Abstract
(1) Background: The study aimed to compare the impact of various natural polymers–sodium alginate, acacia gum, carrageenan, guar gum, xanthan gum, and tragacanth on the formulation and the physical properties of mucoadhesive vaginal tablets containing metronidazole (167 mg/g). (2) Methods: The quality of the tablets prepared by direct compression was evaluated by pharmacopoeia tests (uniformity of mass, resistance to crushing, friability). Mucoadhesion of the tablets was characterized by swelling capacity and mucoadhesive strength, i.e., the force required to detach the tablet from the rabbit mucosa. In vitro drug release was performed by a modified dissolution method in paddle apparatus containing the simulated vaginal fluid (pH 4.5). Scanning electron microscopy observed morphological changes on the swollen tablets’ surface. (3) Results: Pharmacopoeia tests have shown that all prepared tablets met the requirements on quality. The highest mucoadhesive strength was noted in tablets containing guar and xanthan gum. The highest swelling capacity was possessed by tablets containing carrageenan. (4) Conclusions: Summarizing all tests’ results, sodium alginate can be considered the most suitable natural polymer in tablet formulation. The combination of polymers providing higher mucoadhesiveness and at the same time a prolonged release, e.g., xanthan or guar, together with sodium alginate, could also be of interest.
1. Introduction
Bacterial vaginosis is caused by a change in vaginal microbiota, most often by bacterial overgrowth of the common bacteria Gardnerella vaginalis. The most effective treatment includes oral and topical administration of antibiotics, such as metronidazole (MTZ) [1]. It belongs to the synthetic antibiotics derived from nitroimidazole, originally detected in cultures of Streptomyces [2,3]. As a synthetic derivative of azomycin, MTZ has a simple chemical structure (see Figure 1) and a relatively low molecular weight (171.15 g×mol−1) [4]. It was developed in 1959 to treat trichomoniasis, a genital infection caused by Trichomonas vaginalis, which at that time was difficult to cure [5]. Later, it was found out that it is effective not only against Trichomonas but also against other bacteria and protozoa, such as Giardia lamblia, Entamoeba histolytica, Balantidium coli, Clostridium difficile, Helicobacter pylori, etc. [6]. There are no receptors for MTZ. Its uptake is ensured through passive diffusion [2]. The antimicrobial effect most likely lies in nitro group reduction by anaerobic organisms. As a prodrug, it is inactive until it is reduced. Redox potential of electron transport components of microorganism is responsible for nitro group reduction and cause the transformation of MTZ to its cytotoxic metabolites: N-(2-hydroxyethyl) oxamic acid and acetamide, binding to the bacterial DNA molecule and thus inhibiting its synthesis with subsequent cell death [2,3,7]. MTZ is the most commonly administrated orally (standard dosing: 250/500 mg in tablet, 375 mg in capsule) [6]. Specific drug dosage forms can be intended also for intravenous, vaginal, or rectal administration.
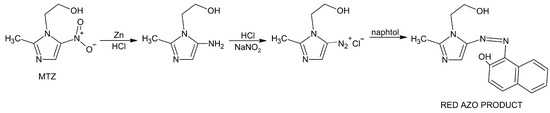
Figure 1.
MTZ reduction followed by diazotization and coupling with β-naphtol resulting in red azo product.
MTZ as active pharmaceutical ingredient (API) is applied in a suitable drug delivery system topically, on the mucous membrane of the vagina, to treat female urogenital trichomonas, candida, or mixed infections. MTZ administered vaginally is available on the pharmaceutical market as a cream, a gel, and vaginal suppositories [8]. The benefits of vaginal administration lie in lower incidence and severity of gastrointestinal side effects, avoiding tissue damage and pain usual for the parenteral route, and possibility of self-insertion [9]. The vagina has a rich blood vessel network and a large surface, providing an ideal way to deliver drugs achieving both systematic and local therapeutic effects [10].
Compared to oral administration, vaginal administration has several advantages. It reduces the occurrence and severity of gastrointestinal and hepatic side effects. In the case of the first undesirable effect appearance, the dosage form can be removed from the action site [11]. Because of non-constant vaginal environment, the anatomy and physiology of the vagina have to be taken into account during the designing of vaginal medicines. Vaginal temperature corresponds to body temperature but increases during ovulation. It can also increase as a result of various diseases. The amount of vaginal mucus varies in the range of 0.3–1.55 g×h−1. It has to be taken into account that absorption of poorly soluble drugs may increase when vaginal secretion is higher, e.g., during ovulation. Mucus is largely made up of water and also contains antimicrobial agents. The current acidity of the vaginal fluid is variable, fluctuating between pH 4 and 5, depending on the menstrual cycle [12,13,14]. During menstruation, the pH influenced by the blood presence is higher. The enzyme activity in the vagina is low than in gastrointestinal tract, mainly ensured by proteases, β-glucuronidase, acid and alkaline phosphatase, and esterase [14]. Drug absorption depends on drug solubility, ionization, relative molecular weight, and possible interactions with vaginal tissue. The drug to be absorbed has to dissolve in the vaginal mucus. Excessive secretion is counterproductive since part of the drug drains away. Therefore, bioadhesive substances find application as the excipients in vaginal formulation, ensuring prolonged contact of the medicine with the vaginal epithelium.
Tablets belonging to the solid vaginal preparations, similarly as vaginal capsules, pessaries, and tampons, have one huge benefit compared to liquid and semi-solid vaginal preparations (e.g., lotions, gels, creams, foams, etc.). They do not expulse so rapidly from the vagina by self-cleaning. Despite all the benefits of vaginal application, the changes during menstruation and the post-menopausal cycle due to hormonal fluctuation have to be taken into account [15]. In women after menopause, the reduction in vaginal epithelial thickness may significantly modify the initial rate of drug absorption.
The future of vaginal dosage forms lies in the application of mucoadhesive polymers and microparticles providing a long-term therapeutic drug delivery. A suitable mucoadhesive polymer should be non-toxic, non-irritating, capable of swelling, ensure quick and sufficient adhesion to the mucous membrane, and have the required pH and viscous elastic properties [16]. Vaginal pH, mucus viscosity, and other features vary depending on a woman’s age, sexual activity, hormone levels, and so it is important to find a stable mucoadhesive agent capable of resisting changes in the internal environment, especially pH [17]. Žigrayová et al. [18] report that pH fluctuations change the mucoadhesive behaviour of a medicine and the release rate of antiviral drugs. On the other hand, some mucoadhesive polymers are capable of inhibiting local enzymes, which can facilitate API penetration to the mucosa [19].
Mucoadhesion describes the attractive force between a natural or synthetic polymer and the mucous membrane. The mucoadhesive polymer in vaginal formulation ensures improved contact between vaginal mucosa and a drug dosage form, i.e., prolong in situ residence of a vaginal dosage form. Mucus lubricates and protects epithelial tissue from mechanical and chemical damage. Mucus secretion can vary depending on age, gender, body parts, and pathologies. The main components of mucus are glycosylated proteins–mucins. They consist of a protein with covalently attached carbohydrate chains along its entire length. These glycoproteins are responsible for the gel-like properties of mucus.
Generally, a tensile strength or shear stress test can be used to determine the mucoadhesion of vaginal medicines. The principle is to measure the force required to separate the dosage form from the membrane model or, conversely. For semi-solid dosage forms, an alternative method is to characterize the change in rheological properties and texture on the addition of mucin based on the interpenetration extension between polymer gels and their mixtures with mucin [20]. Machado et al. [21] found out that the bioadhesion of semi-solids can be predicted by adhesiveness, which can be easily determined by texture analysis without the application of biological material. It would be a simplification of measurement if a similar correlation is found for solid dosage forms.
