LC-HRMS-Based Profiling: Antibacterial and Lipase Inhibitory Activities of Some Medicinal Plants for the Remedy of Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
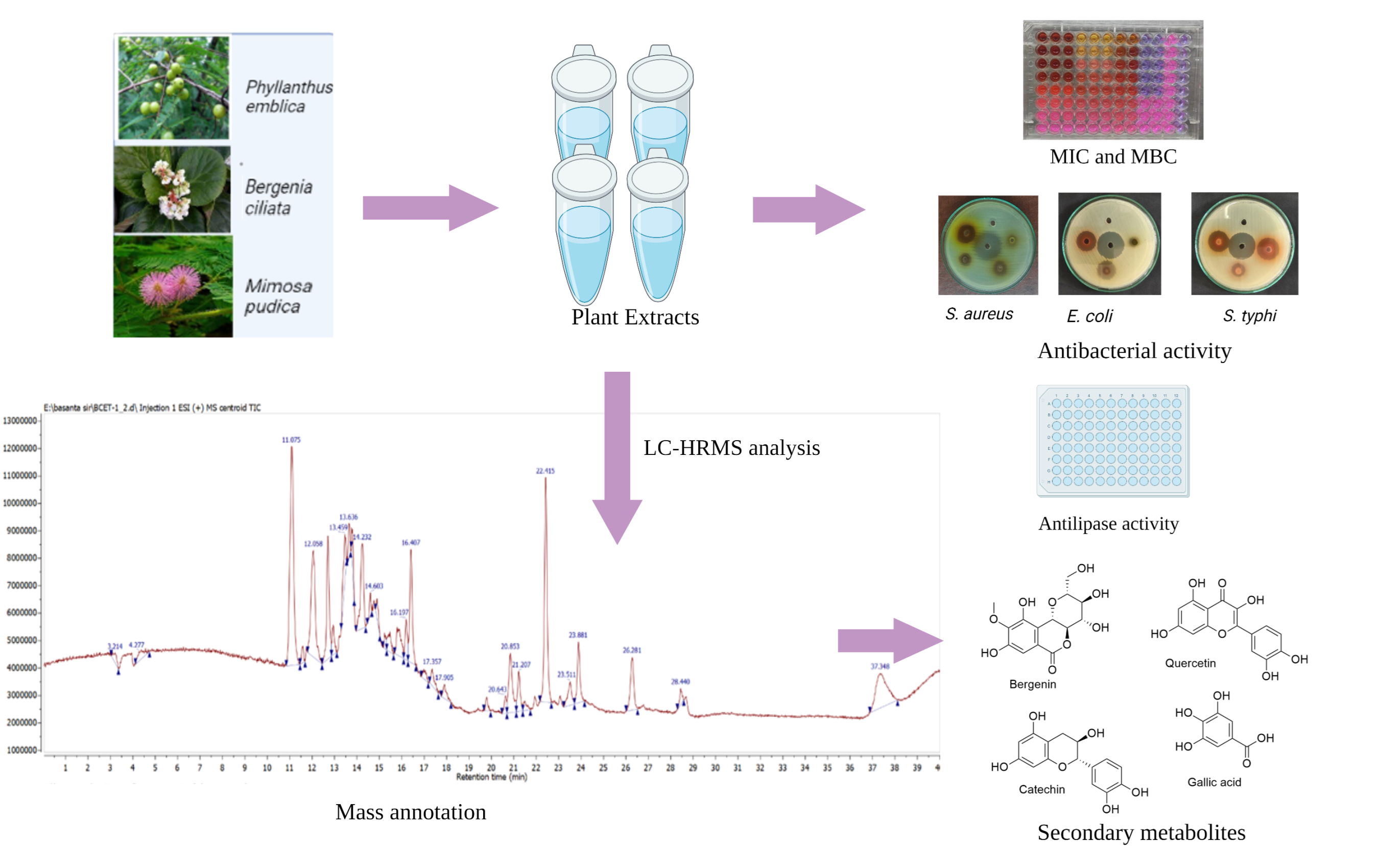
2.2. Plant Collection and Extract Preparation
2.3. Lipase Assay
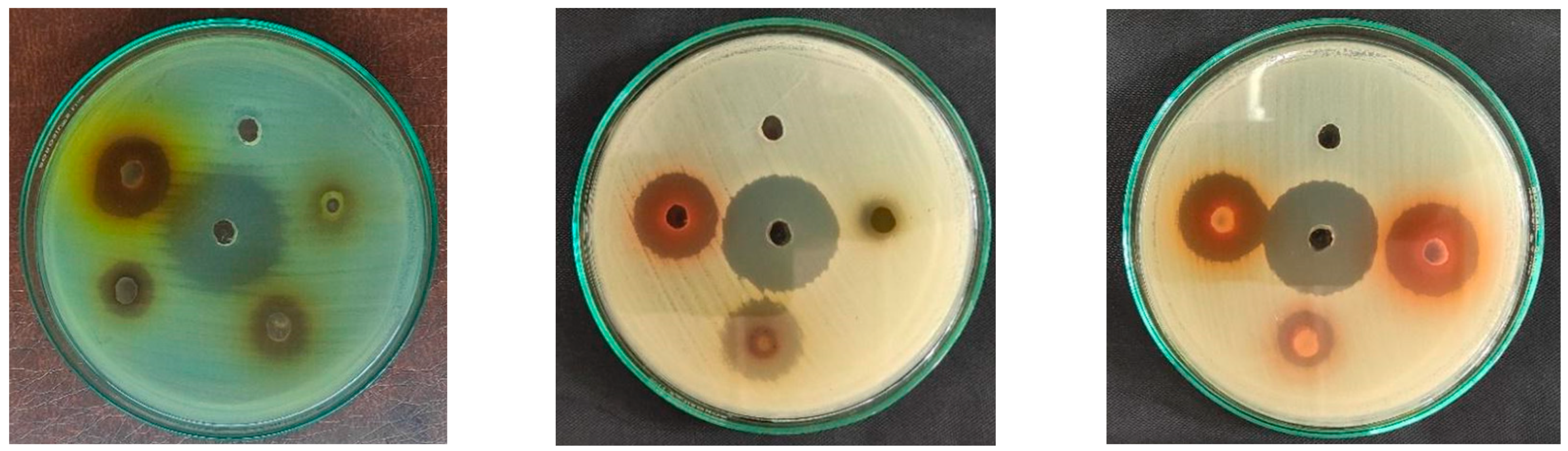
2.4. Antibacterial Assays
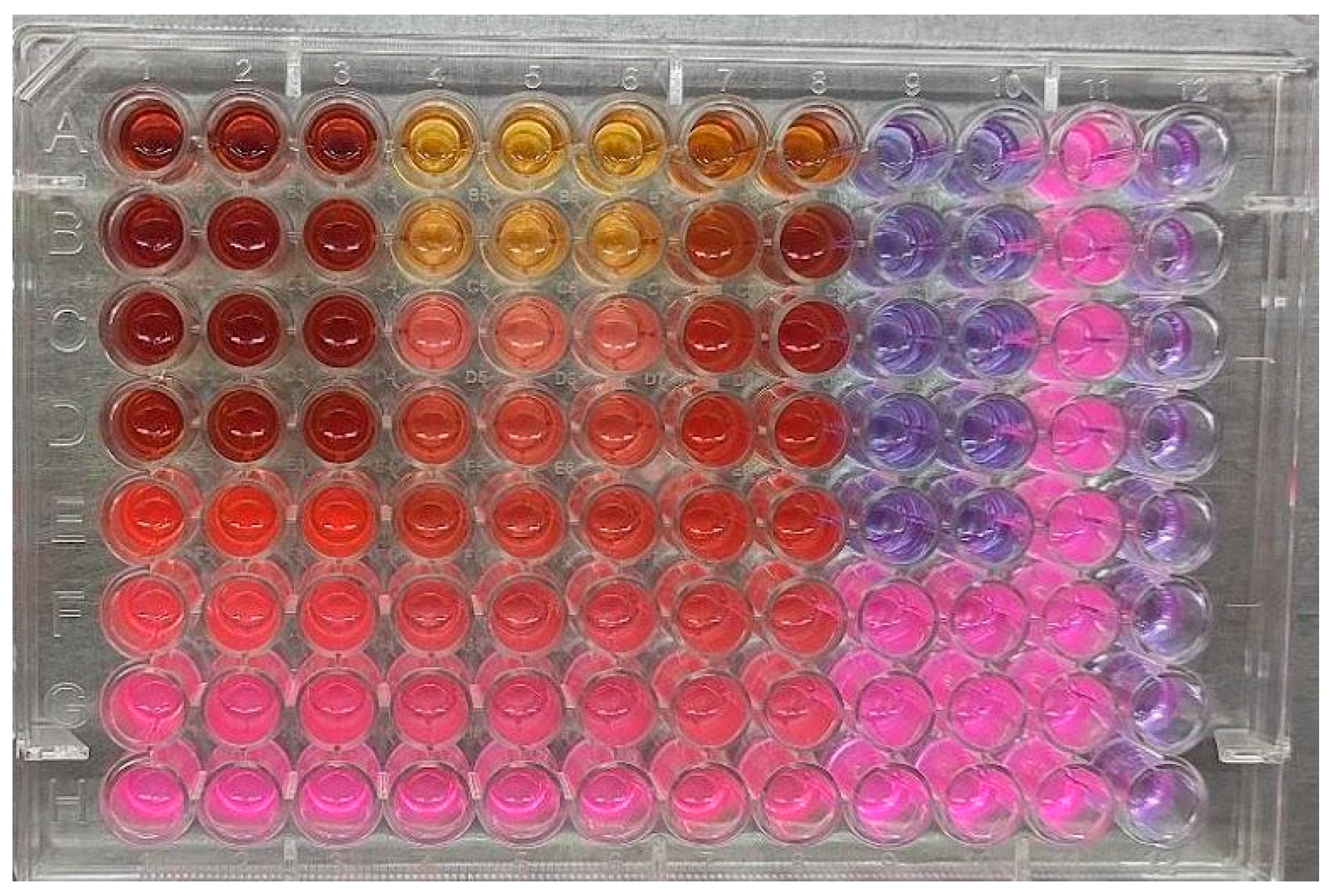
2.5. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.6. Statistical Analysis
2.7. LC-HRMS Analysis
3. Results
3.1. Lipase Inhibition
3.2. Analysis of Antimicrobial Activity
3.3. Determination of MIC and MBC
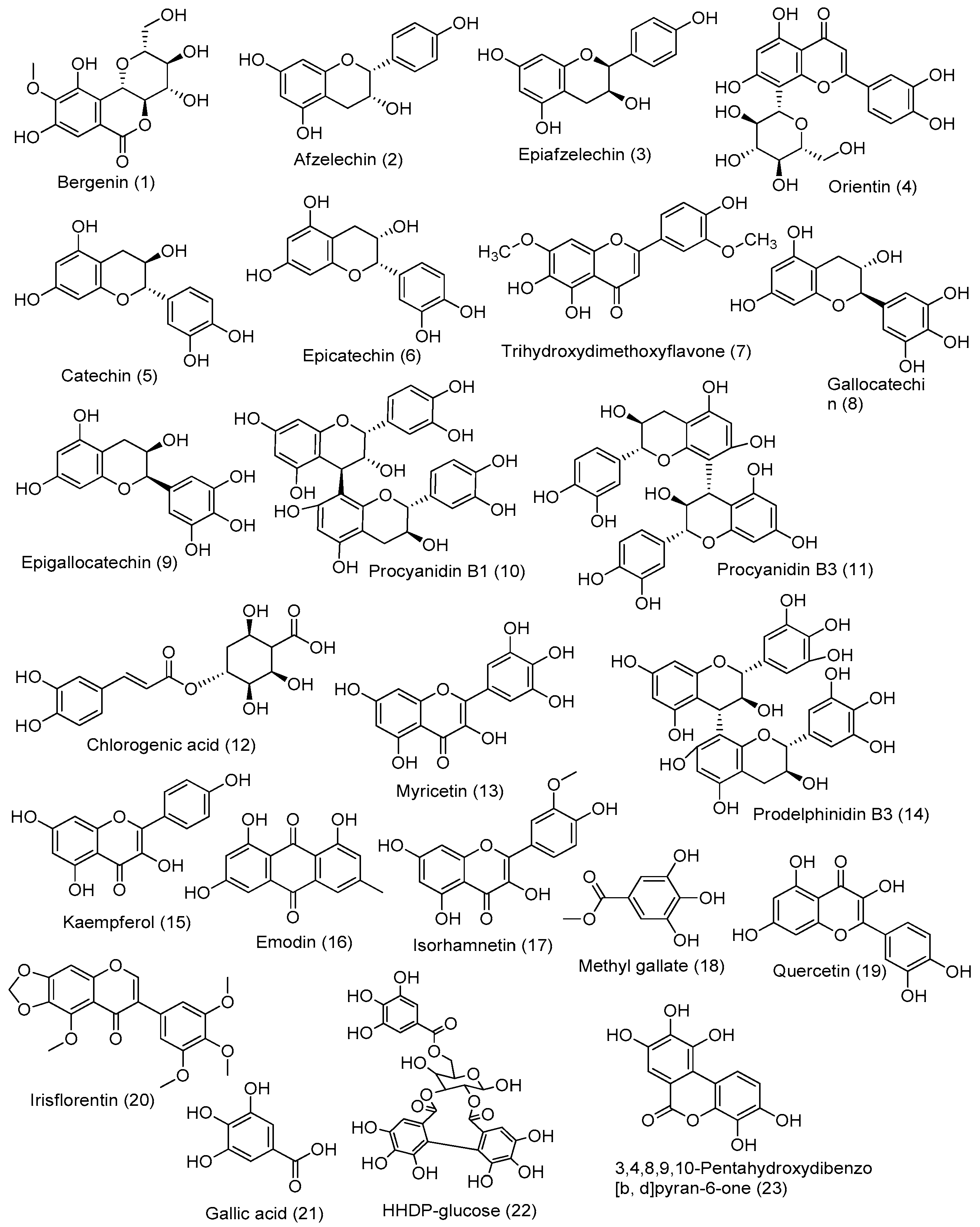
3.4. LC-HRMS-Based Molecular Annotation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Can We Prevent Obesity-Related Metabolic Diseases by Dietary Modulation of the Gut Microbiota? Adv. Nutr. 2016, 7, 90–101. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Federation. Global Obesity Observatory. Available online: https://data.worldobesity.org/?_ga=2.125462479.490317358.1656440210-1950410177.1656440210 (accessed on 29 June 2022).
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.T.; Shimada, Y. Lipases. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Oxford, UK, 2009; pp. 385–392. ISBN 978-0-12-373944-5. [Google Scholar]
- Inthongkaew, P.; Chatsumpun, N.; Supasuteekul, C.; Kitisripanya, T.; Putalun, W.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and Pancreatic Lipase Inhibitory Activities and Glucose Uptake Stimulatory Effect of Phenolic Compounds from Dendrobium formosum. Rev. Bras. Farmacogn. 2017, 27, 480–487. [Google Scholar] [CrossRef]
- Srivastava, G.; Apovian, C.M. Current Pharmacotherapy for Obesity. Nat. Rev. Endocrinol. 2018, 14, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a New Lipase Inhibitor for the Management of Obesity. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2000, 20, 270–279. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M.; Elkhayat, E.S.; El Dine, R.S. Natural Anti-Obesity Agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef]
- Sun, N.-N.; Wu, T.-Y.; Chau, C.-F. Natural Dietary and Herbal Products in Anti-Obesity Treatment. Molecules 2016, 21, 1351. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Zaid, A.N.; Hussein, F.; Zaqzouq, M.; Aljammal, H.; Ayesh, O. Anti-Lipase Potential of the Organic and Aqueous Extracts of Ten Traditional Edible and Medicinal Plants in Palestine; a Comparison Study with Orlistat. Medicines 2017, 4, 89. [Google Scholar] [CrossRef]
- Gaire, B.P.; Subedi, L. Phytochemistry, Pharmacology and Medicinal Properties of Phyllanthus emblica Linn. Chin. J. Integr. Med. 2014. [Google Scholar] [CrossRef]
- Ahmad, H.; Sehgal, S.; Mishra, A.; Gupta, R. Mimosa pudica L. (Laajvanti): An Overview. Pharmacogn. Rev. 2012, 6, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Raj, V.; Kim, Y.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Chitosan-Gum Arabic Embedded Alizarin Nanocarriers Inhibit Biofilm Formation of Multispecies Microorganisms. Carbohydr. Polym. 2022, 284, 118959. [Google Scholar] [CrossRef]
- Patra, A.K. An Overview of Antimicrobial Properties of Different Classes of Phytochemicals. In Dietary Phytochemicals and Microbes; Patra, A.K., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. ISBN 978-94-007-3926-0. [Google Scholar]
