Abstract
Wound healing comprises organized events involving tissue repair and regeneration. The discovery of toll-like receptors (TLRs) sheds recent light on the mechanisms involved in initiating inflammatory responses throughout the healing cascades. Hibiscus sabdariffa (HS) components may exhibit a wound healing action, owing to their antioxidant and anti-inflammatory activities. This study was designed to investigate the early effects of HS loaded in an ointment base on wound healing, antioxidant, antimicrobial effects, burning intensity, and histopathological features on the rat burn model in comparison to the standard treatment, Iruxol® ointment. A burn injury model was used to evaluate the wound healing potency of the preparation. Rats were treated with ointments three times on the day of the induction of the burn. Findings revealed that the strong antioxidant properties of the HS-loaded ointment augmented the skin healing potential by stimulating biomarkers required for skin regeneration. HS repressed the burning-induced inflammation by the effective reduction in the levels of tumor necrosis factor α (TNF-α) and IL-6 through TLR4 protein inhibition. Topical HS downregulates transforming growth factor-beta (TGF-β) levels. HS extract possesses a potential bactericidal activity against highly resistant clinical isolates of Pseudomonas aeruginosa. Overall, this study proclaims that HS-loaded topical preparations could be a valuable product that serves as adjuvants to accelerate burn wound healing through inactivating the TLR4 pathway.
1. Introduction
Burn wounds are physical injuries caused by the opening or breaking of the skin. Burn wound healing comprises a cascade of biochemical and cellular events that include tissue repair and renewal [1,2]. Inflammatory cytokines stimulate growth factors, such as TGF-β and a group of fibroblast growth factors, leading to infiltration of stimulated fibroblasts to the wounded area. Although an accurate level of cytokines is vital for healing, abnormal levels of proinflammatory cytokines lead to disproportionate fibroblast production, thereby initiating hypertrophic scarring, which leads to substantial deformity in skin tissue [3]. Therefore, proinflammatory cytokines and growth factors play a pivotal part in the consequences of inflammation [4,5,6]. In spite of the substantial role of macrophages in wound healing, they produce more pro-inflammatory cytokines (TNF-α) and interleukin (IL-6) in early wounds and less TGF-β, whereas the opposite is observed in later stages of wound healing [7,8].
All TLRs have an extremely homologous cytoplasmic toll/interleukin (IL)-1 receptor domain (TIR), a transmembrane domain, and an extracellular domain. TLR4 signaling is one of the vital mechanisms for inflammation [7,9] and is a major receptor involved in different physiological circumstances, including wounds, shock, and autoimmunity. TLR4 has been explored as a target to inhibit interactions, deliver signaling molecules that initiate inflammatory responses [10,11], sustain epithelial cell integrity, and encourage recovery from injury [12]. Furthermore, TLR4 stimulation enhances TGF-β activity through resident accumulation of endogenous TLR4 ligands named “damage-associated molecular patterns (DAMPs), which, in sequence, stimulate innate immune signaling in local fibroblasts via TLR4 [9], resulting in augmented matrix and TGF-β production [13]. There are two TLR signaling pathways: the MyD88-dependent pathway and the MyD88-independent pathway (The TIR domain-containing adapter-inducing interferon (IFN)-β-dependent pathway) [14]. The MyD88-dependent pathway caused the induction of NFκβ- dependent transcription of inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-8, IL-12, and MIP2 [15]. The MyD88-independent pathway promotes the Nfκβ and IFN regulatory factors, which in turn upregulate the inflammatory cytokines and IFN genes, respectively [16].
Burn wounds are easily infected with microbes which slow down the healing process and may badly deteriorate into systemic infections [17,18]. Topical antiseptics and antimicrobial therapy are crucial to control microbial colonization, thus preventing the development of invasive infection [19]. P. aeruginosa is a leading cause of burn wound infection, leading to fatal issues if not properly treated. P. aeruginosa is inherently resistant to most antibiotics [20,21], which represents a further challenge to managing burn wounds infected with such bacteria.
Hibiscus sabdariffa L. (roselle) (HS) is commonly used in traditional remedies for its abundant content of phytochemicals, such as polyphenols. HS is used in the treatment of several progressive diseases such as hyperlipidemia, hypertension, cancer, and inflammatory diseases of the kidney and liver. HS active constituents are known to have a preventive effect against chronic and degenerative diseases associated with oxidative stress [22]. Its polyphenol content, particularly anthocyanins, may be responsible for its antioxidant capability. Among its anthocyanins, 85% comprise delphinidin-3-sambubioside (Dp3-Sam), which is the major source of the antioxidant effect of roselle extract and could inhibit the proliferation of human leukemia cells by inducing apoptosis, which was characterized by the activation of caspase-3, -8, and -9 and cell morphology, DNA fragmentation, and inactivation of poly (ADP) ribose polymerase (PARP). In addition, recent studies show that anthocyanin aglycons, especially cyanidin (Cy) and delphinidin (Dp), could reverse the in vitro expression of inflammatory mediators, such as prostaglandin (PG) E2 and cyclooxygenase (COX-2) [23]. The antibacterial potential of HS extracts was previously evaluated against broad spectrum of bacterial strains [24,25], however, it is necessary to test this activity against P. aeruginosa clinical isolates obtained from wound burn infection.
The emergency of burn cases is crucial in the first few hours and would, in fact, account for morbidity and mortality. This study investigates whether HS has the potential to perform as an active wound healing agent, and to test whether such potential has antioxidant and anti-inflammatory activities in an in vivo model and evaluates the bactericidal activity against wound isolates of P. aeruginosa.
2. Materials and Methods
2.1. Collection of Plant and Preparation of the Extract
Fresh flowers of HS were obtained from Aswan cultivars, and then seeded in the botanical garden of the Faculty of Pharmacy, Delta University for Science and Technology. Extraction was performed as described [26]. Briefly, the flowers were shade-dried at 25 °C, milled at room temperature using the grinder from a mortar and pestle, and sieved by making them pass over a 50 µm mesh size sieve. Next, 372.58 g of fine flower powder was suspended in 1000 mL of 70% ethyl alcohol for 48 h at room temperature. The residue was removed by filtration (1-mm pore size), and then the extract was effectively concentrated using a rotary evaporator for 1 h. The solid residue was weighed, then the extract was aliquoted and kept at −20 °C for further experiments.