Interest in the development of mucoadhesive vaginal products is still high. Scientific studies from recent years have been devoted to investigating the qualitative parameters of dosage forms, methods of characterization of the mucoadhesion, and release mechanisms depending on the presence of the mucoadhesive agent. However, they either focus on the effect of mucoadhesive polymer concentration [22,23] or study different combinations of at least two mucoadhesive polymers [24,25,26,27]. There are no studies that summarize the mucoadhesive effect of various natural polymers, which was our main intention. The mucoadhesive properties of the pharmaceutical excipients of various origin are extensively discussed in Osmałek’s et al. [17] paper. Some studies [27,28] demonstrate the positive effect of polymer agent concentration on mucoadhesion, i.e., higher polymer concentration increases the mucoadhesive force. The most preferred mucoadhesive polymers are cellulose derivatives, chitosan and polyacrylic acid derivatives because chitosan shows antifungal and antibacterial activity, and Carbopol® for its acid-buffering capacity [27]. Combining natural and synthetic/semi-synthetic polymers in a formulation may have a positive effect on mucoadhesion. E.g., Sánches et al. [27] studied the combination of Carbopol® and chitosan. Pacheco-Quito et al. [24] studied the combination of iota-carrageenan and hydroxypropyl methylcellulose. Abruzzo et al. [25] studied mucoadhesion of chitosan/alginate vaginal inserts. Cirri et al. [26] developed alginate microspheres coated with chitosan for vaginal administration. The authors notify colligative mucoadhesive properties of the dosage form if a couple of polymers was used.
As Osmanek et al. summarize, carrageenan, alginate, and chitosan are commonly used natural polymers in vaginal delivery systems to provide mucoadhesion, which is why they were chosen as the main excipient in the formulations we tested. The other three polymers (guar gum, acacia gum, tragacanth) are less common in vaginal products despite possessing mucoadhesive abilities. Our intention was to compare the traditional natural polymers with the less traditional ones and their effect on the quality and other properties of the tablets. As mentioned earlier, the scientific community lacks studies that compare the effect of different natural mucoadhesive polymers in a composition of tablets. Rather, they focus on examining the effect of the concentration of a single polymer or a combination of multiple polymers in a single formulation. Since current trends in pharmaceutical technology incline the application of the excipients of natural origin, the polymers mentioned above were chosen. The main benefit of natural polymers as the excipients in drug delivery systems is their biocompatibility, biodegradability, low toxicity, and in many cases, low cost.
2. Materials and Methods
Metronidazole (MTZ) was purchased from Penta (Prague, Czech Republic). Sodium alginate (CAS No.: 9005-38-3; Kinematic viscosity: 15–25 cps; Molecular weight: 120,000–190,000 g/mol), acacia gum (CAS No.: 9000-01-5), κ/λ-Carrageenan (CAS No.: 9000-07-1), Guar gum (CAS No.: 9000-30-0), xanthan gum (CAS No.: 11138-66-2), and tragacanth (CAS No.: 9000-65-1) were purchased from Sigma Aldrich (Darmstadt, Germany). Magnesium stearate was purchased from Tachyon Technology Pharm (Bratislava, Slovakia). Microcrystalline cellulose (VIVAPUR® 102) was purchased from JRS PHARMA GmbH&Co.KG (Rosenberg, Germany). Lactic acid, Naphthol, and Sulphanilic acid were purchased from PENTA, s.r.o. (Prague, Czech Republic). Sodium nitrite and powdered Zinc were purchased from Microchem Trade (Pezinok, Slovakia). Sodium hydroxide was purchased from CentralChem (Bratislava, Slovakia). The purified water was prepared at the Department of Galenic Pharmacy by distillation apparatus Kavalier (Prague, Czech Republic).
2.1. Tablets Formulation
The dry mixtures consisting of MTZ, different natural polymers, and the other excipients recorded in Table 1 were prepared by sieving (250 µm) of each ingredient individually, weighing and 15 min’ homogenization in a homogenization cube. Six series of vaginal tablets were prepared by direct compression (eccentric tablet press, Korsch, Berlin, Germany). The mass of the individual tablets was set to 300 mg. The punch with a diameter of 9 mm was used to produce a tablet of cylindrical shape.

Table 1.
The composition of six series of tablets–ingredients in grams per tablet.
2.2. Quality Assessment of Prepared Tablets
2.2.1. Uniformity of Mass
Twenty tablets were weighed individually on the analytical scale (HZY A200, Libra, Bratislava, Slovakia) [29]. In addition, to the uniformity of mass and the tablet height was also controlled using a digital calliper (type 14016458 KS, Somet, Hradec Králové, Czech Republic). The average mass/height ± S.D. (n = 20) was determined.
2.2.2. Resistance to Crushing
Ten tablets were inserted radially between the jaws of the hardness tester (Schleuniger 2E, Solothurn, Switzerland). The force needed for crushing was read from the device expressing the hardness of the tablet in Newton (N) [29]. The average force ± S.D. (n = 10) for each type of formulation was found.
2.2.3. Friability
Twenty tablets were previously sieved through a sieve (1000 µm). Subsequently, they were weighed and placed into a rotating disc with an internal diameter of 286 mm and a width of 39 mm, made of translucent synthetic polymer (Tablet Friability Test Apparatus, Erweka GmbH, Heusenstamm, Germany). The tablets turned 100 times in a rotating disc. Then they were dusted off and weighed again [29]. The weight loss was expressed as friability F (%):
where m1 is the tablet’s weight before stress and m2 is the tablet’s weight after stress. The measurement was repeated in triplicate for each formulation (n = 3). The friability was expressed as the mean ± S.D.
2.2.4. Mucoadhesive Strength
To compare the bioadhesion of the formulated vaginal tablets, the force necessary to pull the tested tablet away from the biological material was measured by texture analyser TA.XT Plus (Stable Micro Systems Ltd., Godalming, UK). Vaginal epithelia were excised from a rabbit vaginal tube that was obtained from a five-month-old rabbit post-mortem. The animals came from domestic breeding. The tissue was taken, processed, and stored according to the Machado et al. [21] procedure with the only exception, that the normal saline was used for rinsing instead of Hank’s balanced salt solution. The tablet was fixed in the upper jaw and it was moving down at a speed of 1 mm/s to the lower jaw, where the mucous membrane moistened with 50 μL of lactic acid solution was fixed. When the contact force was 0.5 N, and the specified contact time (120 s), the force required to pull the tablet away from the vaginal epithelia at a speed of 2 mm/s was measured [30]. The mucoadhesive strength Fm (N) was calculated using Equation (2):
where w is the weight required to detach the tablet from the mucosa (g), and g is the acceleration due to gravity (9.81 m.s−2) [31]. The measurement was repeated in triplicate for each formulation (n = 3). The mucoadhesive strength was expressed as the mean ± S.D.
2.2.5. Swelling Capacity
The determination of the swelling capacity is another way how to characterize the bioadhesion of the tablets. Absorption potency described the ability of a tablet to uptake a certain amount of a liquid and thus increase its volume and weight. The swelling capacity was determined by a “Petri dish” method according to Alzainy and Boateng with minor modification [10]. It expresses the ability of the tablet to absorb water and swell. The tablet was embedded in the bottom of a Petri dish and immersed in 20 mL of a lactic acid solution (pH 4.5). At certain time intervals, the solution from the Petri dish was removed, its surface dried and together with the tablet weighed. From the mass values obtained, swelling capacity S (%) was calculated according to Equation (3):
where m0 is tablet mass before testing and mt is tablet mass at the time t. The measurement was repeated in triplicate for each formulation (n = 3). The swelling capacity was expressed as the mean ± S.D.