- Nair, A.; Balasaravanan, T.; Jadhav, S.; Mohan, V.; Kumar, C. Harnessing the Antibacterial Activity of Quercus infectoria and Phyllanthus emblica against Antibiotic-Resistant Salmonella typhi and Salmonella Enteritidis of Poultry Origin. Vet. World 2020, 13, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Tiwari, S.; Bhandari, K.; Biswal, A.K.; Rawat, A.K.S. Novel Derivatives of Plant Monomeric Phenolics: Act as Inhibitors of Bacterial Cell-to-Cell Communication. Microb. Pathog. 2020, 141, 103856. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Lee, J.-H.; Shim, J.-J.; Lee, J. Recent Findings and Future Directions of Grafted Gum Karaya Polysaccharides and Their Various Applications: A Review. Carbohydr. Polym. 2021, 258, 117687. [Google Scholar] [CrossRef]
- Méndez-López, L.F.; Garza-González, E.; Ríos, M.Y.; Ramírez-Cisneros, M.Á.; Alvarez, L.; González-Maya, L.; Sánchez-Carranza, J.N.; Camacho-Corona, M.d.R. Metabolic Profile and Evaluation of Biological Activities of Extracts from the Stems of Cissus trifoliata. Int. J. Mol. Sci. 2020, 21, 930. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Jones, O.A.H.; Beale, D.J.; Boughton, B.A.; Benheim, D.; Kouremenos, K.A.; Wolfender, J.-L.; Wishart, D.S. Current and Future Perspectives on the Structural Identification of Small Molecules in Biological Systems. Metabolites 2016, 6, 46. [Google Scholar] [CrossRef]
- Strano-Rossi, S.; Odoardi, S.; Castrignanò, E.; Serpelloni, G.; Chiarotti, M. Liquid Chromatography–High Resolution Mass Spectrometry (LC–HRMS) Determination of Stimulants, Anorectic Drugs and Phosphodiesterase 5 Inhibitors (PDE5I) in Food Supplements. J. Pharm. Biomed. Anal. 2015, 106, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Zafar, R.; Ullah, H.; Zahoor, M.; Sadiq, A. Isolation of Bioactive Compounds from Bergenia ciliata (Haw.) Sternb Rhizome and Their Antioxidant and Anticholinesterase Activities. BMC Complement. Altern. Med. 2019, 19, 296. [Google Scholar] [CrossRef]
- Saini, R.; Sharma, N.; Oladeji, O.S.; Sourirajan, A.; Dev, K.; Zengin, G.; El-Shazly, M.; Kumar, V. Traditional Uses, Bioactive Composition, Pharmacology, and Toxicology of Phyllanthus emblica Fruits: A Comprehensive Review. J. Ethnopharmacol. 2022, 282, 114570. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Pinto, J.; Castilho, P.C. Hypoglycemic, Anti-Glycation and Antioxidant In Vitro Properties of Two Vaccinium Species from Macaronesia: A Relation to Their Phenolic Composition. J. Funct. Foods 2018, 40, 595–605. [Google Scholar] [CrossRef]
- Daoud, A.; Malika, D.; Bakari, S.; Hfaiedh, N.; Mnafgui, K.; Kadri, A.; Gharsallah, N. Assessment of Polyphenol Composition, Antioxidant and Antimicrobial Properties of Various Extracts of Date Palm Pollen (DPP) from Two Tunisian Cultivars. Arab. J. Chem. 2019, 12, 3075–3086. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Willcott, M.R. MestRe Nova. J. Am. Chem. Soc. 2009, 131, 13180. [Google Scholar] [CrossRef]
- Li, B.-H.; Wu, J.-D.; Li, X.-L. LC–MS/MS Determination and Pharmacokinetic Study of Bergenin, the Main Bioactive Component of Bergenia Purpurascens after Oral Administration in Rats. J. Pharm. Anal. 2013, 3, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ma, X.; Gu, Y.; Huang, H.; Zhang, G. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, B.; Li, Z.; Hong, T.; Chen, M.; Tan, Y.; Jiang, J.; Huang, C. Metabolite Identification of Myricetin in Rats Using HPLC Coupled with ESI-MS. Chromatographia 2012, 75, 655–660. [Google Scholar] [CrossRef]
