Abstract
Lichens, a diverse group of organisms, have a unique structure consisting of fungal filaments and photosynthetic partner cells. This research conducted a comprehensive chemical analysis and evaluation of the anti-inflammatory and antioxidant properties of methanolic and acetone extracts from Umbilicaria crustulosa lichen, along with its isolated metabolites. The process involved separating atranorin and chloratranorin fractions, physodic acid, and gyrophoric acid. Secondary metabolites were identified using chromatographic and spectroscopic data. The total polyphenols content was determined spectrophotometrically. This study examined the antioxidant activity of extracts of the lichen U. crustulosa and the isolated fractions using three methods: DPPH scavenging activity, ABTS scavenging activity, and reducing power. This study also evaluated the acute oral toxicity and the anti-inflammatory activity of the extracts in Wistar albino rats. A higher content of the total phenolic compounds was found in the acetone extract, but antioxidant and anti-inflammatory activities were more prominent in the methanolic extract. The isolated atranorin and chloratranorin fractions and compound physodic acid showed the highest antioxidant activity. No toxic effects were noted in the acute oral toxicity study. This study highlights the potential of the investigated lichen as a valuable source of novel biological agents.
1. Introduction
Lichens have a unique dual structure consisting of fungal filaments intertwined with the cells of the photosynthetic partner (microalga or cyanobacteria). This structure forms thalli, which can vary in shape, size, and color [1]. Lichens represent a diverse and abundant group of organisms that remain relatively understudied. Within the scientific community, they are recognized as a distinct phylogenetic group within the broader biodiversity of the planet, with their nomenclature and taxonomy governed by the regulations outlined in the International Code of Botanical Nomenclature [2]. The morphological features of lichen thalli exhibit considerable variability, posing challenges for identification. Therefore, studying chemotaxonomy becomes crucial, as it aids in the identification of chemical constituents and potential pharmaceutical applications. The primary organic compounds extracted from lichens are secondary metabolites, predominantly sourced from the fungal component residing on the hyphae surface rather than within the cell wall [3]. These compounds typically exhibit low solubility in water and are often extracted using organic solvents. Lichens biosynthesize a diverse array of secondary metabolites, many of which are unique to this symbiotic organism. These metabolites encompass monoaromatics, depsides, depsidones, depsones, pulvinates, dibenzofurans, anthraquinones, and xanthones [3,4,5]. However, their structural resemblance poses challenges in their identification process [5]. Lichens often contain notable levels of secondary metabolites, typically ranging from 0.1 to 10% of their dry weight, though in certain instances, concentrations may extend up to 30% [6].
Lichens exhibit a broad spectrum of biological potential; however, they have historically been overlooked by mycologists and pharmaceutical industries due to their slow growth and challenges associated with artificial cultivation. The primary agents responsible for their biological activity are their unique metabolites [3,7]. Lichens are recognized as a significant source of natural antioxidants, particularly phenols renowned for their potent antioxidative and antiradical properties. As a result, lichens are attributed with various therapeutic effects, including antibacterial, anti-inflammatory, antiallergic, antimutagenic, antiviral, and anticancer activities [3,7,8].
The Umbilicaria genus encompasses foliose lichens traditionally employed in folk medicine for their purgative (laxative) properties. The US National Park Service, Anchorage, AK, in 1987, referenced them in the literature as “rock tripe” [9]. The Umbilicaria genus is widely distributed across the northern hemisphere, including North America, Europe, and Antarctica. Considering that almost all species of Umbilicaria contain gyrophoric acid, in the past the Indians used them as a coloring agent. Some species are used as food or form part of traditional medicine in China [10].
Given its unique chemical composition, the Umbilicaria genus has demonstrated significant biological activity in our research and that of others [11,12]. Due to the limited data available on Umbilicaria crustulosa species, we conducted a comprehensive chemical analysis and evaluation of the anti-inflammatory and antioxidant properties of the methanolic and the acetone extracts from U. crustulosa lichen, along with its isolated metabolites. This research holds particular significance as there is scarce information regarding the relationship between the chemical composition and antioxidant/anti-inflammatory effects of U. crustulosa lichen. Additionally, this research identified previously unidentified compounds in the U. crustulosa species through isolation and characterization processes. This is the first time that the anti-inflammatory activity of U. crustulosa lichen extracts has been investigated.
2. Materials and Methods
2.1. The Process of Collecting and Identifying Lichen Samples
Lichen specimens of the type U. crustulosa were collected from the eastern slope of the Stara Planina mountain in the Republic of Serbia. The Department of Biology and Ecology at the Faculty of Natural Sciences and Mathematics, University of Niš, identified the specimens using relevant keys and monographs [13], and archived them in the herbarium (Voucher number: 9373).
2.2. The Process of Preparing the Lichen Extracts
The dried U. crustulosa lichen material was ground into a powder (2–6 mm). Then, separate extractions were conducted using acetone and methanol, employing conventional techniques such as maceration. The material was placed in each solvent for 3 days in a dark place at room temperature, with occasional shaking. The extraction process utilized 50 g of the lichen sample with 500 milliliters of the solvents. Evaporation of the solvents was performed under reduced pressure on the rotary vacuum evaporator. Dry extracts (acetone: 1.57 g; methanol: 1.01 g) were obtained and stored in dark glass bottles for further testing.
2.3. Isolation Process of Lichen Metabolites and Fractions
The fraction containing atranorin and chloratranorin was isolated according to the procedure described in the work of Lukač and Onderk [14]. The dry acetone extract was washed three times with toluene, the resulting solution was allowed to stand overnight (5–7 °C), and the crystallized fraction was filtered off and allowed to dry. The obtained fraction was recrystallized from acetone. The crystals were then dissolved in a mixture of chloroform/methanol, and by standing in the cold, white crystals appeared which were separated, determined, and the presence of the atranorin and chloratranorin fraction was determined.
The isolation of physodic acid was performed by dissolving the dry acetone extract of U. crustulosa in benzene, the residue that did not dissolve was washed again with benzene and did not dissolve, it separated in the form of a white precipitate. The precipitate was dissolved in the acetone and then it was subjected to centrifugation at 12,000 rpm for 10 min. The supernatant was separated, evaporated, and dissolved in a mixture of methanol/water, left to stand in the cold overnight (5–7 °C) and white crystals appeared in the form of needles, which were separated and determined.
The isolation of gyrophoric acid was performed using the dry acetone of U. crustulosa which was extracted with the benzene (3 times). To maximize the yield, the benzene phase was centrifuged at 12,000 rpm for 10 min. The resulting supernatant was then mixed with the remaining precipitate from the benzene extraction. The mixture was then dried at room temperature and dissolved again in fresh acetone, after which it underwent recrystallization.
The identification of secondary metabolites was conducted by comparing chromatographic and spectroscopic data with the standards. The identification of the substance was also performed on the basis of the melting point [15].
