Caucasian patients with skin types I–IV and at least five AKs of the face and scalp. Results showed that more patients treated with MAL-PDT were satisfied than those treated with DHA (59% versus 6%, p < 0.0001) [92]. Diclofenac was less effective than 5-FU or MAL-PDT in the topical treatment of AK.
A study by Lang et al. (2024) [93] evaluated multiple therapies for AK, such as surgical methods, daylight PDT, cryotherapy, conventional PDT, and topical treatment with ingenol mebutate, imiquimod, 5-FU, and diclofenac, using a questionnaire for treatment satisfaction. Topical diclofenac in hyaluronic acid received the lowest overall satisfaction score (53.03). The effectiveness of diclofenac 3% topical treatment was 54.54 (±21.85) and differed statistically significantly from surgical methods, which had the highest effectiveness (81.95 ± 14.14). A percentage of 63.44% of all the evaluated patients preferred the topical treatment, especially cream or tincture.
In an observational study by Zhao HJ [94] regarding the international trend in the use of topical treatment for AK and SCC prevalence, diclofenac sodium 3% gel is less commonly used. Diclofenac is used mainly in 14 high-income countries (13 of them in the Northern Hemisphere) and in no middle-income countries, being more expensive and better tolerated, but with lower efficacy. Association of medication utilization with SCC prevalence was not clear. For every percent increase in SCC prevalence there was a decrease in diclofenac use.
A questionnaire-based study by Koch et al. [95,96], disseminated by two publications evaluated the adherence to topical treatment in AK. In this study, 47.8% of the patients received diclofenac and the rest received imiquimod, 5-FU, 5-FU and salicylic acid, and PDT. A high non-adherence rate was found (46.9%) with a poor clearance rate. Only 30.9% of the patients followed the treatment according to the summary of product characteristics. The study emphasizes the role of a longer pretreatment consultation for increased adherence to treatment.
A case control study by Jedlowski et al. [97] conducted between 2012 and 2020 investigated the association of SCC development after AK treatment with some topical substances like ingenol mebutate, diclofenac, and imiquimod in the FAERS system (FDA Adverse Event Reporting System). A total of 7912 adverse reactions were reported as “squamous cell carcinoma”, from which 110 cases were linked to a topical medication used for AK (85 ingenol mebutate, 15 fluorouracil, 9 imiquimod, and 1 diclofenac).
The tendency in prescribing topical treatment for AK was evaluated among 110 dermatologists in Italy by Moretta et al. [98]. The treatment choices were in the following order: cryotherapy and PDT, topical treatment with diclofenac (14.5%), imiquimod 3.75% (9.1%), imiquimod 5% (5.5%), ingenol mebutate (5%), and 5-FUl (7.3%). It was observed that 20% of dermatologists never recommended diclofenac.
A study by Perino et al. [99] regarding patient adherence to diclofenac topical treatment in AK evaluated the standard of care versus an integrated low-intensity intervention program efficacy that included additional visits at 30 and 60 days from baseline. There was no difference in adherence between the two groups.
Treatment adherence, treatment satisfaction, and health-related quality of life in patients with topical treatment for AK in Denmark and Sweden was analyzed using a subset of the RAPID-ACT (Real-Life Topical Field Treatment of Actinic Keratosis, observational study) data by Norrlid et al. [100]. Ingenol mebutate patients reported a higher satisfaction with treatment effectiveness compared to patients treated with diclofenac (p = 0.006), and the difference between diclofenac and imiquimod was borderline significant (p = 0.061). Ingenol mebutate patients reported better treatment adherence compared to both diclofenac (p < 0.001) and imiquimod patients (p = 0.007). Diclofenac patients reported a higher health-related quality of life impairment compared to patients treated with imiquimod (p = 0.048) or ingenol mebutate (p = 0.017). Local skin reactions were less common in patients treated with diclofenac (42%) compared to those treated with imiquimod (79%; p < 0.001) or ingenol mebutate (89%; p < 0.001).
In a study by Calzavara-Pinton et al. [101] it was shown that one tube of diclofenac sodium (Solaraze®) can treat a cancerization field area of 33.3 cm2. It is considered that evaluation of the treatment cost for multiple AK is better when referred to the area treated than to the number of lesions. This was a pharmacoeconomic study that presented a new method of evaluation of topical treatment cost, taking into account the surface of area treated.
A study by Mazella et al. [102] of 30 patients with AK who were treated with diclofenac sodium 3% presented a statistically significant improved outcome when compared with 5-FU at T1 (15 days). All histological and immunohistochemical parameters considered were reduced, especially CD31. The results sustained a possible chemo-preventive action of COX inhibition in the skin (see Table 4).

Table 4.
Topical diclofenac research in actinic keratosis and field cancerization.
A multicentered, prospective, real-life study by Neri et al. [107] regarding treatment satisfaction evaluated 1136 adult patients that were treated with a topical field directed treatment (961 received ingenol mebutate while 175 received either diclofenac 3% or imiquimod 5%). The clearance rate at 1 month was 84% and communication clarity was associated with higher treatment satisfaction and lower risk of non-adherence.
In a pharmacoeconomic study by Nisticò et al. [108], the costs of diclofenac sodium compared to ingenol mebutate and imiquimod in the treatment of AK were assessed for a period of 52 weeks. A comparison between 500 patients treated with diclofenac sodium, 500 patients with ingenol mebutate, and 500 patients with imiquimod, was made. Patients in the diclofenac sodium study arm reached a lower total cost than ingenol mebutate or imiquimod arms.
A pharmacoeconomic evaluation by Tolley et al. [109] regarding topical diclofenac sodium 3% versus ingenol mebutate 0.015% concluded that incomplete data are available in the literature. The International League of Dermatological Societies/European Dermatology Forum S3 guidelines for AK treatment ranked ingenol mebutate higher than diclofenac.
In a study by Schmitz et al. [103] of 24 patients treated with diclofenac sodium 3% in hyaluronic acid 2.5% gel, 20 patients showed an improvement in AKASI (Actinic keratosis area and severity index), 2 patients showed a stable AKASI, and 2 patients showed a worsening of AKASI. AKASI is used to assess the AK severity on the head, by using four areas (scalp, forehead, left and right face) and evaluating the percentage of area affected, the distribution, the erythema, and the degree of thickness (see Table 4).
According to Danish nationwide healthcare registries, in a cohort of patients treated for skin diseases in 2019, including individual patient information on all primary care prescriptions and hospital-administered medications, 1595 patients used diclofenac for AK; the median age was 75 and 63% were male users [110]. An important number of elderly patients from Denmark were treated with diclofenac for AK.
In a case series report by Dirschka et al. [111], 12 patients with AK received diclofenac 3% gel (Solaraze) twice daily for 12 weeks, followed by a treatment-free interval of 2 weeks, then once-daily application of Actikerall (5-FU 5 mg/g and salicylic acid 100 mg/g) to any remaining lesions for up to 6 weeks as required. Two patients received Solaraze alone. Complete (clinical and histological) clearance was achieved in 8 of 10 male patients, in 2 of the patients with Solaraze alone and in 6 patients with sequential treatment. In the latter group, most lesions responded to Solaraze and the remaining lesions were cleared with Actikerall.
Treatment with diclofenac sodium reduced the lesion sizes in field cancerization treatment from 67% to 75%, with similar results to piroxicam, in a study by Jetter et al. [112].
A study by Faghihi et al. [104] evaluated the efficacy of topical 1% colchicine gel versus 3% diclofenac sodium gel, twice daily for 6 weeks, for the treatment of AK. One month after the treatment, the size of surface of lesions was reduced significantly compared to pretreatment in the diclofenac group (p < 0.001) and also in the colchicine group (p < 0.001), with no statistical difference between the two groups (see Table 4).
Regarding the effect of diclofenac on AK, a study by Singer et al. [105] analyzed the biopsies of AK by evaluating myeloid and T cell infiltration, immune cell activation, and glucose, amino acid, and Krebs cycle metabolism prior to, during and after topical treatment with diclofenac. The results were compared with biopsies from untreated, sun-exposed, healthy skin as controls. Prior to treatment with diclofenac, AK showed decreased glucose levels and increased lactate, with accelerated glycolysis, elevated Krebs cycle intermediates other than citrate and amino acids, and increased levels of dermal CD8+ T cells. After treatment with diclofenac, the level of lactate and amino acids was reduced, with high IFN-gamma mRNA expression. The study is interesting as it shows that premalignant entities have altered metabolism, but also that diclofenac acts by mechanisms other than COX inhibition (see Table 4).
Topical diclofenac 3% twice daily for 6 weeks was tested in a clinical trial by Husein-El Ahmed et al. [106] that evaluated ingenol mebutate versus imiquimod versus diclofenac for actinic cheilitis. In the diclofenac group, the clinical response was poor and significantly lower than in the ingenol mebutate and imiquimod groups (see Table 4).
A prospective study by Medeiros et al. [113] reviewed three clinical trials with topical diclofenac for actinic cheilitis. The complete clinical response varied from 20% to 71.4%, with good tolerance to treatment [113,114,115].
A systematic review of four studies by Bakirtzi et al. [114] of 62 patients with diclofenac 3% twice daily for 1 to 6 months for actinic cheilitis showed a clinical cure rate of 45.2% and a recurrence rate of 6.5% [114].
The results showed a clinical response between 20% and 71.4% and recurrence rate between 0 and 6.5%; thus, one can consider that the treatment with topical diclofenac could be promising for this particular form of AK.
Topical diclofenac 3% gel, applied twice daily for up to 4 months, was used for periocular AK in a case series by Batra et al. [116] of four patients, with a clinical cure rate of 100% after the treatment and two patients having recurrence [116,117]. The number of patients tested was small and future studies are needed to establish the utility in periocular diseases.
Regarding adverse reactions, a case report of two patients having contact allergic dermatitis after topically applied diclofenac but not after systemic diclofenac was reported by Beutner et al. (2022) [118]. Gulin et al. [119] also reported four cases of diclofenac-sodium-induced allergic contact dermatitis after topical diclofenac 1% gel applications [119].