- Mittal, A.; Kadyan, P.; Gahlaut, A.; Dabur, R. Nontargeted Identification of the Phenolic and Other Compounds of Saraca asoca by High Performance Liquid Chromatography-Positive Electrospray Ionization and Quadrupole Time-of-Flight Mass Spectrometry. ISRN Pharm. 2013, 2013, e293935. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS Profiles of Phenolic Compounds and Antioxidant Activity of Fruits from Three Citrus Species Consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Wang, J.; Yue, Y.; Jiang, H.; Tang, F. Rapid Screening for Flavone C-Glycosides in the Leaves of Different Species of Bamboo and Simultaneous Quantitation of Four Marker Compounds by HPLC-UV/DAD. Int. J. Anal. Chem. 2012, 2012, e205101. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.A.; El Dib, R.A.; Al-Youssef, H.M.; Amina, M. Chemical Composition and Antimicrobial and Cytotoxic Activities of Antidesm abunius L. Pak. J. Pharm. Sci. 2019, 32, 153–163. [Google Scholar]
- Shen, D.; Wu, Q.; Wang, M.; Yang, Y.; Lavoie, E.J.; Simon, J.E. Determination of the Predominant Catechins in Acacia catechu by Liquid Chromatography/Electrospray Ionization-Mass Spectrometry. J. Agric. Food Chem. 2006, 54, 3219–3224. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Nie, Y.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R.; Ye, J.-H. Ultraviolet B (UVB) Photosensitivities of Tea Catechins and the Relevant Chemical Conversions. Molecules 2016, 21, 1345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Z.; Li, M.; Yuan, Y.; Cui, S.; Chen, J.; Li, R. An Integrated Strategy for Profiling the Chemical Components of Scutellariae Radix and Their Exogenous Substances in Rats by Ultra-High-Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry. Rapid Commun. Mass Spectrom. RCM 2020, 34, e8823. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Song, Y.; Jing, W.; Wang, Y.; Yang, X.; Liu, D. Simultaneous Determination of Caffeine, Gallic Acid, Theanine, (−)-Epigallocatechin and (−)-Epigallocatechin-3-Gallate in Green Tea Using Quantitative 1H-NMR Spectroscopy. Anal. Methods 2014, 6, 907–914. [Google Scholar] [CrossRef]
- Friedrich, W.; Eberhardt, A.; Galensa, R. Investigation of Proanthocyanidins by HPLC with Electrospray Ionization Mass Spectrometry. Eur. Food Res. Technol. 2000, 211, 56–64. [Google Scholar] [CrossRef]
- Klausen, K.; Mortensen, A.G.; Laursen, B.; Haselmann, K.F.; Jespersen, B.M.; Fomsgaard, I.S. Phenolic Compounds in Different Barley Varieties: Identification by Tandem Mass Spectrometry (QStar) and NMR; Quantification by Liquid Chromatography Triple Quadrupole-Linear Ion Trap Mass Spectrometry (Q-Trap). Nat. Prod. Commun. 2010, 5, 407–414. [Google Scholar] [CrossRef]
- Ijaz, S.; Shoaib Khan, H.M.; Anwar, Z.; Talbot, B.; Walsh, J.J. HPLC Profiling of Mimosa pudica Polyphenols and Their Non-Invasive Biophysical Investigations for Anti-Dermatoheliotic and Skin Reinstating Potential. Biomed. Pharmacother. 2019, 109, 865–875. [Google Scholar] [CrossRef]
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. Analyses of Chlorogenic Acids and Related Cinnamic Acid Derivatives from Nicotiana tabacum Tissues with the Aid of UPLC-QTOF-MS/MS Based on the in-Source Collision-Induced Dissociation Method. Chem. Cent. J. 2014, 8, 66. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, K.; Zhou, W.; Zhou, J.; Yang, P. Studies on the Active Components and Antioxidant Activities of the Extracts of Mimosa pudica Linn. from Southern China. Pharmacogn. Mag. 2011, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of Flavonoids and Phenolic Acids in Myrcia bella Cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS Combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Guan, Y.; Zhou, Y.; Wang, Y.; Ji, H.; Liu, Z. Detection and Characterization of the Metabolites of Rutaecarpine in Rats Based on Ultra-High-Performance Liquid Chromatography with Linear Ion Trap-Orbitrap Mass Spectrometer. Pharm. Biol. 2016, 55, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; De Pascale, S.; Aponte, M.; Scaloni, A.; Addeo, F.; Caira, S. Polyphenol Profiling of Chestnut Pericarp, Integument and Curing Water Extracts to Qualify These Food By-Products as a Source of Antioxidants. Molecules 2021, 26, 2335. [Google Scholar] [CrossRef]
