Prenylated Chalcones as Anticancer Agents Against Castration-Resistant Prostate Cancer
Abstract
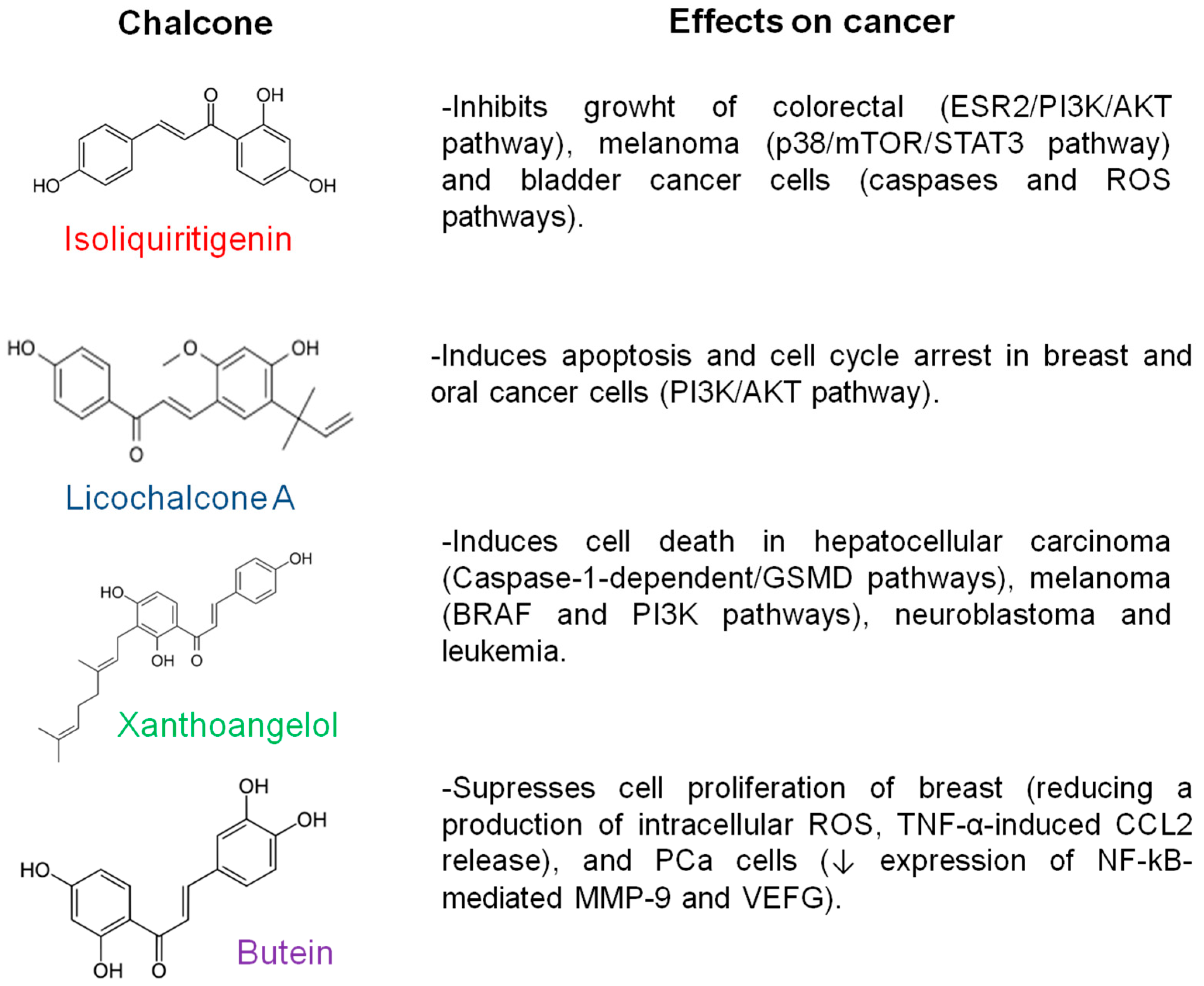
1. Introduction
2. Materials and Methods
2.1. Chalcones
2.2. Cell Lines
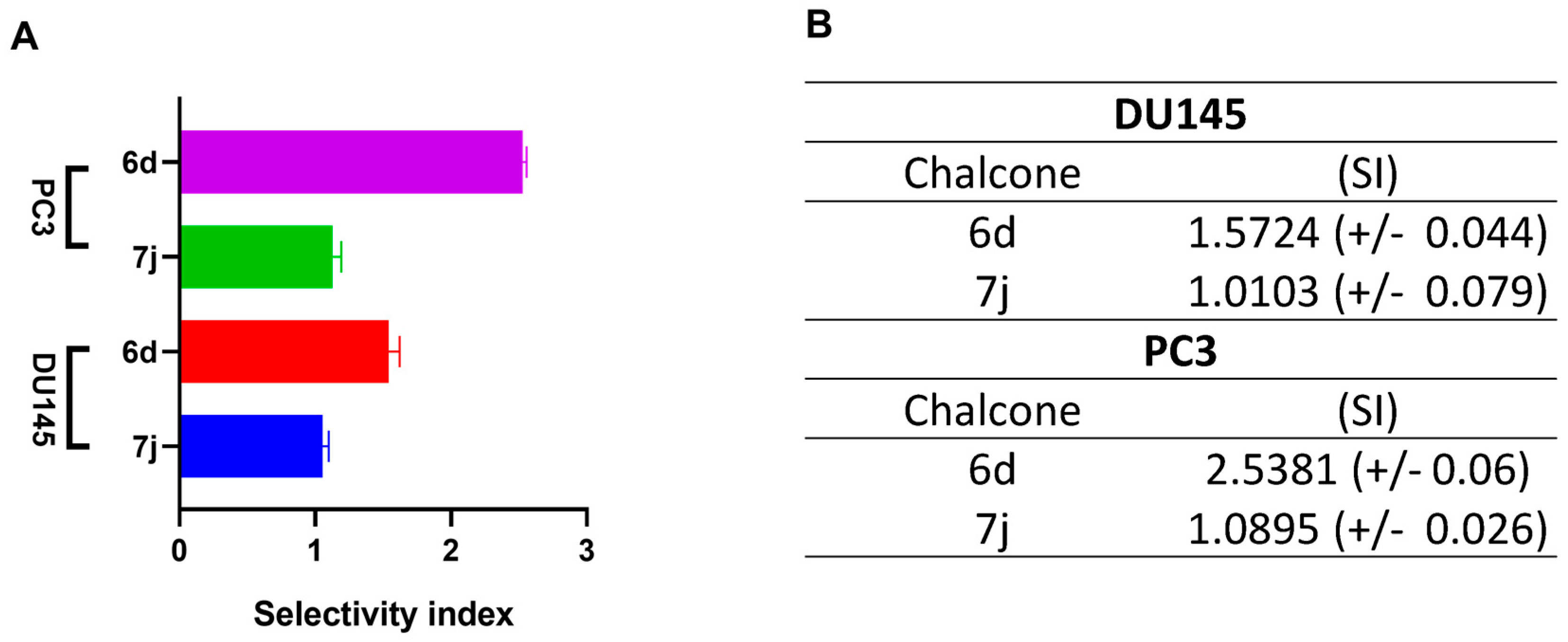
2.3. Cell Viability by MTT Assay and Selectivity Index
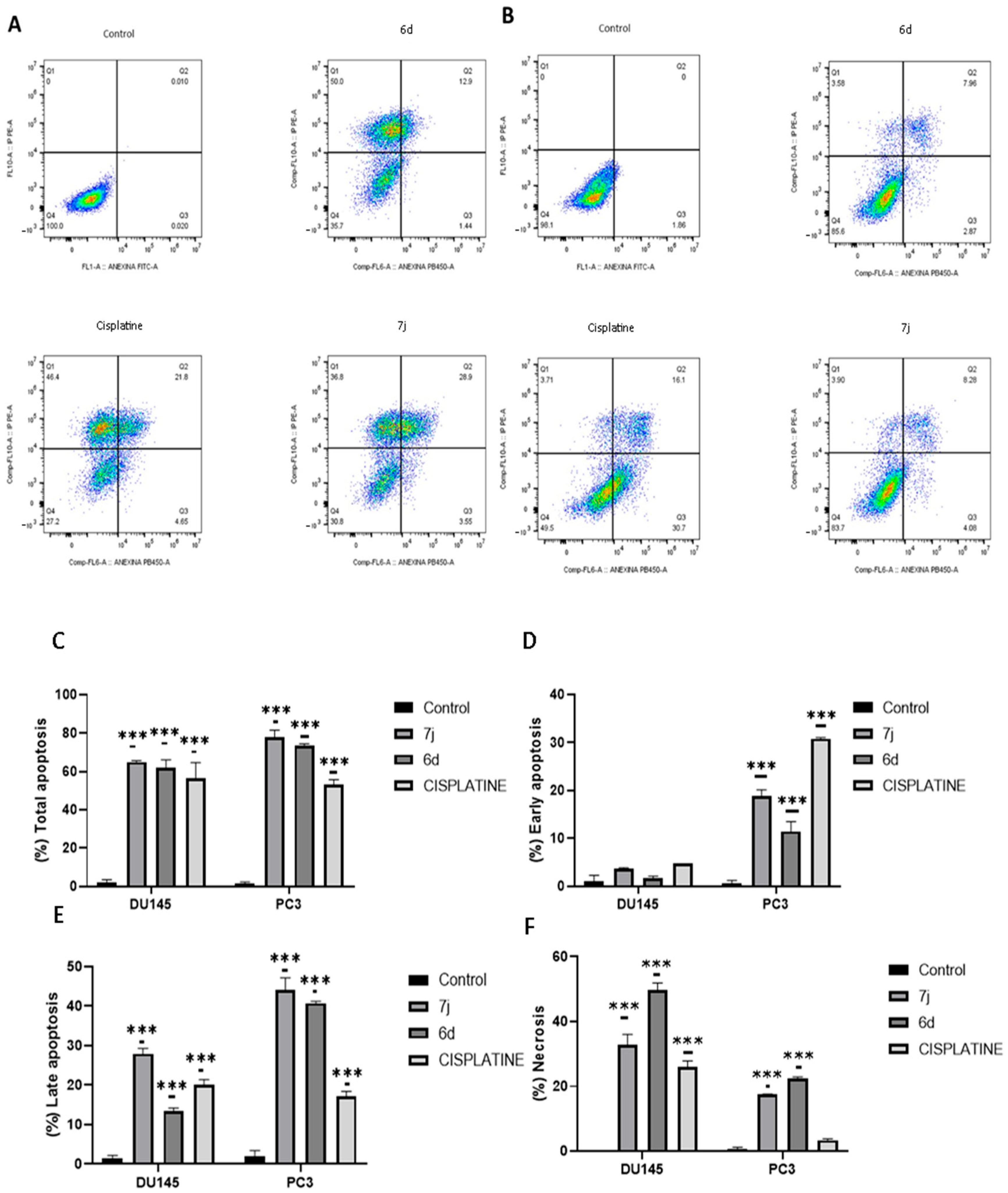
2.4. Apoptosis Assay
2.5. Mitochondrial Membrane Potential Analysis
2.6. Confocal Microscopy
2.7. Statistical Analysis
3. Results
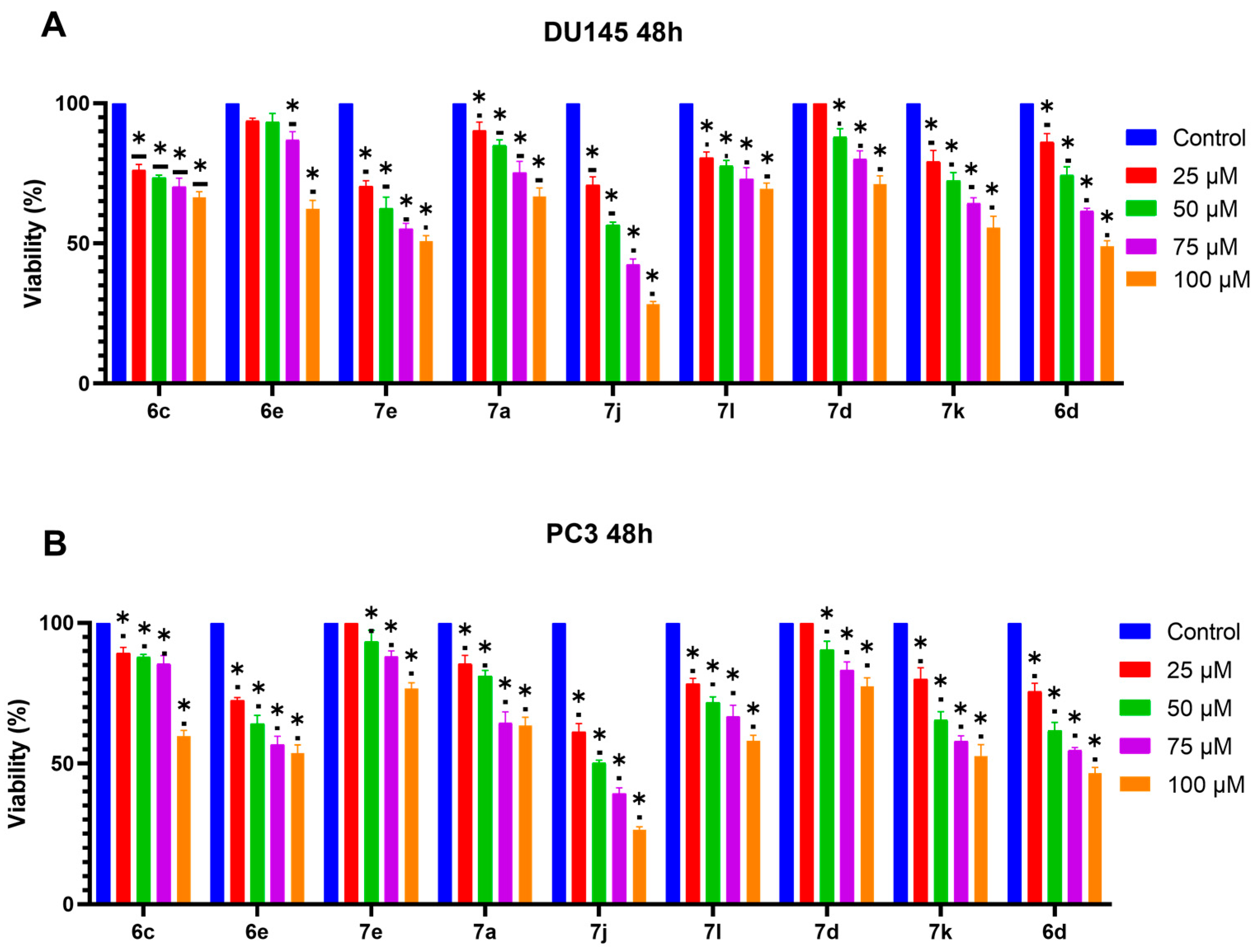
