Orally Administered Prosochit®-Based Nanoparticles of Insulin Ameliorates Alloxan-Induced Diabetes in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Animals
2.3. Preparation of Insulin-Loaded Nanoparticles
2.4. Scanning Electron Microscopy (SEM)
2.5. Examination of the Particle Size Distribution of Loaded and Unloaded Nanoparticles
2.6. Induction of Diabetes
2.7. Study Setting
2.8. Evaluation of the Antidiabetic Effect of the Formulations
2.9. Statistical Analysis
3. Results
3.1. Degree of Drying and Percent Drug Loading
3.2. Scanning Electron Micrographs
3.3. Particle Size Distribution
3.4. Antidiabetic Activity over a 24-h Period
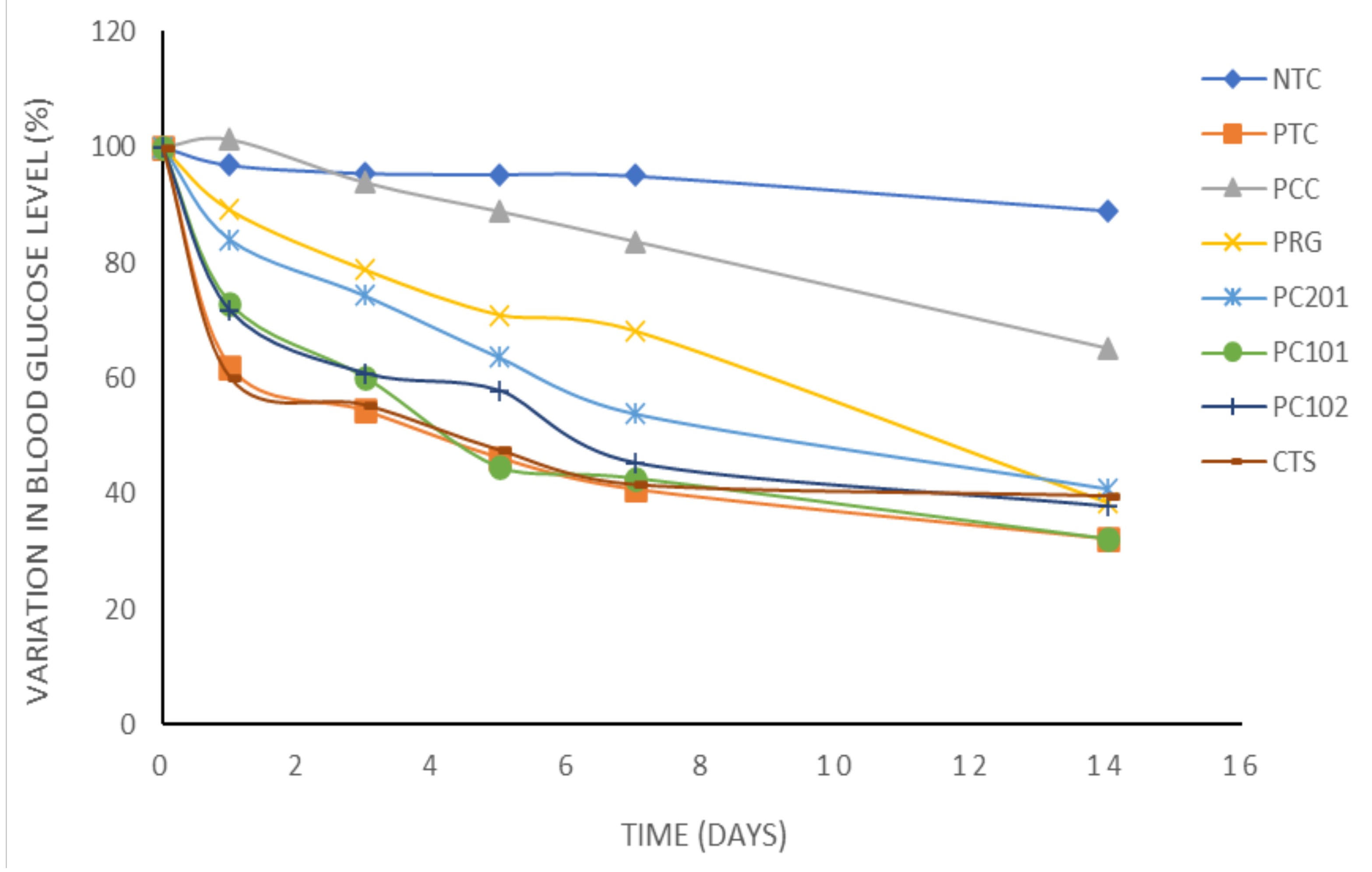
3.5. Antidiabetic Activity over Fourteen Days
4. Discussion
5. Conclusions
6. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, S.; Bennet, D.B.; Cheu, S.; Gray, S.; Seshadri, N.; Parker, J.M.; Sluggett, G.W.; Malcomson, R.; Conley, P.W. Exubera: Pharmaceutical development of a novel product for pulmonary delivery of insulin. Diabetes Technol. Ther. 2005, 7, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Olorunsola, E.O.; Alozie, M.F.; Davies, K.G.; Adedokun, M.O. Advances in the science and technology of insulin delivery: A review. J. Appl. Pharm. Sci. 2021, 11, 184–191. [Google Scholar] [CrossRef]
- Finucane, K.; Ambrey, P.; Narayan, S.; Archer, C.B.; Dayan, C. Insulin injection abscesses caused by Mycobacterium chelonae. Diabetes Care 2003, 26, 2483–2484. [Google Scholar] [CrossRef]