2.4. Analysis Using High-Performance Liquid Chromatography (HPLC)
HPLC with UV detection was used to analyze and identify the individual constituents of the extracts. The analysis was performed using the Agilent 1200 Series (Agilent Technologies, Santa Clara, CA, USA) with a C18 column (ZORBAX Eclipse XDB-C18; 25 cm × 4.6 mm; 5 μm; (Agilent Technologies, Santa Clara, CA, USA)). Detection was carried out using a Diode Array Detector (DAD) at 280, 330, and 350 nm, and the absorption spectra of the components were recorded in the range of 200–400 nm. The dissolved samples were filtered through a 0.45 μm pore size filter. Chromatographic separation was conducted using an acetonitrile–water–phosphoric acid solvent system (90:10:0.1, v/v/v). The mobile phase flow rate was 1 mL/min, and 10 μL of the sample was injected. The column was maintained at a temperature of 30 °C. This procedure was previously described and employed [16,17]. Chromatograms and UV spectral data were collected at a wavelength of 254 nm.
The identification of the secondary metabolites of the acetone and methanolic extracts of the lichen U. crustulosa was made by comparing the retention times (tR) and UV spectra of the metabolites with standards (λ = 200–400 nm). The standards used for HPLC identification were obtained from the following sources: gyrophoric acid (tR = 6.70 min; λmax = 214, 270, 304 nm) from Acarospora fuscata, methyl orsellinate (tR = 3.54 min; λmax = 218, 270, 308 nm) from Usnea longissima, lecanoric acid (tR = 4.22 min; λmax = 220, 270, 312 nm) from Parmotrema tinctorum, methyl lecanorate (tR = 5.62 min; λmax = 228, 270, 308 nm), atranorin (tR = 20.43 min; λmax = 210, 252, 321 nm) and chloroatranorin (tR = 25.90 min; λmax = 213, 252, 315, 350 nm) from lichen Evernia prunastri, physodic acid (tR = 9.61 min; λmax = 212, 263, 314 nm) from Hypogimnia physodes, crustinic acid (tR = 4.51 min; λmax = 220, 268, 308 nm) from Umbilicaria cinereorufescens and haematommic acid (tR = 6.13 min; λmax = 202, 236, 258, 280, 344 nm) from Alectoria sulcata. The standard compounds were previously isolated in our laboratory and their structures were confirmed by mass spectrometry, 1H, and 13C-NMR.
2.5. Measurement of the Total Phenol Content
The total polyphenol content in the extracts was assessed using the Folin–Ciocalteu reagent and the spectrophotometric method [18,19]. A mixture containing 0.5 mL of the extract (1 mg/mL), 2.5 mL of Folin–Ciocalteu reagent, and 2.5 mL of Na2CO3 solution (7.5%) was prepared. The quantitative estimation of polyphenols was conducted by measuring the absorbance at 760 nm. The total polyphenol content was then calculated using an equation derived from a standard gallic acid calibration curve. The parameter was determined from the standard gallic acid curve, as per Equation (1):
Absorbance = 0.0026 × Total phenol + 0.0808 (R2 = 0.9978)
The results are presented as milligrams of gallic acid equivalents per gram of dry extract (mg GA/g).
2.6. DPPH Method
The ability to scavenge free radicals was assessed using 1,1-diphenyl-2-picryl-hydrazyl (DPPH), following the method outlined by Dorman et al. [20] with some modifications. Methanolic solutions of DPPH radical at concentrations of 40 μg/mL were prepared in a dark room. The sample solutions were mixed with 3 mL of the DPPH radical solution, and the mixture was left for 30 min at room temperature in the dark. After incubation, the absorbance was measured at 517 nm. Ascorbic acid and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) from Acros Organics (Fair Lawn, NJ, USA) were employed as positive controls. The capacity to neutralize free radicals was calculated using Equation (2):
Inhibition capacity of the DPPH radical (%) = Ac−As/Ac × 100
Ac represents the absorbance of the control solution (negative control), while As is the absorbance of the sample solution or standard. The IC50 value (μg/mL), which is defined as the concentration of extract required to reduce the DPPH radical concentration by 50%, was determined from the linear regression equation.
2.7. ABTS Method
The ability to neutralize free radicals was tested using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radicals according to the method previously described by Tabassum et al. [21], with modification. During the preparation of the experiment, the mixture of 7 mM ABTS and 2.45 mM potassium persulfate was incubated at room temperature without the presence of light for 24 h. A mixture was prepared by combining 300 μL of extract or standard solution with 600 μL of ABTS solution. The mixture was then incubated at room temperature for 30 min, after which the absorbance was measured at 734 nm. Ascorbic acid and Trolox were employed as positive controls. The concentration of the ABTS radical was calculated using Equation (3):
Inhibition capacity of the ABTS radical (%) = Ac−As/Ac × 100
Ac represents the absorbance of the control, which includes all reagents except for the tested extract or standard, while As is the absorbance of the sample. Using these values, a nonlinear calibration curve was constructed to determine the concentration of the tested sample that inhibits 50% of ABTS radicals (IC50).
2.8. Reduction Capacity
The method previously described by Oyazu et al. was used [22]. One milliliter of each sample was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of K3[Fe(CN)6] (1%). Afterward, 2.5 mL of CCl3COOH was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. A volume of 2.5 mL of the supernatant was extracted, and then 2.5 mL of distilled water and 0.5 mL of FeCl3 were added. The absorbance of the resulting solution was measured spectrophotometrically at 700 nm. Ascorbic acid and Trolox were utilized as positive controls. The rise in absorbance of the solution demonstrates the increase in reducing power.
2.9. Animal Studies–Acute Oral Toxicity and Anti-Inflammatory Activity
In vivo evaluation of the acute oral toxicity and anti-inflammatory activity of UCA and UCM was conducted at the Faculty of Medical Sciences, University of Kragujevac, Serbia. The study was conducted in accordance with the principles of Good Laboratory Practice, the European Council Directive (86/609/EEC), and the regulations of the Committee for the Welfare of Laboratory Animals of the Faculty of Medical Sciences. The experimental study included male Wistar albino rats procured from the Military Medical Academy in Belgrade, Serbia. The rats were kept in a controlled environment at a temperature of 22 ± 2 °C, with 12 h of consistent lighting every day. They were provided with commercial rat food (20% protein rat food; Veterinary Institute Subotica, Subotica, Serbia) and had unrestricted access to water.
The toxicity class was determined using the Organization for Economic Cooperation and Development (OECD) 423 procedure, which is specifically designed for testing acute oral toxicity [23]. Since there is data on the traditional use of lichen U. crustulosa for medicinal purposes, and in order to reduce the number of experimental animals that will be used, it was decided to start with the OECD procedure 423 at a dose of 2000 mg/kg body weight of the experimental animal. The male Wistar albino rats, 10 weeks old at the beginning of the experiment, were used for this part of the investigation.
A total of twelve animals (male Wistar albino rats) were treated with U. crustulosa extracts, more precisely six animals with acetone (UCA) and six animals with methanolic extract (UCM), with three animals in each of two separate cycles at a dose of 2000 mg/kg, which is recommended by the OECD 423 procedure [23]. The extracts were given orally as a solution in 1% sodium–carboxymethylcellulose (CMC) using a gavage method. Following the administration of UCA and UCM, the animals were closely monitored for the initial day and subsequently for the following 14 days, as per the protocol. Observations included alterations in the eyes, mucous membranes, skin, and fur. The animals’ behavior, breathing heart activity, and any neurological issues were monitored. Furthermore, monitoring for tremors, convulsions, diarrhea, lethargy, increased salivation, prolonged sleep duration, and coma was planned. Following the experiment, the animals were euthanized to conduct a necropsy and pathohistological examination of their internal organs, focusing on the liver, kidneys, heart, brain, and testicles.
