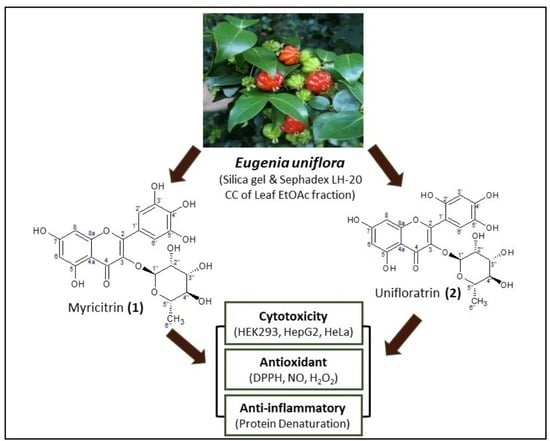
Flavonol Glycosides from Eugenia uniflora Leaves and Their In Vitro Cytotoxicity, Antioxidant and Anti-Inflammatory Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Fractionation
2.4. Isolation of Compounds
2.5. Nuclear Magnetic Resonance (NMR) Analysis
2.6. High-Resolution Mass Spectrometric (HRESI-MS) Analysis
2.7. Cytotoxicity Study
2.7.1. Cell Culture
2.7.2. MTT Assay
2.8. Antioxidant Study
2.8.1. DPPH Radical Scavenging Assay
2.8.2. Nitric Oxide (NO) Radical Inhibition Assay
2.8.3. Hydrogen Peroxide (H2O2) Inhibition Assay
2.9. In Vitro Anti-Inflammatory Study
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Isolated Compounds
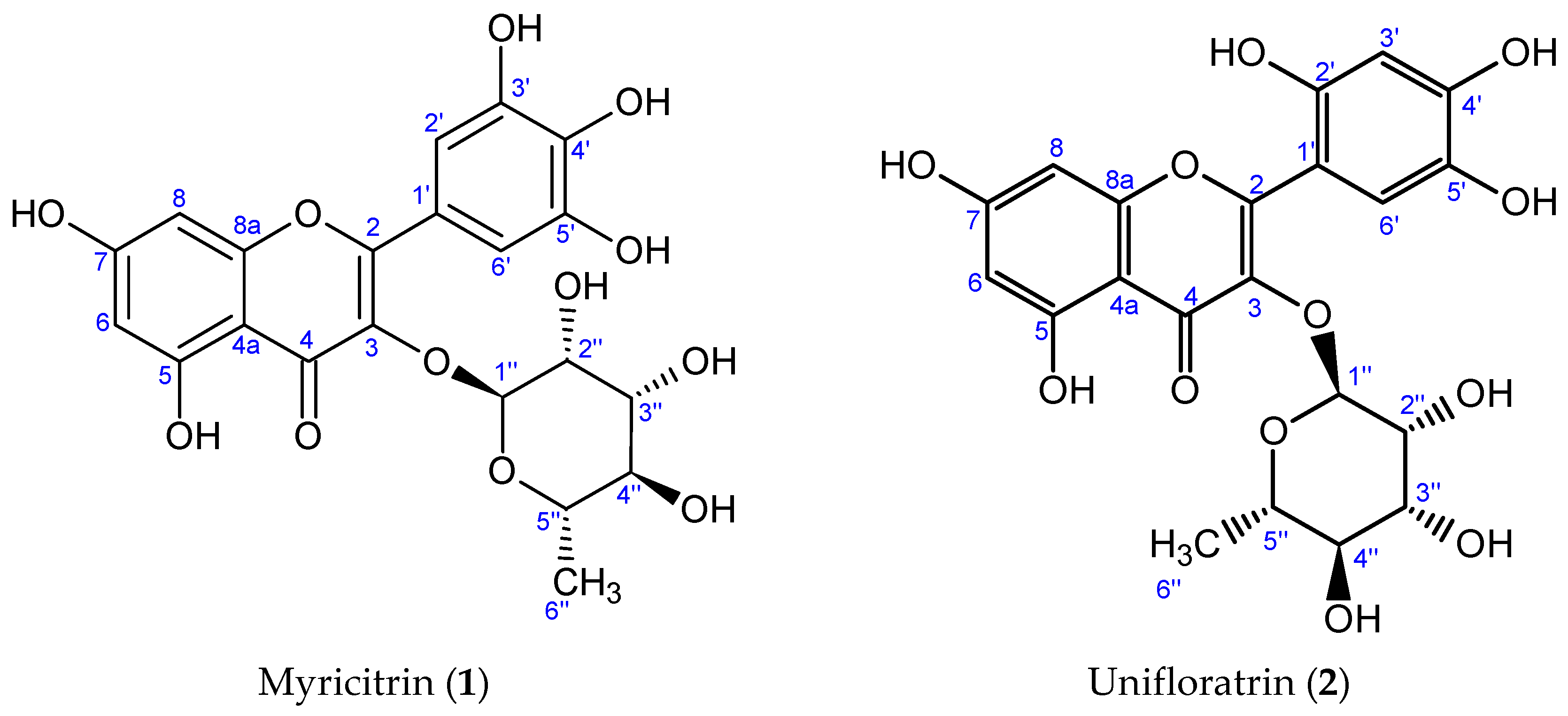
3.1.1. Myricitrin (1), C21H20O12
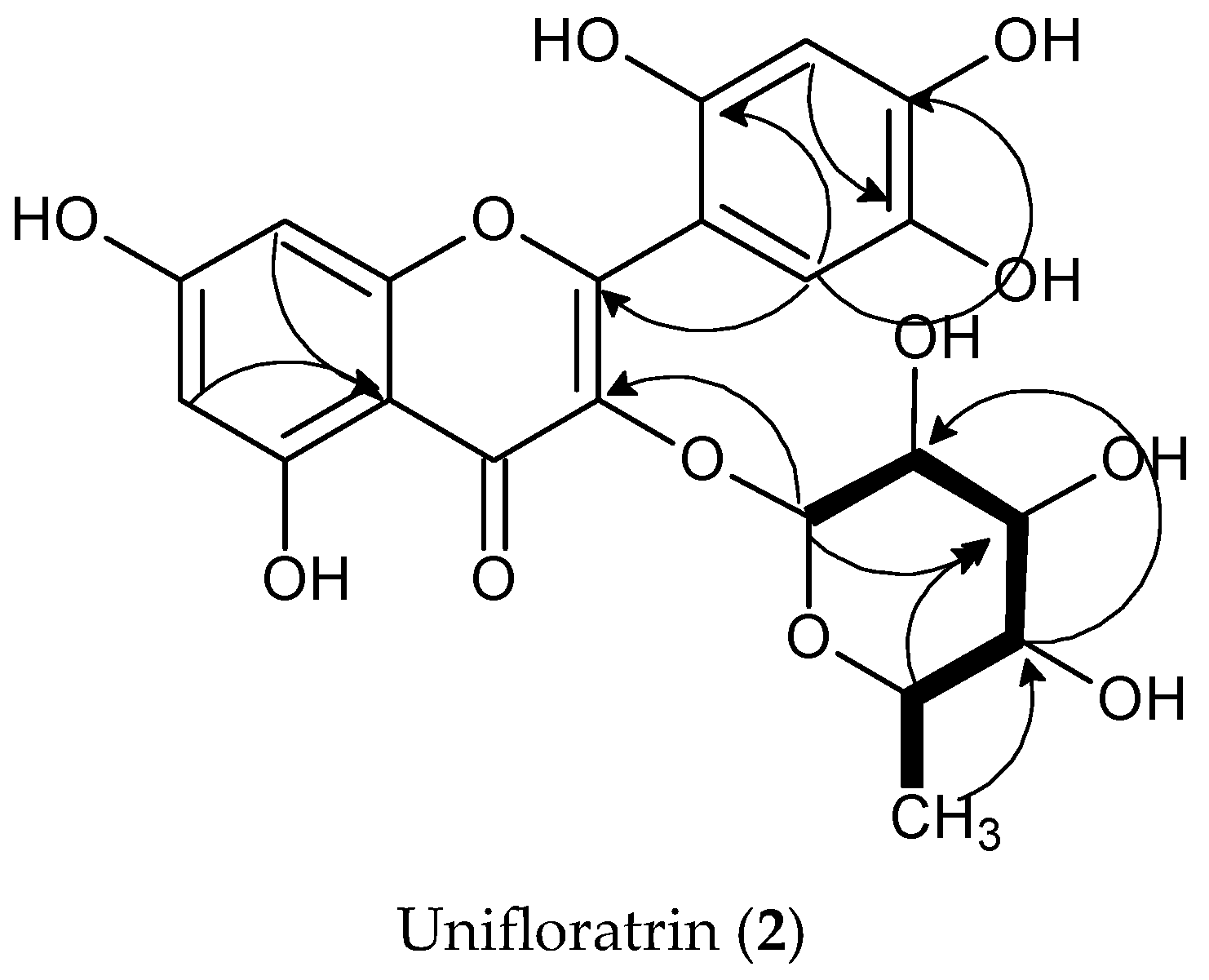
3.1.2. Unifloratrin (2), C21H20O12
3.2. Structure Elucidation of Isolated Compounds
| Position | Unifloratrin (2) | Myricitrin (1) | ||||
|---|---|---|---|---|---|---|
| DEPT135 | δH | HMBC | δc | Acquired δc | Literature δc [31] | |
| 2 | C | 157.6 | 157.5 | 157.0 | ||
| 3 | C | 134.3 | 134.3 | 134.7 | ||
| 4 | C=O | 177.8 | 177.8 | 178.1 | ||
| 4a | C | 104.1 | 104.1 | 104.2 | ||
| 5 | C | 161.4 | 161.4 | 161.7 | ||
| 6 | CH | 6.21, 1H, d, J = 2.2 Hz | C-4a, C-8 | 98.5 | 98.7 | 99.1 |
| 7 | C | 164.2 | 164.2 | 165.6 | ||
| 8 | CH | 6.37, 1H, d, J = 2.6 Hz | C-4a, C-6 | 93.3 | 93.6 | 94.6 |
| 8a | C | 156.5 | 156.5 | 157.8 | ||
| - | 12.68, s | |||||
| 1′ | C | 120.5 | 119.6 | 120.1 | ||
| 2′ | C | 136.5 | 107.9 | 108.4 | ||
| 3′ | CH | 6.92, 1H, d, J = 1.9 Hz | C-5′ | 108.8 | 145.8 | 146.3 |
| 4′ | C | 145.8 | 136.5 | 137.0 | ||
| 5′ | C | 167.5 | 145.8 | 146.3 | ||
| 6′ | CH | 6.88, 1H, d, J = 2.0 Hz | C-2, C-2′, C-4′ | 107.9 | 107.9 | 108.4 |
| L-Rhamnose | ||||||
| 1″ | CH | 5.20, 1H, s | C-3″ | 102.0 | 101.9 | 102.4 |
| 2″ | CH | 3.98, 1H, d, J = 3.3 Hz | 70.1 | 70.0 | 71.8 | |
| 3″ | CH | 3.56, 1H, dd, J = 2.5, 4.4 Hz | 70.4 | 70.4 | 70.9 | |
| 4″ | CH | 3.15, 1H, t, J = 9.3 Hz | C-2″, C-6″ | 71.3 | 71.3 | 71.0 |