- Khafagy, E.S.; Morishita, M.; Onuki, Y.; Takayama, K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Reddy, I.K.; Khan, M.A. Polymethacrylate-based microparticulates of insulin for oral delivery: Preparation and in vitro dissolution stability in the presence of enzyme inhibitors. Int. J. Pharm. 2001, 225, 31–39. [Google Scholar] [CrossRef]
- McCourt, M.; Mielnicki, L.; Hughes, J.; Irving, M.Q.; Schentag, J.; Ghanim, H.; Dandona, P. 1013-P: Modification of inflammatory and Alzheimer markers with intracellular delivery of insulin using Cholestosomes. Diabetes 2020, 69. [Google Scholar] [CrossRef]
- Gupta, R. Diabetes treatment by nanotechnology. J. Biotechnol. Biomater. 2017, 7, 268. [Google Scholar] [CrossRef]
- Yin, L.; Ding, J.; He, C.; Cui, L.; Tang, C.; Yin, C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 2009, 30, 5691–5700. [Google Scholar] [CrossRef]
- Moreton, R.C. Excipient interaction. In Excipient Development for Pharmaceutical, Biotechnology and Drug Delivery Systems; Katdare, A., Chaubal, M.V., Eds.; CRC: Boca Raton, FL, USA, 2006; pp. 93–108. [Google Scholar] [CrossRef]
- Olorunsola, E.O.; Usungurua, S.G. Effect of polymer ratio on the quality of co-processed excipient of prosopis gum and crab shell chitosan. Trop. J. Pharm. Res. 2018, 17, 1693–1699. [Google Scholar] [CrossRef]