The anti-inflammatory activity was assessed using the carrageenan-induced rat paw edema model [24]. Inflammation was induced in all rats by injecting 1 mL of 0.5% carrageenan solution in saline into the left hind paw. A total of eighty male Wistar albino rats weighing between 200 and 260 g and 10 weeks old were categorized into the following groups:
- Ctrl–rats were treated with 1% CMC solution in water, per os, 60 min before inflammation induction.
- 50 UCA–rats were treated with 50 mg/kg of the U. crustulosa acetone extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- 100 UCA–rats were treated with 100 mg/kg of the U. crustulosa acetone extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- 200 UCA–rats were treated with 200 mg/kg of the U. crustulosa acetone extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- 50 UCM–rats were treated with 50 mg/kg of the U. crustulosa methanolic extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- 100 UCM–rats were treated with 100 mg/kg of the U. crustulosa methanolic extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- 200 UCM–rats were treated with 200 mg/kg of the U. crustulosa methanolic extract per os dissolved in 1% CMC solution in water, per os, 60 min before inflammation induction.
- Ind–rats were treated with 10 mg/kg indomethacin, per os, 60 min before inflammation induction.
Indomethacin (as positive control) and carrageenan (as inductor of inflammation) were obtained from Sigma Aldrich (St. Louis, MO, USA).
In order to assess the anti-inflammatory impact, the thickness of the left paw tissue in each rat was measured at specific time intervals: prior to inducing inflammation (moment 0) and at 1, 2, 3, and 4 h (moments 1, 2, 3, and 4) following the onset of inflammation. The thickness of the tissue in the center of the rat paw was measured using a digital vernier caliper (Aerospace, Beijing, China). The percentage of decrease in paw swelling was calculated using Equation (4).
where Yt represents the mean increase in paw thickness in the group of rats that received treatment between two measurements, while Yc represents the mean increase in paw thickness in the group of rats that did not receive treatment between two measurements.
% Inhibition = 100 × [1 − (Yt/Yc)]
3. Results
3.1. HPLC Analysis
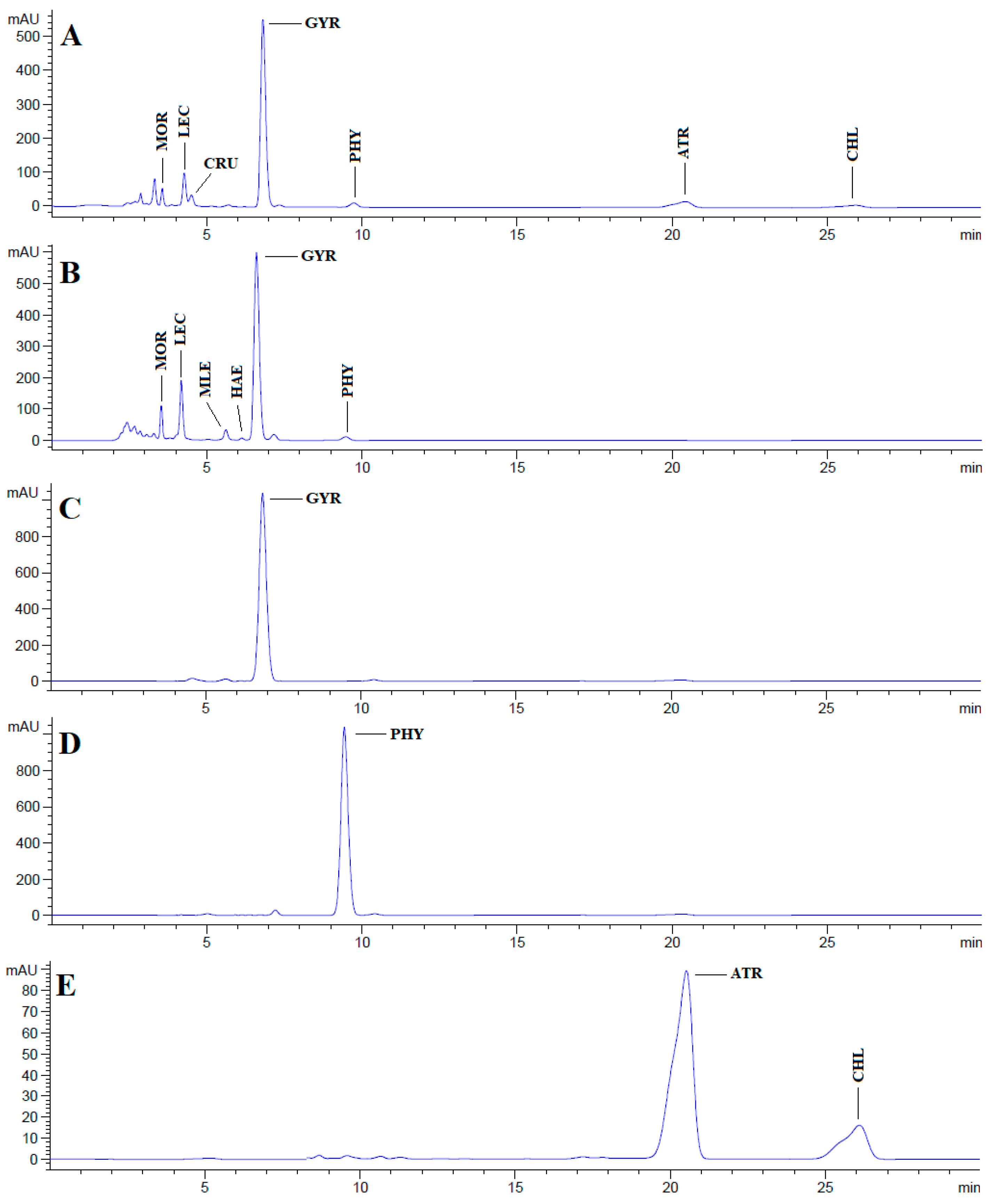
Chromatograms obtained after HPLC analysis of the acetone and methanol extracts of the lichen U. crustulosa, as well as the isolated individual fractions, are presented in Figure 1. A total of nine different compounds were identified in both extracts and individual fractions, with gyrophoric acid being the most abundant compound.
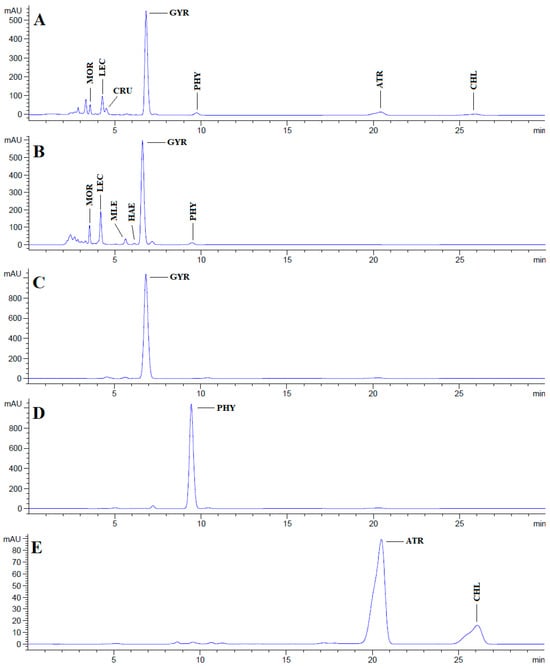
Figure 1.