The precise mechanism of action by which diclofenac acts in AK treatment is not elucidated, but the reduction in COX-2 levels, CD3, and CD8 compared with pretreatment levels and CD31 as a marker of neo-angiogenesis shows that diclofenac has anti-inflammatory, anti-angiogenesis, antiproliferation, and apoptotic properties. The apoptotic effects act on SCC lines by activation of the mitochondrial intrinsic apoptosis pathway and the extrinsic death ligand pathway [120,121].
2.2.3. Malignant Pathologies
Basal Cell Carcinoma
In a study by El-Khalawany et al. [122] conducted on 14 patients diagnosed with high-risk basal cell carcinoma (BCC) considered inoperable (tumor diameter above 5 centimeters, located on the scalp, face and trunk) who received CO2 laser ablation combined with topical diclofenac sodium 3% gel for five days and topical imiquimod 5% for two days, 9 patients had significant improvement [122,123].
In a literature search performed by Tan et al. [124] using a comprehensive PubMed search to evaluate BCC and AK lesion criteria and topical treatment indications, diclofenac 3% gel was indicated for superficial or low-risk BCC.
The efficacy of topically applied 3% diclofenac gel as a single agent, calcitriol 3 μg/g ointment as a single agent, and a combination of diclofenac with calcitriol was tested in superficial BCC (sBCC) and nodular BCC (nBCC), applied twice daily under occlusion, for 8 weeks, in a study by Brinkhuizen et al. [125]. A significant decrease in Ki-67 and Bcl-2 was shown in the diclofenac group and in the combination group. A total of 64.3% of the patients treated with diclofenac and 43.8% with combination therapy had complete histologic regression for sBCC. For nodular BCC, no significant changes were shown (see Table 5).

Table 5.
Topical diclofenac research in basal cell carcinoma.
From a clinical point of view, there is a favorable evolution, but the effect of diclofenac cannot be evaluated, considering that the treatment was combined with other treatments and it was applied for a short period of time. Thus, diclofenac decreased the tumoral markers Ki-67 and Bcl-2, an argument for its antiproliferative effects (see Table 5).
Bowen Disease
In Bowen disease, in some isolated cases, diclofenac 3% gel applied once or twice a day for 8 to 12 weeks led to clinical and histologic clearance with level of evidence IV Oxford Centre for Evidence-Based Medicine (case series, case control studies) [126].
A case report of digital Bowen disease presented by Gracia-Cazaña et al. [127] used MAL-PDT in three therapy sessions, at 8-day intervals, after topical 3% diclofenac in 2.5% hyaluronan, once a day for three months. The resolution of the lesion was complete at 4 months after the treatment ended.
Because there are few case reports and because diclofenac was combined with other treatments, further studies are needed in order to elucidate its effectiveness in digital (acral) Bowen disease.
2.2.4. Darier Disease
Darier disease is caused by a reduced expression of the ATP2A2 gene that codifies SERCA 2 (a sarcoplasmic/endoplasmic reticulum calcium-ATPase) involved in keratinocyte differentiation and intercellular communication. A possible mechanism involved can be the inhibition of PGE-2 that reduces the ATP2A2 gene expression [128,129].
Diclofenac sodium 3% with 2.5% hyaluronic acid in natrosol gel was used in a case report of a patient with Darier disease [130], in topical applications, twice daily for 8 weeks, on the left side of the body. After improvement of the eruption, the application was continued on the right side of the body with significant regression at 4 months follow-up [130].
There are other case reports by Santos-Alarcon et al. [131] regarding the usefulness of topical diclofenac 3% in the treatment of Darier disease, once daily for 6 months, in a patient in whom isotretinoin and doxycycline were ineffective. Another report of two cases of Darier disease used the treatment with diclofenac sodium 3% in hyaluronic acid 2.5% (Solaraze), twice daily. The local treatment was well tolerated, except for irritation.
The case reports of diclofenac sodium used in Darier disease are with diclofenac sodium 3% and 1%. The reported adverse reactions are local irritation and one patient had recurrence of the eruption 3 months after stopping the treatment [129].
In another disease with aberrant keratinocyte differentiation, Darier disease, in a very small number of cases (six) treated with diclofenac 3% and 1% with hyaluronic acid, improvement of the clinical evolution was obtained as compared with the natural evolution of the disease.
2.2.5. Pityriasis Versicolor
In a randomized controlled clinical trial by Swadi et al. [132], with two groups, 20 patients each, diclofenac 1% gel was evaluated in the treatment of pityriasis versicolor. The first group received 1% diclofenac gel, two times per day for one month, and the second group (positive control) received clotrimazole cream, two times daily, for one month. The clotrimazole group had a better complete response than diclofenac gel, 65% versus 20%, respectively (p = 0.008). Four weeks after the treatment, the clotrimazole group maintained a better complete response of 90% [132]. Diclofenac has no clinically significant therapeutic effect in pityriasis versicolor (see Table 6).

Table 6.
Topical diclofenac research in benign pathologies (seborrheic keratosis and pityriasis versicolor).
2.2.6. Seborrheic Keratoses
Regarding topical treatment of seborrheic keratoses with diclofenac, a systematic review conducted by Natarelli et al. [134] showed a good response after topical treatment with diclofenac sodium 1% solution, but also with other topical substances (tazarotene 0.1% cream, hydrogen peroxide, 65% and 80% trichloroacetic acid, maxacalcitol 25 µg/g, urea-based solution).
A study by Afify et al. [133] evaluated 30 patients with seborrheic keratosis treated with diclofenac sodium gel 1% and ibuprofen gel, twice per day for two months. The surface area of the lesions was measured. There was a statistically significant difference between the measured area before and after treatment with diclofenac, but there was not any difference between diclofenac and ibuprofen. The best results were for the diclofenac 1% group (see Table 6) [133]. Taking into account that diclofenac was tested in a small number of patients, further studies are needed.
2.2.7. Porokeratosis
Two studies regarding the efficacy of topical diclofenac 3% gel, twice daily, for three months, were published in treating disseminated superficial porokeratosis, either as a single therapy or in association with a systemic therapy like etretinate [135,136].
In a study by Shimizu et al. (2018) [136] of disseminated superficial porokeratosis, in which diclofenac 1% gel was applied twice a day for one month in association with etretinate systemic treatment, a significant reduction in the scaling and color of the brown lesions was achieved. The efficacy of topical diclofenac in porokeratosis was previously verified in two of eight cases in a study by Vlachou et al. (2008) [137] and in seven of thirteen cases in a study by Marks et al. [135]. In this case, the benefit of using topical diclofenac was for recalcitrant lesions that did not respond to other topical treatments (corticosteroids, vitamin D analogue cream, imiquimod cream, adapalene gel, keratolytic treatments).
In the study by Marks et al. (2009) [135], seventeen adult patients with disseminated superficial actinic porokeratosis applied diclofenac sodium 3% gel to a target area twice daily for 12 weeks, followed by a 4-week follow-up visit, and if the lesions were still present patients continued the treatment for an additional 3 months (24 weeks in total). Two parameters were evaluated: target area after applying diclofenac on the forearm and global body area (untreated). Among the patients who completed 12 weeks of treatment, a mean decrease of 4% in target area lesions with a 12% mean increase in total body lesions was noted. Seven patients of thirteen had a decrease in lesion number and one had a stable number of lesions. Among the patients who completed 24 weeks, a 10% increase in the target area and a 19% mean increase in total body lesions were noted. The target area progressed to a lesser extent when compared to the global lesion count in both groups. The objective parameter, the target area treated with diclofenac versus untreated areas, was improved significantly by diclofenac.
Also in this case, a limited number of patients was evaluated and further studies are needed. Nevertheless, the natural evolution of porokeratosis must be taken into account in interpretation of these results.
2.2.8. Wound Healing
In an experimental wound model study by Costa et al. [138], diclofenac was applied topically in 15 male Wistar rats. Four spherical wounds were performed on the dorsum of all animals, two each side of the median line. In the cranial wounds, topical diclofenac gel (11.6 mg/g) was administrated and caudal wounds were only washed with saline isotonic solution (NaCl 0.9%). Animals were then randomly assigned into three subgroups (N = 5 each): wound repair seven days (WR7), wound repair 14 days (WR14), and wound repair 21 days (WR21). The WR7 group showed a difference between diclofenac wound area and saline solution wound area (p < 0.01); other comparisons were not significant. Gross wound analysis showed significant higher scab formation in diclofenac wounds at seven days, however. Diclofenac wounds showed less hyperemia and phlogistic signals at 14 days [138].
2.2.9. Anti-Aging
In a review by Li Pomi et al. [139] regarding anti-aging effects of topical diclofenac, improvements were described in irregular pigmentation and skin roughness, and in reflectance confocal microscopy features, in 20 patients, for diclofenac 3% gel, applied for two months, and also favorable effects in seborrheic keratoses.
3. Discussion
3.1. Experimental Study
Psoriasis represents a chronic dermatological disease with auto-immune and inflammatory pathogeny, with extracutaneous manifestations. The treatment is challenging, being topical and systemic, as a single therapy or combined [140]. In recent years, various deep learning methods have been utilized to create computer-aided diagnosis systems to detect psoriasis cases and suggest the best treatment options [141].
Regarding the use of diclofenac in psoriasis, our previous article (Nițescu et al., 2022 [10]) showed that topical diclofenac 1% and 2% had anti-psoriatic effects in vivo, by using the tail model for psoriasis [10]. In this model, topical diclofenac 1% and 2%, tretinoin 0.05% (positive control group), and white soft paraffin (negative control group) were applied in the proximal part of the mice tail and histopathological sections were obtained in hematoxylin-eosin staining. Morphometrical assessment of the obtained sections was made using three parameters (orthokeratosis degree, relative drug efficacy, and mean epidermal thickness).
Topical tretinoin 0.05% and 0.01% had evidence regarding its effect in inducing granular layer development, being studied in various dermatologic diseases like acne, psoriasis, vulgar verrucae, ichthyosis, hyperpigmented disorders, and photoaging [142,143,144]. Tretinoin 0.05% ointment was used as positive control as it is a substance known for its effect in inducing normal keratinization, having an anti-psoriatic effect. Two negative control groups, one with untreated mice and one with white soft paraffin as base, were used. The ointment base has occlusive properties, enhancing the skin absorption of the tested substance.