- Agboola, D.A. Prosopis africana (Mimosaceae): Stem, roots and seeds in the economy of the savannah areas of Nigeria. Econ. Bot. 2004, 58, S34–S42. [Google Scholar] [CrossRef]
- Adikwu, M.U.; Yoshikwa, Y.; Kanji, T. Bioadhesive delivery of metformin using prosopis gum with antidiabetic potential. Biol. Pharm. Bull. 2003, 26, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Ezike, C.; Akah, P.; Okoli, O.; Udegbunam, S.; Okwume, N.; Okeke, C.; Iloani, O. Medicinal plants used in wound care: A study of Prosopis africana (Fabaceae) stem bark. Ind. J. Pharm. Sci. 2010, 72, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Henciya, S.; Seturaman, P.; James, A.; Tsai, Y.; Nikam, R.; Wu, Y.; Dahms, H.; Chang, F. Biopharmaceutical potentials of Prosopis spp. (Mimosaceae, Leguminosa). J. Food Drug Anal. 2017, 25, 187–196. [Google Scholar] [CrossRef]
- Jaruszewski, K.M.; Ramakrishnan, S.; Poduslo, J.F.; Kandimalla, K.K. Chitosan enhances the stability and targeting of immune-nanovehicles to cerebrovascular deposits of Alzheimer’s disease amyloid protein. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.R.; Zinman, B.; Bolli, G. Alternative routes of insulin delivery. Diabet. Med. 2003, 20, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Arbit, E.; Kidron, M. Oral insulin delivery in a physiologic context: Review. J. Diabetes Sci. Technol. 2017, 11, 825–832. [Google Scholar] [CrossRef]