- Lobstein, A.; Weniger, B.; Um, B.H.; Steinmetz, M.; Declercq, L.; Anton, R. 4″-Hydroxymaysin and Cassiaoccidentalin B, Two Unusual C-Glycosylflavones from Mimosa pudica (Mimosaceae). Biochem. Syst. Ecol. 2002, 30, 375–377. [Google Scholar] [CrossRef]
- Hernandez, C.; Cadenillas, L.; Maghubi, A.E.; Caceres, I.; Durrieu, V.; Mathieu, C.; Bailly, J.-D. Mimosa tenuiflora Aqueous Extract: Role of Condensed Tannins in Anti-Aflatoxin B1 Activity in Aspergillus flavus. Toxins 2021, 13, 391. [Google Scholar] [CrossRef]
- March, R.E.; Miao, X.-S. A Fragmentation Study of Kaempferol Using Electrospray Quadrupole Time-of-Flight Mass Spectrometry at High Mass Resolution. Int. J. Mass Spectrom. 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Zhan, C.; Xiong, A.; Shen, D.; Yang, L.; Wang, Z. Characterization of the Principal Constituents of Danning Tablets, a Chinese Formula Consisting of Seven Herbs, by an UPLC-DAD-MS/MS Approach. Molecules 2016, 21, 631. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and Quantification by LC-MS/MS of the Chemical Components of the Heating Products of the Flavonoids Extract in Pollen Typhae for Transformation Rule Exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef]
- Scigelova, M.; Hornshaw, M.; Giannakopulos, A.; Makarov, A. Fourier Transform Mass Spectrometry. Mol. Cell. Proteomics MCP 2011, 10, M111.009431. [Google Scholar] [CrossRef]
- Roger, B.; Jeannot, V.; Fernandez, X.; Cerantola, S.; Chahboun, J. Characterisation and Quantification of Flavonoids in Iris germanica L. and Iris pallida Lam. Resinoids from Morocco. Phytochem. Anal. PCA 2012, 23, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Wang, Q.; Qi, L.-W.; Qin, X.-Y.; Qin, M.-J. Characterization and Determination of the Major Constituents in Belamcandae Rhizoma by HPLC–DAD–ESI-MSn. J. Pharm. Biomed. Anal. 2011, 56, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Tamfu, A.N.; Kucukaydin, S.; Yeskaliyeva, B.; Ozturk, M.; Dinica, R.M. Non-Alkaloid Cholinesterase Inhibitory Compounds from Natural Sources. Molecules 2021, 26, 5582. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Pandey, N.; Agnihotri, V.; Singh, K.K.; Pandey, A. Antioxidant, Antimicrobial Activity and Bioactive Compounds of Bergenia ciliata Sternb.: A Valuable Medicinal Herb of Sikkim Himalaya. J. Tradit. Complement. Med. 2017, 7, 152–157. [Google Scholar] [CrossRef]
- Sawant, L.; Pandita, N.; Prabhakar, B. Determination of Gallic Acid in Phyllanthus emblica Linn. Dried Fruit Powder by HPTLC. J. Pharm. Bioallied Sci. 2010, 2, 105. [Google Scholar] [CrossRef]
- Wu, M.; Cai, J.; Fang, Z.; Li, S.; Huang, Z.; Tang, Z.; Luo, Q.; Chen, H. The Composition and Anti-Aging Activities of Polyphenol Extract from Phyllanthus emblica L. Fruit. Nutrients 2022, 14, 857. [Google Scholar] [CrossRef]
- Liu, W.; Huang, J.; Zhang, F.; Zhang, C.-C.; Li, R.-S.; Wang, Y.-L.; Wang, C.-R.; Liang, X.-M.; Zhang, W.-D.; Yang, L.; et al. Comprehensive Profiling and Characterization of the Absorbed Components and Metabolites in Mice Serum and Tissues following Oral Administration of Qing-Fei-Pai-Du Decoction by UHPLC-Q-Exactive-Orbitrap HRMS. Chin. J. Nat. Med. 2021, 19, 305–320. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS Screening of Bioactive Components from Rhus coriaria L. (Sumac) Fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. The Obesity Epidemic: Pathophysiology and Consequences of Obesity. Obes. Res. 2002, 10, 97S–104S. [Google Scholar] [CrossRef]