2.2.6. In Vitro Drug Release
The dissolution profiles of MTZ from vaginal tablets were evaluated by a modified procedure using a paddle type of dissolution apparatus Erweka DT 6 (Langen, Germany). The dissolution vessel was filled up with 500 mL of dissolution medium (lactic acid solution with a pH of 4.5) heated to 37 ± 0.5 °C. A perforated plate was attached to the dissolution vessel so that it was in contact with the level of the dissolution medium and at the same time the tablet placed on the plate was in contact with the dissolution medium. At the bottom of the dissolution vessels, the paddles were rotating at a high speed of 10 rpm. At certain time intervals (0.5, 1, 1.5, 2, 3, 4, 6, 8, 10 h) 10 mL of sample was taken, filtered through a filter paper and prepared to analyse the drug content. To ensure the contact of the tablet and dissolution medium, the same volume of lactic acid solution replaced the dissolution medium taken from the vessels.
The principle of the drug determination in the samples lies in the initial reduction in MTZ as an imidazole derivative by treatment with zinc powder and hydrochloric acid followed by diazotization and coupling of the resulting amine [32]. It means that the selected and filtrated samples were adjusted before spectrophotometric determination of drug concentration as follows: 2 mL of filtrate was reduced by 10 mg of powdered zinc, 1 mL of hydrochloric acid (1M) was added and the mixture was shaken, 1 mL of sodium nitrite solution (1%) was added and the mixture was allowed to stand for 5 min, then 1 mL of sulphamic acid solution (3%) was added with occasional shaking for 3 min. Finally, 5 mL of naphthol (0.1%) was added. The procedure reported by Ibrahim et al. [33] was followed with two modifications: zinc was used for the drug reduction instead of iron and 2-naphthol was used for diazotization instead of N-1-(naphthyl)-ethylenediamine dihydrochloride. The chemical principle of MTZ reduction followed by diazotization and coupling with β-naphtol resulting in red azo product is shown in Figure 1 [34].
The drug concentration c (%) was determined by measuring the absorbance by a spectrophotometer (Genesys10 UV-VIS, Cambridge, UK) at a wavelength 510 nm against the corresponding blank using data from the standard curve inserted in Equation (4):
where A is absorbance, mi is MTZ amount in the tablet (g), and V is the volume of the dissolution medium (L). The standard solutions used for the construction of the standard curve were adjusted in the same way.
2.2.7. In Vitro Drug Release Kinetics
The dissolution data of each formulation were fitted to the kinetic models (zero order, first order, Higuchi, Kormeyer–Peppas, Hixson–Crowell) [35] and the mechanism of the drug release was characterized based on the belonging coefficient of determination (r2).
2.2.8. Characterization of Morphological Changes in Swollen Tablets by SEM
The tablet was placed in the centre of a Petri dish filled with 7 mL of the simulated vaginal fluid (pH 4.5). The tablet was allowed to swell for 15 min, then the liquid was poured off and the Petri dish with the tablet was placed in a laboratory drying oven and dried at 50 °C for 3 h and then at room temperature for another week. The morphological changes on the swollen tablet surface were evaluated by scanning electron microscopy (SEM). The swollen tablet samples were fixed on a metal holder using double-sided carbon tape. The fixed samples were subsequently coated with an approximately 10 nm thick layer of gold in an ion sputter coater MCM-100P (SEC Co., Ltd., Suwon-si, South Korea). Microscopic images of the samples were taken using a scanning electron microscope SNE-4500M Plus (SEC Co., Ltd., Suwon-si, South Korea) under a high vacuum using secondary electron detection mode with an accelerating voltage of 30 kV.
2.2.9. Statistical Analysis
Statistical analysis was performed by one-way ANOVA, using Daniel’s XL Toolbox add-in for Excel. Differences were considered to be significant at p < 0.05. The results were expressed as the mean value ± standard deviation (SD). The graphs indicate either significant (*) or non-significant (NS) differences, cases of which there are fewer. Since no reference formulation was chosen in the experiment and all formulations were compared with each other, i.e., 15 different variations of pairs of formulations, indicating them in the graphs would make them opaque.
3. Results
Six series of tablets containing various natural polymers (40% of the total tablet weight) were prepared after the homogenization of the ingredients by direct compression. As natural polymers, sodium alginate, acacia gum, carrageenan, guar gum, xanthan gum, and tragacanth were selected to monitor their effect on composition of the tablets, mainly on mucoadhesive properties of tablets, which were evaluated through the swelling capacity and mucoadhesive strength. Another comparing parameter was the rate of MTZ release from individual formulations.
3.1. Uniformity of Mass
The average weight of the tablets ranged from 0.297 ± 0.003 g to 0.302 ± 0.003 g (see Table 2). To meet the requirement, among 20 tablets with an average weight of more than 250 mg, only 2 tablets may exceed the permissible deviation of 5%, and none of the tablets may exceed twice the permitted deviation (10%) [29]. None of the formulated tablets exceeded the permissible deviation. The average height of the formulated tablets was 2.99 ± 0.06 mm.

Table 2.
Average mass, height and hardness of the vaginal tablets containing various natural polymers (F1: sodium alginate, F2: acacia gum, F3: carrageenan, F4: guar gum, F5: xanthan gum, F6: tragacanth).
3.2. Resistance to Crushing
The hardness of the tablets was influenced by the natural polymer used and the compression pressure, which was set when compressing the first formulation (F1) and was not changed during compression of the other formulations to allow adequate comparison. The lowest hardness (54.4 ± 2.59 N) was found in the tablets with guar gum (F4). The highest hardness was recorded for the tablets with sodium alginate (F1), where the average force needed to break the tablet was 95.3 ± 17.7 N. The results for all formulations are shown in Table 2.
3.3. Friability
Regarding the friability test, the weight loss on the tablets caused by mechanical stress during rotation in the apparatus might be up to 1% [29]. The weight loss of tablets ranged from 0.33 to 0.64%. All formulations met the requirement for friability. The tablets with sodium alginate (F1) and guar gum (F4) had the lowest friability (see Figure 2).
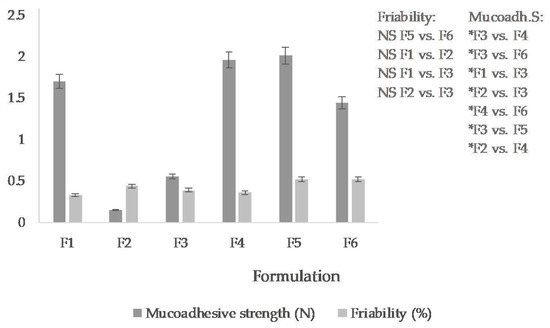
Figure 2.
Mucoadhesive strength (N) and friability (%) of the vaginal tablets (the mean ± S.D., n = 3). (F1: sodium alginate, F2: acacia gum, F3: carrageenan, F4: guar gum, F5: xanthan gum, F6: tragacanth). For a clear illustration, the graph records only non-significant (NS) differences in friability and significant differences (* p ˂ 0.05) in mucoadhesive strength.
3.4. Mucoadhesive Strength
The mucoadhesive properties of the tablets were determined ex vivo on rabbit mucosa. The mucoadhesion expressed as a force required to remove the tablet from the mucosa was in the range from 0.552 N to 2.009 N (see Figure 2), specifically, the highest in the tablets containing xanthan gum (F5), approximately the same in tablets containing guar gum (F4) and the lowest in the acacia gum-containing tablets (F2).