- Mumuni, M.A.; Ernest, O.C.; Ebele, O.; Kenechukwu, F.C.; Salome, C.A.; Chinekwu, N.S.; Aminu, N.; Ben, A. Development and characterization of mucinated chitosan microcomposite for oral insulin delivery. Trop. J. Nat. Prod. Res. 2020, 4, 1000–1006. [Google Scholar] [CrossRef]
- Olorunsola, E.O.; Davies, K.G.; Ibiang, K.P.; Esukpa, P.C.; Uwaechi, E.G.; Ahsan, F. Prosochit®-based nanoparticulate system of insulin for oral delivery: Design, formulation and characterization. J. Appl. Pharm. Sci. 2022. submitted. [Google Scholar]
- Mathews, R.G.; Donald, A.M. Conditions for imaging emulsions in the environmental scanning electron microscope. Scanning 2002, 24, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javamard, R.; Dokhani, A.; Khoasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Olorunsola, E.O.; Uwah, T.O.; Olayemi, O.J.; Etukudo, U.B. Ex-vivo evaluation of crab shell chitosan as absorption enhancer in ciprofloxacin tablet formulation. Afr. J. Biotechnol. 2016, 15, 1930–1935. [Google Scholar] [CrossRef]
- Salama, N.N.; Eddington, N.D.; Fasano, A. Tight junction modulation and its relationship to drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1174–1179. [Google Scholar] [CrossRef]
- Mesiha, M.; Plakogiannis, F.; Vejosoth, S. Enhanced oral absorption of insulin from desolated fatty acid-sodium glycocholate emulsions. Int. J. Pharm. 1994, 111, 213–216. [Google Scholar] [CrossRef]