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L.J. Adipose Tissue Dysfunction in Obesity, Diabetes, and Vascular Diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef]
- Bhutani, K.K.; Birari, R.; Kapat, K. Potential Anti-Obesity and Lipid Lowering Natural Products: A Review. Nat. Prod. Commun. 2007, 2, 1934578X0700200316. [Google Scholar] [CrossRef]
- De la Garza, A.L.; Milagro, F.I.; Boque, N.; Campión, J.; Martínez, J.A. Natural Inhibitors of Pancreatic Lipase as New Players in Obesity Treatment. Planta Med. 2011, 77, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, B.K.; Khadayat, K.; Sharma, K.; Raut, B.K.; Aryal, D.; Thapa, B.B.; Parajuli, N. Phytochemical Analysis and Antioxidant and Antidiabetic Activities of Extracts from Bergenia ciliata, Mimosa pudica, and Phyllanthus emblica. Adv. Pharmacol. Pharm. Sci. 2022, 2022, e4929824. [Google Scholar] [CrossRef] [PubMed]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short, G.F., III; Staunton, J.E.; Jin, X.; et al. Synergistic Drug Combinations Tend to Improve Therapeutically Relevant Selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Li, S.; Pan, J.; Hu, X.; Zhang, Y.; Gong, D.; Zhang, G. Kaempferol Inhibits the Activity of Pancreatic Lipase and Its Synergistic Effect with Orlistat. J. Funct. Foods 2020, 72, 104041. [Google Scholar] [CrossRef]
- George, G.; Paul, A.T. Investigation of Synergistic Potential of Green Tea Polyphenols and Orlistat Combinations Using Pancreatic Lipase Assay-Based Synergy Directed Fractionation Strategy. S. Afr. J. Bot. 2020, 135, 50–57. [Google Scholar] [CrossRef]
- Anyanwu, G.O.; Anzaku, D.; Donwell, C.C.; Usunobun, U.; Adegbegi, A.J.; Ofoha, P.C.; Rauf, K. Chemical Composition and in Vitro Antiobesity and in Vivo Anti-Hyperlipidemic Effects of Ceratotheca Sesamoides, Jatropha tanjorensis, Mucuna flagellipes, Pterocarpus mildbraedii and Piper guineense. Phytomed. Plus 2021, 1, 100042. [Google Scholar] [CrossRef]
- Han, L.-K.; Ninomiya, H.; Taniguchi, M.; Baba, K.; Kimura, Y.; Okuda, H. Norepinephrine-Augmenting Lipolytic Effectors from Astilbe thunbergii Rhizomes. J. Nat. Prod. 1998, 61, 1006–1011. [Google Scholar] [CrossRef]
- Jahromi, M.A.F.; Chansouria, J.P.N.; Ray, A.B. Hypolipidaemic Activity in Rats of Bergenin, the Major Constituent of Flueggea microcarpa. Phytother. Res. 1992, 6, 180–183. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, C.S.; Park, K.-M.; Chang, P.-S. Inhibitory Characteristics of Flavonol-3-O-Glycosides from Polygonum aviculare L. (Common Knotgrass) against Porcine Pancreatic Lipase. Sci. Rep. 2019, 9, 18080. [Google Scholar] [CrossRef]
- Ong, S.L.; Mah, S.H.; Lai, H.Y. Porcine Pancreatic Lipase Inhibitory Agent Isolated from Medicinal Herb and Inhibition Kinetics of Extracts from Eleusine indica (L.) Gaertner. J. Pharm. 2016, 2016, e8764274. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Qiu, M.; Lin, J.; Li, M.; Ma, X.; Ran, F.; Luo, C.; Wei, X.; Xu, R.; Tan, P.; et al. Potential Effect of Tropical Fruits Phyllanthus emblica L. for the Prevention and Management of Type 2 Diabetic Complications: A Systematic Review of Recent Advances. Eur. J. Nutr. 2021, 60, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Bandivadekar, A.; Debjani, D. Inhibition of Propionibacterium acnes Lipase by Extracts of Indian Medicinal Plants. Int. J. Cosmet. Sci. 2012, 34, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, S.R.; Veerappan, K.; Ranjan, A.; Kim, Y.-J.; Chellappan, D.K.; Dua, K.; Lee, J.; Perumalsamy, H. Phyllanthus emblica Fruit Extract Attenuates Lipid Metabolism in 3T3-L1 Adipocytes via Activating Apoptosis Mediated Cell Death. Phytomedicine 2020, 66, 153129. [Google Scholar] [CrossRef]
