Abstract
(1) Background Immune checkpoint inhibitors (ICIs) have recently become an important therapeutic option for patients with advanced urothelial carcinoma (aUC). Avelumab is an anti-PD-L1 (programmed cell death ligand 1) antibody that restores antitumor T-cell immune function by blocking the binding of PD-1 to its ligand PD-L1. (2) Methods: Our study enrolled 60 elderly patients (≥70 years) diagnosed with aUC. The primary endpoints of this study were overall survival (OS), progression free survival (PFS), and objective response rate (ORR); the secondary endpoints were tolerability, pre- and post- treatment reduction in serum Ca 19.9, and quality of life (QoL). (3) Results: Our results showed no statistically significant or clinically relevant differences between the PD-L1-positive and negative groups. Avelumab was well tolerated and resulted in good disease control, with a moderate toxicity profile and significant clinical benefit. The median PFS was 3.6 months (95% CI: 2.3–6.8), and the median OS was 18.6 months (95% CI: 6.3–20.7), with an ORR of 20%. A significant correlation was observed between serum Ca 19.9 reduction and PFS of 0.59 (95% CI: 0.12–0.57), p = 0.007. (4) Conclusions: Avelumab is an immunotherapy treatment that has been shown to be an effective and well tolerated treatment option in elderly patients with aUC.
1. Introduction
Between 20% and 50% of urothelial carcinomas are characterized by a high expression of programmed cell death ligand 1 (PD-L1), a characteristic associated with increased response to immune checkpoint inhibitors in several types of cancer [1]. Advanced stage/metastatic urothelial carcinoma (aUC) has a poor prognosis, although most patients have a good response or stable disease with first line platinum based chemotherapy, usually between four and six cycles, based on their response and tolerability [2,3]. Responses to chemotherapy treatment are generally not durable, and the 5-year progression free survival (PFS) rate is around 10% [4]. Patients with progression, after a period of follow-up, undergo a second line, usually based on vinflunine or taxanes, which are poorly effective and have nonnegligible toxicity [5]. Avelumab is a monoclonal antibody directed against the programmed cell death receptor ligand PD-L1. In the multicenter phase III Javelin 100 study, patients with aUC without progression after a first line regimen of platinum, salts, and gemcitabine for at least four cycles, were randomized to receive maintenance therapy with Avelumab, 800 mg administered intravenously over 60 min every 2 weeks according to the guidelines until unacceptable toxicity, progression, or patient withdrawal of consent [6]. Avelumab showed a median overall survival (OS) of 21.4 months vs. 14.3 months and a median PFS of 3.7 months in the Avelumab group and 2.0 months in the support-only arm [7]. From the subgroup analysis of the Javelin Bladder 100 study, we conducted a retrospective observational study to evaluate the safety and efficacy of Avelumab maintenance in elderly patients treated with first line platinum based chemotherapy—four or six cycles of gemcitabine plus cisplatin or carboplatin chemotherapy—and without disease progression (i.e., ongoing complete response, partial response, or stable disease). Scientific data on the management of elderly patients with aUC are limited and rather scarce in the medical literature due to the underrepresentation of elderly patients in clinical trials [8,9]. In this study, the primary endpoints were OS, PFS, and objective response rate (ORR); the secondary endpoints were tolerability, pre- and post-treatment reduction in serum carcinoembryonic antigen 19.9 (Ca 19.9), and quality of life (QoL) in the overall PD-L1 positive and PD-L1 negative population. Ca 19.9 may be elevated in some patients with advanced or metastatic urothelial carcinoma and may be used for prognostic purposes. Additionally, in the setting of pseudoprogression—when the tumor appears temporarily enlarged due to inflammation or immune activation before responding to treatment—an increase in Ca 19.9 may reflect a temporary change due to treatment.