3.5. Swelling Capacity
The characterization of bioadhesive properties of vaginal tablets was carried out based on a determination of a percentage of swelling (hydration). The percentage of hydration of all series of tablets was moving from 16.3 to 504.3% at its maximum (see in Figure 3). The lowest value was detected in the tablets containing acacia gum (did not even reach 100% in the last time interval). After 1.5 h, the value began to decrease, and the tablets began to crumble. The tablets containing carrageenan had the largest increase in water absorption (up to 510 ± 255%), but the tablets broke down after the first hour. The tablets containing sodium alginate reached the maximum hydration percentage of 359 ± 182% after 6 h. The absorption capacity of tablets containing guar gum gradually increased. After 10 h, it achieved a value of 239 ± 118%. It was noticed the main increase in the tablet weight in the first half hour. The tablets did not crumble for 10 h. The tablets containing xanthan gum showed a significant increase in hydration percentage, achieving a value of 504 ± 250% after 10 h. The hydration curve was almost linear, with the largest increase in the first half hour. The tablets containing tragacanth reached a maximum water absorption of 241 ± 118% after 10 h, whereas the highest increase was observed in the first hour.
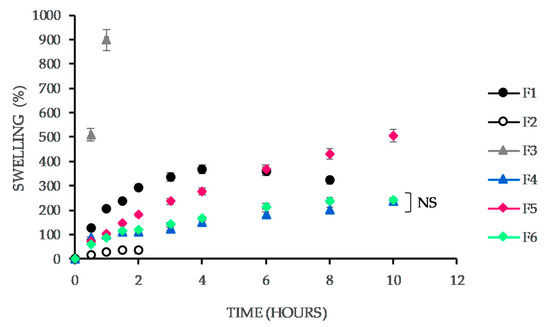
Figure 3.
Swelling capacity (%) of the vaginal tablets vs. time. The formulations vary in the presence of the natural polymer (F1: sodium alginate, F2: acacia gum, F3: carrageenan, F4: guar gum, F5: xanthan gum, F6: tragacanth). For a clear illustration, the graph records only non-significant (NS) difference. Others are considered significant concerning p ˂ 0.05.
3.6. In Vitro Drug Release
The prepared formulations contained only one filler, insoluble microcrystalline cellulose (Vivapur®). MTZ release from the vaginal tablets was detected by dissolution test using a lactic acid solution with a pH of 4.5 as a dissolution medium. The dissolution profiles of MTZ from each formulation are shown in Figure 4. As it is obvious, the natural polymers in the composition affected MTZ release to a great extent. In Figure 4, it is recognizable that the dissolution profiles of MTZ from the tablets containing guar and xanthan gum are similar. From these two formulations, even after 10 h, the entire amount of MTZ was not released, and it can be assumed that the drug would be released gradually until the disintegration of the tablets. Generally, from all formulations except those containing guar gum and xanthan gum, more than 95% of MTZ was released within 6 to 10 h. Therefore, it can assume that the mentioned polymers cause retardation in the drug release.
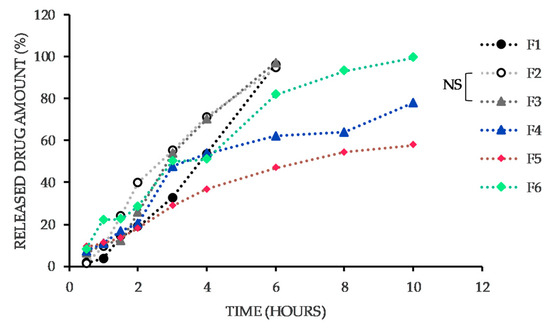
Figure 4.
The dissolution profiles of MTZ from the vaginal tablets. The formulations vary in the presence of the natural polymer (F1: sodium alginate, F2: acacia gum, F3: carrageenan, F4: guar gum, F5: xanthan gum, F6: tragacanth). For a clear illustration, the graph records only non-significant (NS) difference. Others are considered significant concerning p ˂ 0.05.
Summarizing the statistical significance of the data, the difference in the released amounts of MTZ from F2 and F3 is statistically insignificant. All other liberation profiles are statistically different (p ˂ 0.05).
3.7. In Vitro Drug Release Kinetics
The coefficients of determination (r2) and drug release constants (K) for different kinetic models: zero-order, first-order, Higuchi, Kormeyer–Peppas, and Hixson–Crowell, are recorded in Table 3.

Table 3.
Comparison of different kinetic model parameters.
3.8. Characterization of Morphological Changes in Swollen Tablets by SEM
For a more thorough illustration of the morphological changes on the swollen tablets, SEM analysis of the tablets was performed before and after swelling in the simulated vaginal fluid for 15 min. A lower volume of fluid and shorter time than during the swelling test was chosen to avoid complete tablet rupture. The microscopic images show un-swollen tablets F1-F6 on the left (Figure 5), the edge of the tablets after 15 min of swelling at 66-fold magnification (in the middle) and a detail of the tablet surface at 300-fold magnification (right).
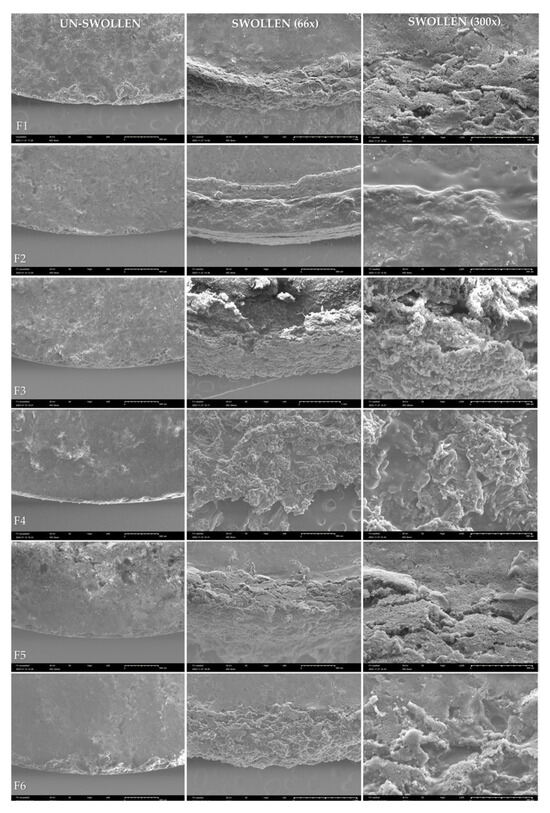
Figure 5.
SEM of the vaginal tablets varying in the type of natural polymer F1–F6; the un-swollen tablets (left); the tablets after 15 min of swelling in the simulated vaginal fluid (pH 4.5) at 66-fold magnification (in the middle) and at 300-fold magnification (right).
4. Discussion
4.1. Physical Parameters of Tablets and Their Mechanical Resistance
Based on the evaluation of the basic physical parameters, it can be summarized that the tablets were uniform and the deviation from the average value in individual batches was minimal, not exceeding the allowed limit of ±5%. Comparing the formulations with each other, it can be evaluated that the effect of natural polymer in the tablet formulation on weight and height is minimal. The average tablet weight for all formulations combined is 0.298 ± 0.002 g and the average height is 2.985 ± 0.061 mm. The tablets containing sodium alginate, acacia gum, and carrageenan, i.e., formulations F1, F2, F3, had approximately the same strength (Table 2), and they were also the strongest among the tested. The other polymers, guar, xanthan, or tragacanth in tablet composition, had the effect of reducing the strength of tablets pressed under the same compression conditions. The friability test showed that guar and sodium alginate in the tablet formulation provide the highest resistance. Thus, the friability and tablet strength results correlate for the sodium alginate formulation. The tablets are both firm and least friable. Paradoxically, tablets containing guar gum are less firm but some of the most resistant to crumbling in the friabilator.
