Purified Clinoptilolite-Tuff as a Trap for Amines Associated with Chronic Wounds: Binding of Cadaverine, Putrescine, Histamines and Polyamines
Abstract
1. Introduction
2. Results
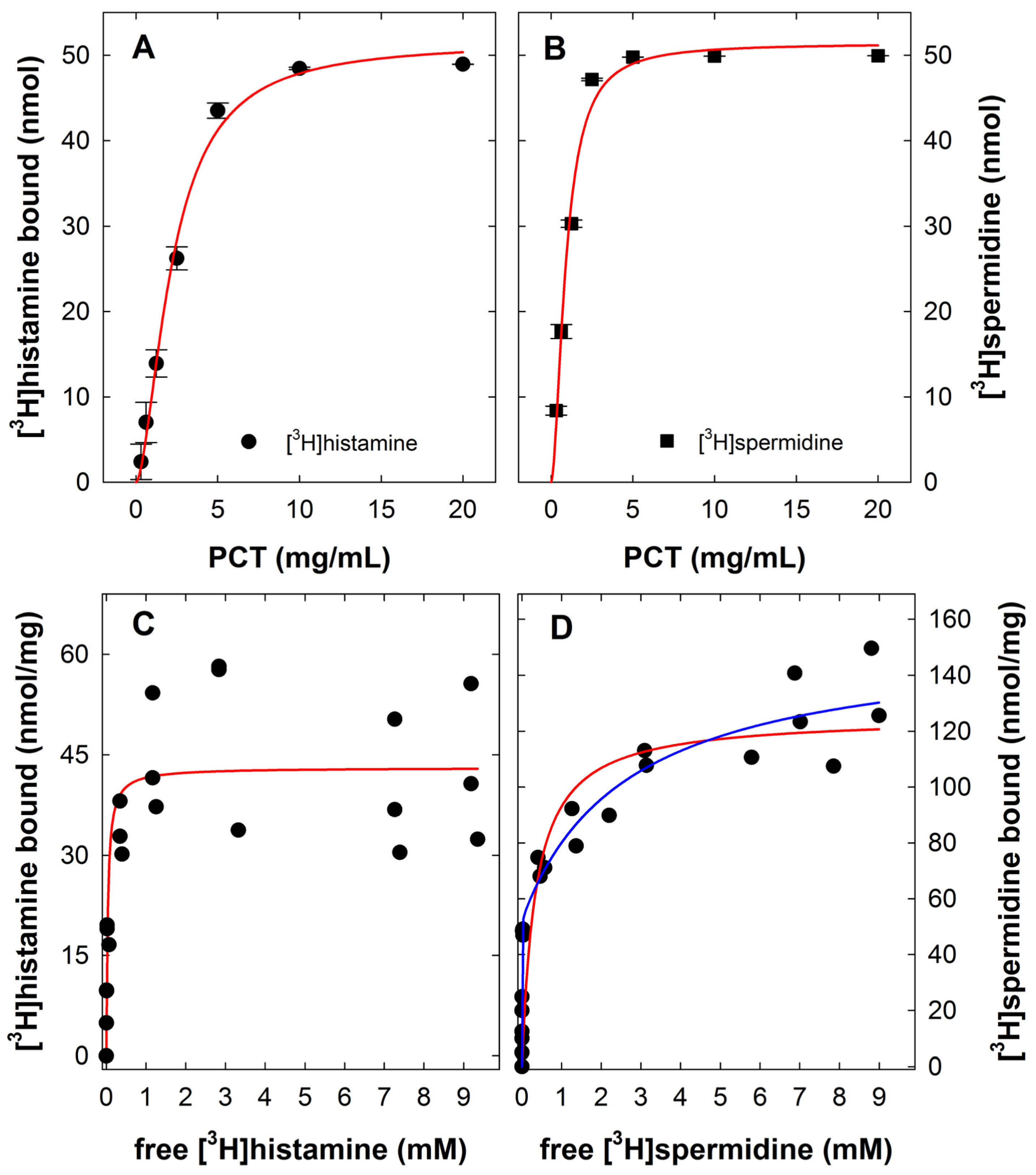
2.1. Binding of [3H]histamine and [3H]spermidine
2.2. Displacement of [3H]histamine and [3H]spermidine
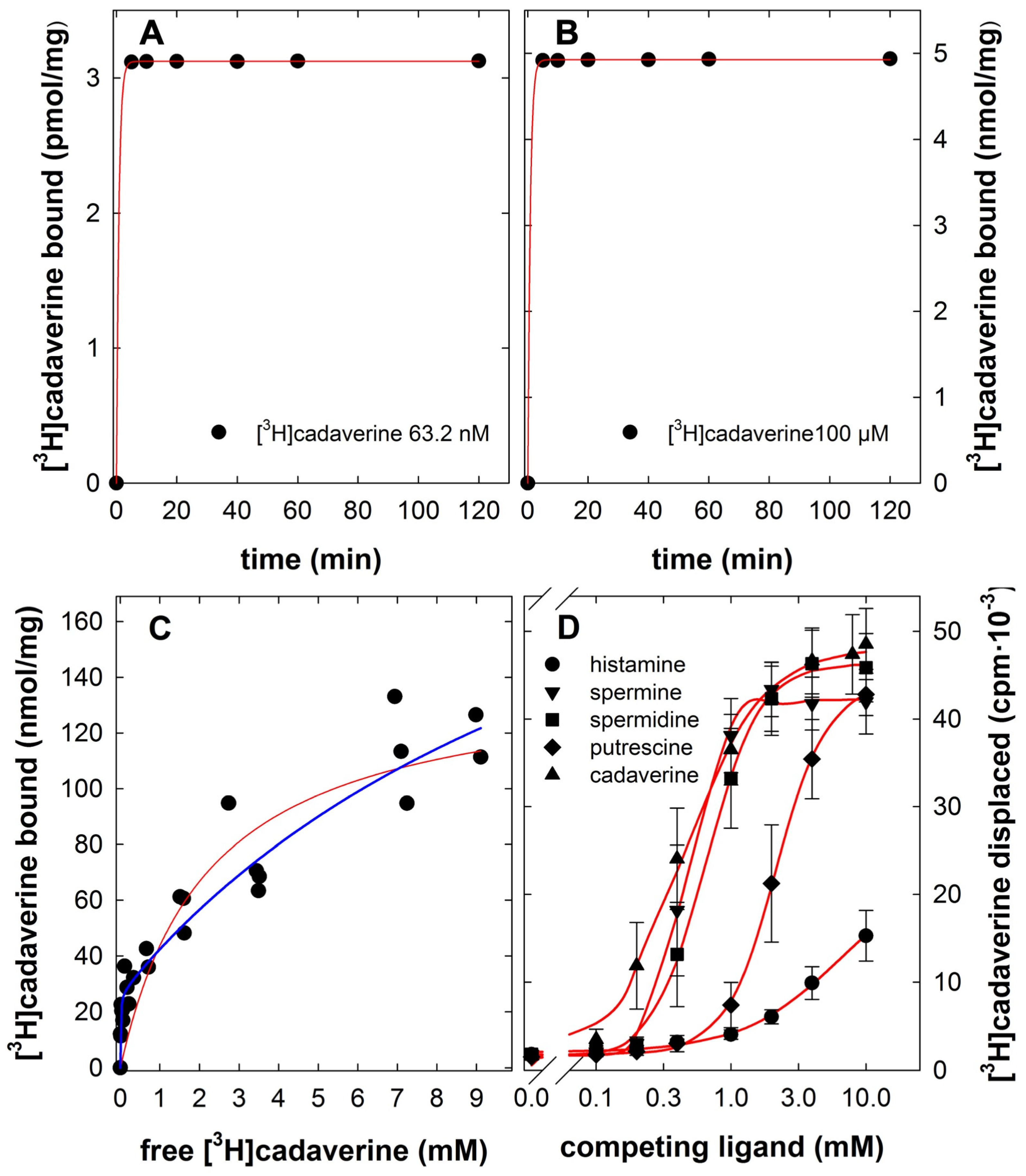
2.3. Binding and Displacement of [3H]cadaverine
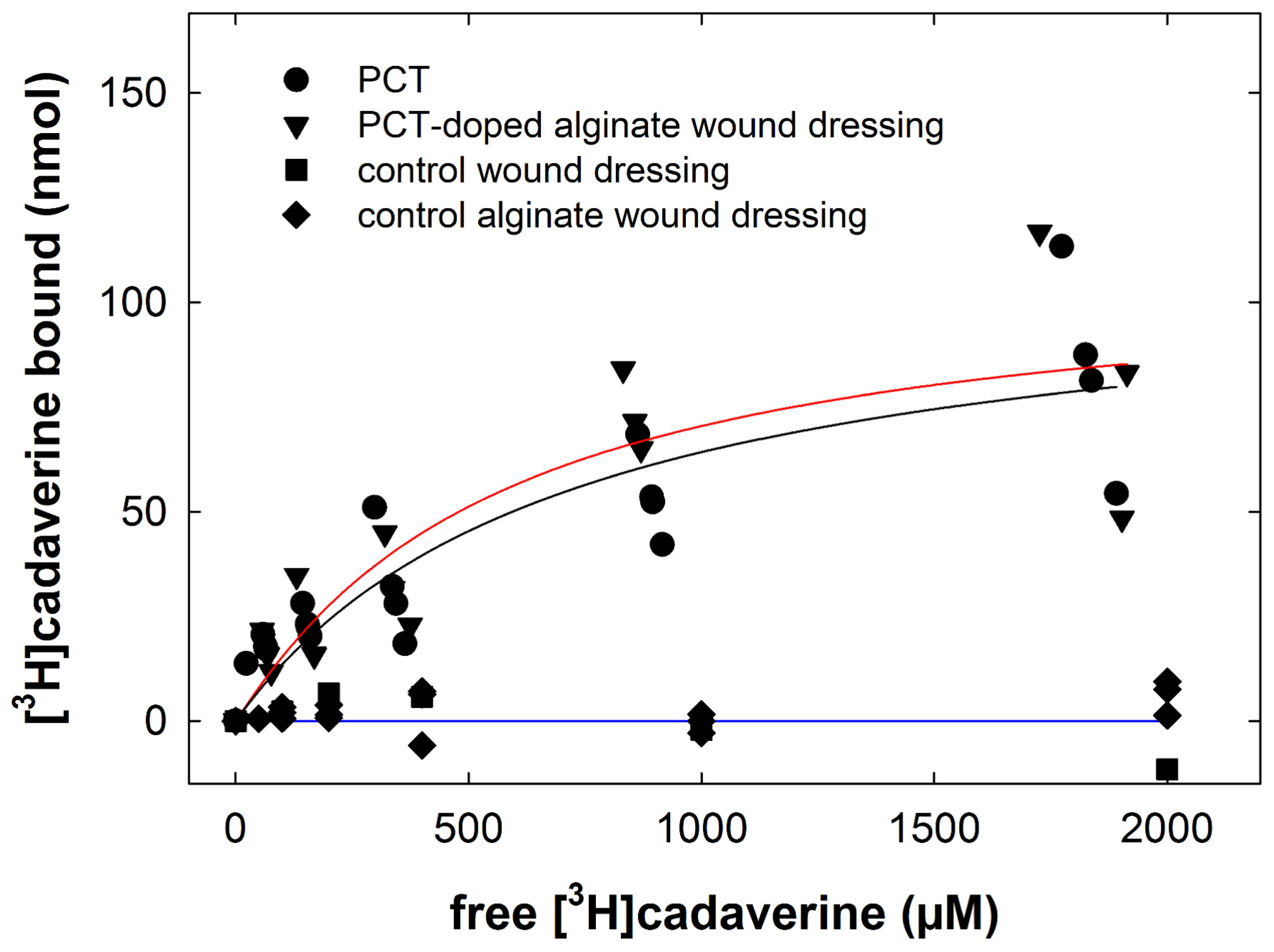
2.4. Binding of [3H]cadaverine in the Presence of Wound Drainage Fluid
2.5. Binding of [3H]cadaverine to PCT-Containing Wound Dressing
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Wound Drainage Fluid
4.3. Binding Experiments

- (i)
- In time course experiments, PCT (10 mg/assay) was mixed either with carrier-free radioactive ligands (resulting in concentrations in the nM range) or with radioactive ligands, which had been isotopically diluted with 100 µM of the unlabeled compound. The incubation time varied between 4 and 119 min. Bound and free radioligand were separated by centrifugation (1 min at 12,000× g). An aliquot of the supernatant (0.25 mL) was withdrawn and transferred to scintillation vials. The bound fraction was calculated by subtracting the free fraction determined in the supernatant from the total radioactivity added to the reaction.