| 5″ | CH | 3.36, 1H, dq, J = 5.2, 6.9 Hz | 70.6 | 70.6 | 70.4 | |
| 6″ | CH3 | 0.85, 3H, d, J = 6.3 Hz | C-4″ | 17.6 | 17.5 | 18.0 |
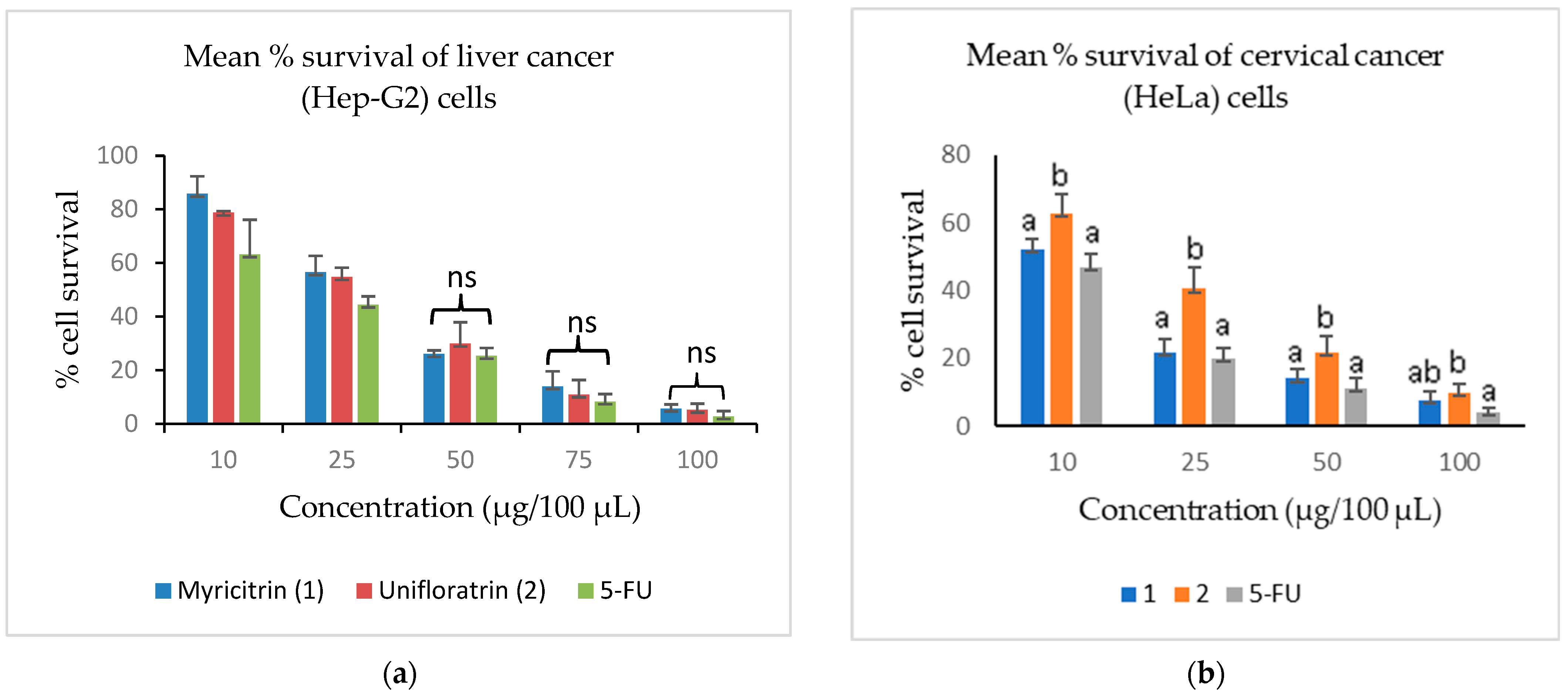
3.3. Evaluation of Cytotoxicity
3.4. Evaluation of Antioxidant Activity
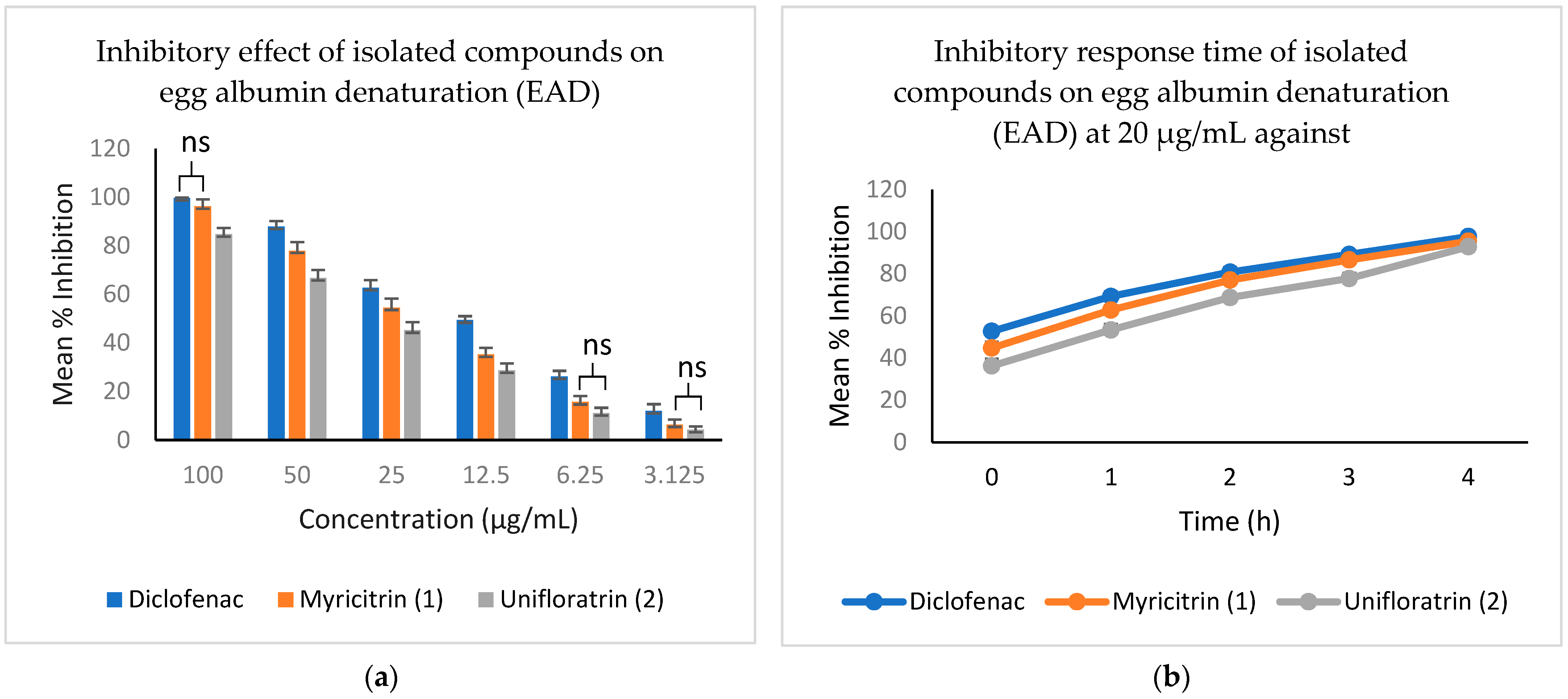
3.5. Evaluation of In Vitro Anti-Inflammatory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engela, M.R.G.d.S.; Furlan, C.M.; Esposito, M.P.; Fernandes, F.F.; Carrari, E.; Domingos, M.; Paoletti, E.; Hoshika, Y. Metabolic and Physiological Alterations Indicate That the Tropical Broadleaf Tree Eugenia uniflora L. Is Sensitive to Ozone. Sci. Total Environ. 2021, 769, 145080. [Google Scholar] [CrossRef] [PubMed]
- McVaugh. The World Flora Online (WFO): Eugenia Uniflora L. Micheli. Nov. Pl. Gen. T 1956, 108, 1729. [Google Scholar]
- Fidelis, E.M.; Savall, A.S.P.; de Oliveira Pereira, F.; Quines, C.B.; Ávila, D.S.; Pinton, S. Pitanga (Eugenia uniflora L.) as a Source of Bioactive Compounds for Health Benefits: A Review. Arab. J. Chem. 2022, 15, 103691. [Google Scholar] [CrossRef]
- Rattmann, Y.D.; De Souza, L.M.; Malquevicz-Paiva, S.M.; Dartora, N.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Analysis of Flavonoids from Eugenia uniflora Leaves and Its Protective Effect against Murine Sepsis. Evid.-Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.G.; Pinheiro, J.D.S.; Silveira, G.F.; Beckenkamp, A.; Buffon, A.; Bruno, A.N. Antineoplastic Potential of the Aqueous Crude Extract of Eugenia uniflora L. In Human Cervical Cancer. Braz. J. Pharm. Sci. 2018, 54, c17267. [Google Scholar] [CrossRef]
- Schapoval, E.E.S.; Silveira, S.M.; Miranda, M.L.; Alice, C.B.; Henriques, A.T. Evaluation of Some Pharmacological Activities of Eugenia uniflora L. J. Ethnopharmacol. 1994, 44, 137–142. [Google Scholar] [CrossRef]
- Lima, E.O.; Gompertz, O.F.; Giesbrecht, A.M.; Paulo, M.Q. In Vitro Antifungal Activity of Essential Oils Obtained from Officinal Plants against Dermatophytes. Mycoses 2009, 36, 333–336. [Google Scholar] [CrossRef]