4.2. Mucoadhesive Strength
Rabbit vaginal mucosa was selected as a model to test the mucoadhesive properties of the tablets based on the recommendation of FDA to be the most suitable for testing the irritancy of vaginal products. Of course, human and rabbit vaginal mucosa throws up morphological and other differences, e.g., rabbit model is lined with columnar epithelium for two-thirds of the vagina compared to the stratified squamous epithelium of humans [36]. Compared to rodent models, it provides the advantages of applying the full strength and dose of the formulation. The primate model is the most similar to the human model, but at the same time the most expensive. McCracken et al. [37] summarized and compared the basic parameters of commonly used vaginal mucosal models, concluding that mini-pig and sheep models are the most suitable choice due to the similarity of anatomy, morphology, pH, and microflora to human. Nevertheless, rabbit vaginal mucosa is a gold standard in vaginal testing due to availability and price.
The highest mucoadhesion was exhibited by formulations F5 > F4 > F1, i.e., containing xanthan gum, guar gum, and sodium alginate (Figure 2). The mucoadhesive properties of the two aforementioned polymers, sodium alginate and xanthan gum, are commonly used in vaginal formulations [17]. More surprising is the finding that guar gum, less frequently used in vaginal application, will provide similar mucoadhesive properties to sodium alginate or xanthan gum in the tablet.
Generally, the mucoadhesive effect is achieved either by physical or secondary chemical bonds. The predominant interactions are hydrogen bonds. As a result, some functional groups, such as hydroxyl groups, carboxyl groups, and other hydrogen bonds, forming functional groups may contribute to mucoadhesion [16]. The drug is released near the membrane, which means improved bioavailability. Although guar gum is more often used as a binder in a tablet production [38] or an excipient for matrix tablet formation [39,40], it also exhibits mucoadhesive ability. It lies mainly in electrostatic interaction between the positive charge of guar and negative charge present in the mucus [41]. Studies have found guar to range from being a relatively poor mucoadhesive to exhibiting good mucoadhesion [42]. This discrepancy in the claim can be explained by the species diversity of guar with different physical properties depending on the molecular weight, but also by differences in the choice of other mucoadhesive polymers compared. The mucoadhesive properties of polymers depend on many factors, in particular the charge, chain flexibility, surface activity, and the number of free groups (carboxyl, hydroxyl, sulphate, amine) [17]. Due to the species diversity of individual polymers given by the diverse number of repeating monomeric units, it is not possible to theoretically predict their mucoadhesive abilities. Therefore, the most relevant comparison is provided by data obtained experimentally.
Acacia-containing tablets showed the lowest mucoadhesion. This result is consistent with the results of a study showing poor acacia mucoadhesion [42], despite the fact that it was not tablets but patches that were tested. Acacia is more likely to be used as a binder in the tablet formulation. Therefore, there is lack of studies comparing the mucoadhesive properties of acacia tablets with traditional, effective natural polymers such as sodium alginate or xanthan gum, e.g., Daneshmehr et al. [43] also focused on comparing the mucoadhesive properties of tablets as a function of acacia concentration, with results suggesting that higher acacia concentration favours the mucoadhesive properties of tablets. Munot et al. [44], in turn, evaluated the positive effect of acacia thiolation, which is also in favour of the mucoadhesive properties of tablets, but whether they are comparable to traditional mucoadhesive natural polymers is unknown.
It is surprising to find that carrageenan is not among the top 3 polymers providing the highest mucoadhesion to the tablet, despite the fact that it is commonly used for this purpose in vaginal preparations. The most likely reason would be species diversity, given that molecular mass of commercial carrageenan gums varies between 100 and 1000 KDa [24].
4.3. Swelling Capacity
The evaluation of swelling capacity is an important parameter both, in terms of adhesion and understanding a way of a drug liberation from tablets. When the tablet comes in contact with the hydrophilic environment, a gradual swelling of the polymer contained in the structure of the tablet and the formation of a gel layer occur, which ensures the adhesion to the biological surface, influencing the drug release from the tablet [30]. Time-dependence of the swelling capacity of the examined formulations illustrates Figure 3. From the graph, it is obvious that the highest increase in hydration is in the first half hour caused by swelling of the polymer in the top layer of the tablet, followed by a subsequent formation of a gel regulating further the supply of the medium to the tablet core, accordingly, the drug rate release. The tablets containing polymers usually producing viscous, semi-solid gels have a slower and longer course of swelling after the initial increase in weight. The tablets containing polymers that form less viscous, non-strong gels are very early and intensely hydrated and also begin to disintegrate very soon which was confirmed by the swelling test of the F2 containing acacia gum.
The results of the swelling capacity test could be summarized as follows: neither extreme is acceptable. The lower swelling capacity found in the tablets containing acacia gum (F2) means that the tablet is not sufficiently capable of mucoadhesion, which is confirmed by the test of mucoadhesive strength. On the other hand, the opposite extreme, too rapid swelling of the polymer in the tablet (F1) can lead to its breaking, which is an intolerable phenomenon in vaginal administration.
It can summarize that in the case of F4, F5, and F6, the gradual, constant swelling of the polymer in the tablet matrix ensures a gradual drug release over 10 h. Moreover, in F5, the course of the swelling profile seems to copy the drug dissolution from this formulation. The swelling course in F4 and F6 containing guar gum or tragacanth is very similar. However, the drug release from these formulations is no longer so constant. It can state that in some cases, the drug release rate from the matrix can predict due to the swelling test. For F1, F2, or F3, which either swell very quickly or was damaged by swelling, almost 100% of the drug released within 6 h. Guar and xanthan gum slowed down the drug release so that within 10 h, the total drug release was still not achieved (only up to 80%). Martín-Illana et al. [45] demonstrated a moderate swelling of xanthan gum resulting to sustained drug release, although it was included in mucoadhesive vaginal films and compared to other polymers (pectin and karaya gum).
4.4. In Vitro Drug Release
Despite the fact that the hardness of the first three formulations (F1, F2, F3) expressed as resistance to crushing was the highest (Table 2), MTZ was released from them slowly during the first hour. However, in the second hour, the cumulative amounts of drugs released were approximately equal to the others. After 6 h, the total drug amount was released from them (F1, F2, F3). The highest mucoadhesive force was determined in F5, F4, F1, F6. With the exception of F1, an extended MTZ release time was also noted from the aforementioned formulations. However, following the drug liberation course only in first four hours, the results correspond to the mucoadhesive strength test—the most mucoadhesive formulation (F5) released the least drug amount. The other formulations (F1, F4, F6) released approximately the same amount of drug (50.95–53.63%), which, however, is still a lower amount than that from the least mucoadhesive formulations (F2, F3) released. This fact proves the considerable influence of mucoadhesive properties of tablets on the drug release rate. Conversely, the variation of natural polymers in the composition did not significantly affect the only physical property of the tablets, namely friability, which was less than 0.52% in all formulations (Figure 2).
However, polymers such as xanthan and guar gum are not the most common choice of mucoadhesive polymer agents in vaginal formulations. The common attribute is their potential in microbiota-activated colon drug delivery [46]. In any case, our results confirm, they can positively influence mucoadhesion. Combining them with proven mucoadhesive polymers, such as Carbopol®, or chitosan, with multiple benefits regarding the vaginal application route and drug bioavailability, could bring positive results. The other strategy enhancing mucoadhesion between a membrane and a dosage form is the application of thiolated polymers, so-called thiomers, which create covalent bonds via disulfide bridges with thiol-rich subdomains of mucin [47]. Cevher et al. state that an increasing thiol conjugates amount enhances the elasticity, cohesiveness, adhesiveness, and mucoadhesion of gels [48].