- (ii)
- In titration experiments, the amount of PCT varied between 0.15 and 10 mg. The concentration of each radioactive ligand (0.05 to 0.1 µCi) was adjusted to 100 µM by isotopic dilution with the unlabeled compound. When added, wound drainage fluid represented 50% of the reaction volume. The incubation was carried out for 2 h or 24 h. We separately determined the extent by which wound drainage fluid quenched liquid scintillation counting of radioactivity: regardless of their color, quenching was not seen if ≤ 50 µL of wound drainage fluid was added to the scintillation vial. Accordingly, in experiments that examined the effect of wound drainage fluid on the binding of [3H]cadaverine to PCT, a 50 µL aliquot was withdrawn to measure the amount of free radioactivity remaining in the supernatant.
- (iii)
- In saturation experiments, PCT (4 mg/reaction) was incubated for 2 h with increasing concentrations of each radioactive ligand. The total radioactivity (0.05 to 0.1 µCi) was kept constant, and the specific activity was progressively diluted by the addition of an unlabeled ligand (0.1 to 10 mM). Saturation experiments were also performed to compare the binding of [3H]cadaverine (0.08 µCi carrier-free and diluted with 0.05 to 2 mM unlabeled cadaverine) to 0.7 mg PCT in suspension with the binding to squares (1 × 1 cm) of PCT-doped wound dressing, alginate wound dressing (PCT free) or control wound dressing.
- (iv)
- Finally, in competition experiments, PCT (4 mg/reaction) was incubated in the presence of each radioactive ligand (0.05 to 0.1 µCi) and increasing concentrations of unlabeled cadaverine, histamine, putrescine, spermidine or spermine for 2 h. Preliminary experiments (with total concentrations increasing by one order magnitude from 1 nM to 10 mM) were carried out to identify the concentration range, where the compounds competed effectively with the radiolabeled tracers. Because total concentrations ≤ 0.1 mM failed to displace the bound radiotracers, logarithmically concentrations covering the range of 0.1 to 10 mM were subsequently to determine the inhibitory potency of competitors.
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abrams, H.L.; Sprio, R.; Goldstein, N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950, 3, 74–85. [Google Scholar] [CrossRef]
- McWhorter, J.E.; Cloud, A.W. Malignant tumors and their metastases: A summary of the necropsies on eight hundred sixty-five cases performed at the Bellevue hospital of New York. Ann. Surg. 1930, 92, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Gates, O. Cutaneous Metastases of Malignant Disease. Am. J. Cancer 1937, 30, 718–730. [Google Scholar]
- Lookingbill, D.P.; Spangler, N.; Helm, K.F. Cutaneous metastases in patients with metastatic carcinoma: A retrospective study of 4020 patients. J. Am. Acad. Dermatol. 1993, 29, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Probst, S.; Arber, A.; Faithfull, S. Malignant fungating wounds: A survey of nurses’ clinical practice in Switzerland. Eur. J. Oncol. Nurs. 2009, 13, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Tilley, C.P.; Fu, M.R.; Qiu, J.M.; Comfort, C.; Crocilla, B.L.; Li, Z.; Axelrod, D. The microbiome and metabolome of malignant fungating wounds: A systematic review of the literature from 1995 to 2020. J. Wound Ostomy Cont. Nurs. 2021, 48, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, M.; Flaminio, Z.; Sapru, S.; Tilley, C.P.; Fu, M.R.; Comfort, C.; Li, X.; Saxena, D. The microbiome, malignant fungating wounds, and palliative care. Front. Cell. Infect. Microbiol. 2019, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.A. Fighting odor in wounds. Adv. Ski. Wound Care 2006, 19, 242–244. [Google Scholar] [CrossRef]