HPLC chromatograms of the acetone and methanol extracts of the lichen U. crustulosa and the isolated compounds obtained at 254 nm. (A) Chromatogram of U. crustulosa acetone extract; (B) chromatogram of U. crustulosa methanolic extract; (C) chromatogram of isolated compound gyrophoric acid; (D) chromatogram of isolated physodic acid; (E) chromatogram of isolated fraction (compounds atranorin/chloroatranorin); MOR—methyl orsellinate; LEC—lecanoric acid; CRU—crustinic acid; GYR—gyrophoric acid; PHY—physodic acid; ATR—atranorin; CHL—chloroatranorin; MLE—methyl lecanorate; HAE—haematommic acid.
Comprehensive data regarding the retention time and absorption maxima of the identified secondary metabolites are provided in Table 1. The content of these metabolites is also included.

Table 1.
Retention time, absorbance maxima, and relative abundance of the examined lichen substances.
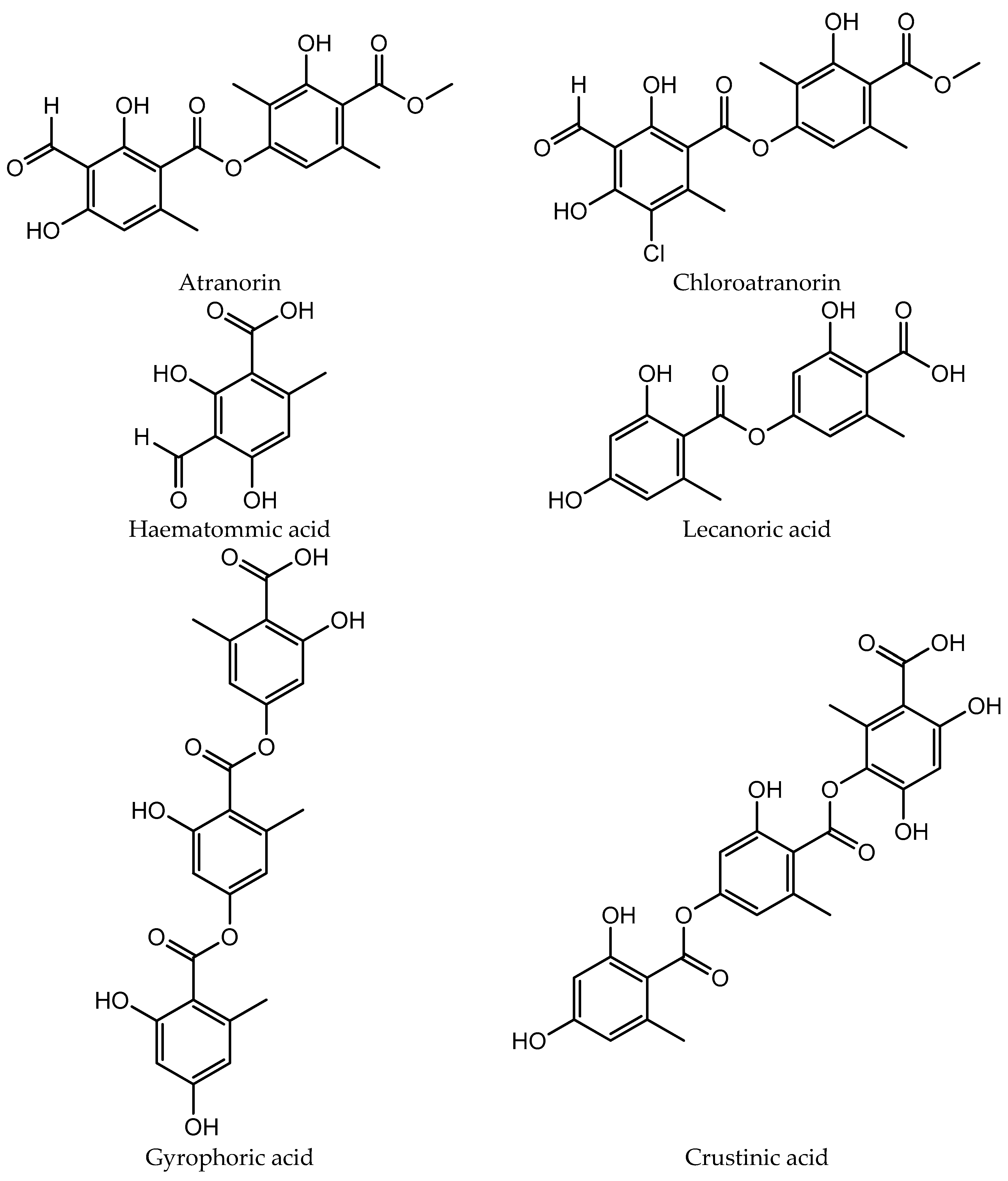
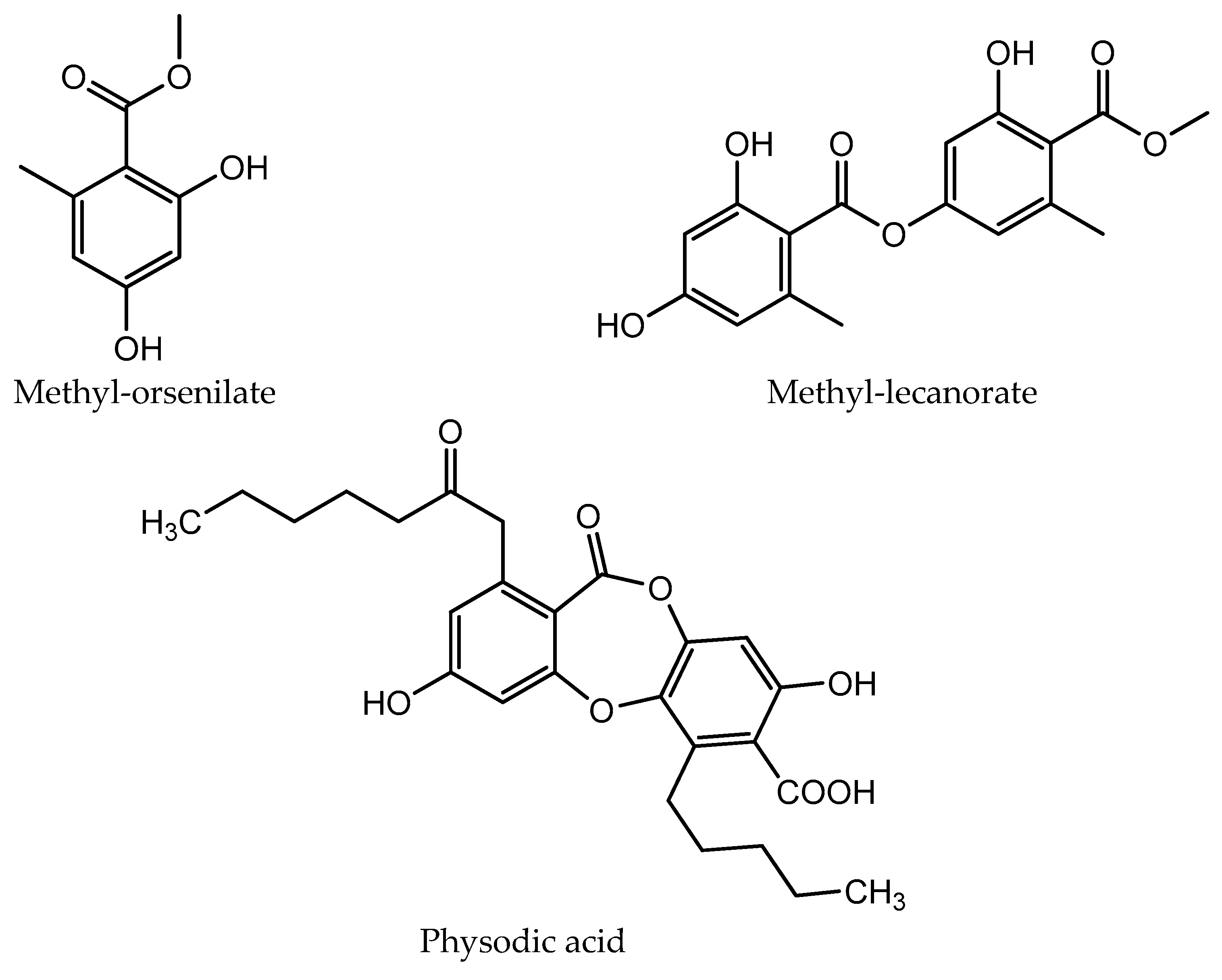
The chemical structures of the compounds identified in extracts and isolated fractions are presented in Figure 2.