3.1. 6d and 7j Chalcones Reduce Viability in DU145 and PC3 Cells
3.2. Chalcones 6d and 7j Induce Apoptosis on Prostate Cancer Cell Lines
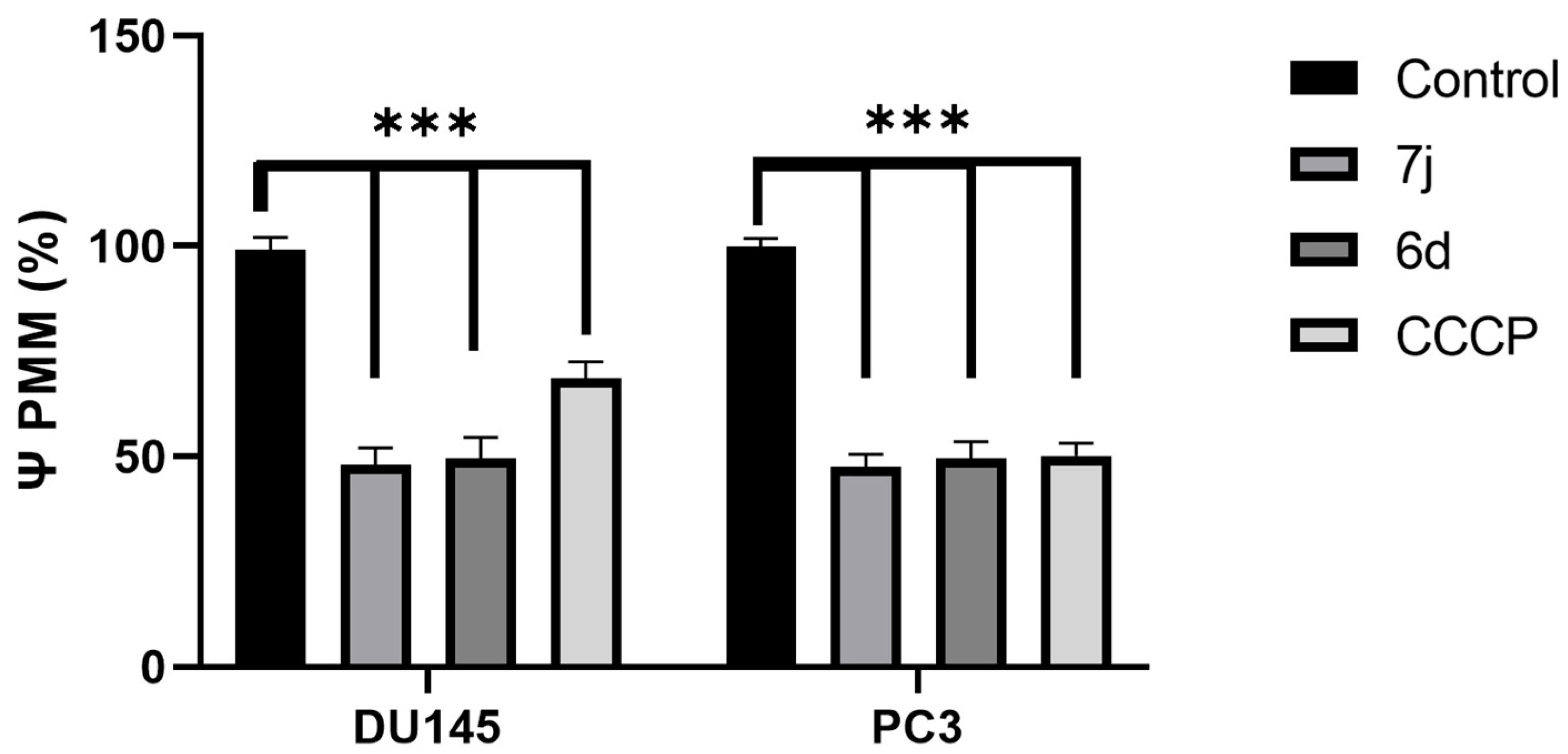
3.3. DU145 and PC3 Cell Lines Change Its Membrane Potential Under 7j and 6d Treatment
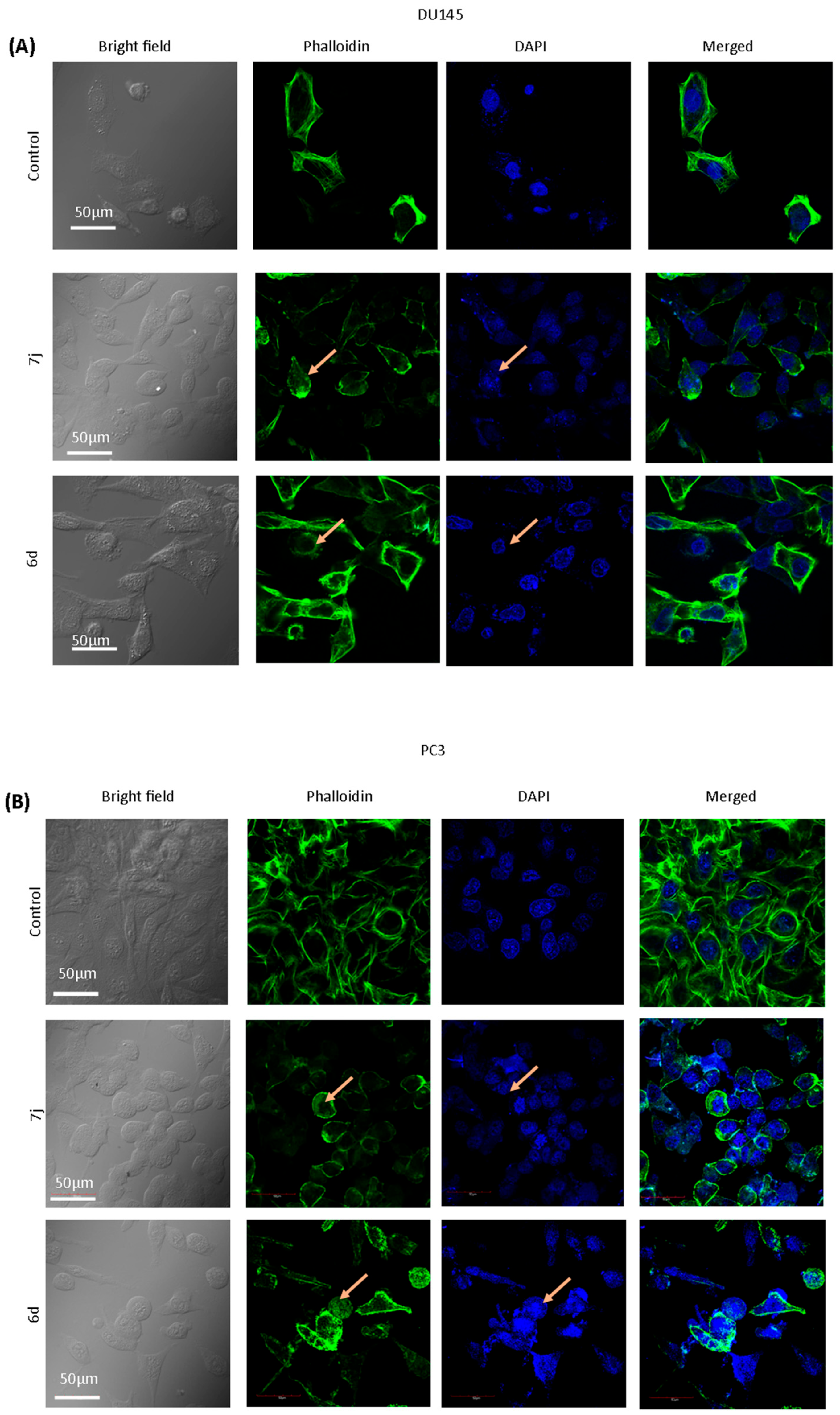
3.4. Chalcones 7j and 6d Induce Changes in the Morphology of DU145 and PC3 Cell Lines
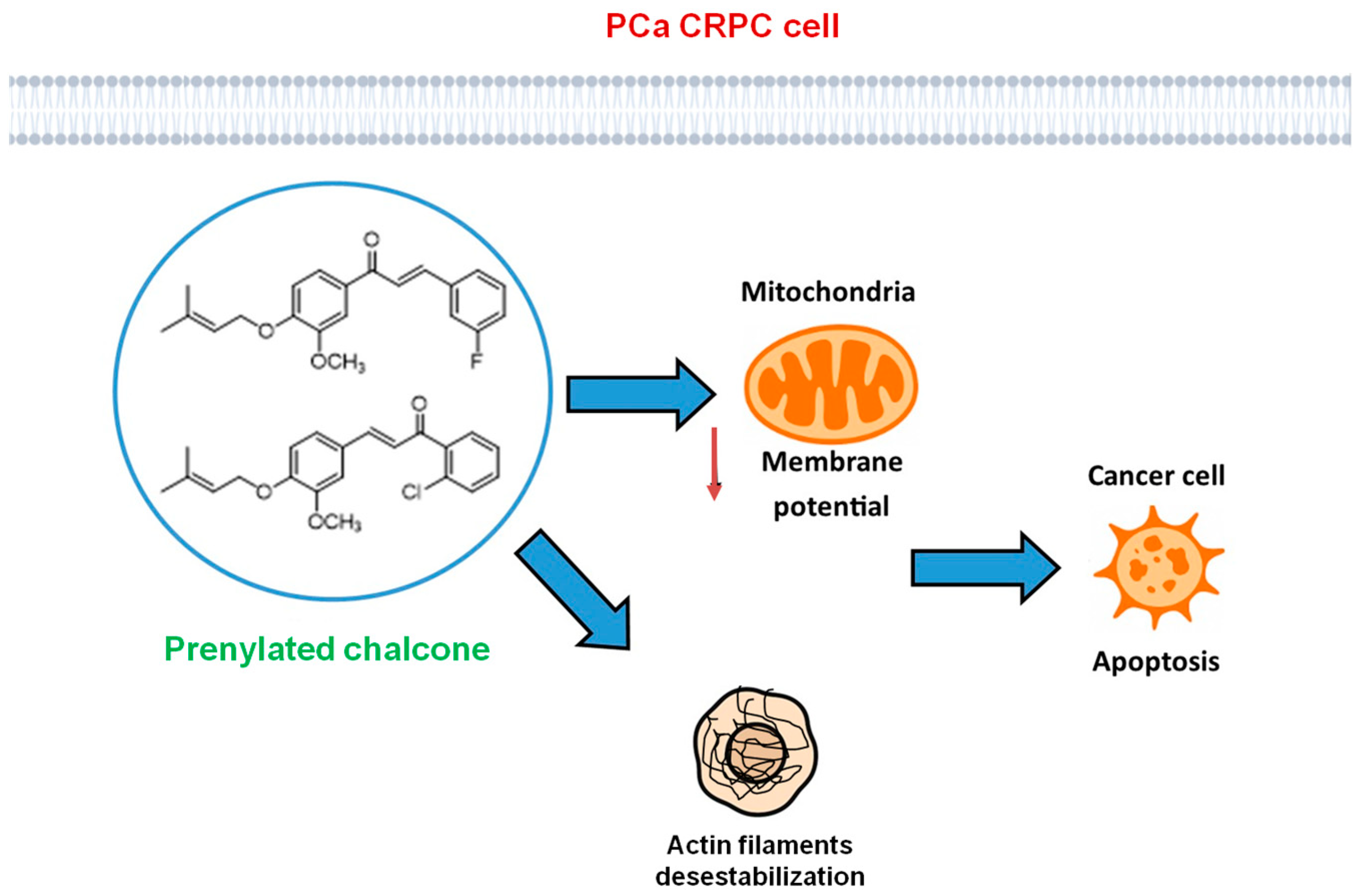
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCa | Prostate Cancer |
| CRPC | castration-resistant prostate cancer |
| 6c | (E)-3-(2-fluorophenyl)-1-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
| 6e | (E)-3-(4-fluorophenyl)-1-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
| 7e | (E)-1-(3-fluorophenyl)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
| 7a | (E)-3-(3-Methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)-1-(2-nitrophenyl)prop-2-en-1-one |
| 7j | (E)-1-(2-chlorophenyl)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
| 7l | (E)-1-(4-chlorophenyl)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
| 7d | (E)-1-(2-fluorophenyl)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
| 7k | (E)-1-(3-chlorophenyl)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
| 6d | (E)-3-(3-fluorophenyl)-1-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
References
- International Agency for Research on Cancer (IARC). Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 22 February 2022).