- Adebajo, A.C.; Oloke, K.J.; Aladesanmi, A.J. Antimicrobial Activities and Microbial Transformation of Volatile Oils of Eugenia uniflora. Fitoterapia 1989, 60, 451–455. [Google Scholar]
- Consolini, A.E.; Sarubbio, M.G. Pharmacological Effects of Eugenia uniflora (Myrtaceae) Aqueous Crude Extract on Rat’s Heart. J. Ethnopharmacol. 2002, 81, 57–63. [Google Scholar] [CrossRef]
- Aladesanmi, A.J.; Famuyiwa, F.G.; Oriola, A.O.; Oguntimehin, S.A.; Aiyedun, P.O.; Arthur, G. Cytotoxic Activity of Selected Nigerian Medicinal Plants. J. Herbs Spices Med. Plants 2020, 26, 203–217. [Google Scholar] [CrossRef]
- Victoria, F.N.; Lenardão, E.J.; Savegnago, L.; Perin, G.; Jacob, R.G.; Alves, D.; Silva, W.P.; da Motta, A.d.S.; da Nascente, P.d.S. Essential Oil of the Leaves of Eugenia uniflora L.: Antioxidant and Antimicrobial Properties. Food Chem. Toxicol. 2012, 50, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, R.-Q.; Guo, Y.; Chen, J.-X.; Jin, Q.; Chen, M.-H.; Chen, B.-Y.; Tu, Z.-C.; Ye, W.-C.; Wang, L. Eugenilones A−N: Sesquiterpenoids from the Fruits of Eugenia uniflora. Phytochemistry 2023, 211, 113699. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Lopes, R.B.; Cabral, F.A.; Eberlin, M.N. Volatile Compounds from Pitanga Fruit (Eugenia uniflora L.). Food Chem. 2006, 99, 1–5. [Google Scholar] [CrossRef]
- da Cunha, F.A.B.; Waczuk, E.P.; Duarte, A.E.; Barros, L.M.; Elekofehinti, O.O.; Matias, E.F.F.; da Costa, J.G.M.; Sanmi, A.A.; Boligon, A.A.; da Rocha, J.B.T.; et al. Cytotoxic and Antioxidative Potentials of Ethanolic Extract of Eugenia uniflora L. (Myrtaceae) Leaves on Human Blood Cells. Biomed. Pharmacother. 2016, 84, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Kasai, M.; Hayashi, T.; Arisawa, M.; Momose, Y.; Arai, I.; Amagaya, S.; Komatsu, Y. α-Glucosidase Inhibitors from Paraguayan Natural Medicine, Nangapiry, the Leaves of Eugenia uniflora. Pharm. Biol. 2000, 38, 302–307. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Destandau, E.; Fougère, L.; Lafosse, M. Isolation by Pressurised Fluid Extraction (PFE) and Identification Using CPC and HPLC/ESI/MS of Phenolic Compounds from Brazilian Cherry Seeds (Eugenia uniflora L.). Food Chem. 2014, 145, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Migues, I.; Baenas, N.; Gironés-Vilaplana, A.; Cesio, M.V.; Heinzen, H.; Moreno, D.A. Phenolic Profiling and Antioxidant Capacity of Eugenia uniflora L. (Pitanga) Samples Collected in Different Uruguayan Locations. Foods 2018, 7, 67. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Wegierek, D. Flavonoids-Food Sources and Health Benefits. Rocz. Państwowego Zakładu Hig. 2014, 65. [Google Scholar]
- Patil, V.M.; Masand, N. Anticancer Potential of Flavonoids: Chemistry, Biological Activities, and Future Perspectives. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; p. 59. [Google Scholar]
- Dixon, R.A.; Dey, P.M.; Lamb, C.J. Phytoalexins: Enzymology and Molecular Biology. In Advances in Enzymology Related Areas of Molecular Biology; Wiley-Interscience: Hoboken, NJ, USA, 1983; Volume 55. [Google Scholar]
- Sobeh, M.; Hamza, M.S.; Ashour, M.L.; Elkhatieb, M.; El Raey, M.A.; Abdel-Naim, A.B.; Wink, M. A Polyphenol-Rich Fraction from Eugenia uniflora Exhibits Antioxidant and Hepatoprotective Activities In Vivo. Pharmaceuticals 2020, 13, 84. [Google Scholar] [CrossRef]
- Jagaran, K.; Singh, M. Copolymer-Green-Synthesized Copper Oxide Nanoparticles Enhance Folate-Targeting in Cervical Cancer Cells In Vitro. Polymers 2023, 15, 2393. [Google Scholar] [CrossRef]
- Oriola, A.O.; Aladesanmi, A.J.; Idowu, T.O.; Akinwumi, F.O.; Obuotor, E.M.; Idowu, T.; Oyedeji, A.O. Ursane-Type Triterpenes, Phenolics and Phenolic Derivatives from Globimetula braunii Leaf. Molecules 2021, 26, 6528. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Antioxidant and Phytochemical Activities of Amaranthus caudatus L. Harvested from Different Soils at Various Growth Stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef] [PubMed]