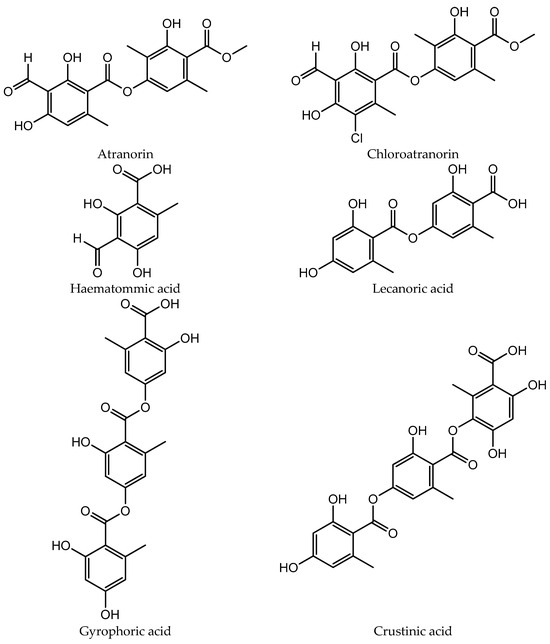
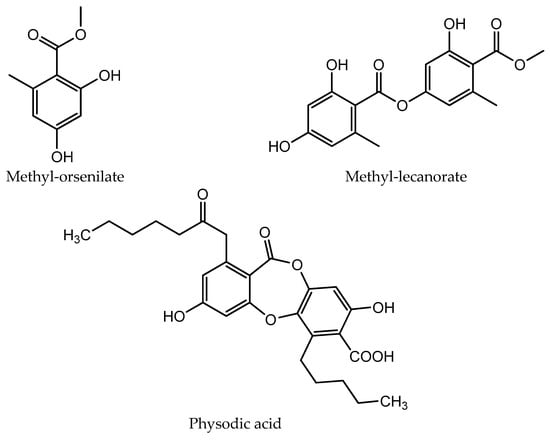
Figure 2.
The chemical structures of compounds identified in extracts and isolated fractions.
3.2. Total Phenolic Content
The results of the total phenolic content (TPC) of the acetone and methanol extracts are presented in Table 2. The acetone extract showed significantly higher values for TPC compared to the other extract.

Table 2.
The total polyphenols content of the extracts of the lichen Umbilicaria crustulosa.
3.3. Antioxidant Activity
The antioxidant activity of two extracts of U. crustulosa and isolated compounds was evaluated using three methods: DPPH scavenging activity, ABTS scavenging activity, and reducing power (Table 3 and Table 4).

Table 3.
The antioxidant activity of the extracts of the lichen U. crustulosa and isolated compounds.

Table 4.
Reducing power of the extracts of lichen U. crustulosa and isolated compounds.
The results of the reducing power of the tested lichen extracts and isolated compounds are shown in Table 4, where the examined extracts had slightly less activity compared to ascorbic acid and Trolox. Physodic acid shows the highest reducing power, while gyrophoric acid shows the weakest at higher concentrations, but at lower concentrations, it is in the same range as the other tested extracts and metabolites.
3.4. Animal Studies
3.4.1. Acute Oral Toxicity Study
The research discovered that giving UCA and UCM at a dosage of 2000 mg/kg did not result in any toxic symptoms or deaths in the animals, and that both extracts can be classified in Class 5 in accordance with the OECD Procedure 423 [23]. No behavioral changes were observed over 14 consecutive days of continuous observation. The histopathological results were within normal limits, and there were no instances of drug-related illness or death. Hence, the UCA and UCM were deemed non-toxic and safe for additional biological activity testing. Data from the acute oral toxicity study are presented in Tables S1–S5.
3.4.2. Anti-Inflammatory Activity
Inflammation caused by carrageenan was observed through swelling and measured by assessing the increase in paw edema. Paw edema enlargement was influenced by the time intervals following carrageenan injection (1, 2, 3, and 4 h) and the dosage of the lichen extract administered (50, 100, and 200 mg/kg), as presented in Table 5.

Table 5.
Anti-inflammatory activity of the extracts of the lichen U. crustulosa in the carrageenan-induced rat paw edema model.
Both obtained extracts at all doses effectively decreased rat paw edema in comparison to the control group. The most significant reduction in edema was seen at two and four hours after carrageenan injection. During the second hour, the acetone extract and methanolic extract of the lichen U. crustulosa at the lowest dose exhibited inhibition percentages of 18.72 and 41.55, respectively. At a dose of 100 mg/kg, acetone extract showed an inhibition percentage of 45.21, while methanolic extract showed an inhibition percentage of 31.96. The most significant effect two hours after carrageenan injection occurred when methanolic was administered at the highest dose of 200 mg/kg. The paw edema inhibition percentage compared to the control was 56.16%. A decrease in paw edema reduction values was observed in the third hour. In the fourth hour, a significant effect could only be observed when the highest dose was applied, resulting in a 46.67% reduction for acetone extract and a 57.78% reduction for methanolic extract.