- Huggins, C.; Hodges, C.V. Studies on Prostatic Cancer: I. The Effect of Castration, of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. 1941. J. Urol. 2002, 168, 9–12. [Google Scholar] [CrossRef]
- Shenoy, D.; Packianathan, S.; Chen, A.M.; Vijayakumar, S. Do African-American Men Need Separate Prostate Cancer Screening Guidelines? BMC Urol. 2016, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.P.; Trevathan, S.; Hammonds, K.A.P.; Speights, V.O.; Hermans, M.R. Gleason Score Evolution and the Effect on Prostate Cancer Outcomes. Am. J. Clin. Pathol. 2021, 155, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.J. Treatment Effects in Prostate Cancer. Mod. Pathol. 2018, 31, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Nevedomskaya, E.; Baumgart, S.; Haendler, B. Recent Advances in Prostate Cancer Treatment and Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1359. [Google Scholar] [CrossRef]
- Ritch, C.; Cookson, M. Recent Trends in the Management of Advanced Prostate Cancer. F1000Research 2018, 7, 1513. [Google Scholar] [CrossRef]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the Castration-Resistant Prostate Cancer Population: A Systematic Review: The Epidemiology of CRPC. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef]
- Amaral, T.M.S.; Macedo, D.; Fernandes, I.; Costa, L. Castration-Resistant Prostate Cancer: Mechanisms, Targets, and Treatment. Prostate Cancer 2012, 2012, 327253. [Google Scholar] [CrossRef]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Joniau, S.; Mason, M.; Matveev, V.; Schmid, H.P.; van der Kwast, T.; Wiegel, T.; Zattoni, F.; et al. Guía de la EAU sobre el cáncer de próstata. Parte II: Tratamiento del cáncer de próstata avanzado, recidivante y resistente a la castración. Actas Urológicas Españolas 2011, 35, 565–579. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer Drug Resistance: An Evolving Paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Rioux, B.; Pinon, A.; Gamond, A.; Martin, F.; Laurent, A.; Champavier, Y.; Barette, C.; Liagre, B.; Fagnère, C.; Sol, V.; et al. Synthesis and Biological Evaluation of Chalcone-Polyamine Conjugates as Novel Vectorized Agents in Colorectal and Prostate Cancer Chemotherapy. Eur. J. Med. Chem. 2021, 222, 113586. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, T.; Yang, J.C.; Gao, A.C.; Evans, C.P. Mechanisms of Resistance in Castration-Resistant Prostate Cancer (CRPC). Transl. Androl. Urol. 2015, 4, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.J.; Henry, I.; Maylin, Z.; Smith, C.; Arunachalam, E.; Pandha, H.; Asim, M. A Compendium of Androgen Receptor Variant 7 Target Genes and Their Role in Castration Resistant Prostate Cancer. Front. Oncol. 2023, 13, 1129140. [Google Scholar] [CrossRef]
- Jamroze, A.; Chatta, G.; Tang, D.G. Androgen Receptor (AR) Heterogeneity in Prostate Cancer and Therapy Resistance. Cancer Lett. 2021, 518, 1–9. [Google Scholar] [CrossRef]
- Obinata, D.; Takayama, K.; Fujiwara, K.; Suzuki, T.; Tsutsumi, S.; Fukuda, N.; Nagase, H.; Fujimura, T.; Urano, T.; Homma, Y.; et al. Targeting Oct1 Genomic Function Inhibits Androgen Receptor Signaling and Castration-Resistant Prostate Cancer Growth. Oncogene 2016, 35, 6350–6358. [Google Scholar] [CrossRef]
- Attar, R.M.; Takimoto, C.H.; Gottardis, M.M. Castration-Resistant Prostate Cancer: Locking up the Molecular Escape Routes. Clin. Cancer Res. 2009, 15, 3251–3255. [Google Scholar] [CrossRef]
- Jandial, D.; Blair, C.; Zhang, S.; Krill, L.; Zhang, Y.-B.; Zi, X. Molecular Targeted Approaches to Cancer Therapy and Prevention Using Chalcones. Curr. Cancer Drug Targets 2014, 14, 181–200. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Wijayanti, L.W.; Swasono, R.T.; Lee, W.; Jumina, J. Synthesis and Evaluation of Chalcone Derivatives as Novel Sunscreen Agent. Molecules 2021, 26, 2698. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-P.; Teng, Q.; Yang, Y.-S.; Cao, J.-Q.; Xue, J.-J. Aggregation-Induced Emission Properties of Triphenylamine Chalcone Compounds. J. Fluoresc. 2021, 31, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, G.; Bhanu, D.; Aruchamy, B.; Ramani, P.; Pandurangan, N.; Bobba, K.N.; Oh, E.J.; Chung, H.Y.; Gangadaran, P.; Ahn, B.-C. Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry. Pharmaceuticals 2022, 15, 1250. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, M.L.; Pauli, I.; Dutra, L.A.; Cruz, R.S.; Valli, M.; Paracatu, L.C.; de Faria, C.M.Q.G.; Ximenes, V.F.; Regasini, L.O.; Andricopulo, A.D.; et al. Identification of a Prenyl Chalcone as a Competitive Lipoxygenase Inhibitor: Screening, Biochemical Evaluation and Molecular Modeling Studies. Molecules 2021, 26, 2205. [Google Scholar] [CrossRef]