- Fromantin, I.; Seyer, D.; Watson, S.; Rollot, F.; Elard, J.; Escande, M.C.; De Rycke, Y.; Kriegel, I.; Larreta Garde, V. Bacterial floras and biofilms of malignant wounds associated with breast cancers. J. Clin. Microbiol. 2013, 51, 3368–3373. [Google Scholar] [CrossRef]
- Michael, A.J. Polyamines in eukaryotes, bacteria, and archaea. J. Biol. Chem. 2016, 291, 14896–14903. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Saraiva, L.R.; Ferrero, D.M.; Ahuja, G.; Krishna, V.S.; Liberles, S.D.; Korsching, S.I. High-affinity olfactory receptor for the death-associated odor cadaverine. Proc. Natl. Acad. Sci. USA 2013, 110, 19579–19584. [Google Scholar] [CrossRef]
- Jia, L.; Li, S.; Dai, W.; Guo, L.; Xu, Z.; Scott, A.M.; Zhang, Z.; Ren, J.; Zhang, Q.; Dexheimer, T.S.; et al. Convergent olfactory trace amine-associated receptors detect biogenic polyamines with distinct motifs via a conserved binding site. J. Biol. Chem. 2021, 297, 101268. [Google Scholar] [CrossRef] [PubMed]
- Wisman, A.; Shrira, I. The smell of death: Evidence that putrescine elicits threat management mechanisms. Front. Psychol. 2015, 6, 1274. [Google Scholar] [CrossRef] [PubMed]
- Gethin, G.; Grocott, P.; Probst, S.; Clarke, E. Current practice in the management of wound odour: An international survey. Int. J. Nurs. Stud. 2014, 51, 865–874. [Google Scholar] [CrossRef]
- Gethin, G.; Murphy, L.; Sezgin, D.; Carr, P.J.; Mcintosh, C.; Probst, S. Resigning oneself to a life of wound-related odour—A thematic analysis of patient experiences. J. Tissue Viability 2023, 32, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Tamai, N.; Akase, T.; Minematsu, T.; Higashi, K.; Toida, T.; Igarashi, K.; Sanada, H. Association between components of exudates and periwound moisture-associated dermatitis in breast cancer patients with malignant fungating wounds. Biol. Res. Nurs. 2016, 18, 199–206. [Google Scholar] [CrossRef]
- Wen, J.; Almurani, M.; Liu, P.; Sun, Y. Aldehyde-functionalized cellulose as reactive sorbents for the capture and retention of polyamine odor molecules associated with chronic wounds. Carbohydr. Polym. 2023, 316, 121077. [Google Scholar] [CrossRef] [PubMed]
- Akhmetova, A.; Saliev, T.; Allan, I.U.; Illsley, M.J.; Nurgozhin, T.; Mikhalovsky, S. A Comprehensive review of topical odor-controlling treatment options for chronic wounds. J. Wound Ostomy Cont. Nurs. 2016, 43, 598–609. [Google Scholar] [CrossRef]
- Haemmerle, M.; Fendrych, J.; Matiasek, E.; Tschegg, C. Adsorption and release characteristics of purified and non-purified clinoptilolite-tuffs towards health-relevant heavy metals. Crystals 2021, 11, 1343. [Google Scholar] [CrossRef]
- Haemmerle, M.M.; Tschegg, C. Sorption of Natural Siderophores onto clinoptilolite-tuff and its controlled-release characteristics. Minerals 2023, 13, 611. [Google Scholar] [CrossRef]
- Ranftler, C.; Röhrich, A.; Sparer, A.; Tschegg, C.; Nagl, D. Purified clinoptilolite-tuff as an efficient sorbent for gluten derived from food. Int. J. Mol. Sci. 2022, 23, 5143. [Google Scholar] [CrossRef] [PubMed]
- Ranftler, C.; Zehentner, M.; Pengl, A.; Röhrich, A.; Tschegg, C.; Nagl, D. Purified clinoptilolite-tuff as an efficient sorbent for food-derived peanut allergens. Int. J. Mol. Sci. 2024, 25, 6510. [Google Scholar] [CrossRef]
- Nizet, S.; Rieger, J.; Sarabi, A.; Lajtai, G.; Zatloukal, K.; Tschegg, C. Binding and inactivation of human coronaviruses, including SARS-CoV-2, onto purified clinoptilolite-tuff. Sci. Rep. 2023, 13, 4673. [Google Scholar] [CrossRef]