2.2. Qualitative Phytochemical Analysis
Qualitative phytochemical analysis was carried out to identify different phytochemicals present in ethanol extracts of Hibiscus sabdariffa flowers [27]. The main phytochemical groups were free amino acids, proteins, carbohydrates, gums, alkaloids, glycosides, fixed oil, phytosterols, phenolic compounds, mucilage, saponins, lignins, and flavonoids with a high degree of precipitation (+++), while terpenoids and resins were determined to be present with a lesser amount (+) in ethanol extracts.
2.3. Chemicals and Reagents
Liquid paraffin and white soft paraffin were supplied from the Pharmaceutics Department, Delta University for Science and Technology. A commercially available ointment (Iruxol® ointment) containing 1.2 IU of collaginase, Smith nephew, Knoll Farmaceutica SpA, Muggio, Italy) was used as a standard ointment for comparing the wound healing potential of the plant extract. Analytical grade chemicals and reagents were used.
2.4. Formulation of the Ointment
The ointment formulation (10% (w/w)) was prepared by infusing 10 g of the extract into 100 g of a simple ointment base (British Pharmacopoeia) [28]. It was prepared using a simple ointment base (liquid paraffin (33.32 g), and white soft paraffin (144.44 g), to which 22.22 g of the extract was added. First, all the extract and liquid paraffin were supplemented and mixed with constant shaking in a water bath at 60 °C to achieve a gel-like uniformity. White soft paraffin was allowed to melt, then the drug and liquid paraffin mixture were added portion-by-portion into the melted white soft paraffin and mixed with constant stirring.
2.5. Evaluation of Ointment Stability
The freshly prepared, cooled ointment was evaluated for color and appearance by visualization, and its consistency and homogeneity were also evaluated by rubbing within the fore and first fingers. Each ointment sample was located in a wide-mouthed plastic container and kept at −5 °C, 25 °C, and 40 °C. The homogeneity, color/appearance, and consistency of these samples were checked daily for 14 days for any alterations. The pH values of the freshly prepared ointment were determined by dispensing one gram of the extract in 100 mL of distilled water using a pH meter (accumet research AR10, Singapore). Ointment samples were put in storage at room temperature, and their pH was checked daily for 14 days.
2.6. Testing for Acute Skin Irritation and Toxicity of the Ointment
The ointment was used for evaluating acute irritation and toxicity of the skin at 10% w/w according to the OECD guideline 402. The ointment was applied after shaving the dorsal hair of rats (N = 6). The treated region was checked for adverse dermal reactions such as erythema, irritation, inflammation, and itching, and compared to the control group treated with the ointment base but without the drug (placebo). The ointment stability, skin irritation, and toxicity assays regarding erythema, irritation, inflammation and itching were confirmed to use the preparation before starting our study.
2.7. Animals and Experimental Protocols
Experimental protocols followed the ARRIVE guidelines of the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs). Adult male Wistar rats, with a typical weight of 200–250 g, were obtained from the animal facility at the faculty of pharmacy, Delta University (FPDU), and were accommodated in polypropylene cages with ad-lib access to basal diet and water. The animals were allowed to acclimatize for one week. Furthermore, all procedures were approved by the Institutional Animal Care and Use Committee at the FPDU (FPDU 10/2021). Rats were anesthetized with thiopental anesthesia (40 mg/kg body weight) before commencing experimental wounds, and surgical interventions were aseptically performed. Only healthy animals free from infections were used. Wound models were commonly used to evaluate the wound’s healing activity [29]. Animals were divided into four groups of six, each as follows: Normal, the ointment base was topically applied to rat skin (Negative control, placebo); 2nd degree burn, burns were topically applied with the ointment base (Positive control, placebo); 2nd degree burn-Iruxol, burns were topically applied with the standard Iruxol® ointment; 2nd degree burn-Iruxol, burns were topically applied with the prepared roselle extract ointment.
2.8. Induction of Burn
Burn wounds were done as described by Cai et al. [30]. Briefly, the dorsal back of rats was smoothly shaved. A 100-g cylindrical stainless-steel rod (1cm-diameter) was heated to 100 °C in boiling water. Contact burns were induced by placing the heated rod on the dorsum for 8 s. The full 1 cm-diameter burned tissue was collected, including epidermis, dermis, and hypodermis, in which there was only one wound per animal. This procedure has been confirmed histologically to induce full-thickness injuries. The simple ointment base, formulated extract ointment, and standard drug were applied to the entire burn area three times/day.
2.9. Collection of Tissues
Tissues from control and test groups were harvested after 36-h of exposure to the wound and treatments. Tissues were washed in cold saline (0.9% NaCl) to remove blood. The harvested cylinder-like tissue was collected [23] and cut longitudinally into two halves. One portion was fixed in 10% formalin for 24 h for histopathological examinations. The second half was stored at −80 °C until further determination of antioxidant and inflammatory markers. To determine antioxidant and inflammatory markers, the harvested skin tissue was homogenized in Tris Buffered Saline (TBS) [50 mM Tris-HCl: 150 mM NaCl, 1:2] at pH 7.4 in Ultra-Turax homogenizer. The homogenates were then centrifuged at 4 °C (1500× g, 15 min).
2.10. Assessment of Burn Intensity
Burn intensity was assessed based on a collective arbitrary scale depending on the following aspects: blistering, swelling, redness, crust, and secretion. A score was given to each clinical feature when being established as follows: None, 0; mild, 1; moderate, 2; and severe, 3. The scoring criteria were blindly visualized by a physician who is an experienced dermatologist in the field of skin care. A score for each feature was given to each experimental rat and added to every other individual feature score to produce the total burn intensity value for each rat. The burn intensity value in a rat group is the average of the calculated scores.
2.11. Histological and Immunohistochemical Analysis
A standard histological technique was conducted [31]. Tissue samples were stored in 10% formalin buffer solution, dehydrated, and then blocked with paraffin wax. Each blocked skin sample was cut into 5-μm-thick sections and stained with hematoxylin and eosin (H&E) stain. The skin sections were checked under light microscope, and microphotographs were captured. Tissues obtained from the test group animals were also stained with Masson’s trichrome, which is used to identify collagen deposition. Areas were quantified as area (A) % using the Image J 1.52n software (NIH, Bethesda, MD, USA). The extent of collagen production was expressed as the % of stained area relative to the total area. IL-6 immunolabeling was developed using IL-6 antibodies (NB600-1131, Novus Biologicals, Littleton, CO, USA). The immunoreactivity was indicated by calculating the immunopositive cells/1000 cell count.