- Khan, N.; Abbasi, A.M.; Dastagir, G.; Nazir, A.; Shah, G.M.; Shah, M.M.; Shah, M.H. Ethnobotanical and Antimicrobial Study of Some Selected Medicinal Plants Used in Khyber Pakhtunkhwa (KPK) as a Potential Source to Cure Infectious Diseases. BMC Complement. Altern. Med. 2014, 14, 122. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. The in Vitro Antibacterial Activity of Dietary Spice and Medicinal Herb Extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Sripriya, N.; Bhagya, R.; Radhika, B.; Prameela, L.; Udayaprakash, N. Phytochemical Screening, Antibacterial and Free Radical Scavenging Effects of Artemisia nilagirica, Mimosa pudica and Clerodendrum siphonanthus—An in–Vitro Study. Asian Pac. J. Trop. Biomed. 2012, 2, S601–S604. [Google Scholar] [CrossRef]
- Goud, M.J.P.; Komraiah, A.; Rao, K.; Ragan, A.; Raju, V.S.; Charya, M.S. Antibacterial Activity of Some Folklore Medicinal Plants from South India. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 421–426. [Google Scholar] [CrossRef][Green Version]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Snook, M.E.; Widstrom, N.W.; Wiseman, B.R.; Byrne, P.F.; Harwood, J.S.; Costello, C.E. New C-4″-Hydroxy Derivatives of Maysin and 3′-Methoxymaysin Isolated from Corn Silks (Zea Mays). J. Agric. Food Chem. 1995, 43, 2740–2745. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumar, B. Profiling of Gallic and Ellagic Acid Derivatives in Different Plant Parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 1934578X1601100227. [Google Scholar] [CrossRef]
| Medicinal Plant | Family | Voucher Specimen | Indigenous Uses | Pharmacological Studies |
|---|---|---|---|---|
| Bergenia ciliata | Saxifragaceae | BS-02 | Treatment of diarrhea, vomiting, fever, cough, diabetes, cancer, pulmonary disorders, and wound healing [23]. | B. ciliata has antibacterial, anti-inflammatory, anticancer, antitussive, antidiabetic, antilithotriptic, antidiabetic, and antimicrobial properties [23]. |
| Mimosa pudica | Fabaceae | BS-04 | Treatment of urogenital disorders, piles, dysentery, sinusitis, and wounds [13]. | Pharmacological activity as an antidiabetic, antitoxin, antihepatotoxic, antioxidant, and wound healer [13]. |
| Phyllanthus emblica | Phyllanthaceae | BS-05 | It is used to treat diarrhea, jaundice, and inflammation, and as a powerful Rasayana (life-extension technique) [24]. | P. emblica has previously been reported to have antimicrobial, antioxidant, anti-inflammatory, analgesic, antipyretic, adaptogenic, hepatoprotective, antitumor, and antiulcerogenic potential [24] |
| Standard/Plants | Fractions | Concentration | % Inhibition | IC50 Value |
|---|---|---|---|---|
| Orlistat (µg/mL) | - | 500 | 65.66 ± 0.40 | 179.70 ± 3.60 |
| 250 | 54.76 ± 1.38 | |||
| 125 | 44.61 ± 1.73 | |||
| 62.5 | 33.64 ± 3.81 | |||
| Bergenia ciliata (mg/mL) | Crude | 2.5 | 79.05 ± 1.18 | 1.07 ± 0.03 |
| 1.25 | 57.42 ± 1.21 | |||
| 0.625 | 28.60 ± 2.26 | |||
| Hexane | 5 | 62.49 ± 0.63 | 1.55 ± 0.02 | |
| 2.5 | 54.54 ± 1.41 | |||
| 1.25 | 48.00 ± 0.44 | |||
| DCM | 10 | 93.33 ± 3.88 | 3.11 ± 0.10 | |