Diclofenac 1% and 2% ointment significantly increased the orthokeratosis degree, based on granular layer development, when compared with both negative control groups represented by the untreated mice group and the white soft paraffin group (p = 0.006 and p = 0.004, respectively, using the Kruskal–Wallis test). Diclofenac 1% and 2% had an anti-psoriatic effect comparable with the tretinoin group used as positive control (p = 0.109 and p = 0.262, respectively, using the Kruskal-Wallis test). Although diclofenac did not significantly increase the mean epidermal thickness, it induced orthokeratosis and had an antiproliferative effect [10].
In the present study, diclofenac in the concentration of 4% and 8% significantly increased the degree of orthokeratosis compared to the negative controls. There was no statistically significant difference between the two diclofenac concentrations tested (4% and 8%). Diclofenac 4% and 8% induced a similar degree of orthokeratosis (71.3% and 71.27%, respectively) with similar intensity of drug action (66.16% and 66.12%, respectively) and mean epidermal thickness (29.34 µm and 27.08 µm, respectively). Diclofenac 4% can be used with optimal results in terms of the degree of orthokeratosis (71.3%), with higher relative drug efficacy (66.16%), and with a lower risk of adverse reactions in long-term use. The mean epidermal thickness was increased for the diclofenac groups but not significantly when compared to untreated mice.
The relative drug efficacy represents a valuable parameter that permits the comparison of the anti-psoriatic effect of a substance tested using the mice tail model in different experimental conditions. This parameter permits the comparison with other substances in various experimental settings. Diclofenac 4% and 8% had higher relative drug efficacy (66.16% and 66.12%, respectively) than tretinoin 0.05% (48.54%), diclofenac 1% (28.12%), diclofenac 2% (27.1%), and beech tar 5% (19%), and lower than retinoic acid 1% (79%) and dithranol 3% (75%) [10,145].
Diclofenac increased the orthokeratosis degree for all tested concentrations (1%, 2%, 4%, and 8%). As the relative drug efficacy for diclofenac 4% and 8% is similar, diclofenac 4% might be a more appropriate concentration for the anti-psoriatic effect. Still, diclofenac in all concentrations tested had a lower orthokeratosis degree compared to retinoic acid 1% and dithranol 3%.
Topical diclofenac was tested in multiple concentrations in order to observe the existence of a possible dose-dependent effect and to compare with the 3% diclofenac gel already approved for the treatment of AK and cancerization field. Following these tests, a ceiling effect was obtained at 4% diclofenac concentration, close to the 3% approved in clinical use. Diclofenac induced a significant effect in terms of normalizing the epidermal keratinization process, objectified by the presence of the granular layer and consequently by orthokeratosis degree, in the experimental tail model in mice. The mouse normally has an alternation of orthokeratosis and parakeratosis, and the increase in the orthokeratosis degree may be an indirect sign of an anti-psoriasis effect of a certain substance.
The main therapeutic classes used for the topical treatment of psoriasis are represented by corticosteroids, which have local anti-inflammatory and immunomodulatory effects; retinoids, which normalize cell differentiation and turnover, although they are not considered first-line in psoriasis, as they have important irritant effects, but can be applied to small skin areas in inverse psoriasis or on the face; topical calcineurin inhibitors, which have immunomodulatory and anti-inflammatory effects; vitamin D analogues, which modulate keratinocyte differentiation and stop hyperproliferation; anthralin with keratolytic and antiproliferative effects and salicylic acid with keratolytic and anti-inflammatory effects. The three main mechanisms of action implied are the anti-inflammatory effect, the immunomodulatory effect, and the keratinocyte differentiation modulation effect. Diclofenac, as a basic representative of non-steroidal anti-inflammatory drugs, is already approved in multiple topical pharmaceutical forms. Through the literature review part, this paper aims to evaluate the trend regarding the use of this substance in dermatology and to evaluate the utility of future clinical studies with topical diclofenac in psoriasis.
Limitation of the Experimental Study
Psoriasis is a systemic disease with complex pathogenesis, with multiorgan involvement, and until now there has not been an experimental model that evaluates all aspects of skin pathology and the systemic response, which is a limitation of this study. The tail model is limited in mimicking human psoriatic plaques, and lacks histological inflammation scoring or immune cell quantification, facts that can be considered other important limitations. A possible advantage of topical application over systemic application of diclofenac would be an increased bioavailability at the keratinocyte level, with reduced systemic effects.
3.2. Literature Review
The articles published in the last 10 years regarding the uses of topical diclofenac in dermatology are mainly regarding AK, actinic cheilitis, and field cancerization, but also examine other diseases characterized by aberrant keratinocyte differentiation, proliferation, or inflammation, such as basal cell carcinoma, Bowen disease, Darier disease, seborrheic keratoses, and porokeratosis.
Diclofenac and also piroxicam are used in the topical treatment of AK, entities characterized by keratinocyte proliferation and aberrant keratinocyte differentiation, with the potential for malignant transformation into SCC [146].
The antiproliferative role of diclofenac obtained in the studies is explained by the blockade of COX-2, with a reduction in angiogenesis and an apoptotic effect. The decrease in the level of PG, especially PGE-2, through inhibition of COXs, is directly involved in nociception and inflammation. By acting on EP1, the receptor for PGE-2, coupled with Gq proteins, with the inositol triphosphate/phospholipase C pathway as second messenger, calcium is released from the stores, with the activation of protein kinases. By activating the EP4 receptors, which are coupled with Gs proteins, the adenylyl cyclase/protein kinase A pathway is activated.
Diclofenac slows the progression and treats AK by inhibiting pro-inflammatory mediators and neo-angiogenesis through involvement in the metabolism of arachidonic acid [146,147]. Recent studies (2019) have shown that diclofenac has anti-tumoral properties by inhibiting glycolysis and myc proto-oncogenes, effects demonstrated in vivo (carcinoma, melanoma, glioma, and lymphoma) [148]. The involvement of diclofenac in carbohydrate metabolism occurs through the accumulation of intracellular lactate or through the inhibition of the cellular and mitochondrial proton-monocarboxylate transporter [149]. Diclofenac inhibits GLUT-1 (glucose transporter), LDHA (lactate dehydrogenase A), and MCT-1 (monocarboxylate transporter), with decreased lactate secretion and decreased glucose reuptake, and inhibits the myc oncogene.
The application of diclofenac in AK influences the composition in immune cells, with a higher level of IL-10, which is implicated in the limitation of inflammatory responses and in regulation of the differentiation of some cells, like keratinocytes, endothelial cells, and immune cells. Also, in the skin of patients with AK treated with diclofenac, the levels of lactate and amino acids were normalized. Diclofenac also affects tryptophan metabolism in AK. Modifications in tryptophan metabolism are common in patients with SCC, atopic dermatitis, and psoriasis [105].
Following the analysis of COX-1 and COX-2 levels in AK, it was observed that the level of messenger RNA corresponding to each enzyme is not significantly changed, but the ratio is changed in favor of COX-2 [105]. By inhibiting COX-2, antiproliferative effects occur in human cancer cell lines [150].
Regardless of COX-2 inhibition and the apoptotic effect of diclofenac, which could explain its efficacy in premalignant, malignant, and inflammatory skin diseases, diclofenac showed an interesting effect on different ion channels. Diclofenac acts on ion channels: voltage-dependent sodium [8,9,10,11], L-type calcium [105,151], voltage-dependent potassium (Kv1.2, Kv1.5, Kv2.1, KV 1.3) [152,153], transient external potassium channels (IA) Kv4.x [154], muscarinic potassium channels (M-type or Kv7. 2/3) [153], vascular potassium channels (Kv7.4, Kv7.5) [155], ATP-sensitive potassium channels (KATP) [156], calcium-activated potassium channels K(Ca), calcium-gated potassium channels (EAG) [157], acid-sensitive ion channels (ASIC) [158], TRP (transient receptor potential) channels [159,160], and other non-selective channels (activated by stress or phenamates) [161]. Diclofenac can also act on sodium channels on a common site with carbamazepine [162]. This effect of diclofenac is independent of its action on COXs [163]. Diclofenac acts by inhibiting Kv1.3 potassium channels in activated macrophages, with decreased IL-2 and decreased channel protein gene expression. Thus, a decrease in macrophage migration is produced, and consequently, diclofenac can modulate immune responses in addition to the anti-inflammatory effect [152].
This part of the article shows that topical diclofenac is of great interest regarding a potential use in dermatological diseases associated with inflammation and aberrant keratinocyte proliferation or differentiation. Multiple clinical and non-clinical research studies tried to elucidate the mechanism underlying its effectiveness in these diseases but the results have been inconsistent. Due to the complexity of their pathogenicity and the diversity of molecular disturbances implicated in the initiation and progression of these pathological phenomena, clinical findings were limited to a small number of patients or case reports, as in vivo or in vitro studies are scarce.
Diclofenac 3% is approved for the treatment of AKs according to international guidelines, although for this indication it has a recommendation grade A of non-use, compared to other indicated substances. In this regard, in the literature there is a series of reviews as well as clinical studies on efficacy, safety, and adherence, but also pharmacoeconomic studies. From their analysis, we can conclude that diclofenac is less effective in AK therapy than imiquimod 5%, 5-FU, PDT, or cryotherapy, but it has a superior safety profile to most of them. In contrast, adherence to therapy is low due to the long administration schedule (2 times a day, for at least 3 months), and, in this context, the costs of therapy are relatively high.
The use of diclofenac for the topical therapy of malignant pathologies, in this case BCC, is relatively limited, with one study demonstrating a favorable effect in superficial BCC but not in nodular BCC.
In other dermatological diseases, the topical use of diclofenac has been anecdotal, is not supported by preclinical research, and requires clinical trials on larger groups of patients, which should be multicentric due to the low incidence of these pathologies.
Although the mechanism of action of diclofenac has not yet been well defined in these dermatological entities, there is some scientific proof of its efficacy in AK.
4. Conclusions
Diclofenac 4% induced granular layer development in mice, with optimal results in terms of orthokeratosis degree, with higher relative drug efficacy than diclofenac 8% and with a possible lower risk of adverse reactions in long-term usage.
Taking into account the fact that diclofenac was not thoroughly researched for the topical treatment of psoriasis, neither in animals nor in humans, the results obtained in the present experimental study can be considered interesting in opening new perspectives into the topical treatment of psoriasis.