- Luo, F.; Tang, Y.; Zheng, L.; Yang, Y.; Gao, H.; Tian, S.; Chen, H.; Tang, C.; Tang, S.; Man, Q.; et al. Isoliquiritigenin Inhibits the Growth of Colorectal Cancer Cells through the ESR2/PI3K/AKT Signalling Pathway. Pharmaceuticals 2023, 17, 43. [Google Scholar] [CrossRef]
- Bortolotto, L.F.B.; Barbosa, F.R.; Silva, G.; Bitencourt, T.A.; Beleboni, R.O.; Baek, S.J.; Marins, M.; Fachin, A.L. Cytotoxicity of Trans-Chalcone and Licochalcone A against Breast Cancer Cells Is Due to Apoptosis Induction and Cell Cycle Arrest. Biomed. Pharmacother. 2017, 85, 425–433. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, C.; Sun, Y.; Xu, H. Licochalcone A Inhibits Cell Proliferation, Migration, and Invasion through Regulating the PI3K/AKT Signaling Pathway in Oral Squamous Cell Carcinoma. OncoTargets Ther. 2019, 12, 4427–4435. [Google Scholar] [CrossRef]
- Pang, X.; Gao, X.; Liu, F.; Jiang, Y.; Wang, M.; Li, Q.; Li, Z. Xanthoangelol Modulates Caspase-1-Dependent Pyroptotic Death among Hepatocellular Carcinoma Cells with High Expression of GSDMD. J. Funct. Foods 2021, 84, 104577. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Q.; Fredimoses, M.; Gao, G.; Wang, K.; Chen, H.; Wang, T.; Oi, N.; Zykova, T.A.; Reddy, K.; et al. The Ashitaba (Angelica keiskei) Chalcones 4-Hydroxyderricin and Xanthoangelol Suppress Melanomagenesis By Targeting BRAF and PI3K. Cancer Prev. Res. 2018, 11, 607–620. [Google Scholar] [CrossRef]
- Tabata, K.; Motani, K.; Takayanagi, N.; Nishimura, R.; Asami, S.; Kimura, Y.; Ukiya, M.; Hasegawa, D.; Akihisa, T.; Suzuki, T. Xanthoangelol, a Major Chalcone Constituent of Angelica Keiskei, Induces Apoptosis in Neuroblastoma and Leukemia Cells. Biol. Pharm. Bull. 2005, 28, 1404–1407. [Google Scholar] [CrossRef]
- Mendonca, P.; Horton, A.; Bauer, D.; Messeha, S.; Soliman, K.F.A. The Inhibitory Effects of Butein on Cell Proliferation and TNF-α-Induced CCL2 Release in Racially Different Triple Negative Breast Cancer Cells. PLoS ONE 2019, 14, e0215269. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, Y.; Hua, Z.-C. Apoptosis and Apoptotic Body: Disease Message and Therapeutic Target Potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, M.; Chen, W.; Hui, J.; Ji, J.; Hu, S.; Zhou, J.; Wang, Y.; Liang, G. Chemopreventive Effect of Chalcone Derivative, L2H17, in Colon Cancer Development. BMC Cancer 2015, 15, 870. [Google Scholar] [CrossRef]
- Dong, N.; Liu, X.; Zhao, T.; Wang, L.; Li, H.; Zhang, S.; Li, X.; Bai, X.; Zhang, Y.; Yang, B. Apoptosis-Inducing Effects and Growth Inhibitory of a Novel Chalcone, in Human Hepatic Cancer Cells and Lung Cancer Cells. Biomed. Pharmacother. 2018, 105, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Z.; Jiang, M.; Zhen, Y.-S. Correlation of HERG K+ channel protein expression to chemosensitivity of tumor cells to doxorubicin and its modulation by erythromycin. Ai Zheng = Aizheng = Chin. J. Cancer 2005, 24, 924–929. [Google Scholar]
- Alaaeldin, R.; Abuo-Rahma, G.E.-D.A.; Zhao, Q.-L.; Fathy, M. Modulation of Apoptosis and Epithelial-Mesenchymal Transition E-Cadherin/TGF-β/Snail/TWIST Pathways by a New Ciprofloxacin Chalcone in Breast Cancer Cells. Anticancer. Res. 2021, 41, 2383–2395. [Google Scholar] [CrossRef]
- Santos, M.M.M.; Moreira, R. Michael Acceptors as Cysteine Protease Inhibitors. Mini Rev. Med. Chem. 2007, 7, 1040–1050. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Fu, D.-J.; Yue, X.-X.; Liu, Y.-C.; Song, J.; Sun, H.-H.; Liu, H.-M.; Zhang, Y.-B. Design, Synthesis and Structure-Activity Relationships of Novel Chalcone-1,2,3-Triazole-Azole Derivates as Antiproliferative Agents. Molecules 2016, 21, 653. [Google Scholar] [CrossRef]
- Fernandes, N.A.R.; Camilli, A.C.; Maldonado, L.A.G.; Pacheco, C.G.P.; Silva, A.F.; Molon, R.S.; Spolidorio, L.C.; Ribeiro de Assis, L.; Regasini, L.O.; Rossa Junior, C.; et al. Chalcone T4, A Novel Chalconic Compound, Inhibits Inflammatory Bone Resorption In Vivo and Suppresses Osteoclastogenesis In Vitro. J. Periodont Res. 2021, 56, 569–578. [Google Scholar] [CrossRef]
- Hernández-Rivera, J.L.; Espinoza-Hicks, J.C.; Chacón-Vargas, K.F.; Carrillo-Campos, J.; Sánchez-Torres, L.E.; Camacho-Dávila, A.A. Synthesis, Characterization and Evaluation of Prenylated Chalcones Ethers as Promising Antileishmanial Compounds. Mol. Divers. 2023, 27, 2073–2092. [Google Scholar] [CrossRef]
- Becton, Dickinson and Company (BD Life Sciences). FlowJoTM, V10.8 Software; FlowJo, LLC: Ashland, OR, USA, 2025.
