Evaluation of the Efficacy of Methylene Blue Administration in SARS-CoV-2-Affected Patients: A Proof-of-Concept Phase 2, Randomized, Placebo-Controlled, Single-Blind Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Clinical Trial Schedule
2.3. Statistical Analysis
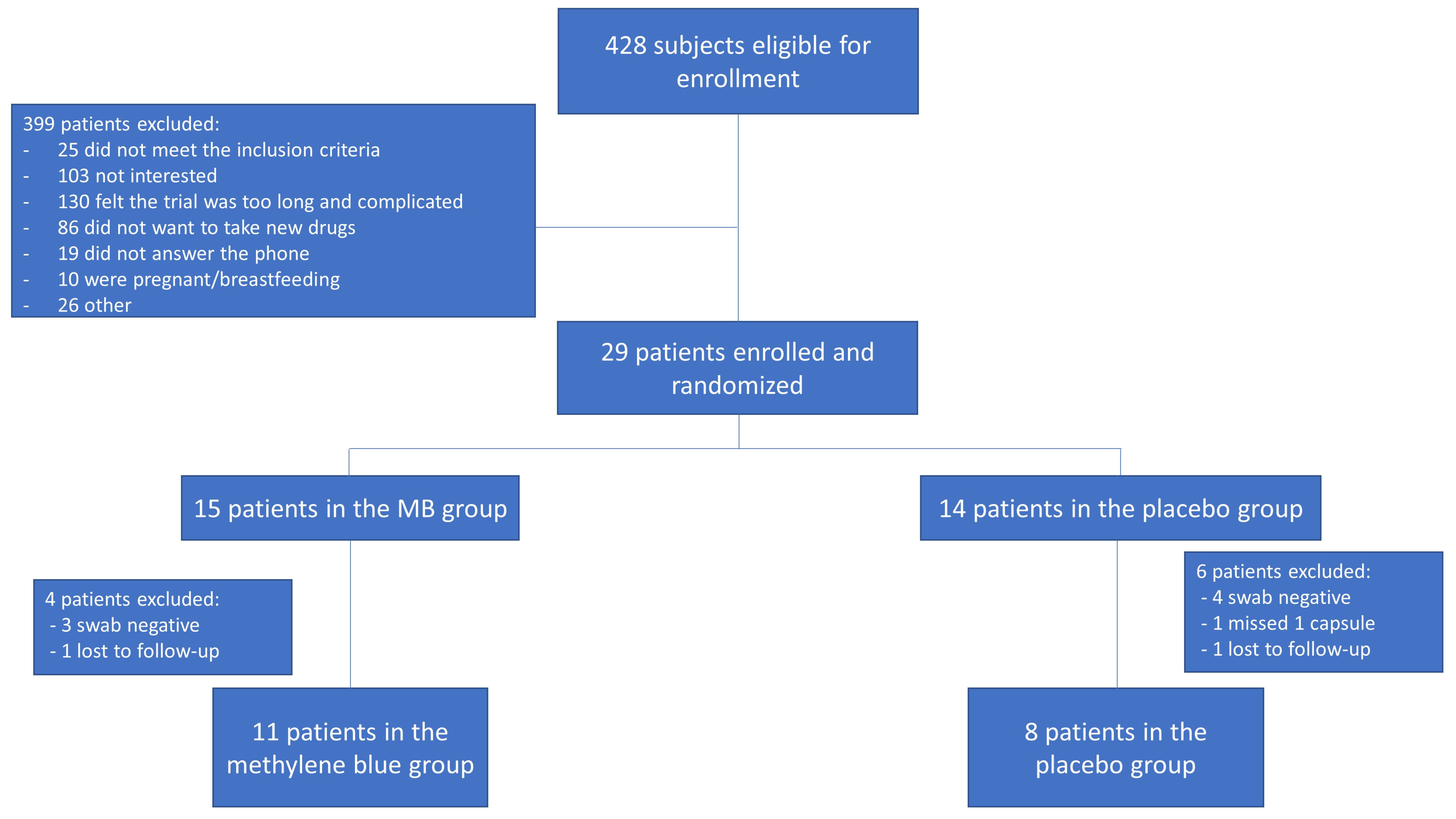
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Knoll, M.D.; Wonodi, C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Nuzhath, T.; Callaghan, T.; Colwell, B. COVID-19 vaccine decisions: Considering the choices and opportunities. Microbes Infect. 2021, 23, 104811. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Klonoff, D.C.; Akram, J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4539–4547. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Chauhan, P.; Kakkar, A.K. Hydroxychloroquine for the treatment and prophylaxis of COVID-19: The journey so far and the road ahead. Eur. J. Pharmacol. 2020, 890, 173717. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M. Paxlovid as a potential treatment for long COVID. Expert Opin. Pharmacother. 2023, 24, 1839–1843. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Gao, W.; Bao, H.; Feng, H.; Mei, S.; Chen, P.; Gao, Y.; Cui, Z.; Zhang, Q.; Meng, X.; et al. VV116 versus Nirmatrelvir–Ritonavir for Oral Treatment of COVID-19. N. Engl. J. Med. 2023, 388, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Summa, M.; Patrick, L.; Schwartz, L. A cohort of cancer patients with no reported cases of SARS-CoV-2 infection: The possible preventive role of Methylene Blue. Substantia 2020, 4, 888. [Google Scholar] [CrossRef]
- Lobo, C.S.; Rodrigues-Santos, P.; Pereira, D.; Núñez, J.; Trêpa, J.C.D.; Sousa, D.L.; Lourenço, J.V.; Coelho, M.F.; de Almeida, L.P.; da Cunha, J.S.; et al. Photodynamic disinfection of SARS-CoV-2 clinical samples using a methylene blue formulation. Photochem. Photobiol. Sci. 2022, 21, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Radicioni, M.; Vaccani, A.; Fransioli, A.; Longo, L.; Moro, L.; Repici, A. Methylene blue MMX® tablets for chromoendoscopy. Bioavailability, colon staining and safety in healthy volunteers undergoing a full colonoscopy. Contemp. Clin. Trials 2018, 71, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Nagbanshi, M.; Goldau, N.; Jorge, M.M.; Meissner, P.; Jahn, A.; Mockenhaupt, F.P.; Müller, O. Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med. 2018, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Schultze, J.L.; Aschenbrenner, A.C. COVID-19 and the human innate immune system. Cell 2021, 184, 1671–1692. [Google Scholar] [CrossRef] [PubMed]
| Methylene Blue Group (n = 15) | Placebo Group (n = 14) | Statistical Test Result | |
|---|---|---|---|
| Gender | |||
| Woman | 8 | 8 | χ2(1) = 0.909 |
| Man | 7 | 9 | |
| Age | |||
| Median (IQR) | 50.0 (21.0) | 46.5 (18.3) | z = 0.481 |
| Body mass index | |||
| Median (IQR) | 26.8 (6.5) | 26.0 (9.1) | z = 0.349 |
| Ethnicity | |||
| White | 14 | 14 | χ2(1) = 0.007 |
| Asian | 1 | 0 |
| Symptom | Methylene Blue Group (n = 11) | Placebo Group (n = 8) | Statistical Test Result |
|---|---|---|---|
| Fever | 9 | 4 | χ2(1) = 2.89 * |
| Cough | 9 | 10 | χ2(1) = 0.41 |
| Fatigue | 13 | 9 | χ2(1) = 1.981 |
| Nasal congestion | 11 | 6 | χ2(1) = 2.77 * |
| Smell alteration | 5 | 3 | χ2(1) = 0.51 |
| Taste alteration | 7 | 4 | χ2(1) = 1.00 |
| Dyspnoea | 3 | 4 | χ2(1) = 0.29 |
| Conjunctivitis | 0 | 1 | χ2(1) = 1.11 |
| Diarrhea | 4 | 4 | χ2(1) = 0.01 |
| Muscular exertion | 8 | 6 | χ2(1) = 0.31 |
| Area under the Curve (AUC) | Group | Difference | 95% Confidence Interval of Difference | t-Statistic | |
|---|---|---|---|---|---|
| Methylene Blue | Placebo | ||||
| AUC Mean (SD 1) | 5.1 (5.2) | 3.4 (3.0) | 1.70 2 | (−2.10,5.50) | 0.94 |
| AUC Median (Min;Max) | 3.5 (0.0;12.6) | 2.6 (0.0;6.8) | 0.88 3 | (−2.59,6.10) 3 | - |
| AUC for 2^DCt expressed as % from baseline [Median (Min;Max)] | 271 (121;820) | 125 (100;436) | - | - | - |
| Efficacy Endpoints | Group | Statistical Test Result | ||
|---|---|---|---|---|
| Methylene Blue (n = 11) | Placebo (n = 8) | |||
| Virus clearance after 3 days | Yes | 2 | 1 | χ2(1) = 0.115 |
| Virus clearance after 6 days | Yes | 4 | 2 | χ2(1) = 0.281 |