2. Results
2.1. Patient Descriptive Characteristics
A total of 60 patients were enrolled in this retrospective observational study between March 2021 and June 2024. All patients were older than 70 years of age at enrollment and were all white, 32 males and 28 females, with a median age of 76 years (range between 70 and 88). Only 16 patients were pretreated with cisplatin, 44 with carboplatin, and all with gemcitabine, and all underwent a multidisciplinary geriatric and psychological evaluation to select patients eligible for treatment. Regarding the Eastern Cooperative Oncology Group (ECOG) performance status (PS), evaluated in all 60 patients, [10] was grade 0 in 43 patients (72%), and 17 (28%) had ECOG PS 1. There were 38 patients who underwent primary surgery. PD-L1 positive status was 53%, and PD-L1 negative status was 47%. Site of primary tumor in bladder 85% and urethra 15%. Metastatic sites of disease prior to Avelumab immunotherapy were lymph nodes 62%, liver 12%, lung 14%, peritoneum 23%, and skeletal metastases 12%; some patients had multiple metastatic sites (Table 1). Thirty-eight patients had pretreatment Ca 19.9 values above the normal range. Biopsy specimens were analyzed by the central laboratory and assessed for PD-L1 expression by immunohistochemistry (IHC). PD-L1 expression was classified as the combined PD-L1 positive score (CPS), defined as the percentage of tumor and immune cells expressing PD-L1 relative to the total number of tumor cells.

Table 1.
Characteristics of enrolled patients (n. 60).
2.2. Clinical Outcomes
Avelumab in first line maintenance in aUC in elderly patients was well tolerated, with a moderate toxicity profile after a median follow-up of 21.6 months (95% CI: 9.2–23.4). The median OS was 18.6 months (95% CI: 6.3–20.7) (Figure 1), and the median PFS was 3.6 months (95% CI: 2.3–6.8) (Figure 2). Response analysis using iRECIST criteria—immunotherapy response criteria—showed that treatment was well tolerated in all patients with good disease control. The median response time in all patients was 4.2 months (95% CI: 2.7–7.9), which had a significant impact on the QoL improvement. No patients had a complete response (CR); 16 patients (27%) had a partial response (PR); 38 patients (63%) had stable disease (SD), and six patients (10%) had progression (PD). Treatment with Avelumab was well tolerated and resulted in a good level of disease control DCR (CR + PR + SD) > 90% and an ORR > 20% (Table 2). The Pearson correlation coefficient (r) showed a good correlation between PFS and Ca 19.9 reduction with an r-value of 0.59 (95% CI: 0.12–0.57), p = 0.007 (Table 3). In particular, the Ca 19.9 reduction was >40%, and this reduction was associated with an increase in PFS. Ca 19.9 reduction was shown in patients with a greater response to treatment. Our results showed no statistically significant or clinically relevant differences between the PD-L1 positive and negative groups; survival and response benefits of therapy were observed in all patients. PD-L1 expression does not appear to be a predictor of treatment response.
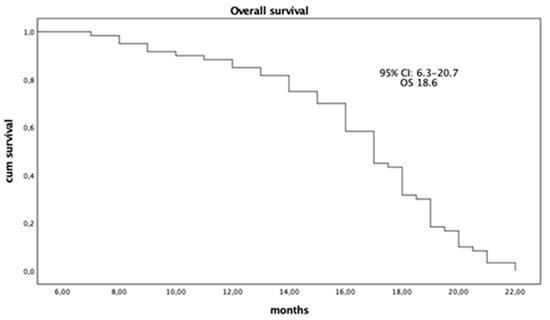
Figure 1.
Overall Survival (OS) (n. 60).
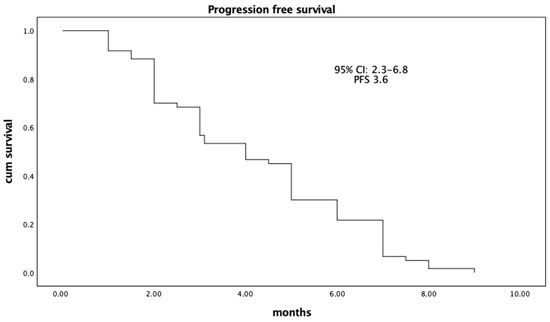
Figure 2.