| 5 | 57.39 ± 1.49 | |||
| 2.5 | 46.00 ± 1.54 | |||
| EA | 2.5 | 54.90 ± 0.39 | 2.01 ± 0.08 | |
| 1.25 | 38.74 ± 2.06 | |||
| 0.625 | 22.23 ± 3.90 | |||
| Aqueous | 5 | 59.37 ± 1.42 | 1.99 ± 0.17 | |
| 2.5 | 52.83 ± 1.56 | |||
| 1.25 | 45.26 ± 0.61 | |||
| Mimosa pudica (mg/mL) | Crude | 2.5 | 79.35 ± 1.70 | 1.33 ± 0.05 |
| 1.25 | 44.86 ± 2.81 | |||
| 0.625 | 19.94 ± 3.76 | |||
| Hexane | 1 | 73.68 ± 1.49 | 0.49 ± 0.02 | |
| 0.5 | 49.42 ± 0.75 | |||
| 0.25 | 26.18 ± 3.34 | |||
| DCM | 10 | 77.32 ± 1.06 | 5.37 ± 0.07 | |
| 5 | 45.02 ± 1.16 | |||
| 2.5 | 20.49 ± 0.96 | |||
| EA | 1.25 | 71.51 ± 4.71 | 0.82 ± 0.05 | |
| 0.625 | 34.17 ± 0.22 | |||
| 0.3125 | 18.78 ± 1.82 | |||
| Aqueous | 5 | 68.85 ± 1.73 | 1.84 ± 0.09 | |
| 2.5 | 55.72 ± 0.97 | |||
| 1.25 | 42.68 ± 1.74 | |||
| Phyllanthus emblica (mg/mL) | Crude | 10 | 34.68 ± 0.14 | - |
| 5 | 22.15 ± 1.48 | |||
| 2.5 | 17.66 ± 1.79 | |||
| Hexane | 5 | 73.02 ± 1.09 | 2.45 ± 0.03 | |
| 2.5 | 45.90 ± 0.97 | |||
| 1.25 | 34.82 ± 1.35 | |||
| DCM | 5 | 53.88 ± 0.85 | 4.19 ± 0.09 | |
| 2.5 | 37.56 ± 2.39 | |||
| 1.25 | 14.39 ± 3.67 | |||
| EA | 10 | 82.87 ± 1.22 | 3.64 ± 0.12 | |
| 5 | 60.86 ± 3.92 | |||
| 2.5 | 36.80 ± 1.49 | |||
| Aqueous | 10 | 32.10 ± 1.63 | - | |
| 5 | 19.67 ± 0.47 | |||
| 2.5 | 10.38 ± 1.15 |
| Microorganism | Zone of Inhibition (mm) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. ciliata | M. pudica | P. emblica | Neomycin | 50% DMSO | |||||||||||||
| C | H | D | E | A | C | H | D | E | A | C | H | D | E | A | |||
| S. aureus | 20 | 13 | 13 | 21 | 20 | 19 | 8 | - | 27 | 12 | 18 | 15 | 19 | 28 | 17 | 27 | - |
| E. coli | 18 | 13 | 9 | 21 | 18 | 8 | - | - | 12 | - | - | 9 | - | 11 | - | 17 | - |
| S. typhi | 13 | 10 | 9 | 14 | 11 | 12 | - | - | 17 | 12 | 11 | 7 | 14 | 14 | 8 | 23 | - |
| S. sonnei | 23 | 15 | 10 | 25 | 22 | 23 | 12 | - | 30 | 21 | 23 | 17 | 21 | 28 | 21 | 30 | - |
| Microorganism | Concentration (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| B. ciliata | M. pudica | P. emblica | Neomycin | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| S. aureus | 1562.5 | 12,500 | 3125 | 12,500 | 6250 | 12,500 | 1.56 | 12.5 |
| E. coli | 1562.5 | 6250 | 1562.5 | 12,500 | 6250 | 12,500 | 15.63 | 62.5 |
| S. typhi | 3125 | 6250 | 1562.5 | 12,500 | 3125 | 6250 | 1.56 | 12.5 |
| S. sonnei | 1562.5 | 12,500 | 3125 | 12,500 | 3125 | 12,500 | 1.56 | 6.25 |
| Annotated Compounds | Calculated Mass | Observed Mass (m/z) | Formula | DBE | Absolute Error (ppm) | Rt Minute | Fragment Peak | Source | References |
|---|---|---|---|---|---|---|---|---|---|
| Bergenin | 328.08 | 329.08 | C14H16O9 | 7.0 | 2.84 | 11.20 | 314.78; 251.05; 237.07; 194.40 | B. ciliata | [29] |
| Afzelechin | 274.08 | 275.08 | C15H14O5 | 9.0 | 0.29 | 13.28 | 257.17, 233.08 | B. ciliata | [30] |
| Epiafzelechin | 274.08 | 275.08 | C15H14O5 | 9.0 | 0.29 | 13.28 | 257.17, 233.08 | B. ciliata | [31,32,33] |
| Orientin | 448.10 | 449.10 | C21H20O11 | 12.0 | 3.18 | 16.34 | 329.36; 299.30 | B. ciliata | [34] |
| Catechin | 290.07 | 291.08 | C15H14O6 | 9 | 1.25 | 12.22 | 313.07 [M + Na] +, and 139.03 | M. pudica | [35,36] |
| Epicatechin | 290.07 | 291.08 | C15H14O6 | 9 | 1.25 | 12.22 | 313.07 [M + Na] +, and 139.03 | M. pudica | [35,36,37] |
| Trihydroxydimethoxyflavone | 330.07 | 331.08 | C17H14O7 | 11 | 0.90 | 15.83 | 301.08, and 315.09 | B. ciliata | [38] |
| Gallocatechin | 306.07 | 307.08 | C15H14O7 | 9 | 0.77 | 10.16 | 329.07 [M + Na] +, 289.07, 139.03 | M. pudica | [36] |
| Epigallocatechin | 306.07 | 307.08 | C15H14O7 | 9 | 2.26 | 7.15 | 329.07 [M + Na] +, 289.07, 139.03 | M. pudica | [36,37,39] |