The drug release from vaginal mucoadhesive tablets is based on its diffusion through swollen polymer network and erosion of the tablet matrix. It can be either surface erosion, bulk erosion, or combination of the two (see in Graphical Abstract). In sustained drug delivery systems, polymers ensuring surface erosion are preferred because they maintain stable, near zero-order drug release [49]. Polymers producing hydrophilic matrices usually occupy 20–80% of the mass depending on the drug concentration and the desired drug release characteristics. As it was mentioned, hydration and swelling are the key processes. Polymer swelling allows a mechanical entanglement by exposing the bioadhesive sites for hydrogen bonding or electrostatic interaction between the polymer and the mucus [50]. The expanded nature of the polymer network contributes significantly to the mucoadhesive force [51]. If the created gel layer is constant, zero-order kinetic is observed. Conversely, if the gel layer tends to increase in thickness, there is a decrease in the drug release rate. Besides that, the drug release mechanism depends on drug solubility and mechanical attrition of the matrix in dissolution medium [52]. Furthermore, the release of API can be modified by a combination of soluble diluents (e.g., lactose, sorbitol, mannitol, xylitol, amylose, and dextrose) and insoluble diluents (e.g., microcrystalline cellulose, calcium phosphate, calcium sulphate) and their ratio in the composition [53].
The daily production of vaginal fluid is approximately 6 mL, while 0.5–0.75 mL is continually present in the vagina. Despite that, most of the studies (59%) state larger volume of dissolution media (from 150 to 900 mL) for in vitro drug release testing. It could be helpful to design and validate a unified technique for a drug dissolution testing from vaginal tablets, because traditional dissolution apparatus does not represent the correct vaginal environment [54,55].
4.5. In Vitro Drug Release Kinetics
Kinetic models are important tools for predicting the release mechanisms and over-time drug concentration in the body [56]. To investigate the release kinetics of MTZ, data obtained from in vitro drug release study were plotted into various kinetic models including zero-order, first-order, Higuchi, Kormeyer–Peppas, and Hixson–Crowell. Zero-order as a cumulative amount of drug released versus time describes concentration-independent drug release from a formulation. The first-order as a logarithm of a cumulative percentage of the drug remaining versus time describes concentration-dependent drug release from a formulation. Higuchi model expresses a linearity between a cumulative percentage of drug released versus square root of time. Kormeyer–Peppas model describes the dependence between the logarithm of percentage drug release and the logarithm of time (only the first 60% of drug release data were plotted to the kinetic model) [35,57]. The Hixson–Crowell model as a cube root of drug percentage remaining in matrix versus time describes drug release from a matrix that degrades via surface erosion [35].
From the data (Table 3), it is obvious that zero-order, Higuchi, Kormeyer–Peppas, or Hixson–Crowell were found to be best fitting models for the examined formulations. As Jackson et al. [58] refer, zero-order kinetic describes an ideal controlled drug release, recorded in the formulations containing sodium alginate and carrageenan (F1, F3). Similarly, as in Jackson’s et al. study [58], the drug release from the investigated vaginal tablets containing acacia gum and xanthan gum (F2 and F5) followed Higuchi model characterizing the drug release from matrix system [59]. The drug release from the tablets with guar gum (F4) followed Kormeyer–Peppas model. A value of n between 0.45 and 0.89 indicates an anomalous transport: Fickian diffusion-mediated transport facilitated by relaxation of polymer chains [24,60]. The drug release from tragacanth-containing tablets (F6) followed Hixson-Crowell kinetic typical for a matrix degrading via surface erosion during which the polymer inside erodes after degradation of the polymer from exterior surface. Usually, the tablet shrinks by dissolution while maintaining its geometric shape [35].
4.6. Characterization of Morphological Changes in Swollen Tablets by SEM
In almost all samples, surface erosion can be observed at the edge, accompanied by breakage of the compact structure from the edge of the tablet towards the center (Figure 5). Only in the case of F2 containing acacia gum, this process is slowed down. The microscopic image only shows the initial formation of a gel layer, which ultimately correlates with the results of the swelling test, where the tablets containing acacia gum swelled to the least extent and the slowest. The edge image of tablet F3 shows the greatest erosion of the material over the indicated experiment time, which again correlates with the swelling test result, demonstrating that carrageenan in the formulation was able to swell the most significantly and subsequently provide almost 100% drug release within 6 h.
4.7. Summary
The scheme (Figure 6) illustrates the results for the examined parameter, i.e., the order of top three formulations in a specific characteristic. As can be noticed, formulation F1 occurs between “most successful” formulations in each characteristic evaluated. Although the positive effect of the highest swelling ability of the tablets on drug release could be disputed, the other characteristics listed are crucial in tablet formulation, especially intended for vaginal administration. Mechanical resistance of tablets is crucial in handling, insertion, as well as post-processing. Strength and friability tests provide the most adequate assessment. Therefore, F1 and F3 can be considered the most resistant in this respect. The natural polymers xanthan gum (in F5) and guar gum (in F4) gave even higher mucoadhesive abilities than sodium alginate, although the differences are minimal. For F1 and F5 formulations, there is a correlation between swelling ability and mucoadhesive ability. The tablets more capable of swelling (F1 and F5), also showed the highest mucoadhesive properties. On the other hand, the tablets with the highest swelling ability also showed the highest drug release during the first 6 h. Considering the results of all the aforementioned tests, sodium alginate can be considered as the most effective natural polymer in the composition of vaginal tablets. The combination of polymers providing higher mucoadhesiveness and at the same time prolonged release, i.e., xanthan or guar with sodium alginate, could also be of interest.
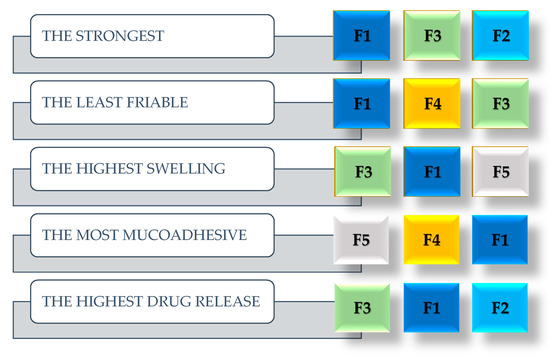
Figure 6.
Summary of tablet quality and physical properties test results. The most successful trio of formulations. (F1: sodium alginate, F2: acacia gum, F3: carrageenan, F4: guar gum, F5: xanthan gum, F6: tragacanth).
5. Conclusions
In addition to evaluating the basic quality parameters of tablets, this study focused mainly on the influence of selected natural polymers (40%; w/w) on the mucoadhesive properties of tablets, swelling capacity, and the resulting consequences on the MTZ release mechanism. The swelling capacity test and the mucoadhesive force test confirmed that the presence of xanthan gum or guar gum gives the tablet the best mucoadhesive properties. A higher percentage of them could lead to an accentuation of the effect, as well as their combination. However, our intention was not the investigation of colligative effect nor the polymer concentration impact but to find the polymer exhibiting the highest mucoadhesive properties at a given concentration and the consequences for the drug release. Due to the findings, it is possible to select the most optimal polymer, which works effectively even at low concentrations, thus saving the producing cost of the final mucoadhesive vaginal product. Taking into consideration all the test results, sodium alginate seems to be the most suitable in the formulation of vaginal tablets, providing the best quality parameters, drug release, and adequate mucoadhesion. The second most optimal polymer in the composition given the test results is carrageenan. Finally, guar gum has the ability to increase the mucoadhesion of tablets despite not being among the common excipients in vaginal application.