Median Progression Free Survival (PFS) (n. 60).

Table 2.
Overall Response Rate.

Table 3.
Average score Ca19.9 ante- and post-treatment with Avelumab (n. 60).
2.3. Quality of Life Value
At baseline, the EORTC (European Organization for Research and Treatment of Cancer) QLQ-C30 questionnaires described a generally low QoL (the score for global health status was 56.4); the follow up score was higher (69.8). In the symptom scales, patients reported sleeping problems (41.4), fatigue (32.6), and pain (48.6) at baseline. An improvement in QoL was observed at follow-up, with a reduction of pain symptoms and an improvement of general health status in 67% of patients (Table 4). Patients reported pain due to skeletal metastases, and they were treated with nonopioid analgesics and, in some cases, with radiation therapy. Furthermore, the follow-up questionnaires reported the disappearance of chemotherapy-related toxicities (mucositis, nausea, vomiting, diarrhea, anemia, neutropenia, and fever).

Table 4.
Quality of Life Score (n. 60).
2.4. Tolerability
In general, adverse events related to immunotherapy—and, therefore, in this case, to Avelumab—are due to the hyperactivation of the immune system. Avelumab inhibits the binding between the PD-1 receptor and the ligand PD-L1, reactivating the immune response against the tumor, but, in some cases, it can also lead to a reaction against healthy tissue, giving rise to autoimmune reactions.
Side effects were mainly immune-related and, therefore, linked to the stimulation of the immune system that can develop autoimmune reactions against the body. All toxicities due to immunotherapy treatment were treated with the immediate discontinuation of Avelumab and administration of corticosteroids with doses gradually increased according to the severity of the reaction and implementation of supportive therapies. Overall, Avelumab was well tolerated at each cycle of therapy. All patients continued treatment until progression or unacceptable toxicity. No patient died from treatment-related adverse events, but two patients had immune-related adverse events (AEs) that led to treatment discontinuation. AEs of any grade occurred in 32%, including grade ≥ 3 AEs in 6%. Three patients initially experienced an AE after the first administration of Avelumab, with facial flushing, tachypnoea, cough, and hypertension that resolved after the administration of antihistamines and steroids. Endocrine toxicities were statistically the most significant. Thyroid dysfunction in four patients was treated by the endocrinologist with levothyroxine, and one patient had grade 2 adrenal insufficiency. Grade 2 to 3 immune-related colitis occurred in six patients and required steroids, rifaximin, loperamide, and electrolyte infusion therapies. Two patients had urinary tract sepsis that resolved with antibiotic therapy. Other more common non-immune-related AEs occurring with Avelumab were fatigue 10%, nausea 9%, 8% decreased appetite, 4% weight decreased, dyspnea (2%), and anemia (3%).