The data show that the examined extracts begin to show a significant effect already after the first hour and that this effect persists until the end of the experiment compared to the control group. Also, time dependence and dose dependence can be clearly observed, that is, the effects of UCA and UCM administration are most pronounced after 4 h from the moment of administration and in the highest administered dose of 200 mg/kg. The effect of UCM administration in the highest dose is almost comparable to indomethacin administration after 2, 3, and 4 h of administration, when the percentage of inhibition was greater than 50%.
4. Discussion
HPLC analysis of the acetone and methanol extracts of U. crustulosa lichen showed that the main components included two monoaromatic compounds, four didepsides, two tridepsides, and depsidone. The predominant phenolic compound in both extracts was tridepside gyrophoric acid (GYR). Apart from gyrophoric acid, methyl orsellinate (MOR), lecanoric acid (LEC), crustinic acid (CRU), physodic acid (PHY), atranorin (ATR), chloroatranorin (CHL), methyl lecanorate (MLE), and haematommic acid (HAE) were also identified in the chromatograms. Gyrophoric acid, the characteristic lichen substance of the Umbilicaria species, was detectable in 31 out of 33 investigated species. Orselinic acid (monoaromatic compound) and lecanoric acid (didepside) are biochemical precursors of gyrophoric acid [25]. The simultaneous presence of gyrophoric and lecanoric acids suggests that lecanoric acid may be a hydrolysis product of gyrophoric acid [26]. While crustinic acid (tridepside) is a common component of the Umbilicaria species [25], in this research, it was only detected in the acetone extract. Methyl orsellinate (monoaromatic compound) and methyl lecanorate (didepside) are known to be an intermediate product in depside biosynthesis [26]. Physodic acid (depsidone) has been previously documented in the lichen Hypogymnia physodes and other lichen genera [27]. Nonetheless, this study marks the first confirmation of its presence and isolation in the lichen U. crustulosa. The presence or absence of depsidones is also important for classification purposes [25]. Atranorin (didepside) and chloratranorin (didepside) were identified in the acetone extract but not found in the methanol extract. In the Umbilicaria species, atranorin and chloratranorin are typically present in small quantities. [11,26]. Atranorin is a frequent companion of the presence of gyrophoric acid in the Umbilicaria species [25]. Haematommic acid (monoaromatic compound) was identified as a satellite peak in the chromatogram of the methanol extract. The ester form of this acid (ethyl haematommate) has already been found in some Umbilicaria species [11]. In addition, the isolation of gyrophoric acid as the most abundant component in the extract, as well as the isolation of the less abundant component in the extract of physodic acid and the fraction of extract which contains atranorin and chlorotranorin (ATR/CHL) (Figure 1), were reported, and these samples were used for further investigations.
In comparison to previous studies on Umbilicaria species [28], the examined extracts demonstrated significantly elevated levels of the total phenols. Various factors such as extraction method, climatic conditions, soil quality, timing of lichen collection, lichen age, and developmental stage may have contributed to these observed differences [29].
Several studies have examined the antioxidant potential of various lichen species [8,11,30,31]. Recent studies have focused on identifying the specific secondary metabolites within lichen extracts responsible for their antioxidant activity. Unlike previous research that utilized whole lichen extracts, these studies aim to pinpoint the individual metabolites contributing to the antioxidant properties [32,33]. In some earlier studies, the antioxidant activity of U. crustulosa extracts was investigated [12], but the antioxidant activity of its isolated metabolites has not been investigated so far. Precisely this correlation will help us to discover on what basis the antioxidant activity of the extracts originates. The assessment of the antioxidant activity showed that the tested extracts and isolated compounds were able to scavenge DPPH radicals (Table 3).
While several reports have demonstrated a positive correlation between the total phenolic content of lichens and their antioxidant activity [8,11,27,28], it is important to acknowledge that this relationship is not consistently observed in all studies [34,35]. In this study, no correlation was found between the antioxidant activity and the total phenolic content of the extracts. Although the acetone extract of U. crustulosa had higher values of total phenols, it showed a weaker antioxidant activity than the methanol extract in all experiments. The variation in correlation may be attributed to the differing antioxidant activities of individual phenols and potential interactions, whether antagonistic or synergistic, with other compounds within the lichens. The isolated fraction ATR/CHL and compound physodic acid showed the highest DPPH free radical scavenging activity (IC50 = 150.77 μg/mL; IC50 = 158.97 μg/mL). These metabolites showed higher antioxidant activity than the tested extracts, while the isolated gyrophoric acid showed the lowest activity (IC50 = <500 μg/mL). The ABTS method shows similar results. Fraction ATR/CHL and physodic acid showed the highest ABTS radical scavenging activity (IC50 = 43.94 μg/mL; IC50 = 107.58 μg/mL), while the isolated gyrophoric acid showed the lowest activity (IC50 = 246.99 μg/mL). As can be seen in Table 3, the DPPH and ABTS scavenging activity of the tested extracts and isolated metabolites were generally lower compared to the standard substances.
These results of the antioxidant activity are in correlation with the previous results of other authors who showed that depsides (norstictic acid and atranorine) and depsidone (fumarprotocetraric acid) show strong antioxidant activity in free radical and superoxide anion scavenging, as well as in reducing power assays. The isolated metabolites showed better antioxidant activity than the extract [7,36]. It has also been shown that depsidones exhibit slightly stronger antioxidant activity than depsides [27,37]. Depsidones in lichens are thought to be formed by the oxidative cyclization of depsides. Furthermore, some researchers have discovered that depsidones exhibit greater DPPH, superoxide anion radicals scavenging activity, and reducing power compared to depsides and dibenzofurans [27]. The increased effectiveness of depsidones may be linked to their greater incorporation into lipidic microdomains [38].
We selected the carrageenan-induced inflammation model for our research experiment because it is commonly used in the literature to evaluate the effectiveness of various agents in reducing local edema [39,40]. This study showed that using both acetone and a methanolic extract of U. crustulosa reduced paw edema in a dose-dependent pattern at 1, 2, 3, and 4 h after carrageenan injection. The results indicate that both extracts at doses of 50, 100, and 200 mg/kg can effectively inhibit acute inflammation, as demonstrated by a significant reduction in paw edema in rats 4 h after carrageenan injection. Also, both extracts at the highest dosage showed the highest efficiency four hours after inflammation induced by carrageenan.
The literature indicates that the acute inflammatory response consists of two phases: the initial phase and the latter phase [24]. Following carrageenan administration, the first phase begins around 1–2 h later and involves mast cells producing histamine and serotonin. The later phase is characterized by the involvement of prostaglandin and various cytokines [41]. NSAIDs demonstrate anti-inflammatory effects in the later stage of carrageenan-induced inflammation by inhibiting cyclooxygenase and suppressing prostaglandin production through COX enzyme inhibition [42].
Atranorin was previously found to inhibit the enzyme cyclooxygenase 1 in a dose-dependent manner [43]. Atranorin was also the only compound from those identified in the extracts that were tested in a carrageenan-induced paw edema reduction model, where it showed a significant effect [44]. Also, there is data on in-silico studies of the effects of atranorin and lecanoric acid on enzymes associated with the inflammatory response [45]. The anti-inflammatory potential of physodic acid has been demonstrated in in vitro studies [46] but has never been investigated in vivo in a model of carrageenan-induced paw edema.