2.12. Determination of Total Antioxidant Capacity
Total antioxidant capacity (TAC) was determined using an enzymatic colorimetric method. All measurements followed the instructions of the manufacturer of the kit (Biodiagnostic, Cairo, Egypt). The antioxidant capacity was determined based on the reaction of antioxidants in the sample with a defined amount of exogenously provided H2O2. The antioxidants present in the sample reduce a certain H2O2 amount. The remaining H2O2 is calorimetrically assayed in an enzymatic reaction that comprises the conversion of 3,5-dichloro-2-hydroxybenzenesulfonate into a colored product.
2.13. Determination of Inflammatory Markers
The protein concentrations of TNF-α and TGF-β were quantified using ELISA according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). Absorbance was read against a blank in a Spectrum Max Plus 384 spectrophotometer (Sunnyvale, CA, USA), at dual wave lengths of 450 nm and 570 nm. Cytokine’s concentrations were calculated using standard curves obtained from recombinant cytokine activities.
2.14. Western Blot Analysis for TLR4 Expression
Protein extraction from the skin tissues was performed using a radioimmunoprecipitation assay buffer (RIPA) (Bio Basic Inc., Markham, ON, Canada). Protein concentrations were estimated using the Bradford method. For each lane, 20 µg of protein was used and separated by 10% Sodium dodecyl sulphate-Polyacrylamide gel electrophoresis SDS-PAGE (Bio-Rad Laboratories, TNC, Hercules, CA, USA) and then transferred electrophoretically to pol-vinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were then incubated at RT for 2 h with a blocking solution consisting of 5% non-fat dried milk in TBST (10 mM Tris-Cl, 100 mM NaCl, and 0.1% Tween 20 (pH 7.5)). Next, the membranes were incubated overnight at 4 °C with monoclonal antibodies specific for TLR4 and GAPDH and then with rabbit anti-rat secondary antibody conjugated to horseradish peroxidase (Thermo Fisher Scientific Inc., Waltham, MA, USA) at RT for 2 h. This was followed by washing four times with TBST at RT after each incubation. A chemiluminescent substrate (Clarity™ Western ECL substrate, BIO-RAD, Hercules, CA, USA) was used to detect chemiluminescence according to the manufacturer’s instructions. Then, the chemiluminescent signals were captured using a CCD camera-based imager, followed by image analysis to read the band intensity of the target proteins against the control sample after normalization by GAPDH on the Chemi Doc MP imager.
2.15. Antibiotic Sensitivity and Evaluation of Antibacterial Activity
The clinical isolates of P. aeruginosa used in this study were obtained from the collection bank of the Microbiology and Immunology Lab, Faculty of Pharmacy, University of Zagazig. Bacterial isolates were recovered from human burn wound exudates and were purified and identified by morphology, pigmentation, Gram stain, biochemical tests, and growth on selective media. Antibiotic sensitivity was determined for the clinical isolates of P. aeruginosa by the disk diffusion method (DDM) [32] according to the Clinical and Laboratory Standard Institute. Bacterial culture media were purchased from Oxoid, (Hampshire, UK), and Hi-Media, (India), antibiotic discs were obtained from Oxoid, (Hampshire, UK) including Amikacin (AK, 30 μg), ampicillin (AM, 10 μg), ceftazidime (CAZ, 30 μg), cefoperazone (CFP, 75 μg), ciprofloxacin (CIP, 5 μg), gentamicin (CN, 10 μg), cefepime (FEP, 30 μg), gatifloxacin (GAT, 5 μg), and piperacillin/tazobactam (TPZ, 110 μg).
In vitro antibacterial activity of HS ethanolic extract was determined using cut-well agar diffusion [33]. Briefly, each bacterial isolate was grown in 20 mL of tryptone soya broth medium (TSB) at 37 °C for 2 days with constant shaking at 150 rpm. A total of 100 uL of 0.5 McFarland turbidity standards (1.0 × 108 CFU/mL) of each strain were streaked over a Mueller–Hinton agar plate using a cotton swab, and wells of 6 mm were prepared. Then, 150 μL of HS ethanolic extract at a concentration of 250 mg/mL was loaded into the well. The plate was incubated for 24 h at 37 °C. The experiment was done in triplicates and the mean diameter of the zone of inhibition around the well was scored.
2.16. Statistical Analysis
Data were expressed as the mean ± standard deviation (SD). Statistical analysis was conducted using the GraphPad Prism version 6 (GraphPad Software Inc., La Jolla, CA, USA). Differences between groups were examined using one-way ANOVA, followed by Tukey’s Kramer multiple comparison test. Scores of burn intensity were expressed as the median ± interquartile range and were compared using the Kruskal-Wallis test, followed by Dunn’s multiple comparison test.
3. Results
3.1. Effect of HS on the Intensity of 2nd Degree Burn Wound
The effect of HS on the intensity of 2nd degree burns is presented in Figure 1. At the end of the experiment, the burn group showed a significant elevation in the intensity of the burn compared with that of the normal unexposed group. The Iruxol-treated group showed that the intensity was significantly decreased to 40% compared to the burn group, whereas it was significantly increased only to 20% compared to the normal group. The HS-treated group showed that the intensity was significantly decreased by 50% compared to the burn group and was significantly increased to 30% compared with the normal group.
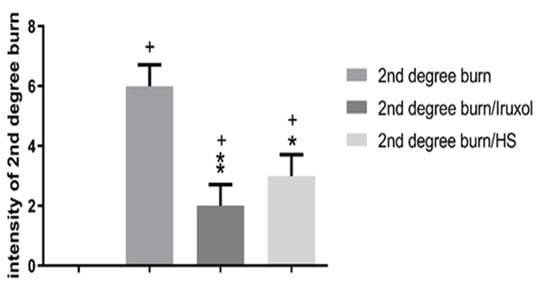
Figure 1.
Intensity of 2nd degree burn in different groups. HS: Hibiscus sabdariffa, +, p < 0.05 vs. normal group; *, p < 0.05 vs. 2nd degree burn group; **, p < 0.01 vs. 2nd degree burn group. Differences between groups were examined by Kruskal-Wallis followed by Dunn’s multiple comparison test. n = 6.