3. Discussion
The incidence of cancer increases with age, although with a different trend between the two sexes. The biological bases of this correlation include the progressive accumulation of carcinogenic factors in the body and the decrease in cellular repair mechanisms [11]. Age is no longer a limit for increasingly effective and less toxic cancer medical therapies; increasingly, older people enjoy good health, and this makes them able to tolerate cancer treatments better and for longer. In elderly patients with aUC, treatment is aimed at symptom control, maintenance of good QoL, and improved survival. The literature does not provide enough data on elderly patients because they are often underrepresented due to the risk of serious toxicities or treatment discontinuations [12]. We conducted this retrospective observational study only in elderly patients with aUC with no disease progression after platinum to understand how they responded to Avelumab. Our results showed that the maintenance of Avelumab significantly prolonged OS with a good ORR and manageable toxicity. In the descriptive analysis, we monitored serum Ca 19.9 in our patients and found a decrease in Ca 19.9 enzyme levels in patients who responded to treatment and an increase in those with progressive disease; in fact, the result showed a good correlation between PFS and Ca 19.9 reduction with an r-value of 0.59 (95% CI: 0.12–0.57), p = 0.007. This observation suggests that serum Ca 19.9 response could be an important predictor of treatment response and a method of treatment monitoring [13]. Treatment was continued until iRECIST-defined disease progression, development of an unacceptable level of toxic effects, or withdrawal of consent. The most frequent and serious adverse reactions during treatment with Avelumab were predominantly immune-related and resolved with treatment interruption and corticosteroid administration, consistent with published data from the multicenter phase III Javelin 100 study [6]. Adverse events among elderly patients in this study were consistent with those in younger patients in other studies. This study suggests that Avelumab may be one of the therapeutic options in elderly patients with a favorable risk/benefit ratio. The primary benefit is associated with disease stabilization rather than tumor shrinkage, even considering that objective response to treatment was observed in only a few patients. These results showed no statistically significant or clinically relevant differences between the PD-L1 positive and negative groups. PD-L1 expression does not appear to be a predictor of treatment response. To achieve optimal results, all patients underwent a multidisciplinary geriatric and psychological evaluation before starting treatment. Survival and response benefits were observed in all patient categories, regardless of tumor expression of the PD-L1 drug target. A comprehensive assessment of the elderly patient leads to a better overall assessment and determines the best individual treatment strategies for optimal outcomes. We considered cognitive and social function with the administration of the QoL questionnaire by the psycho-oncologist. Until recently, there were not many treatment options available in elderly patients with aUC after platinum therapy due to the frequent toxicity. Limitations of this analysis include the inconsistent number of elderly patients enrolled and the nonrandomized sampling. Despite the retrospective nature of our analysis and the limitations listed above, we can state that our results can provide valuable hypotheses for future studies.
4. Materials and Methods
4.1. Study Design
This retrospective observational study was conducted to evaluate the safety and efficacy of Avelumab monotherapy in patients over 70 with aUC treatment who have received a single previous platinum-containing chemotherapy and who, at the end of the same, had presented a complete response, a partial response, or stability. Sixty elderly patients (aged > 70) were enrolled and followed up at the Medical Oncology Unit of the University of Palermo and the Medical Oncology Unit of the Civico Hospital of Palermo. The Ethics Committee of the Policlinic Hospital of Palermo approved our study. This study was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. All enrolled patients signed an informed consent form stating the risks and benefits of treatment with Avelumab.
4.2. Patient Selection
Eligible patients included in this study had to meet the following entry criteria: age ≥ 70 years, performance status ≤ 2 according to the ECOG scale, pathologically confirmed diagnosis of aUC, no progression after a single line of platinum based chemotherapy, chemotherapy permissive blood counts, and renal and liver functions. Metastatic disease also had to be measurable according to the RECIST criteria [14] and iRECIST. Hypersensitive to Avelumab and its excipients or patients with an autoimmune disease were excluded; patients were ineligible If they had received anti-PD-1, anti-PD-L1, or anti-CTLA-4 previous therapy; patients with a history of other cancers except basal cell skin cancer or curatively treated carcinoma in situ of the cervix as well as severe and uncontrolled metabolic, infectious, cardiological, or neurological disease were also excluded.
4.3. Method of Administration
All patients received Avelumab monotherapy administered intravenously at 800 mg over 60 min every 2 weeks according to the guidelines [15]. To prevent the occurrence of infusion related reactions, patients were premedicated with an antihistamine and paracetamol. Spiral CT was performed every 8 weeks for 12 months and then every 12 weeks until disease progression. Baseline head imaging was required in patients with a history of brain metastases or suspected brain metastases. Electrocardiogram (ECG) and echocardiogram were performed at baseline and every three months. Blood tests were always performed at baseline and approximately every two weeks before subsequent treatment, while safety assessments were performed during the treatment phase. Treatment was continued until disease progression or unacceptable toxicity.