Most of the reviewed studies regarding the topical use of diclofenac in dermatology described favorable effects of diclofenac, mainly in AK, actinic cheilitis, and field cancerization, and less in seborrheic keratoses, Darier disease, Bowen disease, porokeratosis, pityriasis versicolor, wound healing, and basal cell carcinoma.
Consequently, we consider that diclofenac represents a promising topical treatment for some dermatologic pathologies with inflammatory, auto-immune, or proliferative components, characterized by aberrant epidermal proliferation. Future experimental and clinical studies are needed in order to establish the mechanism of action implicated and also to prove the efficacy and safety of topical diclofenac in these pathologies.
Author Contributions
D.A.-M.N. writing—original draft preparation, H.P. writing—review and editing. M.C. formal analysis B.N. visualization. L.C. writing, I.F. writing—review and editing O.A.C. supervision, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The protocol was approved by the Ethics Committee of “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania (13310/27 May 2021) for studies involving animals, in conformity with 43/2014 Law regarding animal protection used in scientific purposes, with further completions and 86/609/CEE Directive from 24 November 1986 regarding acts with power of law and administrative acts of member states for animal protection used in experimental purposes and other scientific purposes.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grosser, T. Biological basis for the cardiovascular consequences of COX-2 inhibition: Therapeutic challenges and opportunities. J. Clin. Investig. 2005, 116, 4–15. [Google Scholar] [CrossRef]
- Hernandez-Diaz, S.; Varas-Lorenzo, C.; Garcia Rodriguez, L.A. Non-Steroidal Antiinflammatory Drugs and the Risk of Acute Myocardial Infarction. Basic Clin. Pharmacol. Toxicol. 2006, 98, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Bort, R.; Ponsoda, X.; Jover, R.; Gómez-Lechón, M.J.; Castell, J.V. Diclofenac toxicity to hepatocytes: A role for drug metabolism in cell toxicity. J. Pharmacol. Exp. Ther. 1999, 288, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hickey, E.J.; Raje, R.R.; Reid, V.E.; Gross, S.M.; Ray, S.D. Diclofenac induced in vivo nephrotoxicity may involve oxidative stress-mediated massive genomic DNA fragmentation and apoptotic cell death. Free Radic. Biol. Med. 2001, 31, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Gautam, L.; Shukla, P.K.; Kaur, P.; Sharma, S.; Singh, T.P. Current Perspectives in NSAID-Induced Gastropathy. Mediat. Inflamm. 2013, 2013, 258209. [Google Scholar] [CrossRef]
- Patrignani, P.; Tacconelli, S.; Bruno, A.; Sostres, C.; Lanas, A. Managing the adverse effects of nonsteroidal anti-inflammatory drugs. Expert. Rev. Clin. Pharmacol. 2011, 4, 605–621. [Google Scholar] [CrossRef]
- Hajjar, B.; Zuo, J.; Park, C.; Azarmi, S.; Silva, D.A.; Bou-Chacra, N.A.; Löbenberg, R. In Vitro Evaluation of a Foamable Microemulsion Towards an Improved Topical Delivery of Diclofenac Sodium. AAPS PharmSciTech 2022, 23, 102. [Google Scholar] [CrossRef]
- Martin, G.M.; Stockfleth, E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J. Drugs Dermatol. 2012, 11, 600–608. [Google Scholar]
- Bakry, O.; Samaka, R.; Shoeib, M.; Abdel Aal, S. Nuclear factor kappa B and cyclo-oxygenase-2: Two concordant players in psoriasis pathogenesis. Ultrastruct. Pathol. 2015, 39, 49–61. [Google Scholar] [CrossRef]
- Nițescu, D.A.M.; Păunescu, H.; Ștefan, A.E.; Coman, L.; Georgescu, C.C.; Stoian, A.C.; Gologan, D.; Fulga, I.; Coman, O.A. Anti-Psoriasis Effect of Diclofenac and Celecoxib Using the Tail Model for Psoriasis. Pharmaceutics 2022, 14, 885. [Google Scholar] [CrossRef]
- Lapidus, A.H.; Lee, S.; Liu, Z.F.; Smithson, S.; Chew, C.Y.; Gin, D. Topical Calcipotriol Plus 5-Fluorouracil in the Treatment of Actinic Keratosis, Bowen’s Disease, and Squamous Cell Carcinoma: A Systematic Review. J. Cutan. Med. Surg. 2024, 28, 375–380. [Google Scholar] [CrossRef]
- Bakirtzi, K.; Papadimitriou, I.; Vakirlis, E.; Lallas, A.; Sotiriou, E. Photodynamic Therapy for Field Cancerization in the Skin: Where Do We Stand? Dermatol. Pract. Concept. 2023, 13, e2023291. [Google Scholar] [CrossRef]
- Kirchberger, M.C.; Gfesser, M.; Erdmann, M.; Schliep, S.; Berking, C.; Heppt, M.V. Tirbanibulin 1% Ointment Significantly Reduces the Actinic Keratosis Area and Severity Index in Patients with Actinic Keratosis: Results from a Real-World Study. J. Clin. Med. 2023, 12, 4837. [Google Scholar] [CrossRef] [PubMed]
- Rosan, T.; Ljubojević Hadžavdić, S. Ketoprofen-induced Photoallergic Reaction. Acta Dermatovenerol. Croat. 2022, 30, 197–198. [Google Scholar] [PubMed]
- Miller, A.C.; Adjei, S.; Temiz, L.A.; Tyring, S.K. Tirbanibulin for the Treatment of Actinic Keratosis: A Review. Ski. Ther. Lett. 2022, 27, 4–7. [Google Scholar]
- Barakat, L.; Dereure, O.; Raison-Peyron, N. A police case: Finding propylene glycol guilty as culprit allergen. Contact Dermat. 2021, 85, 475–476. [Google Scholar] [CrossRef]
- Agozzino, M.; Russo, T.; Franceschini, C.; Mazzilli, S.; Garofalo, V.; Campione, E.; Bianchi, L.; Milani, M.; Argenziano, G. Effects of topical piroxicam and sun filters in actinic keratosis evolution and field cancerization: A two-center, assessor-blinded, clinical, confocal microscopy and dermoscopy evaluation trial. Curr. Med. Res. Opin. 2019, 35, 1785–1792. [Google Scholar] [CrossRef]
- Wollina, U.; Gaber, B.; Koch, A. Photodynamic Treatment with Nanoemulsified 5-Aminolevulinic Acid and Narrow Band Red Light for Field Cancerization Due to Occupational Exposure to Ultraviolet Light Irradiation. Georgian Med. News 2018, 274, 138–143. [Google Scholar]
- Adil, M.; Amin, S.S.; Arif, T. Nicolau’s syndrome: A rare but preventable iatrogenic disease. Acta Dermatovenerol. Croat. 2017, 25, 251–253. [Google Scholar]
- Garofalo, V.; Ventura, A.; Mazzilli, S.; Diluvio, L.; Bianchi, L.; Toti, L.; Tisone, G.; Milani, M.; Campione, E. Treatment of Multiple Actinic Keratosis and Field of Cancerization with Topical Piroxicam 0.8% and Sunscreen 50+ in Organ Transplant Recipients: A Series of 10 Cases. Case Rep. Dermatol. 2017, 9, 211–216. [Google Scholar] [CrossRef]
- Damps, T.; Laskowska, A.K.; Kowalkowski, T.; Prokopowicz, M.; Puszko, A.K.; Sosnowski, P.; Czuwara, J.; Konop, M.; Różycki, K.; Borkowska, J.K.; et al. The effect of wool hydrolysates on squamous cell carcinoma cells in vitro. Possible implications for cancer treatment. PLoS ONE 2017, 12, e0184034. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Rivers, J.K. ActikerallTM (5-Fluorouracil 0.5% and Salicylic Acid 10%) Topical Solution for Patient-directed Treatment of Actinic Keratoses. Ski. Ther. Lett. 2016, 21, 1–3. [Google Scholar]
- Pruitt, L.G.; Hsia, L.L.B.; Burke, W.A. Disseminated superficial porokeratosis involving the groin and genitalia in a 72-year-old immunocompetent man. JAAD Case Rep. 2015, 1, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Babino, G.; Diluvio, L.; Bianchi, L.; Orlandi, A.; Di Prete, M.; Chimenti, S.; Milani, M.; Campione, E. Long-term use of a new topical formulation containing piroxicam 0.8% and sunscreen: Efficacy and tolerability on actinic keratosis. A proof of concept study. Curr. Med. Res. Opin. 2016, 32, 1345–1349. [Google Scholar] [CrossRef]
- Herbig, M.E.; Houdek, P.; Gorissen, S.; Zorn-Kruppa, M.; Wladykowski, E.; Volksdorf, T.; Grzybowski, S.; Kolios, G.; Willers, C.; Mallwitz, H.; et al. A custom tailored model to investigate skin penetration in porcine skin and its comparison with human skin. Eur. J. Pharm. Biopharm. 2015, 95, 99–109. [Google Scholar] [CrossRef]
- Russo, J.; Fiegel, J.; Brogden, N.K. Effect of Salt Form on Gelation and Drug Delivery Properties of Diclofenac-Loaded Poloxamer Gels for Delivery to Impaired Skin. Pharm. Res. 2022, 39, 2515–2527. [Google Scholar] [CrossRef]
- Do, L.H.D.; Law, R.M.; Maibach, H.I. Dose response effect of chemical surface concentration on percutaneous penetration in human: In vivo + in vitro. Regul. Toxicol. Pharmacol. 2022, 132, 105186. [Google Scholar] [CrossRef]
- Heppt, M.V.; Dykukha, I.; Graziadio, S.; Salido-Vallejo, R.; Chapman-Rounds, M.; Edwards, M. Comparative Efficacy and Safety of Tirbanibulin for Actinic Keratosis of the Face and Scalp in Europe: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2022, 11, 1654. [Google Scholar] [CrossRef]