It is clear that the extracts of lichen have the potential to have an anti-inflammatory effect; however, it is up to future research to determine the precise molecular mechanism that underlies the anti-inflammatory effect.
Given that the effect of the applied extracts can be observed already after the first hour of inducing the inflammatory process with carrageenan, but the effect remains until the very end of the experiment, it can be assumed that the components of the extract are involved in the inhibition of the inflammatory response both in the initial and in the later phase, but additional research is necessary to examine in detail the mechanism of anti-inflammatory action of both U. crustulosa extracts and their individual components.
5. Conclusions
This study marks the first detailed chemical analysis of the lichen U. crustulosa collected from Stara Planina mountain in Serbia and the identification of physodic acid from this lichen. This study’s significance lies in its pioneering evaluation of pure compounds isolated from the extract of the lichen U. crustulosa for its antioxidant activity. Notably, certain compounds exhibited substantial antioxidant effects, marking a noteworthy discovery. This is the first time that the anti-inflammatory activity of U crustulosa lichen extracts has been investigated. Furthermore, the acetone and methanolic extracts demonstrated dose-dependent anti-inflammatory effects in a carrageenan-induced inflammation model. The highest dose of both extracts (200 mg/kg) resulted in the most significant reduction in paw edema, similar to the standard drug indomethacin. Additional research is needed to clarify the fundamental mechanisms of its biological effects and to identify and describe particular active compounds for potential drug development and therapeutic uses. This research highlights the potential of lichens as a valuable source of novel biological agents, which could find applications across various industries such as food, cosmetics, and pharmaceuticals. Future investigations will be focused on isolating larger amounts of pure compounds and determining their anti-inflammatory activity as well as other biological activities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/scipharm92020027/s1.
Author Contributions
Conceptualization, J.T., M.S., P.V. and N.M.; methodology, J.T., A.K., J.B., M.S., P.V. and N.M.; software, J.T., A.K. and J.B.; validation, J.T., A.K., J.B., V.J., M.S., P.V. and N.M.; formal analysis, J.T., A.K., M.A., J.B., N.Z., M.S., P.V. and N.M.; investigation, J.T., A.K., M.A., J.B., V.J., M.S., P.V. and N.M.; resources, V.J., M.S., P.V. and N.M.; data curation, J.T., A.K., J.B., M.S., P.V. and N.M.; writing—original draft preparation, J.T., A.K., J.B., M.S. and N.M.; writing—review and editing, J.T., A.K., J.B., M.S. and N.M.; visualization, J.T., A.K., J.B., M.S. and N.M.; supervision, V.J. and N.M.; project administration, V.J. and N.M.; funding acquisition, V.J. and N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Agreement No: 451-03-65/2024-03/200111 and 451-03-65/2024-03/200124), and by the Science Fund of the Republic of Serbia, The Program Ideas, Grant No. 7743504, NES.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board for the welfare of laboratory animals of the Faculty of Medical Sciences (decision number 01-7540 from 6 July 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sanders, W.B.; Masumoto, H. Lichen algae: The photosynthetic partners in Lichen Symbioses. Lichenologist 2021, 53, 347–393. [Google Scholar] [CrossRef]
- DePriest, P.T. Early molecular investigations of lichen-forming symbionts: 1986–2001*. Annu. Rev. Microbiol. 2004, 58, 273–301. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M. Lichens as a Potential Source of Bioactive Secondary Metabolites. In Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential, 2nd ed.; Ranković, B., Ed.; Springer Nature: Cham, Switzerland, 2019; Volume 1, pp. 1–29. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Gargas, A. Phylogenetic distribution and evolution of secondary metabolites in the lichenized fungal genus Lepraria (Lecanorales: Stereocaulaceae). Nova Hedwig. 2008, 86, 115–131. [Google Scholar] [CrossRef]
- Nash, T.H. Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 104–133. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E. Metabolic diversity of lichen-forming ascomycetous fungi: Culturing, polyketide and shikimate metabolite production, and PKS genes. Nat. Prod. Rep. 2008, 25, 188–200. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M.; Stanojković, T.; Vasiljević, P.; Manojlović, N. Biological activities of Toninia candida and Usnea barbata together with their norstictic acid and usnic acid constituents. Int. J. Mol. Sci. 2012, 13, 14707–14722. [Google Scholar] [CrossRef]
- Tomović, J.; Kosanić, M.; Ranković, B.; Vasiljević, P.; Najman, S.; Manojlović, N. Phytochemical analysis and biological activity of extracts of lichen Physcia semipinnata: As a new source of pharmacologically active compounds. Farmacia 2019, 67, 346–353. [Google Scholar] [CrossRef]
- Kari, P.R. Tanaina plantlore. In Dena’ina K’et’una: An Ethnobotany of the Dena’ina Indians of Southcentral Alaska, 2nd ed.; US National Park Service: Anchorage, AK, USA, 1987. [Google Scholar]
- Huneck, S. The significance of lichens and their metabolites. Naturwissenschaften 1999, 86, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Manojlovic, N.T.; Vasiljevic, P.J.; Maskovic, P.Z.; Juskovic, M.; Bogdanovic-Dusanovic, G. Chemical Composition, Antioxidant, and Antimicrobial Activities of Lichen Umbilicaria cylindrica (L.) Delise (Umbilicariaceae). Evid. Based Complement. Alternat. Med. 2012, 2012, 452431. [Google Scholar] [CrossRef] [PubMed]
- Zlatanović, I.; Stanković, M.; Stankov Jovanović, V.; Mitić, V.; Zrnzevic, I.; Đorđević, A.; Stojanović, G. Biological activities of Umbilicaria crustulosa (Ach.) Frey acetone extract. J. Serb. Chem. Soc. 2017, 82, 141–150. [Google Scholar] [CrossRef]
- Dobson, F.S. Lichens. In An Illustrated Guide to the British and Irish Species, 6th ed.; Richmond Publishing Co.: London, UK, 2011. [Google Scholar]
- Lukač, M.; Onderka, M. Aplicaton of DDQ (2,3-dichloro-5,6-dicyano-benzoquinone) in detection of lichen acids. Acta Fac. Pharm. Univ. Comen. 2006, 53, 160–166. [Google Scholar]
- Huneck, S.; Yoshimura, I. Identification of Lichen Substances, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 1996; pp. 125–446. [Google Scholar] [CrossRef]
- Manojlović, N.T.; Rančić, A.B.; Décor, R.; Vasiljević, P.; Tomović, J. Determination of chemical composition and antimicrobial, antioxidant and cytotoxic activities of lichens Parmelia conspersa and Parmelia Perlata. J. Food Meas. Charact. 2020, 15, 686–696. [Google Scholar] [CrossRef]