- Okeleye, B.I.; Nongogo, V.; Mkwetshana, N.T.; Ndip, R.N. Polyphenolic Content and In Vitro Antioxidant Evaluation of the Stem Bark Extract of Peltophorum africanum Sond (Fabaceae). Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 1–8. [Google Scholar] [CrossRef]
- Miya, G.M.; Oriola, A.O.; Payne, B.; Cuyler, M.; Lall, N.; Oyedeji, A.O. Steroids and Fatty Acid Esters from Cyperus sexangularis Leaf and Their Antioxidant, Anti-Inflammatory and Anti-Elastase Properties. Molecules 2023, 28, 3434. [Google Scholar] [CrossRef] [PubMed]
- Motlhatlego, K.E.; Abdalla, M.A.; Leonard, C.M.; Eloff, J.N.; McGaw, L.J. Inhibitory Effect of Newtonia Extracts and Myricetin-3-O-Rhamnoside (Myricitrin) on Bacterial Biofilm Formation. BMC Complement. Med. Ther. 2020, 20, 358. [Google Scholar] [CrossRef]
- Kalabin, G.; Vasil’ev, V.; Ivlev, V.; Babkin, V. Fast Screening of Some Flavonoids Content in Raw Plant Materials: Opportunities of 1HNMR Spectroscopy. E3S Web Conf. 2020, 169, 02006. [Google Scholar] [CrossRef]
- de Souza Farias, S.A.; da Costa, K.S.; Martins, J.B.L. Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef]
- Hvattum, E.; Ekeberg, D. Study of the Collision-Induced Radical Cleavage of Flavonoid Glycosides Using Negative Electrospray Ionization Tandem Quadrupole Mass Spectrometry. J. Mass Spectrom. 2003, 38, 43–49. [Google Scholar] [CrossRef]
- Kassem, M.E.S.; Ibrahim, L.F.; Hussein, S.R.; El-Sharawy, R.; El-Ansari, M.A.; Hassanane, M.M.; Booles, H.F. Myricitrin and Bioactive Extract of Albizia amara Leaves: DNA Protection and Modulation of Fertility and Antioxidant-Related Genes Expression. Pharm. Biol. 2016, 54, 2404–2409. [Google Scholar] [CrossRef]
- Banerjee, C.; Nandy, S.; Chakraborty, J.; Kumar, D. Myricitrin-a Flavonoid Isolated from the Indian Olive Tree (Elaeocarpus floribundus)-Inhibits Monoamine Oxidase in the Brain and Elevates Striatal Dopamine Levels: Therapeutic Implications against Parkinson’s Disease. Food Funct. 2022, 13, 6545–6559. [Google Scholar] [CrossRef]