- Wisuitiprot, V.; Ingkaninan, K.; Chakkavittumrong, P.; Wisuitiprot, W.; Neungchamnong, N.; Chantakul, R.; Waranuch, N. Effects of Acanthus ebracteatus Vahl. extract and verbascoside on human dermal papilla and murine macrophage. Sci. Rep. 2022, 12, 1491. [Google Scholar]
- Dao, D.P.D.; Sahni, V.N.; Sahni, D.R.; Balogh, E.A.; Grada, A.; Feldman, S.R. 1% Tirbanibulin Ointment for the Treatment of Actinic Keratoses. Ann. Pharmacother. 2022, 56, 494–500. [Google Scholar] [CrossRef]
- Kash, N.; Silapunt, S. A review of emerging and non-US FDA-approved topical agents for the treatment of basal cell carcinoma. Future Oncol. 2021, 17, 3111–3132. [Google Scholar] [CrossRef]
- Russo, J.; Fiegel, J.; Brogden, N.K. Rheological and Drug Delivery Characteristics of Poloxamer-Based Diclofenac Sodium Formulations for Chronic Wound Site Analgesia. Pharmaceutics 2020, 12, 1214. [Google Scholar] [CrossRef]
- O’Grady, C.; Flynn, A.; Mulligan, N.; Moloney, F.J. Pemphigus foliaceous triggered by topical diclofenac. Australas. J. Dermatol. 2020, 61, e442–e443. [Google Scholar] [CrossRef]
- Steeb, T.; Heppt, M.V.; Becker, L.; Kohl, C.; French, L.E.; Berking, C. Long-term efficacy of interventions for actinic keratosis: Protocol for a systematic review and network meta-analysis. Syst. Rev. 2019, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Savas, J.; Doerfler, L. Nonsurgical Treatments for Nonmelanoma Skin Cancer. Dermatol. Clin. 2019, 37, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Nemer, K.M.; Council, M.L. Topical and Systemic Modalities for Chemoprevention of Nonmelanoma Skin Cancer. Dermatol. Clin. 2019, 37, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Puviani, M.; Galloni, C.; Marchetti, S.; Sergio Pavone, P.; Lovati, S.; Pistone, G.; Caputo, V.; Tilotta, G.; Scarcella, G.; Campione, E.; et al. Efficacy of a film-forming medical device containing sunscreen (50+) and piroxicam 0.8% in actinic keratosis and field cancerization: A multicenter, assessor-blinded, 3 month trial. Curr. Med. Res. Opin. 2017, 33, 1255–1259. [Google Scholar] [CrossRef]
- Asche, C.V.; Zografos, P.; Norlin, J.M.; Urbanek, B.; Mamay, C.; Makin, C.; Erntoft, S.; Chen, C.C.; Hines, D.M.; Mark Siegel, D. Evaluation of Resource Utilization and Treatment Patterns in Patients with Actinic Keratosis in the United States. Value Health 2016, 19, 239–248. [Google Scholar] [CrossRef]
- Zarchi, K.; Jemec, G.B.E. Ingenol mebutate: From common weed to cancer cure. Curr. Probl. Dermatol. 2015, 46, 136–142. [Google Scholar][Green Version]
- Philipp-Dormston, W.G. Field cancerization: From molecular basis to selective field-directed management of actinic keratosis. Curr. Probl. Dermatol. 2015, 46, 115–121. [Google Scholar][Green Version]
- Altenburg, A.; El-Haj, N.; Micheli, C.; Puttkammer, M.; Abdel-Naser, M.; Zouboulis, C.C. The Treatment of Chronic Recurrent Oral Aphthous Ulcers. Dtsch. Arztebl. Int. 2014, 111, 665–673. [Google Scholar] [CrossRef]
- Javor, S.; Cozzani, E.; Parodi, A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: Review of the literature about the cumulative evidence of its efficacy and safety. G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2016, 151, 275–280. [Google Scholar]
- Ulrich, M.; Pellacani, G.; Ferrandiz, C.; Lear, J.T. Evidence for field cancerisation treatment of actinic keratoses with topical diclofenac in hyaluronic acid. Eur. J. Dermatol. 2014, 24, 158–167. [Google Scholar] [CrossRef]
- Messerschmidt, A.; Schultheis, K.; Ochsendorf, F. Topische Therapie von Infektionen, Hauttumoren und Hyperkeratosen. Hautarzt 2014, 65, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Esmann, S.; Jemec, G.B.E. Patients’ perceptions of topical treatments of actinic keratosis. J. Dermatol. Treat. 2014, 25, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Jeter, J.M.; Curiel-Lewandrowski, C.; Stratton, S.P.; Myrdal, P.B.; Warneke, J.A.; Einspahr, J.G.; Bartels, H.G.; Yozwiak, M.; Bermudez, Y.; Hu, C.; et al. Phase IIB Randomized Study of Topical Difluoromethylornithine and Topical Diclofenac on Sun-Damaged Skin of the Forearm. Cancer Prev. Res. 2016, 9, 128–134. [Google Scholar] [CrossRef]
- Kovács, A.; Falusi, F.; Gácsi, A.; Budai-Szűcs, M.; Csányi, E.; Veréb, Z.; Monostori, T.; Csóka, I.; Berkó, S. Formulation and investigation of hydrogels containing an increased level of diclofenac sodium using risk assessment tools. Eur. J. Pharm. Sci. 2024, 193, 106666. [Google Scholar] [CrossRef] [PubMed]
- Birngruber, T.; Vought, K.; Schwingenschuh, S.; Reisenegger, P.; Maibach, H.; Lissin, D. Topical Delivery Systems Effectively Transport Analgesics to Areas of Localized Pain via Direct Diffusion. Pharmaceutics 2023, 15, 2563. [Google Scholar] [CrossRef]
- Michelini, M. Photodynamic therapy activated by intense pulsed light in the treatment of actinic keratosis. G. Ital. Dermatol. Venereol. 2020, 155, 470–476. [Google Scholar] [CrossRef]
- Jolaoye, O.O. A Rare Manifestation of Sunburn Rash: A Case Report of Brachioradial Pruritus. Cureus 2025, 17, e82806. [Google Scholar] [CrossRef]
- Chaiyabutr, C.; Dawe, R.; Lesar, A.; Ibbotson, S.H. Topical Photodynamic Therapy in a Medical Centre: The Scottish Dermatology Experience. Photodermatol. Photoimmunol. Photomed. 2025, 41, e70010. [Google Scholar] [CrossRef]
- Bera, S.; Datta, H.K.; Dastidar, P. An injectable supramolecular hydrogel as a self-drug-delivery system for local chemoimmunotherapy against melanoma. Biomater. Sci. 2023, 11, 5618–5633. [Google Scholar] [CrossRef] [PubMed]
- Albanell-Fernández, M.; Luque-Luna, M.; López-Cabezas, C.; Alamon-Reig, F.; Espinosa-Villaseñor, N.; Barboza-Guadagnini, L.; Mascaró, J.M. Treatment of Porokeratosis Ptychotropica with a Topical Combination of Cholesterol and Simvastatin. JAMA Dermatol. 2023, 159, 458. [Google Scholar] [CrossRef] [PubMed]
- Ngo, J.L.; Ramirez Quizon, M.; Balagat, R. A rare case of Acrodermatitis continua of Hallopeau successfully treated with topical calcipotriol/betamethasone dipropionate ointment associated with Jaccaud’s arthropathy: A case report. SAGE Open Med. Case Rep. 2022, 10, 2050313X221136766. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Pozo-Román, T.; Sánchez-Velicia, L.; Vega-Gutiérrez, J.; Arias-Santiago, S.; Molina-Leyva, A. Ustekinumab in the treatment of patients with hidradenitis suppurativa: Multicenter case series and systematic review. J. Dermatol. Treat. 2022, 33, 348–353. [Google Scholar] [CrossRef]
- Peterson, H.; Luke, J. Ingenol Mebutate. J. Dermatol. Nurses Assoc. 2021, 13, 63–66. [Google Scholar] [CrossRef]
- Tater, K.C.; Gwaltney-Brant, S.; Wismer, T. Dermatological topical products used in the US population and their toxicity to dogs and cats. Vet. Dermatol. 2019, 30, 474. [Google Scholar] [CrossRef]
- Masnec, S.; Vidas Pauk, S.; Jurilj, M.; Kalauz, M.; Kuzman, T.; Škegro, I.; Jukić, T.; Jandroković, S.; Seiwerth, S.; Barišić Kutija, M. Enterococcus Faecalis Corneal Ulcers with Endophthalmitis and Consequent Bilateral Blindness as a Result of Unrecognised Intentional Self-Injury—A Case Report. Psychiatr. Danub. 2021, 33, 676–680. [Google Scholar]
- Alonso, C.; Carrer, V.; Espinosa, S.; Zanuy, M.; Córdoba, M.; Vidal, B.; Domínguez, M.; Godessart, N.; Coderch, L.; Pont, M. Prediction of the skin permeability of topical drugs using in silico and in vitro models. Eur. J. Pharm. Sci. 2019, 136, 104945. [Google Scholar] [CrossRef]
- Kirby, J.S.; Silva, C.F.; Ferguson, S.B.; Shupp, D.; Marks, J.G.; Miller, J.J. Bundled payment for actinic keratosis management: Pilot evaluation of developed models. J. Am. Acad. Dermatol. 2019, 80, 679–684. [Google Scholar] [CrossRef]
- Poulin, P.; Collet, S.H.; Atrux-Tallau, N.; Linget, J.M.; Hennequin, L.; Wilson, C.E. Application of the Tissue Composition–Based Model to Minipig for Predicting the Volume of Distribution at Steady State and Dermis-to-Plasma Partition Coefficients of Drugs Used in the Physiologically Based Pharmacokinetics Model in Dermatology. J. Pharm. Sci. 2019, 108, 603–619. [Google Scholar] [CrossRef]
- Zito, P.M.; Murgia, R.D. Pentoxifylline (Trental) in Venous Insufficiency and Venous Leg Ulcers. J. Dermatol. Nurses Assoc. 2018, 10, 294–296. [Google Scholar] [CrossRef]
- Wen, X.; Li, Y.; Hamblin, M.R. Photodynamic therapy in dermatology beyond non-melanoma cancer: An update. Photodiagn. Photodyn. Ther. 2017, 19, 140–152. [Google Scholar] [CrossRef]
- Kostovic, K.; Gulin, S.J.; Mokos, Z.B.; Ceovic, R. Topical Ingenol Mebutate: A New Treatment Modality for Multiple Actinic Keratoses and Field Cancerization. Anti-Cancer Agents Med. Chem. 2017, 17, 1304–1311. [Google Scholar] [CrossRef]