3.2. Effect of HS on Collagen Deposition
The effect of HS on collagen deposition is shown in Figure 2. Collagen deposition significantly increased in the burn group compared with that of the normal group. Collagen deposition was significantly decreased in the Iruxol-treated group compared to the burn group (p < 0.01). Additionally, the HS-treated group showed a significant decrease in collagen deposition compared to that of the burn group (p < 0.05).
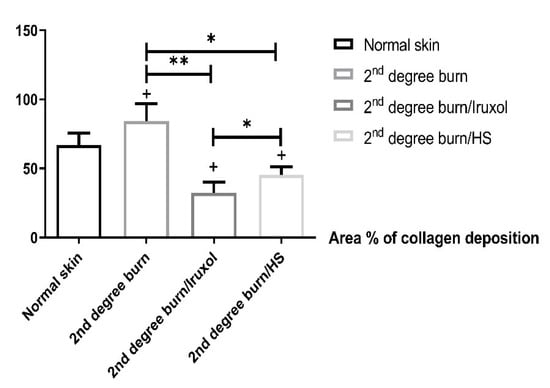
Figure 2.
Percentage of collagen deposition. HS: Hibiscus sabdariffa, +, (p < 0.05) vs. normal group; *, **, significant difference between indicated pairs (p < 0.05, p < 0.01, respectively). Differences between groups were examined by ANOVA/Tukey’s Kramer multiple comparison test. n = 6.
3.3. Histopathological Examination of Skin Tissues
The photomicrographs of the H&E and the Masson’s trichrome staining revealed a normal epidermal layer formed of stratified epithelium with marked keratinization, and a normal dermal layer contained numerous hair follicles in the normal skin group. The 2nd degree burn group exhibited a minimal degree of epithelialization with subepithelial edema. However, treatment with either Iruxol or HS resulted in a marked epithelialization degree and normal dermal hair follicle appendages. Masson’s trichrome staining indicated that the normal dermal layer consisted primarily of parallel collagen. The burn group was associated with dermal collagen formation. In contrast, Iruxol or HS treatments displayed minimal dermal collagen formation and resulted in a marked epithelialization degree with normal dermal hair follicle appendages (Figure 3).
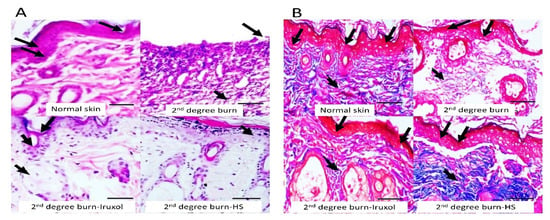
Figure 3.
Histopathological examination of skin tissues from different groups by H&E staining. The left panel (A) shows histopathological examination of skin tissues from different groups by H&E staining, ×200. The normal epidermal layer formed of stratified epithelium with marked keratinization and normal dermal layer (arrows). The 2nd degree burn group exhibited a minimal degree of epithelialization (arrow) with subepithelial edema (short arrow). In contrast, treatment with Iruxol resulted in marked epithelialization degree (arrow) and normal dermal hair follicle appendages (short arrow). Treatment with HS resulted in marked epithelialization (short arrow). Bar = 100 µm. The right panel (B) illustrates the Masson’s trichrome staining, ×200. Masson’s trichrome staining indicated the normal dermal layer (arrows) and primary parallel collagen (short arrow). The 2nd degree burn group was associated with disturbed epithelia (arrows) and massive dermal collagen formation (short arrow). In contrast, treatment with Iruxol resulted in a less disturbed dermal layer (arrows) and minimal dermal collagen formation (short arrow). Additionally, treatment with HS resulted in a less disturbed dermal layer (arrows) and minimal dermal collagen formation (short arrow). Bar = 100 µm.
3.4. Immunostaining of IL-6
A marked expression of IL-6 within the dermal layer of the burn group, as indicated upon calculation of the % immunopositive cells/1000 counted cell was clearly displayed. While the treated groups with either Iruxol or HS showed a marked decrease in the expression of IL-6 (Figure 4).
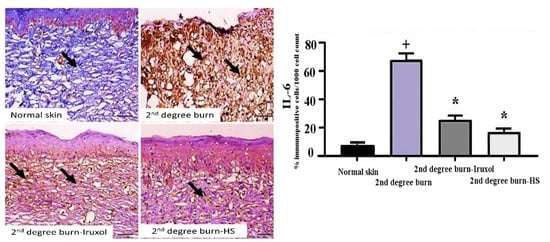
Figure 4.
Immunohistochemical detection of IL-6 in skin tissues. Immunohistochemical detection of IL-6 in skin tissues, ×200. Arrows indicate expression of IL-6 within the dermal layers of different groups. Marked immunolabeling was detected in the 2nd degree burn group of rats, while decreased expression was detected in the treatment groups. Bar = 100 µm. +, p < 0.05 vs. normal group; *, p < 0.05 vs. 2nd degree burn group.
3.5. Effect of HS on Total Antioxidant Capacity (TAC)
The burn group showed a significant (p < 0.05) decrease in the level of TAC compared to the normal group. In the Iruxol-treated groups, there was a non-significant change in the levels of TAC compared to the burn group. However, in the HS-treated groups, there was a significant increase in the levels of TAC compared to the burn group (p < 0.05) Figure 5.
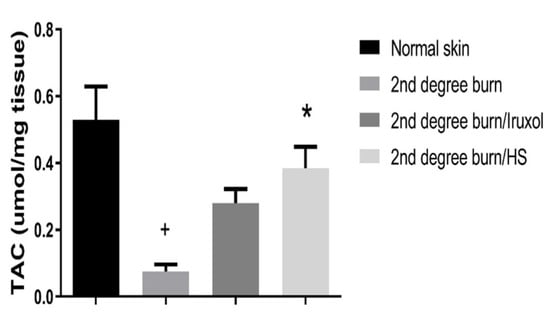
Figure 5.
Effect of HS on total antioxidant capacity (TAC). TAC: Total antioxidant capacity; HS: Hibiscus sabdariffa; +, (p < 0.05) vs. normal group; *, (p < 0.05) vs. 2nd degree burn group. Differences between groups were examined by ANOVA/Tukey-Kramer’s multiple comparison test. n = 6.