- Sarabi, A.; Nizet, S.; Röhrich, A.; Tschegg, C. Unveiling the broad-spectrum virucidal potential of purified clinoptilolite-tuff. Microorganisms 2024, 12, 1572. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I.; Bernhardt, R. Interactions of natural polyamines with mammalian proteins. Biomol. Concepts 2011, 2, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Deinsberger, J.; Marquart, E.; Nizet, S.; Meisslitzer, C.; Tschegg, C.; Uspenska, K.; Gouya, G.; Niederdöckl, J.; Freissmuth, M.; Wolzt, M.; et al. Topically administered purified clinoptilolite-tuff for the treatment of cutaneous wounds: A prospective, randomised phase I clinical trial. Wound Repair Regen. 2022, 30, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Krell, T.; Gavira, J.A.; Velando, F.; Fernández, M.; Roca, A.; Monteagudo-Cascales, E.; Matilla, M.A. Histamine: A bacterial signal molecule. Int. J. Mol. Sci. 2021, 22, 6312. [Google Scholar] [CrossRef]
- Cooper, R.A.; Morwood, J.M.; Burton, N. Histamine production by bacteria isolated from wounds. J. Infect. 2004, 49, 39. [Google Scholar] [CrossRef]
- Nanoff, C.; Yang, Q.; Hellinger, R.; Hermann, M. Activation of the calcium-sensing receptor by a subfraction of amino acids contained in thyroid drainage fluid. ACS Pharmacol. Transl. Sci. 2024, 7, 1937–1950. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef]
- Tschegg, C.; Rice, A.H.N.; Grasemann, B.; Matiasek, E.; Kobulej, P.; Dzivák, M.; Berger, T. Petrogenesis of a large-scale miocene zeolite tuff in the Eastern Slovak Republic: The Nižný Hrabovec open-pit clinoptilolite mine. Econ. Geol. 2019, 114, 1177–1194. [Google Scholar] [CrossRef]
- Tschegg, C.; Hou, Z.; Rice, A.H.N.; Fendrych, J.; Matiasek, E.; Berger, T.; Grasemann, B. Fault zone structures and strain localization in clinoptilolite-tuff (Nižný Hrabovec, Slovak Republic). J. Struct. Geol. 2020, 138, 104090. [Google Scholar] [CrossRef]
- Samekova, K.; Firbas, C.; Irrgeher, J.; Opper, C.; Prohaska, T.; Retzmann, A.; Tschegg, C.; Meisslitzer, C.; Tchaikovsky, A.; Gouya, G.; et al. Concomitant oral intake of purified clinoptilolite-tuff (G-PUR) reduces enteral lead uptake in healthy humans. Sci. Rep. 2021, 11, 14796. [Google Scholar] [CrossRef] [PubMed]
- Ranftler, C.; Nagl, D.; Sparer, A.; Röhrich, A.; Freissmuth, M.; El-Kasaby, A.; Nasrollahi Shirazi, S.; Koban, F.; Tschegg, C.; Nizet, S. Binding and neutralization of C. difficile toxins A and B by purified clinoptilolite-tuff. PLoS ONE 2021, 16, e0252211. [Google Scholar] [CrossRef]
- Nizet, S.; Muñoz, E.; Fiebich, B.L.; Abuja, P.M.; Kashofer, K.; Zatloukal, K.; Tangermann, S.; Kenner, L.; Tschegg, C.; Nagl, D.; et al. Clinoptilolite in dextran sulphate sodium-induced murine colitis: Efficacy and safety of a microparticulate preparation. Inflamm. Bowel Dis. 2017, 24, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Yoshikawa, K. Cutaneous wound healing: An update. J. Dermatol. 2001, 28, 521–534. [Google Scholar] [CrossRef]
- Singh, A.V.; Gemmati, D.; Kanase, A.; Pandey, I.; Misra, V.; Kishore, V.; Jahnke, T.; Bill, J. Nanobiomaterials for vascular biology and wound management: A review. Veins Lymphat. 2018, 7, 7196. [Google Scholar] [CrossRef]
- Numata, Y.; Terui, T.; Okuyama, R.; Hirasawa, N.; Sugiura, Y.; Miyoshi, I.; Watanabe, T.; Kuramasu, A.; Tagami, H.; Ohtsu, H. The accelerating effect of histamine on the cutaneous wound-healing process through the action of basic fibroblast growth factor. J. Investig. Dermatol. 2006, 126, 1403–1409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ashida, Y.; Denda, M.; Hirao, T. Histamine H1 and H2 receptor antagonists accelerate skin barrier repair and prevent epidermal hyperplasia induced by barrier disruption in a dry environment. J. Investig. Dermatol. 2001, 116, 261–265. [Google Scholar] [CrossRef]