- Bettencourt, M.S. Tolerability of Ingenol Mebutate Gel, 0.05%, for Treating Patients with Actinic Keratosis on the Scalp in a Community Dermatology Practice. J. Clin. Aesthetic Dermatol. 2016, 9, 20–24. [Google Scholar]
- Nagelreiter, C.; Kratochvilova, E.; Valenta, C. Dilution of semi-solid creams: Influence of various production parameters on rheological properties and skin penetration. Int. J. Pharm. 2015, 478, 429–438. [Google Scholar] [CrossRef]
- Alchin, D.R. Ingenol Mebutate: A Succinct Review of a Succinct Therapy. Dermatol. Ther. 2014, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, M.S. Use of ingenol mebutate gel for actinic keratosis in patients in a community dermatology practice. J. Drugs Dermatol. 2014, 13, 269–273. [Google Scholar] [PubMed]
- Quinton, J.F.; Prélaud, P.; Poujade, A.; Cochet Faivre, N. A Case of Actinic Keratosis in a Rabbit. J. Exot. Pet. Med. 2014, 23, 283–286. [Google Scholar] [CrossRef]
- Javor, S.; Chimenti, S.; Patrizi, A.; Stingeni, L.; Pellacani, G.; Cavicchini, S.; Sala, R.; Rongioletti, F.; Parodi, A. Relapsed actinic keratosis evaluation: An observational Italian multicenter prospective study. Does Gend. Have a Role? G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2014, 149, 199–204. [Google Scholar]
- Cantisani, C.; Paolino, G.; Faina, V.; Frascani, F.; Cantoresi, F.; Bianchini, D.; Fazia, G.; Calvieri, S. Overview on Topical 5-ALA Photodynamic Therapy Use for Non Melanoma Skin Cancers. Int. J. Photoenergy 2014, 2014, 304862. [Google Scholar] [CrossRef]
- Peris, K.; Neri, L.; Calzavara Pinton, P.; Catricalà, C.; Pellacani, G.; Pimpinelli, N.; Peserico, A. Physicians’ opinions and clinical practice patterns for actinic keratosis management in Italy. G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2014, 149, 185–192. [Google Scholar]
- Willenbrink, T.J.; Ruiz, E.S.; Cornejo, C.M.; Schmults, C.D.; Arron, S.T.; Jambusaria-Pahlajani, A. Field cancerization: Definition, epidemiology, risk factors, and outcomes. J. Am. Acad. Dermatol. 2020, 83, 709–717. [Google Scholar] [CrossRef]
- Eisen, D.B.; Asgari, M.M.; Bennett, D.D.; Connolly, S.M.; Dellavalle, R.P.; Freeman, E.E.; Goldenberg, G.; Leffell, D.J.; Peschin, S.; Sligh, J.E.; et al. Guidelines of care for the management of actinic keratosis. J. Am. Acad. Dermatol. 2021, 85, e209–e233. [Google Scholar] [CrossRef]
- Gutzmer, R.; Wiegand, S.; Kölbl, O.; Wermker, K.; Heppt, M.; Berking, C. Actinic Keratosis and Cutaneous Squamous Cell Carcinoma. Dtsch. Ärzteblatt Int. 2019, 116, 616. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Amici, J.M.; Basset-Seguin, N.; Cribier, B.; Claudel, J.P.; Richard, M.A. Management of actinic keratosis: A practical report and treatment algorithm from AKTeamTM expert clinicians. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Kandolf, L.; Peris, K.; Malvehy, J.; Mosterd, K.; Heppt, M.V.; Fargnoli, M.C.; Berking, C.; Arenberger, P.; Bylaite-Bučinskiene, M.; Del Marmol, V.; et al. European consensus-based interdisciplinary guideline for diagnosis, treatment and prevention of actinic keratoses, epithelial UV-induced dysplasia and field cancerization on behalf of European. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1024–1047. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Lesiak, A.; Narbutt, J.; Owczarczyk-Saczonek, A.; Pastuszczak, M.; Sobjanek, M.; Szepietowski, J.; Walecka, I.; Owczarek, W. Actinic keratosis–diagnostic and therapeutic recommendations of the Polish Dermatological Society. Dermatol. Rev. 2024, 111, 81–96. [Google Scholar] [CrossRef]
- Stockfleth, E.; Bastian, M. Pharmacokinetic and pharmacodynamic evaluation of ingenol mebutate for the treatment of actinic keratosis. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 911–918. [Google Scholar] [CrossRef]
- Bernal Masferrer, L.; Gracia Cazaña, T.; Bernad Alonso, I.; Álvarez-Salafranca, M.; Almenara Blasco, M.; Gallego Rentero, M.; Juarranz De La Fuente, Á.; Gilaberte, Y. Topical Immunotherapy for Actinic Keratosis and Field Cancerization. Cancers 2024, 16, 1133. [Google Scholar] [CrossRef]
- Maltusch, A.; Röwert-Huber, J.; Matthies, C.; Lange-Asschenfeldt, S.; Stockfleth, E. Modes of action of diclofenac 3%/hyaluronic acid 2.5% in the treatment of actinic keratosis: Diclofenac 3%/hyaluronic acid 2.5% and actinic keratosis. J. Dtsch. Dermatol. Ges. 2011, 9, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Wang, J.; Bitterman, D.; Mineroff, J.; Austin, E.; Jagdeo, J. Systematic review of randomized controlled trials of topicals for actinic keratosis field therapy. Arch. Dermatol. Res. 2024, 316, 108. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, I.; Puyana, C.; Chandan, N.; Jetter, N.; Tsoukas, M. Field Cancerization Therapies for the Management of Actinic Keratosis: An Updated Review. Am. J. Clin. Dermatol. 2024, 25, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, D.; Ramchatesingh, B.; Lagacé, F.; Iannattone, L.; Netchiporouk, E.; Lefrançois, P.; Litvinov, I.V. Pharmacological Agents Used in the Prevention and Treatment of Actinic Keratosis: A Review. Int. J. Mol. Sci. 2023, 24, 4989. [Google Scholar] [CrossRef]
- Tampucci, S.; Carpi, S.; Digiacomo, M.; Polini, B.; Fogli, S.; Burgalassi, S.; Macchia, M.; Nieri, P.; Manera, C.; Monti, D. Diclofenac-Derived Hybrids for Treatment of Actinic Keratosis and Squamous Cell Carcinoma. Molecules 2019, 24, 1793. [Google Scholar] [CrossRef]
- Collins, L.; Asfour, L.; Stephany, M.; Lear, J.T.; Stasko, T. Management of Non-melanoma Skin Cancer in Transplant Recipients. Clin. Oncol. 2019, 31, 779–788. [Google Scholar] [CrossRef]
- Dodds, A.; Chia, A.; Shumack, S. Actinic Keratosis: Rationale and Management. Dermatol. Ther. 2014, 4, 11–31. [Google Scholar] [CrossRef]
- Micali, G.; Lacarrubba, F.; Nasca, M.R.; Schwartz, R.A. Topical pharmacotherapy for skin cancer: Part I. Pharmacology. J. Am. Acad. Dermatol. 2014, 70, 965.e1–965.e12. [Google Scholar] [CrossRef]
- Piaserico, S.; Mazzetto, R.; Sartor, E.; Bortoletti, C. Combination-Based Strategies for the Treatment of Actinic Keratoses with Photodynamic Therapy: An Evidence-Based Review. Pharmaceutics 2022, 14, 1726. [Google Scholar] [CrossRef]
- Segatto, M.M.; Dornelles, S.I.T.; Silveira, V.B.; Frantz, G.D.O. Comparative study of actinic keratosis treatment with 3% diclofenac sodium and 5% 5-fluorouracil. An. Bras. Dermatol. 2013, 88, 732–738. [Google Scholar] [CrossRef]
- Zane, C.; Facchinetti, E.; Rossi, M.T.; Specchia, C.; Calzavara-Pinton, P.G. A randomized clinical trial of photodynamic therapy with methyl aminolaevulinate vs. diclofenac 3% plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br. J. Dermatol. 2014, 170, 1143–1150. [Google Scholar] [CrossRef]
- Khanna, R.; Bakshi, A.; Amir, Y.; Goldenberg, G. Patient satisfaction and reported outcomes on the management of actinic keratosis. Clin. Cosmet. Investig. Dermatol. 2017, 10, 179–184. [Google Scholar] [CrossRef]
- Lang, B.M.; Zielbauer, S.; Stege, H.; Grabbe, S.; Staubach, P. If patients had a choice–Treatment satisfaction and patients’ preference in therapy of actinic keratoses. J. Dtsch. Dermatol. Ges. 2024, 22, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Ushcatz, I.; Tadrous, M.; Aoki, V.; Chang, A.Y.; Levell, N.J.; Von Schuckmann, L.; Drucker, A.M. International time trends and differences in topical actinic keratosis therapy utilization. JAAD Int. 2024, 16, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.A.T.; Steeb, T.; Bender-Säbelkampf, S.; Busch, D.; Feustel, J.; Kaufmann, M.D.; Maronna, A.; Meder, C.; Ronicke, M.; Toussaint, F.; et al. Poor Adherence to Self-Applied Topical Drug Treatment Is a Common Source of Low Lesion Clearance in Patients with Actinic Keratosis—A Cross-Sectional Study. J. Clin. Med. 2023, 12, 3813. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.A.T.; Wessely, A.; Steeb, T.; Berking, C.; Heppt, M.V. Safety of topical interventions for the treatment of actinic keratosis. Expert. Opin. Drug Saf. 2021, 20, 801–814. [Google Scholar] [CrossRef]
- Jedlowski, P.M. Ingenol Mebutate Is Associated with Increased Reporting Odds for Squamous Cell Carcinoma in Actinic Keratosis Patients, a Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS). J. Cutan. Med. Surg. 2023, 27, 39–43. [Google Scholar] [CrossRef]