4.4. Evaluation of Response and Toxicity
Response rate assessment in terms of measurable reduction in disease, according to iRECIST, [9] was performed at baseline and every 3 months until disease progression. Positron emission tomography was performed in selected cases at the discretion of the physician. In the case of brain metastases, a magnetic resonance imaging scan was performed every 6 to 12 weeks. Treatment interruption was permitted to manage treatment-related adverse events (TRAEs). TRAEs of Avelumab were recommended based on the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 [16]. G3 or G4 toxicity was managed with a reduction or delay of the dose and Prednisone tablets starting at 5 mg. In more severe cases G3–G4, toxicity management also included dose interruption until toxicity resolved at least to G1 or baseline.
4.5. Quality of Life
QoL was assessed by the psycho-oncologists, who provided all patients with the EORTC QLQ-C30 questionnaire at the beginning of the treatment and after three months [17]. The questionnaire is composed of both single-item and multi-item scales. The scaling is organized into five functional domains (physical, role, cognitive, emotional, and social), three-item symptom scales, a global health status/QoL scale, and six single-item scales (dyspnea, loss of appetite, insomnia, constipation, diarrhea, and perceived financial impact). The score is a linear grading scale ranging from 0 to 100. A high score on a functional scale and global health status/QoL represents a high/healthy level of functioning, whereas a high score on a symptom scale/item represents a high level of symptomatology/problems.
4.6. Statistical Analysis
All statistical analyses were performed using the Statistical Package for Social Science (SPSS) version 25 for Mac (IBM Corp., Armonk, NY, USA). The last follow-up was in June 2024. PD-L1 expression was assessed in tumor samples by immunohistochemistry with Ventana PD-L1 assay (SP263, Ventana Medical Systems). Proportions for categorical variables were compared using Student’s t-tests. ORR calculated the percentage of patients who achieved a complete or partial response after treatment. The survival curve was assessed using the Kaplan–Meier method. The Pearson correlation coefficient was used to correlate PFS and Ca 19.9 with a 95% confidence interval (CI). Statistical significance was defined as a p-value less than 0.05.
5. Conclusions
This study showed that maintenance therapy with Avelumab prolongs overall survival in elderly patients with aUC who have not progressed on first line platinum based chemotherapy [18]. Avelumab was well tolerated, with no significant increase in toxicity rates compared to a younger population. Adverse events among elderly patients in this study were not different from those expected from studies in younger patients. Therefore, the therapeutic approach with Avelumab for platinum pretreated elderly patients resembles that of younger patients [19,20]. Avelumab, as shown in the present work, remains a valid treatment option in pretreated elderly patients with aUC. Despite the retrospective study design and the limited number of patients, our results are consistent with those of other studies in the literature.
Author Contributions
Conceptualization, G.C., A.S. and A.P.; Methodology, G.C.; Validation, R.A. and P.V.; Formal analysis, A.P.; Investigation, R.D.L.; Resources, A.S.; Data curation, R.D.L.; Writing—original draft preparation, G.C.; Writing—reviewing and editing, R.A., P.V. and R.S.; Visualization, R.S.; Supervision, G.C.; Project administration, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boll, L.M.; Perera-Bel, J.; Rodriguez-Vida, A.; Arpí, O.; Rovira, A.; Juanpere, N.; de Oca, S.V.M.; Hernández-Llodrà, S.; Lloreta, J.; Albà, M.M.; et al. The impact of mutational clonality in predicting the response to immune checkpoint inhibitors in advanced urothelial cancer. Sci. Rep. 2023, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Carroll, P.R.; Small, E.J. Update on chemotherapy for advanced bladder cancer. J. Urol. 2005, 174, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Von Der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-Term Survival Results of a Randomized Trial Comparing Gemcitabine Plus Cisplatin, with Methotrexate, Vinblastine, Doxorubicin, Plus Cisplatin in Patients With Bladder Cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef] [PubMed]