3.6. Effect of HS on TNF-α
The burn group showed a significant increase in the levels of TNF-α (p < 0.05) compared to the normal group. The Iruxol- or HS-treated group of rats exhibited a significant decrease in TNF-α level (p < 0.05) compared to the burn group (Figure 6).
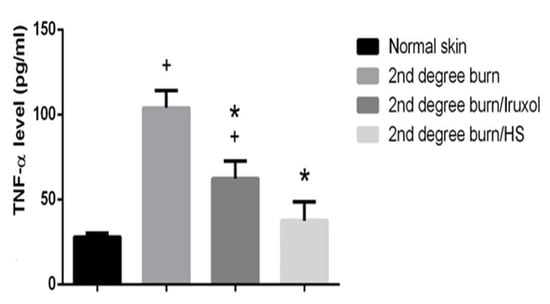
Figure 6.
Effect of HS on TNF-α. TNF-α: Tumor necrosis factor alpha; HS: Hibiscus sabdariffa; +, (p < 0.05) vs. normal group; *, (p < 0.05) vs. 2nd degree burn group. Differences between groups were examined by ANOVA/Tukey/Kramer’s multiple comparison test. n = 6.
3.7. Effect of HS on TGF-β
The burn group showed a significantly increased level of TGF-β compared to the normal group. The Iruxol-treated group showed non-significant changes in the level of TGF-β compared to that of the burn. However, the HS-treated group showed a significant decrease in those levels compared to the burn group (p < 0.05) (Figure 7).
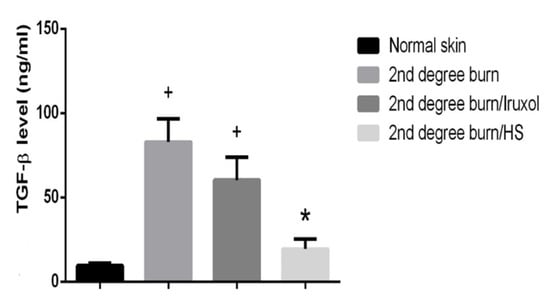
Figure 7.
Effect of HS on transforming growth factor β (TGF-β) level. TGF-β: transforming growth factor-beta; HS: Hibiscus sabdariffa; +, (p < 0.05) vs. normal group; *, (p < 0.05) vs. 2nd degree burn group. Differences between groups were examined by ANOVA/Tukey/Kramer’s multiple comparison test. n = 6.
3.8. Effect of HS on TLR4 Expression in Different Groups
The burn group showed a significant (p < 0.05) increase (8- fold) in the relative expression of TLR4 compared with that of the normal group. The Iruxol- or HS-treated groups showed that TLR4 expression was significantly decreased (p < 0.05) compared to the burn group. However, the HS-treated group exhibited a significant decrease (p < 0.05) in the expression of TLR4 compared to that of the Iruxol-treated group (Figure 8).
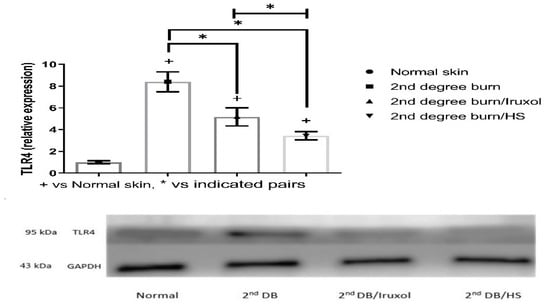
Figure 8.
Effect of HS on Toll-like receptor 4 (TLR4) expression in different groups. TLR4: Toll-like receptor 4; HS: Hibiscus sabdariffa; +, p < 0.05 vs. normal group; *, p < 0.05 vs. 2nd degree burn group. Group differences were examined by ANOVA/Tukey/Kramer’s multiple comparison test. n = 6.
3.9. Antibacterial Activity of HS on Burn Wound Isolates of P. aeruginosa
We finally evaluated the bactericidal activity of HS extract against 2 clinical isolates of P. aeruginosa obtained from burn wound exudates. While the 2 isolates exhibited prominent resistance against different categories of antibiotics that target the bacterial cell wall, the bacterial ribosomes, or the bacterial DNA replicating enzymes, HS ethanolic extract at a concentration of 250 mg/mL clearly exhibits a bactericidal effect against wound isolates of P. aeruginosa, giving a zone of inhibition ranging from (24 ± 5) to (33 ± 3.6) mm in diameter (Figure 9).
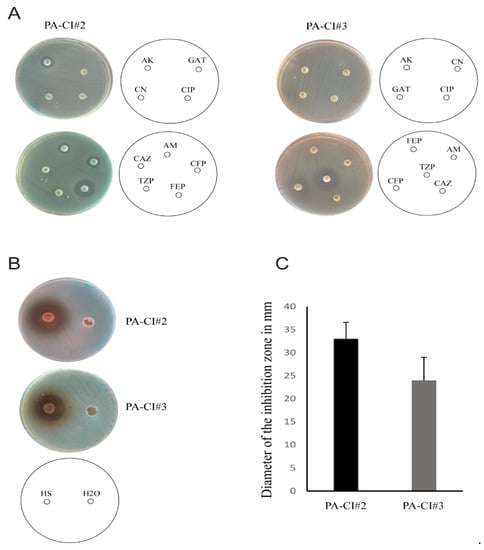
Figure 9.
Antibacterial activity of HS ethanolic extract against multidrug resistant P. aeruginosa. (A) Antibiotic sensitivity by DDM of 2 clinical isolates of P. aeruginosa isolated from burn wound exudates against different antibiotics (bacterial isolate was defined as multidrug resistant when resistance against 3 or more categories of antibiotics was detected. Amikacin (AK, 30 μg), ampicillin (AM, 10 μg), ceftazidime (CAZ, 30 μg), cefoperazone (CFP, 75 μg), ciprofloxacin (CIP, 5 μg), gentamicin (CN, 10 μg), cefepime (FEP, 30 μg), gatifloxacin (GAT, 5 μg), and piperacillin/tazobactam (TPZ, 110 μg). (B) Bactericidal activity of HS ethanolic extract by cut-well agar diffusion against the 2 bacterial isolates in A. (C) Diameters of the zone of inhibitions in B (the bar indicates the mean of 3 independent experiments ± standard deviation).