- Lim, H.K.; Rahim, A.B.; Leo, V.I.; Das, S.; Lim, T.C.; Uemura, T.; Igarashi, K.; Common, J.; Vardy, L.A. Polyamine regulator AMD1 promotes cell migration in epidermal wound healing. J. Invest. Dermatol. 2018, 138, 2653–2665. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Ito, H.; Ideta, T.; Kanbe, A.; Ninomiya, S.; Shimizu, M. Systemic and topical administration of spermidine accelerates skin wound healing. Cell Commun. Signal. 2021, 19, 36. [Google Scholar] [CrossRef]
- Johnson, L.; Mulcahy, H.; Kanevets, U.; Shi, Y.; Lewenza, S. Surface-localized spermidine protects the Pseudomonas aeruginosa outer membrane from antibiotic treatment and oxidative stress. J. Bacteriol. 2012, 194, 813–826. [Google Scholar] [CrossRef]
- Hasan, C.M.; Pottenger, S.; Green, A.E.; Cox, A.A.; White, J.S.; Jones, T.; Winstanley, C.; Kadioglu, A.; Wright, M.H.; Neill, D.R.; et al. Pseudomonas aeruginosa utilizes the host-derived polyamine spermidine to facilitate antimicrobial tolerance. JCI Insight 2022, 7, e158879. [Google Scholar] [CrossRef] [PubMed]
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Community Nurs. 2003, 8 (Suppl. S9), 4–9. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef]
- Shukla, V.K.; Shukla, D.; Tiwary, S.K.; Agrawal, S.; Rastogi, A. Evaluation of pH measurement as a method of wound assessment. J. Wound Care 2007, 16, 291–2944. [Google Scholar] [CrossRef] [PubMed]
- Alavi, A.; Farzanfar, D.; Lee, R.K.; Almutairi, D. The contribution of malodour in quality of life of patients with hidradenitis suppurativa. J. Cutan. Med. Surg. 2018, 22, 166–174. [Google Scholar] [CrossRef]
- Hubner, P.; Donati, N.; Quines, L.K.M.; Tessaro, I.C.; Marcilio, N.R. Gelatin-based films containing clinoptilolite-Ag for application as wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110215. [Google Scholar] [CrossRef] [PubMed]





| Displacement of | ||||||
|---|---|---|---|---|---|---|
| [3H]histamine Binding | [3H]spermidine Binding | [3H]cadaverine Binding | ||||
| IC50 (µM) | Slope Factor | IC50 (µM) | Slope Factor | IC50 (µM) | Slope Factor | |
| cadaverine | 1024 ± 24 | 1.6 ± 0.1 | 5357 ± 1000 | 2.9 ± 0.3 | 470 ± 123 | 1.6 ± 0.2 |
| histamine | 1389 ± 198 | 1.6 ± 0.2 | n.c. | n.c. | n.c. | n.c. |
| putrescine | 1423 ± 310 | 3.0 ± 1.0 | 4569 ± 541 | 2.9 ± 0.3 | 2153 ± 455 | 2.1 ± 0.3 |
| spermidine | 819 ± 59 | 3.5 ± 0.5 | 1127 ± 78 | 2.1 ± 0.1 | 663 ± 212 | 2.2 ± 0.2 |
| spermine | 785 ± 41 | 5.0 ± 0.7 | 662 ± 4 | 2.7 ± 0.1 | 473 ± 194 | 3.7 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Kasaby, A.; Nanoff, C.; Nizet, S.; Tschegg, C.; Freissmuth, M. Purified Clinoptilolite-Tuff as a Trap for Amines Associated with Chronic Wounds: Binding of Cadaverine, Putrescine, Histamines and Polyamines. Sci. Pharm. 2025, 93, 7. https://doi.org/10.3390/scipharm93010007
El-Kasaby A, Nanoff C, Nizet S, Tschegg C, Freissmuth M. Purified Clinoptilolite-Tuff as a Trap for Amines Associated with Chronic Wounds: Binding of Cadaverine, Putrescine, Histamines and Polyamines. Scientia Pharmaceutica. 2025; 93(1):7. https://doi.org/10.3390/scipharm93010007
Chicago/Turabian StyleEl-Kasaby, Ali, Christian Nanoff, Stephane Nizet, Cornelius Tschegg, and Michael Freissmuth. 2025. "Purified Clinoptilolite-Tuff as a Trap for Amines Associated with Chronic Wounds: Binding of Cadaverine, Putrescine, Histamines and Polyamines" Scientia Pharmaceutica 93, no. 1: 7. https://doi.org/10.3390/scipharm93010007
APA StyleEl-Kasaby, A., Nanoff, C., Nizet, S., Tschegg, C., & Freissmuth, M. (2025). Purified Clinoptilolite-Tuff as a Trap for Amines Associated with Chronic Wounds: Binding of Cadaverine, Putrescine, Histamines and Polyamines. Scientia Pharmaceutica, 93(1), 7. https://doi.org/10.3390/scipharm93010007