- Moretta, G.; Samela, T.; Sampogna, F.; Ricci, F.; Carlesimo, F.; Panebianco, A.; D’Erme, A.M.; Di Lella, G.; Pallotta, S.; Dellambra, E.; et al. Attitudes among dermatologists regarding actinic keratosis treatment options. Dermatol. Rep. 2022, 14, 9392. [Google Scholar] [CrossRef]
- Perino, F.; Fattori, A.; Piccerillo, A.; Bianchi, L.; Fargnoli, M.C.; Frascione, P.; Pellacani, G.; Carbone, A.; Campione, E.; Esposito, M.; et al. Treatment adherence with diclofenac 3% gel among patients with multiple actinic keratoses: An integrated low-intensity intervention program versus standard-of-care. Ital. J. Dermatol. Venereol. 2022, 157, 164–172. [Google Scholar] [CrossRef]
- Norrlid, H.; Norlin, J.M.; Holmstrup, H.; Malmberg, I.; Sartorius, K.; Thormann, H.; Jemec, G.B.E.; Ragnarson Tennvall, G. Patient-reported outcomes in topical field treatment of actinic keratosis in Swedish and Danish patients. J. Dermatol. Treat. 2018, 29, 68–73. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Tanova, N.; Hamon, P. Evaluation of the treatment costs and duration of topical treatments for multiple actinic keratosis based on the area of the cancerization field and not on the number of lesions. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Mazzella, C.; Greco, V.; Costa, C.; Scalvenzi, M.; Russo, D.; Savastano, R.; Staibano, S.; Fabbrocini, G. Management of clinical and subclinical actinic keratoses with histological and immunohistochemical assessments by confocal microscopy. Dermatol. Ther. 2018, 31, e12672. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Gupta, G.; Segert, M.H.; Kost, R.; Sternberg, J.; Gambichler, T.; Stockfleth, E.; Dirschka, T. Diclofenac Sodium 3% in Hyaluronic Acid 2.5% Gel Significantly Diminishes the Actinic Keratosis Area and Severity Index. Ski. Pharmacol. Physiol. 2018, 31, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, G.; Elahipoor, A.; Iraji, F.; Behfar, S.; Abtahi-Naeini, B. Topical Colchicine Gel versus Diclofenac Sodium Gel for the Treatment of Actinic Keratoses: A Randomized, Double-Blind Study. Adv. Med. 2016, 2016, 5918393. [Google Scholar] [CrossRef]
- Singer, K.; Dettmer, K.; Unger, P.; Schönhammer, G.; Renner, K.; Peter, K.; Siska, P.J.; Berneburg, M.; Herr, W.; Oefner, P.J.; et al. Topical Diclofenac Reprograms Metabolism and Immune Cell Infiltration in Actinic Keratosis. Front. Oncol. 2019, 9, 605. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Almazan-Fernandez, F.M.; Husein-ElAhmed, S. Ingenol mebutate versus imiquimod versus diclofenac for actinic cheilitis: A 6-month follow-up clinical study. Clin. Exp. Dermatol. 2019, 44, 231–234. [Google Scholar] [CrossRef]
- Neri, L.; Peris, K.; Longo, C.; Calvieri, S.; Frascione, P.; Parodi, A.; Eibenschuz, L.; Bottoni, U.; Pellacani, G.; the Actinic Keratosis–TReatment Adherence INitiative (AK-TRAIN) study group. Physician–patient communication and patient-reported outcomes in the actinic keratosis treatment adherence initiative (AK-TRAIN): A multicenter, prospective, real-life study of treatment satisfaction, quality of life and adherence to topical field-directed therapy for the treatment of actinic keratosis in Italy. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 93–107. [Google Scholar]
- Nisticò, S.; Del Duca, E.; Torchia, V.; Gliozzi, M.; Bottoni, U.; Muscoli, C. Cost-efficacy analysis of 3% diclofenac sodium, ingenol mebutate, and 3.75% imiquimod in the treatment of actinic keratosis. Int. J. Immunopathol. Pharmacol. 2018, 31, 2058738418757925. [Google Scholar] [CrossRef]
- Tolley, K.; Argenziano, G.; Calzavara-Pinton, P.G.; Larsson, T.; Ryttig, L. Pharmacoeconomic evaluations in the treatment of actinic keratoses. Int. J. Immunopathol. Pharmacol. 2017, 30, 178–181. [Google Scholar] [CrossRef]
- Delvin, T.; Bygum, A.; Lund, L.C.; Andersen, J.H.; Hallas, J. Screening of dermatology drugs for aberrant use-patterns: An application of epidemiologic estimates and measures of inequality in drug use. Br. J. Clin. Pharmacol. 2024, 90, 1450–1462. [Google Scholar] [CrossRef]
- Dirschka, T.; Lear, J.T. Sequential Treatment of Multiple Actinic Keratoses with Solaraze and Actikerall. Case Rep. Dermatol. 2014, 6, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Jetter, N.; Chandan, N.; Wang, S.; Tsoukas, M. Field Cancerization Therapies for Management of Actinic Keratosis: A Narrative Review. Am. J. Clin. Dermatol. 2018, 19, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.K.S.; de França, G.M.; Lima JGda, C.; Pinheiro, J.C.; Almeida DRde, M.F.; Santos PPde, A. Use of topical anti-inflammatory and antineoplastic agents in the treatment of young-aged actinic cheilitis: A systematic review. J. Cosmet. Dermatol. 2022, 21, 473–481. [Google Scholar] [CrossRef]
- Bakirtzi, K.; Papadimitriou, I.; Andreadis, D.; Sotiriou, E. Treatment Options and Post-Treatment Malignant Transformation Rate of Actinic Cheilitis: A Systematic Review. Cancers 2021, 13, 3354. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, A.K.G.; De Oliveira, P.T.; Da Silveira, É.J.D.; Queiroz, L.M.G.; De Medeiros, A.M.C. Diclofenac sodium gel therapy as an alternative to actinic cheilitis. Clin. Oral Investig. 2018, 22, 1319–1325. [Google Scholar] [CrossRef]
- Batra, R.; Sundararajan, S.; Sandramouli, S. Topical Diclofenac Gel for the Management of Periocular Actinic Keratosis. Ophthal Plast. Reconstr. Surg. 2012, 28, 1–3. [Google Scholar] [CrossRef]
- Buján Bonino, C.; Rodríguez-Blanco, I.; Sánchez-Aguilar Rojas, D.; Vázquez Veiga, H.A.; Flórez, Á. Topical and Intralesional Immunotherapy for the Management of Skin Cancer in Special Locations: Lips and Eyelids. Cancers 2023, 15, 5018. [Google Scholar] [CrossRef]
- Beutner, C.; Forkel, S.; Kreipe, K.; Geier, J.; Buhl, T. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermat. 2022, 86, 41–43. [Google Scholar] [CrossRef]
- Gulin, S.J.; Chiriac, A. Diclofenac-Induced Allergic Contact Dermatitis: A Series of Four Patients. Drug Saf. Case Rep. 2016, 3, 15. [Google Scholar] [CrossRef]
- Thomas, G.J.; Herranz, P.; Cruz, S.B.; Parodi, A. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: Potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol. Ther. 2019, 32, e12800. [Google Scholar] [CrossRef]
- Costa, C.; Scalvenzi, M.; Ayala, F.; Fabbrocini, G.; Monfrecola, G. How to treat actinic keratosis? An update. J. Dermatol. Case Rep. 2015, 9, 29–35. [Google Scholar] [CrossRef]
- El-Khalawany, M.; Saudi, W.M.; Ahmed, E.; Mosbeh, A.; Sameh, A.; Rageh, M.A. The combined effect of CO2 laser, topical diclofenac 3%, and imiquimod 5% in treating high-risk basal cell carcinoma. J. Cosmet. Dermatol. 2022, 21, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Costescu, M.; Coman, O.; Tampa, M.; Tudose, I.; Coman, L.; Georgescu, S. Axillary basal cell carcinoma–a rare form of a frequent kind of carcinoma. Rom. J. Morphol. Embryol. 2013, 54, 851–856. [Google Scholar]
- Tan, I.J.; Pathak, G.N.; Silver, F.H. Topical Treatments for Basal Cell Carcinoma and Actinic Keratosis in the United States. Cancers 2023, 15, 3927. [Google Scholar] [CrossRef] [PubMed]
- Brinkhuizen, T.; Frencken, K.J.A.; Nelemans, P.J.; Hoff, M.L.S.; Kelleners-Smeets, N.W.J.; Zur Hausen, A.; Van Der Horst, M.P.J.; Rennspiess, D.; Winnepenninckx, V.J.L.; Van Steensel, M.A.M.; et al. The effect of topical diclofenac 3% and calcitriol 3 μg/g on superficial basal cell carcinoma (sBCC) and nodular basal cell carcinoma (nBCC): A phase II, randomized controlled trial. J. Am. Acad. Dermatol. 2016, 75, 126–134. [Google Scholar] [CrossRef]
- Micali, G.; Lacarrubba, F.; Nasca, M.R.; Ferraro, S.; Schwartz, R.A. Topical pharmacotherapy for skin cancer: Part II. Clinical applications. J. Am. Acad. Dermatol. 2014, 70, 979.e1–979.e12. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Cazaña, T.; López, M.T.; Oncins, R.; Gilaberte, Y. Successful treatment of sequential therapy in digital Bowen’s disease with methyl aminolevulinate photodynamic therapy and topical diclofenac 3% in hyaluronan 2.5% gel: Adjuvant therapy with topical diclofenac and photodynamic therapy in Bowen’s disease. Dermatol. Ther. 2015, 28, 341–343. [Google Scholar] [CrossRef]
- Millán-Parrilla, F.; Rodrigo-Nicolás, B.; Molés-Poveda, P.; Armengot-Carbó, M.; Quecedo-Estébanez, E.; Gimeno-Carpio, E. Improvement of Darier disease with diclofenac sodium 3% gel. J. Am. Acad. Dermatol. 2014, 70, e89–e90. [Google Scholar] [CrossRef]
- Haber, R.N.; Dib, N.G. Management of Darier disease: A review of the literature and update. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 14–21. [Google Scholar] [CrossRef]