| Procyanidin B1 | 578.15 | 579.15 | C30H26O12 | 18 | 0.01 | 11.82 | 427.10 [M + H − 152] +, 289.07 (kaempferol) | M. pudica | [36,40] |
| Procyanidin B3 | 578.15 | 579.15 | C30H26O12 | 18 | 0.01 | 11.82 | 427.10 [M + H − 152] +, 289.07 (kaempferol) | M. pudica | [36,41] |
| Chlorogenic acid | 354.09 | 355.10 | C16H18O9 | 8.0 | 0.68 | 11.97 | 193.02 | M. pudica | [42,43] |
| Vitexin | 432.11 | 433.11 | C21H20O10 | 12.0 | 1.81 | 14.30 | 343.04; 313.07; 285.14 | M. pudica | [44] |
| Myricetin | 318.03 | 319.04 | C15H10O8 | 11 | 4.58 | 14.51 | 181.05; 153.01 | M. pudica | [45,46] |
| Isoquercetin | 464.09 | 465.1 | C21H20O12 | 12 | 3.59 | 14.72 | 303.05 (Quercetin), 289.07 (Kaempferol) | P. emblica | [46] |
| Prodelphinidin B3 | 594.13 | 595.14 | C30H26O13 | 18 | 3.23 | 14.79 | 427.08, 169.07, 291.09, 305.07 | P. emblica | [40,46,47] |
| Cassiaoccidentalin B | 576.15 | 577.15 | C27H28O14 | 14.0 | 3.66 | 15.33 | - | P. emblica | [48] |
| Aflotaxin B1 | 328.06 | 329.06 | C17H12O7 | 12.0 | 0.18 | 16.20 | - | P. emblica | [49] |
| Kaempferol | 286.04 | 287.05 | C15H10O6 | 11 | 0.90 | 18.33 | 259.13, 165.09, 153.12 | P. emblica | [50] |
| Emodin | 270.05 | 271.06 | C15H10O5 | 11 | 1.73 | 19.28 | 253.16, 243.17, 229.14, 225.13 and 197.08 | P. emblica | [51] |
| Isorhamnetin | 316.05 | 317.06 | C16H12O7 | 11 | 3.86 | 18.72 | 303.21, 274.20, 153.12 | P. emblica | [52] |
| Methyl gallate | 184.04 | 185.05 | C8H8O5 | 5.0 | 2.38 | 12.43 | 170.97; 127.03 | P. emblica | [44] |
| Quercetin | 302.04 | 303.05 | C15H10O7 | 11.0 | 4.82 | 15.28 | 273.12, 257.13 | P. emblica | [53] |
| Irisflorentin | 386.09 | 387.1 | C20H18O8 | 12 | 1.59 | 11.09 | 357.09 [M + H − CH3 × 2] +, 372.07 [M + H − CH3] + | P. emblica | [54,55,56] |
| Gallic acid | 170.02 | 171.02 | C7H6O5 | 5.0 | 0.62 | 7.30 | 127.03 [M + H − CO2] + | P. emblica | [57] |
| HHDP-glglucose | 482.07 | 483.07 | C20H18O14 | 12.0 | 2.12 | 12.07 | 251.21; 277.03; 303.20; | P. emblica | [58] |
| 2-O-Caffeoylhydroxycitric acid | 370.05 | 371.06 | C15H14O11 | 9.0 | 3.23 | 9.30 | - | P. emblica | [59] |
| 3,4,8,9,10-Pentahydroxydibenzo [b, d]pyran-6-one | 276.04 | 277.06 | C13H8O7 | 10.0 | 1.03 | 13.54 | - | P. emblica | [60] |
| Trigalloyllevoglucosan IX | 618.09 | 619.09 | C20H26O22 | 8.0 | 4.33 | 13.76 | - | P. emblica | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapkota, B.K.; Khadayat, K.; Aryal, B.; Bashyal, J.; Jaisi, S.; Parajuli, N. LC-HRMS-Based Profiling: Antibacterial and Lipase Inhibitory Activities of Some Medicinal Plants for the Remedy of Obesity. Sci. Pharm. 2022, 90, 55. https://doi.org/10.3390/scipharm90030055
Sapkota BK, Khadayat K, Aryal B, Bashyal J, Jaisi S, Parajuli N. LC-HRMS-Based Profiling: Antibacterial and Lipase Inhibitory Activities of Some Medicinal Plants for the Remedy of Obesity. Scientia Pharmaceutica. 2022; 90(3):55. https://doi.org/10.3390/scipharm90030055
Chicago/Turabian StyleSapkota, Basanta Kumar, Karan Khadayat, Babita Aryal, Jyoti Bashyal, Shankar Jaisi, and Niranjan Parajuli. 2022. "LC-HRMS-Based Profiling: Antibacterial and Lipase Inhibitory Activities of Some Medicinal Plants for the Remedy of Obesity" Scientia Pharmaceutica 90, no. 3: 55. https://doi.org/10.3390/scipharm90030055
APA StyleSapkota, B. K., Khadayat, K., Aryal, B., Bashyal, J., Jaisi, S., & Parajuli, N. (2022). LC-HRMS-Based Profiling: Antibacterial and Lipase Inhibitory Activities of Some Medicinal Plants for the Remedy of Obesity. Scientia Pharmaceutica, 90(3), 55. https://doi.org/10.3390/scipharm90030055