- Gavrikova, T.; Nakamura, N.; Davydova, J.; Antonarakis, E.S.; Yamamoto, M. Infectivity-Enhanced, Conditionally Replicative Adenovirus for COX-2-Expressing Castration-Resistant Prostate Cancer. Viruses 2023, 15, 901. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C. Calcium Signaling in Prostate Cancer Cells of Increasing Malignancy. Biomol. Concepts 2022, 13, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wu, H.; Li, C.; Deng, G.; Chen, C. YAP1 Inhibits RSL3-Induced Castration-Resistant Prostate Cancer Cell Ferroptosis by Driving Glutamine Uptake and Metabolism to GSH. Mol. Cell Biochem. 2023, 479, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.F.; de Sousa, N.F.; de Oliveira, B.H.M.; Duarte, G.D.; Ferreira, M.D.L.; Scotti, M.T.; Filho, J.M.B.; Rodrigues, L.C.; de Moura, R.O.; Mendonça-Junior, F.J.B.; et al. Anticancer Activity of Chalcones and Its Derivatives: Review and In Silico Studies. Molecules 2023, 28, 4009. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Q.; Zhang, S. Computation of Structure Activity and Design of Chalcone Derivatives. Comput. Chem. 2019, 7, 51–58. [Google Scholar] [CrossRef]
- Sheikh, K.A.; Gupta, A.; Umar, M.; Ali, R.; Shaquiquzzaman, M.; Akhter, M.; Khan, M.A.; Kaleem, M.; Ambast, P.K.; Charan, S.; et al. Advances in Chalcone Derivatives: Unravelling Their Anticancer Potential through Structure-Activity Studies. J. Mol. Struct. 2024, 1299, 137154. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Sahebkar, A.; Hadizadeh, F.; Behravan, J.; Arabzadeh, S. Structural and Functional Aspects of P-Glycoprotein and Its Inhibitors. Life Sci. 2018, 214, 118–123. [Google Scholar] [CrossRef]
- Rapetti-Mauss, R.; Berenguier, C.; Allegrini, B.; Soriani, O. Interplay Between Ion Channels and the Wnt/β-Catenin Signaling Pathway in Cancers. Front. Pharmacol. 2020, 11, 525020. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Bartmańska, A.; Tronina, T.; Popłoński, J.; Milczarek, M.; Filip-Psurska, B.; Wietrzyk, J. Highly Cancer Selective Antiproliferative Activity of Natural Prenylated Flavonoids. Molecules 2018, 23, 2922. [Google Scholar] [CrossRef]
- Boozari, M.; Soltani, S.; Iranshahi, M. Biologically Active Prenylated Flavonoids from the Genus Sophora and Their Structure-Activity Relationship-A Review: Biological Activity of Prenylated Flavonoids from the Genus Sophora. Phytother. Res. 2019, 33, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Kromann, H.; Larsen, M.; Boesen, T.; Schønning, K.; Nielsen, S.F. Synthesis of Prenylated Benzaldehydes and Their Use in the Synthesis of Analogues of Licochalcone A. Eur. J. Med. Chem. 2004, 39, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Alimirah, F.; Chen, J.; Basrawala, Z.; Xin, H.; Choubey, D. DU-145 and PC-3 Human Prostate Cancer Cell Lines Express Androgen Receptor: Implications for the Androgen Receptor Functions and Regulation. FEBS Lett. 2006, 580, 2294–2300. [Google Scholar] [CrossRef]
- Lafront, C.; Germain, L.; Weidmann, C.; Audet-Walsh, É. A Systematic Study of the Impact of Estrogens and Selective Estrogen Receptor Modulators on Prostate Cancer Cell Proliferation. Sci. Rep. 2020, 10, 4024. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Fairbrother, W.J.; Leverson, J.D.; Souers, A.J. From Basic Apoptosis Discoveries to Advanced Selective BCL-2 Family Inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Birkinshaw, R.W. Challenges in Small-Molecule Target Identification: A Commentary on “BDA-366, a Putative Bcl-2 BH4 Domain Antagonist, Induces Apoptosis Independently of Bcl-2 in a Variety of Cancer Cell Models”. Cell Death Differ. 2021, 28, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Vucic, D. Targeting IAP Proteins for Therapeutic Intervention in Cancer. Nat. Rev. Drug Discov. 2012, 11, 109–124. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Z.; Zhao, X. Targeting Bcl-2 for Cancer Therapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188569. [Google Scholar] [CrossRef]
- Chen, L.B. Mitochondrial Membrane Potential in Living Cells. Annu. Rev. Cell Biol. 1988, 4, 155–181. [Google Scholar] [CrossRef]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef]
- Jin, J.; Qiu, S.; Wang, P.; Liang, X.; Huang, F.; Wu, H.; Zhang, B.; Zhang, W.; Tian, X.; Xu, R.; et al. Cardamonin Inhibits Breast Cancer Growth by Repressing HIF-1α-Dependent Metabolic Reprogramming. J. Exp. Clin. Cancer Res. 2019, 38, 377. [Google Scholar] [CrossRef] [PubMed]
- Meier, D.; Hernández, M.V.; van Geelen, L.; Muharini, R.; Proksch, P.; Bandow, J.E.; Kalscheuer, R. The Plant-Derived Chalcone Xanthoangelol Targets the Membrane of Gram-Positive Bacteria. Bioorganic Med. Chem. 2019, 27, 115151. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-A. Modulation of Signaling between TM4SF5 and Integrins in Tumor Microenvironment. Front. Biosci. 2011, 16, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Kotra, V.; Kola, P.K.; Devi, C.P.; Anusha, N.; Babu, B.H.; Adil, S.F.; Shaik, M.R.; Khan, M.; Al-Warthan, A.; et al. ZnCl2 Catalyzed New Coumarinyl-Chalcones as Cytotoxic Agents. Saudi J. Biol. Sci. 2021, 28, 386–394. [Google Scholar] [CrossRef]