- Fossen, T.; Smund Larsen, A.Ê.; Kiremire, B.T.; Andersen, M. Flavonoids from Blue Flowers of NymphaeÁa caerulea. Phytochemistry 1999, 51, 1133–1137. [Google Scholar] [CrossRef]
- Hidayati, D.N.; Meyta Parusiza, I.; Fauzizah, N. Cytotoxic Activity of Eugenia polyantha Wight Leaves Extract, Purified Extract and Ethyl Acetate Fraction in T47D and Determination of Flavonoid Levels. Indones. J. Chem. Sci. 2022, 11, 16–25. [Google Scholar] [CrossRef]
- Chen, W.; Feng, L.; Shen, Y.; Su, H.; Li, Y.; Zhuang, J.; Zhang, L.; Zheng, X. Myricitrin Inhibits Acrylamide-Mediated Cytotoxicity in Human Caco-2 Cells by Preventing Oxidative Stress. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kumar Sarkar, M.; Kar, A.; Jayaraman, A.; Shanmugam, K.; Vellingiri, V.; Mahapatra, S.K. Apoptotic Mechanisms of Myricitrin Isolated from Madhuca longifolia Leaves in HL-60 Leukemia Cells. Mol. Biol. Rep. 2021, 48, 5327–5334. [Google Scholar] [CrossRef]
- Pradhan, A.B.; Bhuiya, S.; Haque, L.; Das, S. Role of Hydroxyl Groups in the B-Ring of Flavonoids in Stabilization of the Hoogsteen Paired Third Strand of Poly(U).Poly(A)*Poly(U) Triplex. Arch. Biochem. Biophys. 2018, 637, 9–20. [Google Scholar] [CrossRef]
- Sarian, M.N.; Ahmed, Q.U.; So’ad, Z.M.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Syed Mohamad, S.N.A.; Khatib, A.; Latip, J. Antioxidant and Antidiabetic Effects of Flavonoids: A Structure-Activity Relationship Based Study. Biomed Res. Int. 2017, 2017, 8386065. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods That Define Mechanism of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Domitrović, R.; Rashed, K.; Cvijanović, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin Exhibits Antioxidant, Anti-Inflammatory and Antifibrotic Activity in Carbon Tetrachloride-Intoxicated Mice. Chem.-Biol. Interact. 2015, 230, 21–29. [Google Scholar] [CrossRef]
- Wu, J.H.; Huang, C.Y.; Tung, Y.T.; Chang, S.T. Online RP-HPLC-DPPH Screening Method for Detection of Radical-Scavenging Phytochemicals from Flowers of Acacia confusa. J. Agric. Food Chem. 2008, 56, 328–332. [Google Scholar] [CrossRef]