4. Discussion
Burn wound healing requires a well-organized cascade of biochemical and cellular events that involve tissue repair and regeneration. HS (roselle) is a widely used commercial traditional medicine, due to which it was primarily selected in our study. This study proposed that the content of polyphenols, especially anthocyanins, in roselle may be responsible for its antioxidant and anti-inflammatory properties, essential for burn wound healing [34]. We intended to evaluate the effect of HS alcoholic extract loaded in an ointment base on healing based on its potential to regulate the link between TLR4 and TGF-β signaling pathways. Inhibition of these pathways may stimulate the antioxidant and anti-inflammatory effects of HS, which may be relevant to the observed skin regeneration in rats with superficial skin excision wounds.
The authors have preferred the ointment form as a base for hibiscus extract to ensure close contact to the site of application and sustained release of the components of the extract. In addition, the simple preparation and the low cost of ointment compared to other forms, such as spray, encourage the authors to prepare the ointment form.
Iruxol® is a topical collagenase preparation used for enzymatic debridement of necrotic tissue caused by ulcers and burns, and hence it was used for the purpose of comparison as a standard treatment in this study. Iruxol® contains the collagenase proteolytic enzyme derived from Clostridium histolyticum. Collagenase digests denatured collagen, cleanses necrotic tissues, and destroys the strands of endogenous collagen, which attach the necrotic residues to the wound bed. Using Iruxol® in uncomplicated wounds is considered to be well tolerated [35]. In the present study, at the end of Iruxol® treatment, the superficial burns exhibited dramatic healing percentages (40%) with the absence of charring and eschar due to its collagenase activity, which corresponded to the percentage of collagen deposition as evaluated using the Image J software.
In the case of HS ointment, healing occurred significantly but to a lower degree than that of Iruxol® treatment. This may be due to the autolytic debridement caused by topically applied natural products such as roselle. Our findings converge with the autolytic debridement with support from topically applied natural products such as honey, which is believed to work in the same way as the self-healing process to cleanse the wound from tissue debris. The healing activity of honey is attributed to its acidity, suppressing destructive proteases, and osmotic effect on the wound bed [36].
A pivotal crosstalk has been recognized between TLR4 and TGF-β signaling cascades and wound healing. Until now, HS is the first suggestion to be used an inhibitor for both TLR4, TGF-β and collagen deposition upon tissue damage. Hence, it is recognized that oxidative stress has a serious role in the origin and development of wounds. The effects of both Iruxol® and HS on burn wound healing were confirmed by histopathological examination, which revealed marked epithelialization degree, normal dermal hair follicle appendages, and minimal dermal collagen formation in both groups compared with those in the burn untreated group. Burning wounds in rats are similar to human wound injuries in terms of histopathological features, characterized by a minimal degree of epithelialization, which significantly contributes to the progression of subepithelial edema through the production of dermal collagen. Moreover, tissue injury correlates with a strong local immune response that is connected to augmented production of cytokines, particularly TNF-α, that can trigger an oxidative burst [37].
Emerging evidence indicates that TLR4 participates in the stimulation of innate immune response by triggering signaling cascades along with the release of several inflammatory cytokines such as TNF-α [38,39]. Here, we found that HS ointment downregulates TNF-α to <50 pg/mL, as the normal skin TNF-α level is <50 pg/mL [40]. Comparison between Iruxol® and HS treatment results showed that HS treatment resulted in less tissue TNF-α levels than those obtained with Iruxol® ointment treatment, as the latter value was >50 pg/mL. This implies that roselle extract has an anti-inflammatory effect that helps in burn healing. A previous study reported that the levels of IL-6, nitric oxide (NO), and TNF-α in inflammatory cells could be significantly reduced by HS treatment.
These inflammatory cytokines lead to the initiation of growth factor release, leading to the infiltration of stimulated fibroblasts to the wounded area. Although the correct level of each cytokine is important for healing, abnormal levels of proinflammatory cytokines lead to extreme fibroblast proliferation, resulting in hypertrophic scarring, which leads to robust scarring [41].
In this study, the application of either Iruxol® or HS ointments caused the TGF-β level to significantly decrease. Previously, studies reported that new tissue development and scar formation are two crucial steps in the healing process. Fibroblasts are the primary cells in the healing process since they migrate to the wound where they proliferate. Then, these cells produce the extracellular collagen matrix. The functions of fibroblasts are regulated by growth factors [42]. Recently, there has been a focus on the double-edged weapon of TGF-β level through hypertrophic scarring, which is a common occurrence in burn injuries, with an incidence of 70% [43]. Moreover, the importance of the TGF-β level in keeping the vascular activity has been established in several studies, which have shown that increased TGF-β level and its receptors may impair vessels formation [44].
However, the present data shows that HS has a greater antioxidant effect than Iruxol® ointment when topically applied three times/day. Numerous in vitro and in vivo reports have confirmed that extracts of roselle possess actual antioxidant potential [45]. Wound progression is concomitant with oxidative stress that arises because of the disturbance in the balance between ROS production and endogenous antioxidant protection mechanism, which disturbs the biomolecules functioning in a reversible or irreversible manner [46]. It was established that ordinary cellular antioxidants such as superoxide dismutase and catalase participate in oxygen-protection responses in the tissue matrix by producing water and molecular dioxygen from superoxide. Increasing glutathione levels in the tissue matrix results in enhanced glutathione peroxidase, which by turn reduces peroxides [47,48]. The present study’s results indicate a significant increase in TAC in the HS-ointment-applied group as evidenced by the neutralization of additional free radicals in the wound, owing to the phenolic nature of HS, which ultimately encourages a synergistic outcome in wound healing [49].
It has been described that such effects were addressed for both alcoholic and aqueous HS extracts of flowers, seeds, or leaves through shielding effect against tert-butyl hydroperoxide (t-BHP)-induced oxidative damage. This activity of HS is due to the inhibition of xanthine oxidase activity [50], cytoprotection from impairment by lipid peroxidation, inhibition of Cu2+-mediated oxidation of LDL, and the creation of thio-barbituric acid reactive substances (TBARs), maintaining the formation of malondialdehyde content, reduction of GSH exhaustion, and the increase in SOD and CAT activities [51].