| Virus clearance after 9 days | Yes | 9 | 4 | χ2(1) = 2.170 |
| Virus clearance after 12 days | Yes | 10 | 6 | χ2(1) = 0.882 |
| Virus clearance after 15 days | Yes | 8 | 6 | χ2(1) = 0.012 |
| Virus clearance after 21 days | Yes | 11 | 8 | No test performed |
| Viral load reduction of at least 2 log by day 3 | Yes | 0 | 1 | χ2(2) = 2.847 |
| No | 9 | 7 | ||
| VCW2LR 2 | 2 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barda, B.; Di Mari, B.; Soldini, E.; Di Bartolomeo, C.; Bissig, M.; Baserga, A.; Robatto, A.; Magenta, L.; Forlenza, R.; Cerny, A. Evaluation of the Efficacy of Methylene Blue Administration in SARS-CoV-2-Affected Patients: A Proof-of-Concept Phase 2, Randomized, Placebo-Controlled, Single-Blind Clinical Trial. Sci. Pharm. 2024, 92, 56. https://doi.org/10.3390/scipharm92040056
Barda B, Di Mari B, Soldini E, Di Bartolomeo C, Bissig M, Baserga A, Robatto A, Magenta L, Forlenza R, Cerny A. Evaluation of the Efficacy of Methylene Blue Administration in SARS-CoV-2-Affected Patients: A Proof-of-Concept Phase 2, Randomized, Placebo-Controlled, Single-Blind Clinical Trial. Scientia Pharmaceutica. 2024; 92(4):56. https://doi.org/10.3390/scipharm92040056
Chicago/Turabian StyleBarda, Beatrice, Bruno Di Mari, Emiliano Soldini, Claudia Di Bartolomeo, Maurizia Bissig, Adriana Baserga, Antonella Robatto, Lorenzo Magenta, Rossella Forlenza, and Andreas Cerny. 2024. "Evaluation of the Efficacy of Methylene Blue Administration in SARS-CoV-2-Affected Patients: A Proof-of-Concept Phase 2, Randomized, Placebo-Controlled, Single-Blind Clinical Trial" Scientia Pharmaceutica 92, no. 4: 56. https://doi.org/10.3390/scipharm92040056
APA StyleBarda, B., Di Mari, B., Soldini, E., Di Bartolomeo, C., Bissig, M., Baserga, A., Robatto, A., Magenta, L., Forlenza, R., & Cerny, A. (2024). Evaluation of the Efficacy of Methylene Blue Administration in SARS-CoV-2-Affected Patients: A Proof-of-Concept Phase 2, Randomized, Placebo-Controlled, Single-Blind Clinical Trial. Scientia Pharmaceutica, 92(4), 56. https://doi.org/10.3390/scipharm92040056