- Erel, G.; Kotmakc, I.M.; Akbaba, H. Nanoencapsulated chitosan nanoparticles in emulsion-based oral delivery system: In-vitro and in-vivo evaluation of insulin loaded formulation. J. Drug Deliv. Sci. Technol. 2016, 36, 161–167. [Google Scholar] [CrossRef]
- Sharma, G.K.; Wilson, C.F.; Vander, W.; Sattar, N.; Petrie, J.R.; Kumar, M.R. Microemulsions for oral delivery of insulin: Design, development and evaluation in streptozocin-induced diabetic rats. Eur. J. Pharm. Biopharm. 2010, 76, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.L. Gastrointestinal absorption of intact proteins. Annu. Rev. Nutr. 1988, 8, 329–350. [Google Scholar] [CrossRef] [PubMed]
- Olorunsola, E.O.; Tologbonse, A.A.; Onwuka, N.A.; Adikwu, M.U. Enhanced oral delivery of artemether: An application of Prosochit®. J. Appl. Pharm. Sci. 2019, 9, 133–136. [Google Scholar] [CrossRef]
- Amidi, M.E.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef] [PubMed]
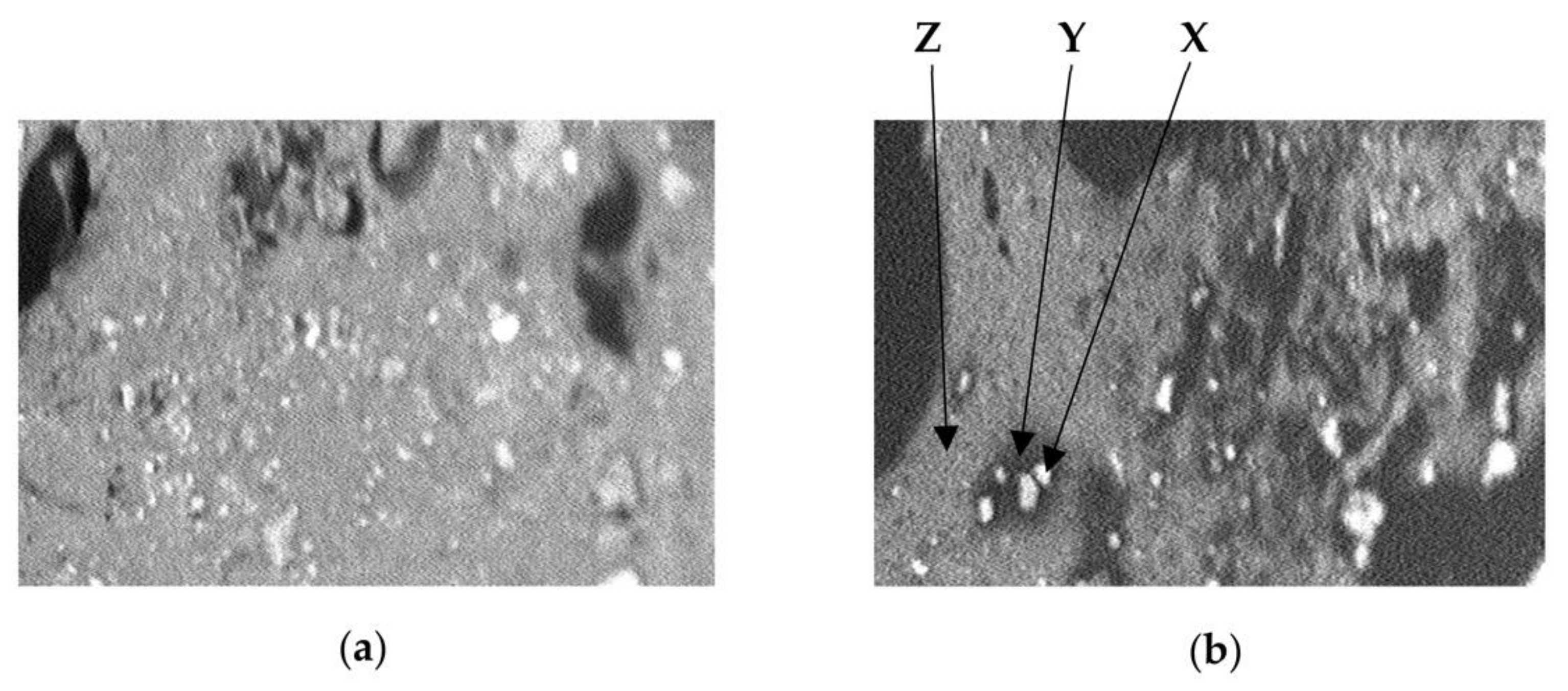
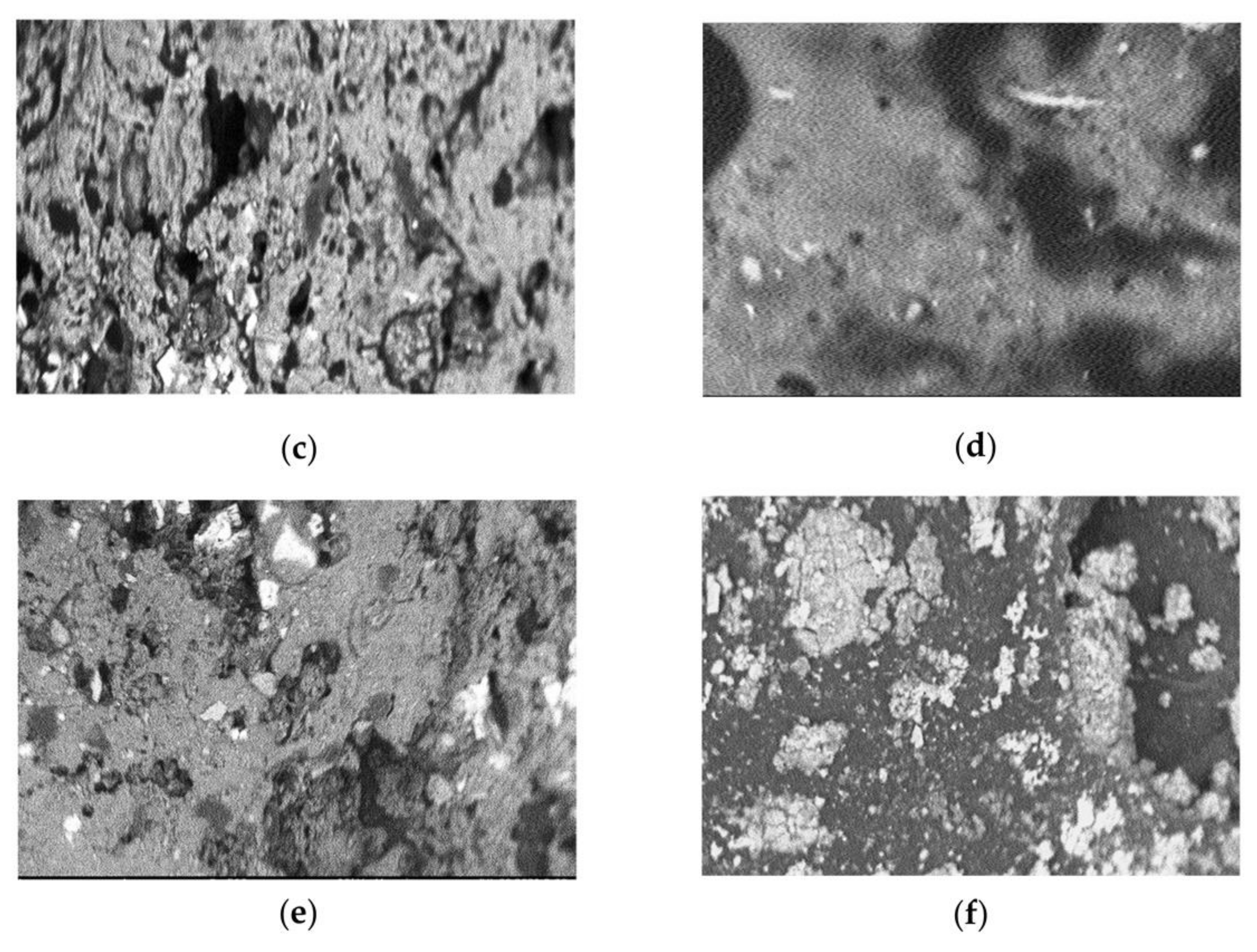
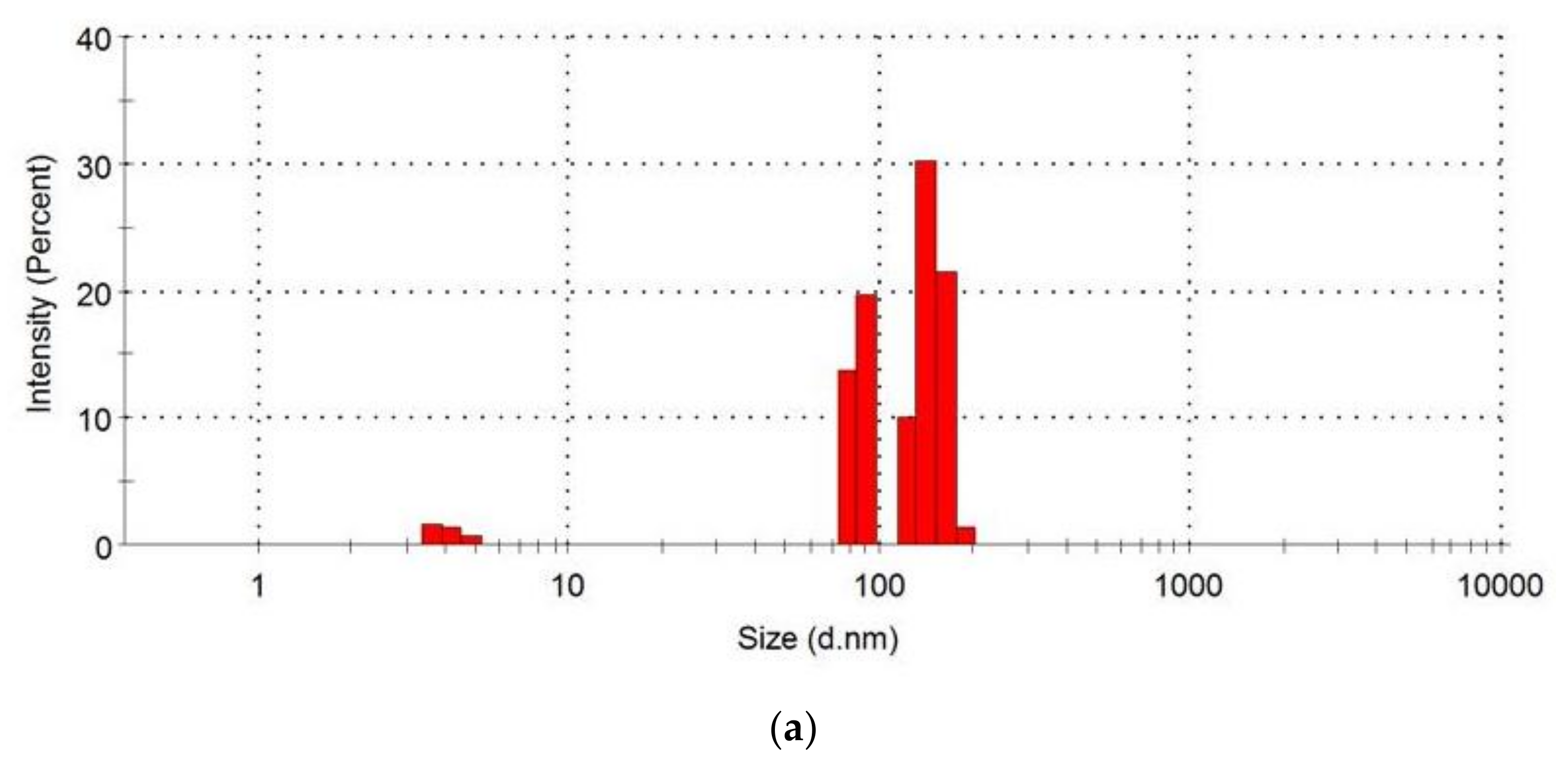
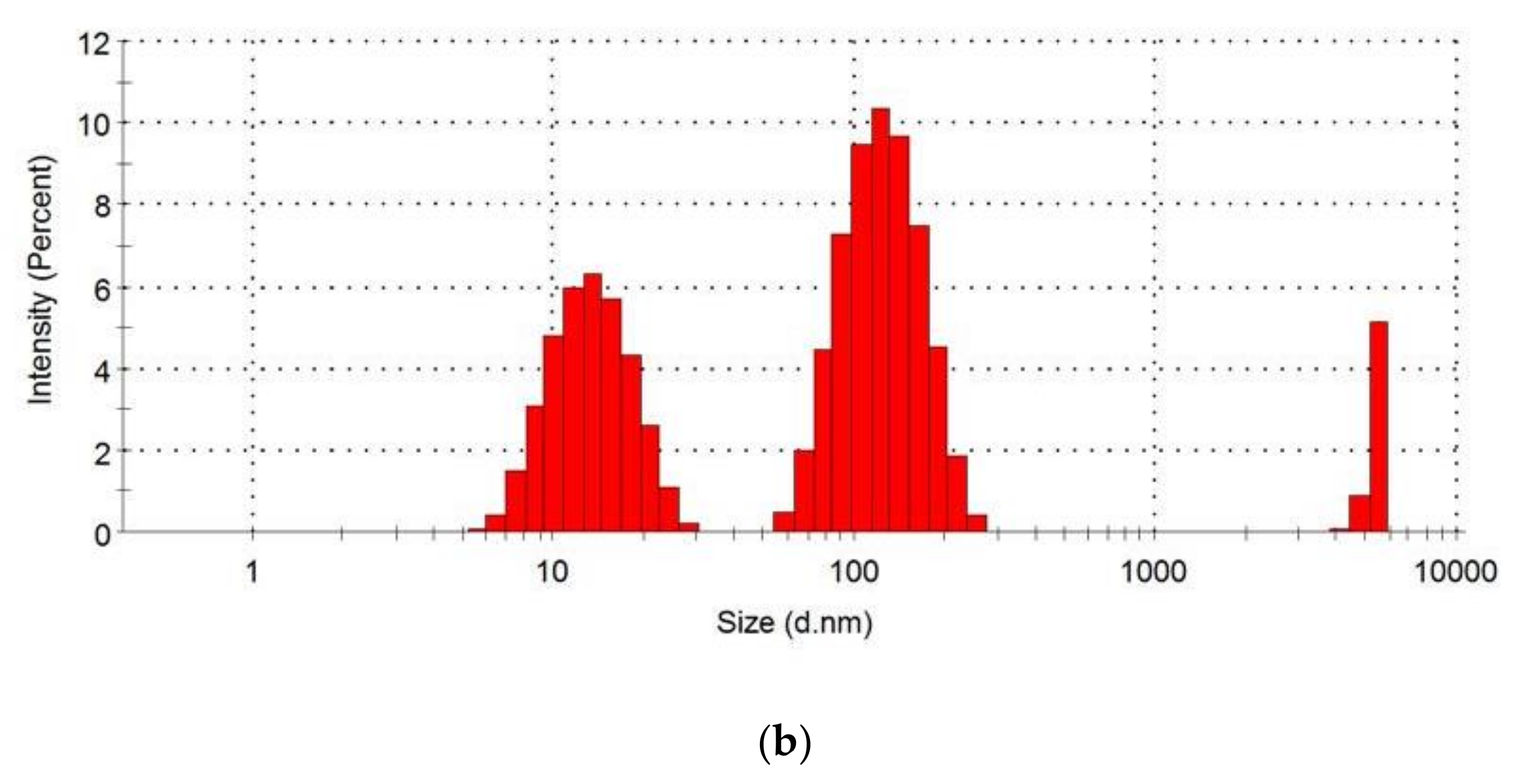

| Group | Formulation Administered |
|---|---|
| 1 | Purified water (F1): 10 mL per kg weight of rat (negative control) |
| 2 | Actrapid soluble insulin (F2): 50 IU per kg weight of rat (positive control) |
| 3 | Unloaded PC101-based nanoparticles (F3): 1.81 g per kg weight of rat |
| 4 | PRG-based insulin nanoparticles (F4): 1.80 g per kg weight of rat |