Microbial contamination of wounds and burns greatly slows down the recovery, interferes with the healing process, complicates the management, and may lead to invasive infection. P. aeruginosa is a gram-negative bacterium that commonly infects wounds and burns, leading to invasive burn sepsis, which requires prompt therapeutic intervention [52]. One of the key aspects of burn care guidelines is to prevent infection and colonization of P. aeruginosa; however, this pathogen is rapidly developing antimicrobial resistance against most commonly used antibiotics. Broad spectrum antibacterial activity of HS ethanolic extract was previously addressed [53], but our study demonstrated a potential bactericidal activity of HS extract in tolerable concentrations against burn wound isolates of P. aeruginosa that exhibit multidrug resistance against different categories of antibiotics. Taken together, HS extract exhibits a dual role in wound healing through its anti-inflammatory activity, which is necessary for skin regeneration, and its antibacterial effect, which prevents microbial colonization and invasive infection.
The definitive challenges and limitations of this study were in confirming the early emergency of HS topical ointment. Other studies with different time points would be helpful to assess the effect of the tested ointments on the outcomes over time.
Additionally, the antimicrobial activity of HS extract against the most common and resistant pathogen infecting wounds and burns (Pseudomonas aeruginosa) as another axis to explain the enhanced wound and burn healing of HS extract. We will need advanced strategies to service the design for burn infection with the same clinical isolates of P. aeruginosa to test whether the in vitro concentration of HS extract in the agar diffusion test would match the in vivo dose with possible requirements and times of follow-up. On the other hand, there is a need to test the possible contents of P. aeruginosa, e.g., protease, hemolysin, phospholipases, catalases, pyocyanin, and biofilm to determine the virulence factors of P. aeruginosa in the early stages of the burn. A supportive point of the present study was that it reflected the minimum inhibitory concentration (MIC) by agar diffusion assay that was done after consistent screening of the MBC by 2-fold serial dilution of the HS extract in broth cultures. The control was the solvent of the HS extract. We selected one antibiotic Meropenem as a control for inhibition of bacterial survival that aimed to fill the missing parts of the antimicrobial study.
5. Conclusions
HS ointment has an important role in healing wounds. HS extract enhanced the skin’s healing capacity by accelerating the healing process itself and stimulating the levels of skin regeneration biomarkers through its strong antioxidant and anti-inflammatory effects. Moreover, HS topical treatment significantly reduced GF-β levels. Therefore, it would be encouraging to discover natural medications that have the possibility to control the link between these TLR4 and TGF-β signaling pathways. In addition, HS extract has remarkable bactericidal activity against wound pathogens. This study suggests that HS is a valuable extract to be used in cosmetics and alternative medicine, which may also be considered for use in combination with Iruxol® as a synergistic product to accelerate wound healing.
Author Contributions
R.K., contributed to conception, data acquisition and analysis, manuscript synthesis; G.Y. and M.S.A.-H., performed the microbiology experiments, collected and wrote the data interpretation; D.J., G.S.E.-T., H.E. and C.M., contributed to data analysis and discussion, manuscript synthesis; W.S.A., conducted the histological examinations, Western blot analysis, image analysis, data validation; S.S. and S.C., performed animal experiments, ELISA, software, conducted statistical analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
All procedures were approved by the Institutional Animal Care and Use Committee at the FPDU (FPDU 10/2021).
Data Availability Statement
The data obtained after analysis in the current study are available from the relevant authors upon reasonable request.
Acknowledgments
Authors appreciate the support and encouragement provided by Delta University students (Esraa E. Borham, Laila A.Ibrahim, Mirvat E. EL shnawye, Hager A. Elrefaei, Asmaa M. Elsayed, Khloud T. Hammad, Amany T. Ali, Nesreen A. Elsaid, Zeinab E. Rizk, and Eman A. Elsaid) who were helpful in this project.
Conflicts of Interest
The authors declared that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
- Yadav, E.; Singh, D.; Yadav, P.; Verma, A. Antioxidant and anti-inflammatory properties of Prosopis cineraria based phenolic rich ointment in wound healing. Biomed. Pharmacother. 2018, 108, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, I.A.; Antonescu, A.; Miere, F.; Fritea, L.; Teușdea, A.C.; Vicaș, L.; Vicaș, S.I.; Brihan, I.; Domuța, M.; Zdrinca, M.; et al. Evaluation of Wound Healing Potential of Novel Hydrogel Based on Ocimum basilicum and Trifolium pratense Extracts. Processes 2021, 9, 2096. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Choucry, M.A.; Kandil, Z.A. Antibacterial, antioxidant, and topical anti-inflammatory activities of Bergia ammannioides: A wound-healing plant. Pharm. Biol. 2016, 54, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.; Shata, A.; El-Kader, E.M.A.; Sharaf, H.; Abdo, W.S.; Amin, N.A.; Saber, S. Vildagliptin, a DPP-4 inhibitor, attenuates carbon tetrachloride-induced liver fibrosis by targeting ERK1/2, p38α, and NF-κB signaling. Toxicol. Appl. Pharmacol. 2020, 407, 115246. [Google Scholar] [CrossRef]