- Edenharder, R.; Grünhage, D. Free Radical Scavenging Abilities of Flavonoids as Mechanism of Protection against Mutagenicity Induced by Tert-Butyl Hydroperoxide or Cumene Hydroperoxide in Salmonella typhimurium TA102. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 540, 1–18. [Google Scholar] [CrossRef]
- Meotti, F.C.; Luiz, A.P.; Pizzolatti, M.G.; Kassuya, C.A.L.; Calixto, J.B.; Santos, A.R.S. Analysis of the Antinociceptive Effect of the Flavonoid Myricitrin: Evidence for a Role of the L-Arginine-Nitric Oxide and Protein Kinase C Pathways. J. Pharmacol. Exp. Ther. 2006, 316, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Yang, L.-L.; Lee, T.J.-F. Oroxylin A Inhibition of Lipopolysaccharide-Induced INOS and COX-2 Gene Expression via Suppression of Nuclear Factor-B Activation. Biochem. Pharmacol. 2000, 59, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Liu, M.; Cheng, X.; Li, W.H.; Zhang, S.S.; Wang, Y.H.; Du, G.H. Myricitrin Blocks Activation of NF-ΚB and MAPK Signaling Pathways to Protect Nigrostriatum Neuron in LPS-Stimulated Mice. J. Neuroimmunol. 2019, 337, 577049. [Google Scholar] [CrossRef]
- Yan, L.J.; Yang, H.T.; Duan, H.Y.; Wu, J.T.; Qian, P.; Fan, X.W.; Wang, S. Myricitrin Inhibits Vascular Adhesion Molecule Expression in TNF-α-Stimulated Vascular Smooth Muscle Cells. Mol. Med. Rep. 2017, 16, 6354–6359. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Park, S.Y.; Seo, Y.; Kong, C.S. Anticatabolic and Anti-Inflammatory Effects of Myricetin 3-O-β-D-Galactopyranoside in UVA-Irradiated Dermal Cells via Repression of MAPK/AP-1 and Activation of TGFβ/Smad. Molecules 2020, 25, 1331. [Google Scholar] [CrossRef]

| Test Sample | CC50 ± SD (µg/100 µL) | ||
|---|---|---|---|
| HEK-293 | Hep-G2 | HeLa | |
| EtOH | 38.2 ± 3.3 bc | 30.8 ± 2.9 cd | 32.6 ± 4.1 d |
| n-Hexane | 42.5 ± 6.8 c | 35.4 ± 4.3 d | 33.8 ± 3.0 d |
| DCM | 42.3 ± 3.9 c | 35.2 ± 3.0 d | 31.3 ± 2.8 d |
| EtOAc | 32.5 ± 6.1 bc | 28.2 ± 3.1 cd | 25.6 ± 3.4 cd |
| Aqueous | 38.0 ± 4.7 c | 29.7 ± 4.0 cd | 30.9 ± 5.7 cd |