- Bamias, A.; Dafni, U.; Karadimou, A.; Timotheadou, E.; Aravantinos, G.; Psyrri, A.; Xanthakis, I.; Tsiatas, M.; Koutoulidis, V.; Constantinidis, C.; et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: A Hellenic Cooperative Oncology Group study (HE 16/03). Ann. Oncol. 2012, 24, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Park, S.H.; Voog, E.; Caserta, C.; Gurney, H.; Bellmunt, J.; Kalofonos, H.; Ullén, A.; Loriot, Y.; Sridhar, S.S.; et al. Avelumab First-line Maintenance Therapy for Advanced Urothelial Carcinoma: Comprehensive Clinical Subgroup Analyses from the JAVELIN Bladder 100 Phase 3 Trial. Eur. Urol. 2023, 84, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Canouï-Poitrine, F.; Lièvre, A.; Dayde, F.; Lopez-Trabada-Ataz, D.; Baumgaertner, I.; Dubreuil, O.; Brunetti, F.; Coriat, R.; Maley, K.; Pernot, S.; et al. Inclusion of Older Patients with Cancer in Clinical Trials: The SAGE Prospective Multicenter Cohort Survey. Oncologist 2019, 24, e1351–e1359. [Google Scholar] [CrossRef] [PubMed]
- Shao, I.H.; Lin, Y.H.; Hou, C.P.; Juang, H.H.; Chen, C.L.; Chang, P.L. Tsui KH Risk factors associated with ineligibility of adjuvant cisplatin-based chemotherapy after nephroureterectomy. Drug Des. Dev. Ther. 2014, 8, 1985–1991. [Google Scholar]
- Azam, F.; Latif, M.F.; Farooq, A.; Tirmazy, S.H.; AlShahrani, S.; Bashir, S.; Bukhari, N. Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) Score for Cancer Pa-tients by Oncology Healthcare Professionals. Case Rep. Oncol. 2019, 12, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Alouini, S. Risk Factors Associated with Urothelial Bladder Cancer. Int. J. Environ. Res. Public Health 2024, 21, 954. [Google Scholar] [CrossRef] [PubMed]
- Jodon, G.; Fischer, S.M.; Kessler, E.R. Treatment of Urothelial Cancer in Elderly Patients: Focus on Immune Checkpoint Inhibitors. Drugs Aging 2018, 35, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.H.; Hwang, J.; Venook, A.P.; Abbruzzese, J.L.; Bergsland, E.K.; A Tempero, M. Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br. J. Cancer 2005, 93, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer. 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Agarwal, N.; Pal, S.; Kalebasty, A.R.; Sridhar, S.S.; Smith, J.; Devgan, G.; Sternberg, C.N.; Bellmunt, J. Avelumab first-line maintenance in locally advanced or metastatic urothelial carcinoma: Applying clinical trial findings to clinical practice. Cancer Treat. Rev. 2021, 97, 102187. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.P.; Fisher, B.T.; Getz, K.D.; Sack, L.; Razzaghi, H.; Seif, A.E.; Bagatell, R.; Adamson, P.C.; Aplenc, R. Unintended consequences of evolution of the Common Terminology Criteria for Adverse Events. Pediatr. Blood Cancer 2019, 66, e27747. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in interna-tional clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Gonzalez, D.E.; Saffati, G.; Salgado-Garza, G.; Patel, S.; Kronstedt, S.; Jones, J.A.; Taylor, J.M.; Yen, A.E. Slawin Novel therapeutic regimens in previously untreated metastatic urothelial carcinoma: A 13systematic review and bayesian network me-ta-analysis. JR. Urol. Oncol. 2024, S1078-1439(24)00544-1. [Google Scholar]
- Lec, P.M.; Venkataramana, A.; Lenis, A.T.; Fero, K.E.; Sharma, V.; Golla, V.; Gollapudi, K.; Blumberg, J.; Chamie, K. Trends in management of ureteral urothelial carcinoma and effects on survival: A hospital-based registry study. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 194.e17–194.e24. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, R.; Cicala, C.M.; Ciccarese, C.; Sacco, E.; Racioppi, M.; Bassi, P.F.; Tortora, G. Management of metastatic urothelial carcinoma: Current approach, emerging agents, and future perspectives. Urol. J. 2022, 90, 3–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).