- Das, A.; Bhattacharya, A.; Chakrabarty, S.; Ganguli, A.; Chakrabarti, G. Smokeless Tobacco Extract (STE)-Induced Toxicity in Mammalian Cells Is Mediated by the Disruption of Cellular Microtubule Network: A Key Mechanism of Cytotoxicity. PLoS ONE 2013, 8, e68224. [Google Scholar] [CrossRef]
- Das, M.; Manna, K. Chalcone Scaffold in Anticancer Armamentarium: A Molecular Insight. J. Toxicol. 2016, 2016, 7651047. [Google Scholar] [CrossRef]
- Lamie, P.F.; Philoppes, J.N. 2-Thiopyrimidine/Chalcone Hybrids: Design, Synthesis, ADMET Prediction, and Anticancer Evaluation as STAT3/STAT5a Inhibitors. J. Enzyme Inhib. Med. Chem. 2020, 35, 864–879. [Google Scholar] [CrossRef]
- de Souza, P.S.; Bibá, G.C.C.; Melo, E.D.D.N.; Muzitano, M.F. Chalcones against the Hallmarks of Cancer: A Mini-Review. Nat. Prod. Res. 2022, 36, 4809–4826. [Google Scholar] [CrossRef]
- Elkhalifa, D.; Alali, F.; Al Moustafa, A.-E.; Khalil, A. Targeting Triple Negative Breast Cancer Heterogeneity with Chalcones: A Molecular Insight. J. Drug Target. 2019, 27, 830–838. [Google Scholar] [CrossRef]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The Role of Chalcones in Suppression of NF-κB-Mediated Inflammation and Cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef]
| Structure | Code | MW | SMILE | IUPAC Name |
|---|---|---|---|---|
 | (6c) | 340.39 | C/C(C)=C\COC1=CC=C(C(/C=C/C2=C(F)C=CC=C2)=O)C=C1OC | (E)-3-(2-fluorophenyl)-1-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
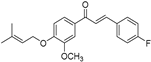 | (6e) | 340.39 | C/C(C)=C\COC1=CC=C(C(/C=C/C2=CC=C(F)C=C2)=O)C=C1OC | (E)-3-(4-fluorophenyl)-1-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
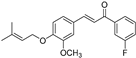 | (7e) | 340.39 | C/C(C)=C\COC1=CC=C(/C=C/C(C2=CC(F)=CC=C2)=O)C=C1OC | (E)-1-(3-fluorophenyl)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
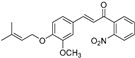 | (7a) | 367.40 | C/C(C)=C\COC1=CC=C(/C=C/C(C2=C([N+]([O-])=O)C=CC=C2)=O)C=C1OC | (E)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)-1-(2-nitrophenyl)prop-2-en-1-one |
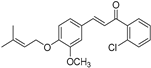 | (7j) | 356.84 | C/C(C)=C\COC1=CC=C(/C=C/C(C2=C(Cl)C=CC=C2)=O)C=C1OC | (E)-1-(2-chlorophenyl)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
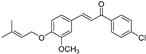 | (7l) | 356.84 | C/C(C)=C\COC1=CC=C(/C=C/C(C2=CC=C(Cl)C=C2)=O)C=C1OC | (E)-1-(4-chlorophenyl)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
 | (7d) | 340.39 | C/C(C)=C\COC1=CC=C(/C=C/C(C2=C(F)C=CC=C2)=O)C=C1OC | (E)-1-(2-fluorophenyl)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
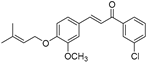 | (7k) | 356.84 | C/C(C)=C\COC1=CC=C(/C=C/C(C2=CC(Cl)=CC=C2)=O)C=C1OC | (E)-1-(3-chlorophenyl)-3-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
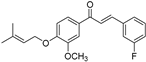 | (6d) | 340.39 | C/C(C)=C\COC1=CC=C(C(/C=C/C2=CC(F)=CC=C2)=O)C=C1OC | (E)-3-(3-fluorophenyl)-1-(3-methoxy-4-((3-methylbut-2-en-1-yl)oxy)phenyl)prop-2-en-1-one |
| Chalcone | Structure | IC50 DU145 (μM) | IC50 PC3 (μM) | IC50 RWPE-1 (μM) |
|---|---|---|---|---|
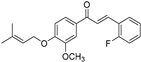
| 6d | 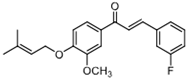 | 88.73 (+/−1.086) | 54.969 (+/−0.56) | 62.34 (+/−0.47) |
| 7j | 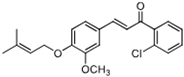 | 61.71 (+/−0.79) | 57.22 (+/−0.85) | 1 139.5 (+/−0.7) |
| Treatment | Control | 7j | 6d | CCCP | |
|---|---|---|---|---|---|
| Cell line | |||||
| DU 145 | 99% | 48% | 49.6% | 68.5% | |
| PC3 | 99.8% | 47.5 | 49.6% | 50.2% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Reyna, M.; Figueroa-Angulo, E.E.; Espinoza-Hicks, J.; Camacho-Dávila, A.; López-Camarillo, C.; Vázquez-Carrillo, L.I.; Salgado-Aguayo, A.; Carlos-Reyes, Á.; Álvarez-Jiménez, V.D.; Puente-Rivera, J.; et al. Prenylated Chalcones as Anticancer Agents Against Castration-Resistant Prostate Cancer. Sci. Pharm. 2025, 93, 25. https://doi.org/10.3390/scipharm93020025
Morales-Reyna M, Figueroa-Angulo EE, Espinoza-Hicks J, Camacho-Dávila A, López-Camarillo C, Vázquez-Carrillo LI, Salgado-Aguayo A, Carlos-Reyes Á, Álvarez-Jiménez VD, Puente-Rivera J, et al. Prenylated Chalcones as Anticancer Agents Against Castration-Resistant Prostate Cancer. Scientia Pharmaceutica. 2025; 93(2):25. https://doi.org/10.3390/scipharm93020025
Chicago/Turabian StyleMorales-Reyna, Marcos, Elisa Elvira Figueroa-Angulo, José Espinoza-Hicks, Alejandro Camacho-Dávila, César López-Camarillo, Laura Isabel Vázquez-Carrillo, Alfonso Salgado-Aguayo, Ángeles Carlos-Reyes, Violeta Deyanira Álvarez-Jiménez, Jonathan Puente-Rivera, and et al. 2025. "Prenylated Chalcones as Anticancer Agents Against Castration-Resistant Prostate Cancer" Scientia Pharmaceutica 93, no. 2: 25. https://doi.org/10.3390/scipharm93020025
APA StyleMorales-Reyna, M., Figueroa-Angulo, E. E., Espinoza-Hicks, J., Camacho-Dávila, A., López-Camarillo, C., Vázquez-Carrillo, L. I., Salgado-Aguayo, A., Carlos-Reyes, Á., Álvarez-Jiménez, V. D., Puente-Rivera, J., & Alvarez-Sánchez, M. E. (2025). Prenylated Chalcones as Anticancer Agents Against Castration-Resistant Prostate Cancer. Scientia Pharmaceutica, 93(2), 25. https://doi.org/10.3390/scipharm93020025