- Campos, M.O.M.Q.D.; Figueiredo, G.A.P.D.; Evangelista, A.C.; Bauk, A.R. Darier’s disease: Treatment with topical sodium diclofenac 3% gel. Bras. Dermatol. 2023, 98, 882–884. [Google Scholar] [CrossRef]
- Santos-Alarcon, S.; Sanchis-Sanchez, C.; Mateu-Puchades, A. Diclofenac sodium 3% gel for darier’s disease treatment. Dermatol. Online J. 2016, 22, 17. [Google Scholar] [CrossRef]
- Swadi, A.A.J.; Jabur, A.H. The value of diclofenac gel 1% in the treatment of pityriasis versicolor in a sample of Iraqi patients. Int. J. Pharm. Res. 2019, 11, 25–28. [Google Scholar] [CrossRef]
- Afify, A.A.; Hana, M.R. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol. Ther. 2020, 33, e14370. [Google Scholar] [CrossRef]
- Natarelli, N.; Krenitsky, A.; Hennessy, K.; Moore, S.; Grichnik, J. Efficacy and safety of topical treatments for seborrheic keratoses: A systematic review. J. Dermatol. Treat. 2023, 34, 2133532. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.; Varma, R.; Cantrell, W.; Chen, S.; Gold, M.; Muellenhoff, M.; Elewski, B. Diclofenac sodium 3% gel as a potential treatment for disseminated superficial actinic porokeratosis. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Takashima, Y.; Hotta, M.; Ito, E.; Moriuchi, R. Inflammatory disseminated superficial porokeratosis successfully controlled with a combination of topical diclofenac gel and systemic etretinate. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e201–e202. [Google Scholar] [CrossRef]
- Vlachou, C.; Kanelleas, A.; Martin-Clavijo, A.; Berth-Jones, J. Treatment of disseminated superficial actinic porokeratosis with topical diclofenac gel: A case series. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1343–1345. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.L.D.S.; Tiussi, L.D.; Nascimento, M.S.; Corrêa, A.C.D.S.; Yasojima, E.Y.; Pires, C.A.A. Diclofenac topical gel in excisional wounds maintain heal quality and reduce phlogistic signals1. Acta Cir. Bras. 2014, 29, 328–333. [Google Scholar] [CrossRef]
- Li Pomi, F.; d’Aloja, A.; Valguarnera, D.; Vaccaro, M.; Borgia, F. Exploring Anti-Aging Effects of Topical Treatments for Actinic Keratosis. Medicina 2025, 61, 207. [Google Scholar] [CrossRef]
- Constantin, M.M.; Bucur, S.; Mutu, C.C.; Poenaru, E.; Olteanu, R.; Ionescu, R.A.; Nicolescu, A.C.; Furtunescu, F.; Constantin, T. The Impact of Smoking on Psoriasis Patients with Biological Therapies in a Bucharest Hospital. J. Pers. Med. 2021, 11, 752. [Google Scholar] [CrossRef]
- Yaseliani, M.; Ijadi Maghsoodi, A.; Hassannayebi, E.; Aickelin, U. Diagnostic clinical decision support based on deep learning and knowledge-based systems for psoriasis: From diagnosis to treatment options. Comput. Ind. Eng. 2024, 187, 109754. [Google Scholar] [CrossRef]
- Bhawan, J.; Gonzalez-Serva, A.; Nehal, K.; Labadie, R.; Lufrano, L.; Thorne, E.G.; Gilchrest, B.A. Effects of tretinoin on photodamaged skin. A histologic study. Arch. Dermatol. 1991, 127, 666–672. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Treatment of photodamage with topical tretinoin: An overview. J. Am. Acad. Dermatol. 1997, 36, S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Bungau, A.F.; Tit, D.M.; Bungau, S.G.; Vesa, C.M.; Radu, A.F.; Marin, R.C.; Endres, L.M.; Moleriu, L.C. Exploring the Metabolic and Endocrine Preconditioning Associated with Thyroid Disorders: Risk Assessment and Association with Acne Severity. Int. J. Mol. Sci. 2024, 25, 721. [Google Scholar] [CrossRef] [PubMed]
- Bosman, B.; Matthiesen, T.; Hess, V.; Friderichs, E. Quantitative Method for Measuring Antipsoriatic Activity of Drugs by the Mouse Tail Test. Ski. Pharmacol. 1992, 5, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Paternò, E.; Candi, E.; Falconi, M.; Constanza, G.; Diluvio, L.; Terrinoni, A.; Bianchi, L.; Orlandi, A. The relevance of piroxicam for the prevention and treatment of nonmelanoma skin cancer and its precursors. Drug Des. Dev. Ther. 2015, 9, 5843–5850. [Google Scholar] [CrossRef]
- Fecker, L.F.; Stockfleth, E.; Braun, F.K.; Rodust, P.M.; Schwarz, C.; Köhler, A.; Leverkus, M.; Eberle, J. Enhanced Death Ligand-Induced Apoptosis in Cutaneous SCC Cells by Treatment with Diclofenac/Hyaluronic Acid Correlates with Downregulation of c-FLIP. J. Investig. Dermatol. 2010, 130, 2098–2109. [Google Scholar] [CrossRef]
- Chirasani, S.R.; Leukel, P.; Gottfried, E.; Hochrein, J.; Stadler, K.; Neumann, B.; Oefner, P.J.; Gronwald, W.; Bogdahn, U.; Hau, P.; et al. Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. Int. J. Cancer 2013, 132, 843–853. [Google Scholar] [CrossRef]
- Gottfried, E.; Lang, S.A.; Renner, K.; Bosserhoff, A.; Gronwald, W.; Rehli, M.; Einhell, S.; Gedig, I.; Singer, K.; Seilbeck, A.; et al. New Aspects of an Old Drug–Diclofenac Targets MYC and Glucose Metabolism in Tumor Cells. Rafael MS, editor. PLoS ONE 2013, 8, e66987. [Google Scholar] [CrossRef]
- Huang, K.H.; Kuo, K.L.; Chen, S.C.; Weng, T.I.; Chuang, Y.T.; Tsai, Y.C.; Pu, Y.S.; Chiang, C.K.; Liu, S.H. Down-Regulation of Glucose-Regulated Protein (GRP) 78 Potentiates Cytotoxic Effect of Celecoxib in Human Urothelial Carcinoma Cells. Ahmad A, editor. PLoS ONE 2012, 7, e33615. [Google Scholar]
- Yarishkin, O.V.; Hwang, E.M.; Kim, D.; Yoo, J.C.; Kang, S.S.; Kim, D.R.; Shin, J.H.J.; Chung, H.J.; Jeong, H.S.; Kang, D.; et al. Diclofenac, a Non-steroidal Anti-inflammatory Drug, Inhibits L-type Ca Channels in Neonatal Rat Ventricular Cardiomyocytes. Korean J. Physiol. Pharmacol. 2009, 13, 437–442. [Google Scholar] [CrossRef]
- Villalonga, N.; David, M.; Bielańska, J.; González, T.; Parra, D.; Soler, C.; Comes, N.; Valenzuela, C.; Felipe, A. Immunomodulatory effects of diclofenac in leukocytes through the targeting of Kv1.3 voltage-dependent potassium channels. Biochem. Pharmacol. 2010, 80, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Degani, N.; Nachman, R.; Uziyel, Y.; Gibor, G.; Shabat, D.; Attali, B. Meclofenamic Acid and Diclofenac, Novel Templates of KCNQ2/Q3 Potassium Channel Openers, Depress Cortical Neuron Activity and Exhibit Anticonvulsant Properties. Mol. Pharmacol. 2005, 67, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Fei, X.W.; Li, Z.M.; Zhang, Z.H.; Mei, Y.A. Diclofenac, a nonsteroidal anti-inflammatory drug, activates the transient outward K+ current in rat cerebellar granule cells. Neuropharmacology 2005, 48, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Brueggemann, L.I.; Mackie, A.R.; Martin, J.L.; Cribbs, L.L.; Byron, K.L. Diclofenac Distinguishes among Homomeric and Heteromeric Potassium Channels Composed of KCNQ4 and KCNQ5 Subunits. Mol. Pharmacol. 2011, 79, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.P.; Tatsuo, M.A.F.; Leite, R.; Duarte, I.D.G. Diclofenac-induced peripheral antinociception is associated with ATP-sensitive K+ channels activation. Life Sci. 2004, 74, 2577–2591. [Google Scholar] [CrossRef]
- Liu, L.Y.; Hu, C.L.; Ma, L.J.; Zhang, Z.H.; Mei, Y.A. ET-1 inhibits B-16 murine melanoma cell migration by decreasing K+ currents. Cell Motil. Cytoskelet. 2004, 58, 127–136. [Google Scholar] [CrossRef]
- Dorofeeva, N.A.; Barygin, O.I.; Staruschenko, A.; Bolshakov, K.V.; Magazanik, L.G. Mechanisms of non-steroid anti-inflammatory drugs action on ASICs expressed in hippocampal interneurons. J. Neurochem. 2008, 106, 429–441. [Google Scholar] [CrossRef]
- Macianskiene, R.; Gwanyanya, A.; Sipido, K.R.; Vereecke, J.; Mubagwa, K. Induction of a novel cation current in cardiac ventricular myocytes by flufenamic acid and related drugs. Br. J. Pharmacol. 2010, 161, 416–429. [Google Scholar] [CrossRef]
- Grigore, A.; Coman, O.A.; Păunescu, H.; Costescu, M.; Fulga, I. Latest Insights into the In Vivo Studies in Murine Regarding the Role of TRP Channels in Wound Healing—A Review. Int. J. Mol. Sci. 2024, 25, 6753. [Google Scholar] [CrossRef]
- Lang, P.A.; Kempe, D.S.; Myssina, S.; Tanneur, V.; Birka, C.; Laufer, S.; Lang, F.; Wieder, T.; Huber, S.M. PGE2 in the regulation of programmed erythrocyte death. Cell Death Differ. 2005, 12, 415–428. [Google Scholar] [CrossRef]
- Yang, Y.C.; Kuo, C.C. An Inactivation Stabilizer of the Na+ Channel Acts as an Opportunistic Pore Blocker Modulated by External Na+. J. Gen. Physiol. 2005, 125, 465–481. [Google Scholar] [CrossRef] [PubMed]
- McCormack, K.; Brune, K. Dissociation between the antinociceptive and anti-inflammatory effects of the nonsteroidal anti-inflammatory drugs: A survey of their analgesic efficacy. Drugs 1991, 41, 533–547. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).