| 5 | PC201-based insulin nanoparticles (F5): 1.89 g per kg weight of rat |
| 6 | PC101-based insulin nanoparticles (F6): 1.82 g per kg weight of rat |
| 7 | PC102-based insulin nanoparticles (F7): 1.93 g per kg weight of rat |
| 8 | CTS-based insulin nanoparticles (F8): 1.87 g per kg weight of rat |
| S/N | Batch | Weight before Drying (g) | Weight after Drying (g) | % Drying | Percent Drug Loading (%) |
|---|---|---|---|---|---|
| 1 | Unloaded | 166.50 | 18.12 | 89.12 | Nil |
| 2 | PRG | 169.34 | 18.02 | 89.36 | 0.97 |
| 3 | PC201 | 181.80 | 18.96 | 89.57 | 0.93 |
| 4 | PC101 | 166.44 | 18.18 | 89.08 | 0.96 |
| 5 | PC102 | 180.52 | 19.34 | 89.29 | 0.90 |
| 6 | CTS | 176.16 | 18.74 | 89.36 | 0.98 |
| Absolute Values of Blood Glucose Level (mg/dL) | |||||||
|---|---|---|---|---|---|---|---|
| Batch | Time 0 h | Time 1 h | Time 2 h | Time 3 h | Time 6 h | Time 12 h | Time 24 h |
| NTC | 316.19 ± 19.25 | 316.40 ± 23.92 | 321.60 ± 23.86 | 326.20 ± 22.27 | 314.00 ± 19.52 | 307.40 ± 19.03 | 306.40 ± 19.74 |
| PTC | 376.75 ± 60.89 | 373.75 ± 51.97 | 347.50 ± 45.95 | 330.00 ± 45.36 | 281.25 ± 33.10 | 242.25 ± 24.03 | 233.75 ± 22.54 * |
| PCC | 207.00 ± 11.79 | 184.00 ± 4.36 | 156.33 ± 8.25 | 149.30 ± 3.18 | 117.33 ± 15.51 * | 114.33 ± 13.53 * | 206.00 ± 36.47 |
| PRG | 318.75 ± 71.22 | 261.69 ± 98.01 | 204.31 ± 97.79 | 206.55 ± 94.88 | 193.48 ± 94.90 * | 193.16 ± 93.83 * | 284.00 ± 63.13 |