Author Contributions
Conceptualization, K.B.; methodology, K.B.; investigation, K.B.; resources, K.B., M.Š. and M.P.; data curation, K.B., M.Š., M.P., M.H. and O.M.; writing—original draft preparation, M.Š.; writing—review and editing, M.Š., M.P., M.H. and O.M.; visualization, M.Š., M.P., M.H. and O.M.; supervision, M.Š.; project administration, K.B. and O.M.; funding acquisition, K.B. and O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Faculty of Pharmacy, Comenius University Bratislava under Grant FaF UK/27/2016 and by grant of The Ministry of Education, Science, Research and Sport of the Slovak Republic KEGA 021 STU-4/2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank Faculty of Pharmacy and the Ministry of Education, Science, Research and Sport of the Slovak Republic for the financial support.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- Utomo, E.; Domínguez-Robles, J.; Anjani, Q.K.; Picco, C.J.; Korelidou, A.; Magee, E.; Donnelly, R.F.; Larrañeta, E. Development of 3D-Printed Vaginal Devices Containing Metronidazole for Alternative Bacterial Vaginosis Treatment. Int. J. Pharm. X 2023, 5, 100142. [Google Scholar] [CrossRef] [PubMed]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An Update on Metabolism, Structure-Cytotoxicity and Resistance Mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Ceruelos, A.H.; Romero-Quezada, L.C.; Ledezma, J.C.R.; Contreras, L.L. Therapeutic Uses of Metronidazole and Its Side Effects: An Update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar] [CrossRef]
- PubChem Metronidazole. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4173 (accessed on 5 February 2023).
- Leitsch, D. A Review on Metronidazole: An Old Warhorse in Antimicrobial Chemotherapy. Parasitology 2019, 146, 1167–1178. [Google Scholar] [CrossRef]
- Weir, C.B.; Le, J.K. Metronidazole; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Żyro, D.; Radko, L.; Śliwińska, A.; Chęcińska, L.; Kusz, J.; Korona-Głowniak, I.; Przekora, A.; Wójcik, M.; Posyniak, A.; Ochocki, J. Multifunctional Silver(I) Complexes with Metronidazole Drug Reveal Antimicrobial Properties and Antitumor Activity against Human Hepatoma and Colorectal Adenocarcinoma Cells. Cancers 2022, 14, 900. [Google Scholar] [CrossRef]
- Metronidazole. Available online: https://go.drugbank.com/drugs/db00916 (accessed on 17 February 2023).
- de Araújo Pereira, R.R.; Bruschi, M.L. Vaginal Mucoadhesive Drug Delivery Systems. Drug Dev. Ind. Pharm. 2012, 38, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Alzainy, A.; Boateng, J. Novel Mucoadhesive Wafers for Treating Local Vaginal Infections. Biomedicines 2022, 10, 3036. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.M.; Rohan, L.C. The Importance of the Vaginal Delivery Route for Antiretrovirals in HIV Prevention. Ther. Deliv. 2011, 2, 1535–1550. [Google Scholar] [CrossRef]
- da Silva, P.B.; dos Santos Ramos, M.A.; Bonifácio, B.V.; Negri, K.M.S.; Sato, M.R.; Bauab, T.M.; Chorilli, M. Nanotechnological Strategies for Vaginal Administration of Drugs—A Review. J. Biomed. Nanotechnol. 2014, 10, 2218–2243. [Google Scholar] [CrossRef]
- Dedeloudi, A.; Siamidi, A.; Pavlou, P.; Vlachou, M. Recent Advances in the Excipients Used in Modified Release Vaginal Formulations. Materials 2022, 15, 327. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Deshkar, S.S.; Mahore, J.G. Chapter 5—Herbal Bioactive–Based Vaginal and Rectal Drug Delivery Systems. In Herbal Bioactive-Based Drug Delivery Systems; Bakshi, I.S., Bala, R., Madaan, R., Sindhu, R.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 111–168. ISBN 978-0-12-824385-5. [Google Scholar]
- Shaikh, R.; Raj Singh, T.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive Drug Delivery Systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Jadach, B.; Tatarek, A.; Gadziński, P.; Falana, A.; Gralińska, K.; Ekert, M.; Puri, V.; Wrotyńska-Barczyńska, J.; et al. Recent Advances in Polymer-Based Vaginal Drug Delivery Systems. Pharmaceutics 2021, 13, 884. [Google Scholar] [CrossRef]
- Žigrayová, D.; Mikušová, V.; Mikuš, P. Advances in Antiviral Delivery Systems and Chitosan-Based Polymeric and Nanoparticulate Antivirals and Antiviral Carriers. Viruses 2023, 15, 647. [Google Scholar] [CrossRef]
- Reddy, P.C.; Chaitanya, K.S.C.; Rao, Y.M. A Review on Bioadhesive Buccal Drug Delivery Systems: Current Status of Formulation and Evaluation Methods. DARU J. Pharm. Sci. 2011, 19, 385. [Google Scholar]
- das Neves, J.; Amaral, M.H.; Bahia, M.F. Vaginal Drug Delivery. In Pharmaceutical Manufacturing Handbook; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 809–878. ISBN 978-0-470-25981-8. [Google Scholar]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Vaginal Semisolid Products: Technological Performance Considering Physiologic Parameters. Eur. J. Pharm. Sci. 2017, 109, 556–568. [Google Scholar] [CrossRef]
- Abidin, I.Z.; Rezoagli, E.; Simonassi-Paiva, B.; Fehrenbach, G.W.; Masterson, K.; Pogue, R.; Cao, Z.; Rowan, N.; Murphy, E.J.; Major, I. A Bilayer Vaginal Tablet for the Localized Delivery of Disulfiram and 5-Fluorouracil to the Cervix. Pharmaceutics 2020, 12, 1185. [Google Scholar] [CrossRef]
- Hani, U.; Shivakumar, H.G.; Osmani, R.A.M.; Srivastava, A.; Kumar Varma, N.S. Development of a Curcumin Bioadhesive Monolithic Tablet for Treatment of Vaginal Candidiasis. Iran. J. Pharm. Res. IJPR 2016, 15, 23–34. [Google Scholar] [PubMed]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Rubio, J.; Tamayo, A.; Veiga, M.-D. Carrageenan-Based Acyclovir Mucoadhesive Vaginal Tablets for Prevention of Genital Herpes. Mar. Drugs 2020, 18, 249. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Saladini, B.; Gallucci, M.C.; Cruciani, F.; Vitali, B.; Luppi, B. Chitosan/Alginate Complexes for Vaginal Delivery of Chlorhexidine Digluconate. Carbohydr. Polym. 2013, 91, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Maestrelli, F.; Scuota, S.; Bazzucchi, V.; Mura, P. Development and Microbiological Evaluation of Chitosan and Chitosan-Alginate Microspheres for Vaginal Administration of Metronidazole. Int. J. Pharm. 2021, 598, 120375. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.T.; Ruiz, M.A.; Castán, H.; Morales, M.E. A Novel Double-Layer Mucoadhesive Tablet Containing Probiotic Strain for Vaginal Administration: Design, Development and Technological Evaluation. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2018, 112, 63–70. [Google Scholar] [CrossRef]