| Myricitrin (1) | 27.3 ± 3.1 b | 22.0 ± 2.7 ab | 8.5 ± 2.2 a |
| Unifloratrin (2) | 27.6 ± 3.2 b | 25.5 ± 4.9 bc | 14.8 ± 2.0 b |
| 5-FU | 6.1 ± 1.2 a | 17.5 ± 2.1 a | 7.5 ± 1.5 a |
| Test Sample | IC50 ± SD (µM) | ||
|---|---|---|---|
| DPPH | NO | H2O2 | |
| EtOH | 33.28 ± 3.17 d | 57.23 ± 3.16 e | 123.37 ± 11.05 f |
| Hexane | 79.14 ± 8.57 e | 101.87 ± 12.30 f | 379.19 ± 22.81 g |
| DCM | 38.91 ± 4.45 d | 51.02 ± 4.22 e | 112.74 ± 5.29 ef |
| EtOAc | 23.52 ± 4.61 c | 39.16 ± 3.56 d | 87.50 ± 4.44 d |
| Aqueous | 31.08 ± 3.87 d | 45.25 ± 2.92 de | 104.27 ± 4.01 e |
| Myricitrin (1) | 6.23 ± 1.09 a | 22.01 ± 2.59 a | 30.46 ± 1.79 a |
| Unifloratrin (2) | 12.53 ± 3.36 b | 34.10 ± 3.69 c | 57.42 ± 3.61 b |
| L-ascorbic acid | 8.32 ± 1.69 a | 21.21 ± 1.44 a | 54.92 ± 2.45 b |
| Quercetin | 7.11 ± 1.55 a | 25.54 ± 1.27 ab | 61.17 ± 2.58 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oriola, A.O.; Miya, G.M.; Singh, M.; Oyedeji, A.O. Flavonol Glycosides from Eugenia uniflora Leaves and Their In Vitro Cytotoxicity, Antioxidant and Anti-Inflammatory Activities. Sci. Pharm. 2023, 91, 42. https://doi.org/10.3390/scipharm91030042
Oriola AO, Miya GM, Singh M, Oyedeji AO. Flavonol Glycosides from Eugenia uniflora Leaves and Their In Vitro Cytotoxicity, Antioxidant and Anti-Inflammatory Activities. Scientia Pharmaceutica. 2023; 91(3):42. https://doi.org/10.3390/scipharm91030042
Chicago/Turabian StyleOriola, Ayodeji Oluwabunmi, Gugulethu Mathews Miya, Moganavelli Singh, and Adebola Omowunmi Oyedeji. 2023. "Flavonol Glycosides from Eugenia uniflora Leaves and Their In Vitro Cytotoxicity, Antioxidant and Anti-Inflammatory Activities" Scientia Pharmaceutica 91, no. 3: 42. https://doi.org/10.3390/scipharm91030042
APA StyleOriola, A. O., Miya, G. M., Singh, M., & Oyedeji, A. O. (2023). Flavonol Glycosides from Eugenia uniflora Leaves and Their In Vitro Cytotoxicity, Antioxidant and Anti-Inflammatory Activities. Scientia Pharmaceutica, 91(3), 42. https://doi.org/10.3390/scipharm91030042