| PC201 | 288.75 ± 73.92 | 300.00 ± 75.54 | 272.00 ± 79.70 | 260.66 ± 82.17 | 237.25 ± 88.59 | 234.75 ± 89.34 | 247.00 ± 87.69 |
| PC101 | 372.75 ± 67.25 | 347.00 ± 59.32 | 314.00 ± 67.69 | 292.50 ± 76.99 | 280.00 ± 79.53 | 269.00 ± 73.19 | 272.03 ± 25.96 |
| PC102 | 324.00 ± 68.82 | 281.85 ± 82.00 | 245.00 ± 91.16 | 267.25 ± 91.81 | 257.50 ± 88.91 | 255.50 ± 89.37 | 236.00 ± 84.79 |
| CTS | 318.00 ± 89.18 | 262.00 ± 95.15 | 260.33 ± 97.48 | 249.66 ± 99.29 | 199.00 ± 74.51 | 196.66 ± 72.65 | 191.00 ± 69.01 * |
| Absolute Values of Blood Glucose Level (mg/dL) | ||||||
|---|---|---|---|---|---|---|
| Batch | Time 0 h | After 1 Day | After 3 Days | After 5 Days | After 7 Days | After 14 Days |
| NTC | 316.19 ± 19.25 | 306.40 ± 19.74 | 302.40 ± 19.87 | 301.60 ± 19.97 | 300.60 ± 19.51 | 281.80 ± 17.73 |
| PTC | 376.75 ± 60.89 | 233.75 ± 22.54 * | 202.00 ± 8.39 * | 173.25 ± 4.83 *** | 159.50 ± 6.06 *** | 121.75 ± 5.51 *** |
| PCC | 207.00 ± 11.79 | 206.00 ± 36.47 | 196.00 ± 42.53 | 185.33 ± 30.28 | 174.33 ± 25.77 | 136.66 ± 23.21 |
| PRG | 318.75 ± 71.22 | 284.00 ± 63.13 | 251.02 ± 23.98 * | 225.99 ± 6.57 * | 217.22 ± 4.09 * | 122.00 ± 3.72 ** |
| PC201 | 288.75 ± 73.92 | 247.00 ± 87.69 | 220.75 ± 70.73 * | 180.00 ± 43.13 * | 155.75 ± 26.54 * | 118.24 ± 9.99 ** |
| PC101 | 372.75 ± 67.25 | 272.03 ± 25.96 | 224.34 ± 28.80 * | 161.75 ± 21.85 *** | 158.75 ± 20.37 *** | 120.02 ± 17.43 *** |