- Rao, D.M.; Zope, A. Formulation and Evaluation of Mucoadhesive Clotrimazole Vaginal Tablet Using Liquisolid Technology. J. Drug Deliv. Ther. 2019, 9, 477–485. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 11th ed.; EDQM: Strasbourg, France, 2023. [Google Scholar]
- Perioli, L.; Ambrogi, V.; Pagano, C.; Scuota, S.; Rossi, C. FG90 Chitosan as a New Polymer for Metronidazole Mucoadhesive Tablets for Vaginal Administration. Int. J. Pharm. 2009, 377, 120–127. [Google Scholar] [CrossRef]
- Li, K.L.; Castillo, A.L. Formulation and Evaluation of a Mucoadhesive Buccal Tablet of Mefenamic Acid. Braz. J. Pharm. Sci. 2020, 56, e18575. [Google Scholar] [CrossRef]
- Abd-Alrassol, K.S.; Al-Salman, H.N.K.; Jasim, E.; Hussein, H.H. Determination and Evaluation of Doses of Metronidazole in Different Quantities and Formulations with Multiple Spectroscopic Methods. Syst. Rev. Pharm. 2020, 11, 130–139. [Google Scholar] [CrossRef]
- Ibrahim, W.H.; Bashir, W.A. Spectrophotometric Determination of Metronidazole by Prior Reduction and Subsequent Diazotisation and Coupling with N-(1-Naphthyl)Ethylenediamine—Application to Pharmaceutical Preparations. Rafidain J. Sci. 2012, 23, 78–93. [Google Scholar] [CrossRef]
- Saffaj, T.; Charrouf, M.; Abourriche, A.; Aboud, Y.; Bennamara, A.; Berrada, M. Spectrophotometric Determination of Metronidazole and Secnidazole in Pharmaceutical Preparations Based on the Formation of Dyes. Dyes Pigment. 2006, 70, 259–262. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Fichorova, R.N.; Mendonca, K.; Yamamoto, H.S.; Murray, R.; Chandra, N.; Doncel, G.F. A Quantitative Multiplex Nuclease Protection Assay Reveals Immunotoxicity Gene Expression Profiles in the Rabbit Model for Vaginal Drug Safety Evaluation. Toxicol. Appl. Pharmacol. 2015, 285, 198–206. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.M.; Calderon, G.A.; Robinson, A.J.; Sullivan, C.N.; Cosgriff-Hernandez, E.; Hakim, J.C.E. Animal Models and Alternatives in Vaginal Research: A Comparative Review. Reprod. Sci. 2021, 28, 1759–1773. [Google Scholar] [CrossRef]
- Karmakar, K. Application of Natural Gum as a Binder in Modern Drug Delivery. J. Anal. Pharm. Res. 2016, 3, 09752366. [Google Scholar] [CrossRef]
- Bisht, T.; Poonam, R. A Comparative Study of Matrix Tablets Designed by Different Methods. Int. J. Pharm. Sci. Nanotechnol. IJPSN 2017, 10, 3645–3652. [Google Scholar] [CrossRef]
- Chavda, H.; Patel, M.S.; Patel, C.N. Preparation and in Vitro Evaluation of Guar Gum Based Triple-Layer Matrix Tablet of Diclofenac Sodium. Res. Pharm. Sci. 2012, 7, 57–64. [Google Scholar] [PubMed]
- Bruschi, M.L.; Borghi-Pangoni, F.B.; Junqueira, M.V.; de Souza Ferreira, S.B. Chapter 12—Nanostructured Therapeutic Systems with Bioadhesive and Thermoresponsive Properties. In Nanostructures for Novel Therapy; Ficai, D., Grumezescu, A.M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 313–342. ISBN 978-0-323-46142-9. [Google Scholar]
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A Food Perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Daneshmehr, M.; Adibpour, H.; Ataie, Z. Formulation and Evaluation of Hydrocortisone Sodium Succinate Mucoadhesive Buccal Tablet. Int. J. Pharm. Investig. 2020, 10, 300–304. [Google Scholar] [CrossRef]
- Munot, N.; Kandekar, U.; Rikame, C.; Patil, A.; Sengupta, P.; Urooj, S.; Bilal, A. Improved Mucoadhesion, Permeation and In Vitro Anticancer Potential of Synthesized Thiolated Acacia and Karaya Gum Combination: A Systematic Study. Molecules 2022, 27, 6829. [Google Scholar] [CrossRef] [PubMed]
- Martín-Illana, A.; Chinarro, E.; Cazorla-Luna, R.; Notario-Perez, F.; Veiga-Ochoa, M.D.; Rubio, J.; Tamayo, A. Optimized Hydration Dynamics in Mucoadhesive Xanthan-Based Trilayer Vaginal Films for the Controlled Release of Tenofovir. Carbohydr. Polym. 2022, 278, 118958. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.; Shivapooja, A.; Muthyala, J.; Pandya, P. Effect of Guar Gum and Xanthan Gum Compression Coating on Release Studies of Metronidazole in Human Fecal Media for Colon Targeted Drug Delivery Systems. Asian J. Pharm. Clin. Res. 2013, 6, 315–318. [Google Scholar]
- Baus, R.A.; Innerhofer, J.; Rohrer, J.; Lupo, N.; Bernkop-Schnürch, A. Anhydrous Thiomers: Strategy for Enhanced Mucoadhesion. Eur. J. Pharm. Biopharm. 2018, 129, 273–281. [Google Scholar] [CrossRef]
- Cevher, E.; Sensoy, D.; Taha, M.A.M.; Araman, A. Effect of Thiolated Polymers to Textural and Mucoadhesive Properties of Vaginal Gel Formulations Prepared with Polycarbophil and Chitosan. AAPS PharmSciTech 2008, 9, 953–965. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive Drug Delivery System: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef]
- Yermak, I.M.; Davydova, V.N.; Volod’ko, A.V. Mucoadhesive Marine Polysaccharides. Mar. Drugs 2022, 20, 522. [Google Scholar] [CrossRef]
- Aulton, M.E.; Taylor, K. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; ISBN 978-0-7020-4290-4. [Google Scholar]
- Acarturk, F. Mucoadhesive Vaginal Drug Delivery Systems. Recent Pat. Drug Deliv. Amp Formul. 2009, 3, 193. [Google Scholar] [CrossRef] [PubMed]
- Abidin, I.Z.; Murphy, E.J.; Fehrenbach, G.W.; Rezoagli, E.; Gately, N.; Major, I. A Systematic Review of Mucoadhesive Vaginal Tablet Testing. Drug Target Insights 2023, 17, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.F.; Yuan, A.; Gao, Y. Vaginal Drug Distribution Modeling. Adv. Drug Deliv. Rev. 2015, 92, 2–13. [Google Scholar] [CrossRef]
- Pourtalebi Jahromi, L.; Ghazali, M.; Ashrafi, H.; Azadi, A. A Comparison of Models for the Analysis of the Kinetics of Drug Release from PLGA-Based Nanoparticles. Heliyon 2020, 6, e03451. [Google Scholar] [CrossRef]
- Arora, G.; Malik, K.; Singh, I.; Arora, S.; Rana, V. Formulation and Evaluation of Controlled Release Matrix Mucoadhesive Tablets of Domperidone Using Salvia Plebeian Gum. J. Adv. Pharm. Technol. Res. 2011, 2, 163–169. [Google Scholar] [CrossRef]
- Jackson, T.C.; Agboke, A.A.; Udofa, E.J.; Ucheokoro, A.S.; Udo, B.E.; Ifekpolugo, N.L. Characterization and Release Kinetics of Metronidazole Loaded Silver Nanoparticles Prepared from Carica Papaya Leaf Extract. Adv. Nanopart. 2019, 08, 47–54. [Google Scholar] [CrossRef]
- Paul, D.R. Elaborations on the Higuchi Model for Drug Delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Rosu, M.-C.; Bratu, I. Promising Psyllium-Based Composite Containing TiO2 Nanoparticles as Aspirin-Carrier Matrix. Prog. Nat. Sci. Mater. Int. 2014, 24, 205–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).