- Aoussar, N.; Achmit, M.; Es-Sadeqy, Y.; Vasiljević, P.; Rhallabi, N.; Ait Mhand, R.; Zerouali, K.; Manojlović, N.; Mellouki, F. Phytochemical constituents, antioxidant and antistaphylococcal activities of Evernia prunastri (L.) Ach., Pseudevernia furfuracea (L.) Zopf. and Ramalina farinacea (L.) Ach. from Morocco. Arch. Microbiol. 2021, 203, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.; Bachmayer, O.; Kosar, M.; Hiltunen, R. Antioxidant Properties of Aqueous Extracts from Selected Lamiaceae Species Grown in Turkey. J. Agric. Food Chem. 2004, 52, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Ahmad, S.; Rehman Khan, K.U.; Tabassum, F.; Khursheed, A.; Zaman, Q.U.; Bukhari, N.A.; Alfagham, A.; Hatamleh, A.A.; Chen, Y. Phytochemical profiling, antioxidant, anti-inflammatory, thrombolytic, hemolytic activity in vitro and in silico potential of Portulacaria afra. Molecules 2022, 27, 2377. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- The Organization for Economic Cooperation and Development (OECD). Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals; Test No. 423: Acute Oral toxicity; Section 4; OECD Publishing: Paris, France, 2002. [Google Scholar] [CrossRef]
- Mićović, T.; Katanić Stanković, J.S.; Bauer, R.; Nöst, X.; Marković, Z.; Milenković, D.; Jakovljević, V.; Tomović, M.; Bradić, J.; Stešević, D.; et al. In vitro, in vivo and in silico evaluation of the anti-inflammatory potential of Hyssopus officinalis L. subsp. aristatus (Godr.) Nyman (Lamiaceae). J. Ethnopharmacol. 2022, 293, 115201. [Google Scholar] [CrossRef] [PubMed]
- Posner, B.; Feige, G.B.; Huneck, S. Studies on the chemistry of the lichen genus Umbilicaria Hoffm. Z. Naturforsch. C 1992, 47, 1–9. [Google Scholar] [CrossRef]
- Elix, J.A.; Gaul, K.L. The interconversion of the lichen depsides para-and meta-scrobiculin, and the biosynthetic implications, Aust. J. Chem. 1986, 39, 613–624. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M.; Manojlović, N.; Rančić, A.; Stanojković, T. Chemical composition of Hypogymnia physodes lichen and biological activities of some its major metabolites. Med. Chem. 2014, 23, 408–416. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Antioxidant, antimicrobial, and anticancer activity of 3 Umbilicaria species. J. Food Sci. 2012, 77, 20–25. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Gudžinskas, Z.; Kovalyov, V.; Desenko, V.; Ivanauskas, L.; Bezruk, I.; Georgiyants, V. Effect of ecological factors on the accumulation of phenolic compounds in Iris species from Latvia, Lithuania and Ukraine. Phytochem. Anal. 2020, 31, 545–563. [Google Scholar] [CrossRef]
- Aslan, A.; Güllüce, M.; Sökmen, M.; Adιgüzel, A.; Sahin, F.; Özkan, H. Antioxidant and Antimicrobial Properties of the Lichens Cladonia foliacea., Dermatocarpon miniatum., Everinia divaricata., Evernia prunastri., and Neofuscella pulla. Pharm. Biol. 2006, 44, 247–252. [Google Scholar] [CrossRef]
- Kumar, J.; Dhar, P.; Tayade, A.B.; Gupta, D.; Chaurasia, O.P.; Upreti, D.K.; Arora, R.; Srivastava, R.B. Antioxidant capacities, phenolic profile and cytotoxic effects of saxicolous lichens from trans-Himalayan cold desert of Ladakh. PLoS ONE 2014, 9, e98696. [Google Scholar] [CrossRef] [PubMed]
- Maulidiyah, M.; Darmawan, A.; Usman, U.; Musdalifah, A.; Ode, L.; Salim, A.; Nurdin, M. Antioxidant activity of secondary metabolite compounds from lichen Teloschistes flavicans. Biointerface Res. Appl. Chem. 2021, 11, 13878–13884. [Google Scholar] [CrossRef]
- Elečko, J.; Vilková, M.; Frenák, R.; Routray, D.; Ručová, D.; Bačkor, M.; Goga, M. A comparative study of isolated secondary metabolites from lichens and their antioxidative properties. Plants 2022, 11, 1077. [Google Scholar] [CrossRef]
- Stanly, C.; Ali, D.M.H.; Keng, C.L.; Boey, P.L.; Bhatt, A. Comparative evaluation of antioxidant activity and total phenolic content of selected lichen species from Malaysia. J. Pharm. Res. 2011, 4, 2824–2827. [Google Scholar] [CrossRef]
- Odabasoglu, F.; Aslan, A.; Cakir, A.; Suleymand, H.; Karagoza, Y.; Bayira, Y.; Haliciaet, M. Antioxidant activity, reducing power phenolic content of some lichen species. Fitoterapia 2005, 76, 216–219. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T.; Rančić, A.; Manojlović, N. Cladonia lichens and their major metabolites as possible natural antioxidant, antimicrobial and anticancer agents. LWT—Food Sci. Technol. 2014, 59, 518–525. [Google Scholar] [CrossRef]
- Thadhani, V.M.; Choudhary, M.I.; Ali, S.; Omar, I.; Siddique, H.; Karunaratne, V. Antioxidant activity of some lichen metabolites. Nat. Prod. Res. 2011, 25, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.E.; Fernández, E.; Quilhot, W.; Lissi, E. Antioxidant activity of depsides and depsidones. Phytochemistry 1994, 37, 1585–1587. [Google Scholar] [CrossRef] [PubMed]
- William, C.M.; Rao, N.V.; Kumar, B.R.; Mohan, G.K. Anti-inflammatory and analgesic activities of methanolic extract of Kigelia pinnata DC flower. J. Ethnopharmacol. 2010, 130, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Paredes, A.; Olivares, A.; Bravo, J. Acute oral toxicity and anti-inflammatory activity of hydroalcoholic extract from Lampaya medicinalis Phil in rats. Biol. Res. 2014, 47, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Su, J.; Wang, G.; Zheng, L.; Wang, G.; Sun, Y.; Bao, Y.; Wang, S.; Huang, Y. Discovery of phenolic glycoside from Hyssopus cuspidatus attenuates LPS-induced inflammatory responses by inhibition of iNOS and COX-2 expression through suppression of NF-κB activation. Int. J. Mol. Sci. 2021, 22, 12128. [Google Scholar] [CrossRef] [PubMed]
- Komakech, R.; Kim, Y.G.; Matsabisa, G.M.; Kang, Y. Anti-inflammatory and analgesic potential of Tamarindus indica Linn.(Fabaceae): A narrative review. Integr. Med. Res. 2019, 8, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Studzinska-Sroka, E.; Galanty, A.; Bylka, W. Atranorin-an interesting lichen secondary metabolite. Mini-Rev. Med. Chem. 2017, 17, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen Depsides and Tridepsides: Progress in Pharmacological Approaches. J. Fungus 2023, 9, 116. [Google Scholar] [CrossRef]
- Joshi, T.; Sharma, P.; Joshi, T.; Chandra, S. In silico screening of anti-inflammatory compounds from Lichen by targeting cyclooxygenase-2. J. Biomol. Struct. Dyn. 2020, 38, 3544–3562. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Dubino, A. Lichens as a source of chemical compounds with anti-inflammatory activity. Herba Pol. 2018, 64, 56–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).