| PC102 | 324.00 ± 68.82 | 236.00 ± 84.79 | 185.25 ± 43.52 * | 127.75 ± 21.77 ** | 146.75 ± 23.59 ** | 122.25 ± 8.14 ** |
| CTS | 318.00 ± 89.18 | 191.00 ± 69.01 * | 176.00 ± 62.79 * | 139.00 ± 28.59 ** | 132.33 ± 27.09 ** | 126.01 ± 32.00 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olorunsola, E.O.; Davies, K.G.; Essien, E.B.; Alozie, M.F.; Adedokun, M.O.; Ahsan, F. Orally Administered Prosochit®-Based Nanoparticles of Insulin Ameliorates Alloxan-Induced Diabetes in Rats. Sci. Pharm. 2022, 90, 66. https://doi.org/10.3390/scipharm90040066
Olorunsola EO, Davies KG, Essien EB, Alozie MF, Adedokun MO, Ahsan F. Orally Administered Prosochit®-Based Nanoparticles of Insulin Ameliorates Alloxan-Induced Diabetes in Rats. Scientia Pharmaceutica. 2022; 90(4):66. https://doi.org/10.3390/scipharm90040066
Chicago/Turabian StyleOlorunsola, Emmanuel O., Koofreh G. Davies, Enomfon B. Essien, Mfonobong F. Alozie, Musiliu O. Adedokun, and Fakhrul Ahsan. 2022. "Orally Administered Prosochit®-Based Nanoparticles of Insulin Ameliorates Alloxan-Induced Diabetes in Rats" Scientia Pharmaceutica 90, no. 4: 66. https://doi.org/10.3390/scipharm90040066
APA StyleOlorunsola, E. O., Davies, K. G., Essien, E. B., Alozie, M. F., Adedokun, M. O., & Ahsan, F. (2022). Orally Administered Prosochit®-Based Nanoparticles of Insulin Ameliorates Alloxan-Induced Diabetes in Rats. Scientia Pharmaceutica, 90(4), 66. https://doi.org/10.3390/scipharm90040066