- Saber, S.; Youssef, M.E.; Sharaf, H.; Amin, N.A.; El-Shedody, R.; Aboutouk, F.H.; El-Galeel, Y.A.; El-Hefnawy, A.; Shabaka, D.; Khalifa, A.; et al. BBG enhances OLT1177-induced NLRP3 inflammasome inactivation by targeting P2X7R/NLRP3 and MyD88/NF-κB signaling in DSS-induced colitis in rats. Life Sci. 2021, 270, 119123. [Google Scholar] [CrossRef]
- El-Rous, M.A.; Saber, S.; Raafat, E.M.; Ahmed, A.A.E. Dapagliflozin, an SGLT2 inhibitor, ameliorates acetic acid-induced colitis in rats by targeting NFκB/AMPK/NLRP3 axis. Inflammopharmacology 2021, 29, 1169–1185. [Google Scholar] [CrossRef]
- Portou, M.J.; Baker, D.; Abraham, D.; Tsui, J. The innate immune system, toll-like receptors and dermal wound healing: A review. Vasc. Pharmacol. 2015, 71, 31–36. [Google Scholar] [CrossRef]
- Miere, F.; Teușdea, A.C.; Laslo, V.; Cavalu, S.; Fritea, L.; Dobjanschi, L.; Zdrinca, M.; Zdrinca, M.; Ganea, M.; Pașc, P.; et al. Evaluation of In Vitro Wound-Healing Potential, Antioxidant Capacity, and Antimicrobial Activity of Stellaria media (L.) Vill. Appl. Sci. 2021, 11, 11526. [Google Scholar] [CrossRef]
- Youssef, M.E.; El-Fattah, E.E.A.; Abdelhamid, A.M.; Eissa, H.; El-Ahwany, E.; Amin, N.A.; Hetta, H.F.; Mahmoud, M.H.; Batiha, G.E.-S.; Gobba, N.; et al. Interference with the AMPKα/mTOR/NLRP3 Signaling and the IL-23/IL-17 Axis Effectively Protects Against the Dextran Sulfate Sodium Intoxication in Rats: A New Paradigm in Empagliflozin and Metformin Reprofiling for the Management of Ulcerative Colitis. Front Pharmacol. 2021, 12, 719984. [Google Scholar] [CrossRef]
- Zohny, M.H.; Cavalu, S.; Youssef, M.E.; Kaddah, M.M.Y.; Mourad, A.A.E.; Gaafar, A.G.A.; El-Ahwany, E.; Amin, N.A.; Arakeep, H.M.; Shata, A.; et al. Coomassie brilliant blue G-250 dye attenuates bleomycin-induced lung fibrosis by regulating the NF-κB and NLRP3 crosstalk: A novel approach for filling an unmet medical need. Biomed. Pharmacother. 2022, 148, 112723. [Google Scholar] [CrossRef]
- Saber, S.; Abd El-Fattah, E.E.; Yahya, G.; Gobba, N.A.; Maghmomeh, A.O.; Khodir, A.E.; Mourad, A.A.; Saad, A.S.; Mohammed, H.G.; Nouh, N.A.; et al. A Novel Combination Therapy Using Rosuvastatin and Lactobacillus Combats Dextran Sodium Sulfate-Induced Colitis in High-Fat Diet-Fed Rats by Targeting the TXNIP/NLRP3 Interaction and Influencing Gut Microbiome Composition. Pharmaceuticals 2021, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Lafyatis, R.; Farina, A. New insights into the mechanisms of innate immune receptor signalling in fibrosis. Open Rheumatol. J. 2012, 6, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Midwood, K.S.; Yin, H.; Varga, J. Toll-Like Receptor-4 Signaling Drives Persistent Fibroblast Activation and Prevents Fibrosis Resolution in Scleroderma. Adv. Wound Care 2017, 6, 356–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saber, S.; Yahya, G.; Gobba, N.A.; Sharaf, H.; Alshaman, R.; Alattar, A.; Amin, N.A.; El-Shedody, R.; Aboutouk, F.H.; El-Galeel, Y.A.; et al. The Supportive Role of NSC328382, a P2X7R Antagonist, in Enhancing the Inhibitory Effect of CRID3 on NLRP3 Inflammasome Activation in Rats with Dextran Sodium Sulfate-Induced Colitis. J. Inflamm. Res. 2021, 14, 3443–3463. [Google Scholar] [CrossRef]
- Youssef, M.E.; El-Azab, M.F.; Abdel-Dayem, M.A.; Yahya, G.; Alanazi, I.S.; Saber, S. Electrocardiographic and histopathological characterizations of diabetic cardiomyopathy in rats. Environ. Sci. Pollut. Res. 2022, 29, 25723–25732. [Google Scholar] [CrossRef]
- Chen, L.; DiPietro, L.A. Toll-Like Receptor Function in Acute Wounds. Adv. Wound Care 2017, 6, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Coban, Y.K. Infection control in severely burned patients. World J. Crit. Care Med. 2012, 1, 94–101. [Google Scholar] [CrossRef]
- Palmieri, T.L.; Greenhalgh, D.G. Topical Treatment of Pediatric Patients with Burns. Am. J. Clin. Dermatol. 2002, 3, 529–534. [Google Scholar] [CrossRef]
- Momtaz, S.; Dibaj, M.; Abdollahi, A.; Amin, G.; Bahramsoltani, R.; Abdollahi, M.; Mahdaviani, P.; Abdolghaffari, A.H. Wound healing activity of the flowers of Lilium candidum L. in burn wound model in rats. JMPIR 2020, 19, 109–118. [Google Scholar] [CrossRef]
- Manuel, R.G.; Fleuchot, B.; Lauciello, L.; Jafari, P.; Lee, A.A.; Raffoul, W.; Que, Y.-A.; Perron, K.; Blokesch, M. Effect of Human Burn Wound Exudate on Pseudomonas aeruginosa virulence. mSphere 2016, 1, e00111-15. [Google Scholar]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Adewuni, T.M.; Ademiluyi, A.O.; Olasehinde, T.A.; Ademosun, A.O. Phenolic Constituents and Inhibitory Effects of Hibiscus sabdariffa L. (Sorrel) Calyx on Cholinergic, Monoaminergic, and Purinergic Enzyme Activities. J. Diet. Suppl. 2018, 15, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Jabeur, I.; Pereira, E.; Caleja, C.; Calhelha, R.C.; Soković, M.; Catarino, L.; Barros, L.; Ferreira, I. Exploring the chemical and bioactive properties of Hibiscus sabdariffa L. calyces from Guinea-Bissau (West Africa). Food Funct. 2019, 10, 2234–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, E.M. Antibacterial efficiency of the Sudanese Roselle (Hibiscus sabdariffa L.), a famous beverage from Sudanese folk medicine. J. Intercult. Ethnopharmacol. 2016, 5, 186–190. [Google Scholar] [CrossRef]
- Abdallah, E.M. Antibacterial activity of Hibiscus sabdariffa L. calyces against hospital isolates of multidrug resistant Acinetobacter baumannii. J. Acute Dis. 2016, 5, 512–516. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, A.; Nithya, V. Evaluation of the wound-healing activity of Hibiscus rosa sinensis L. (Malvaceae) in Wistar albino rats. Indian J. Pharmacol. 2012, 44, 694–698. [Google Scholar] [CrossRef] [Green Version]
- Cranwell, P.B.; Harwood, L.M.; Moody, C.J. Experimental Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Kodym, A.; Bujak, T. Physicochemical and microbiological properties as well as stability of ointments containing aloe extract (Aloe arborescens Mill.) or aloe extract associated to neomycin sulphate. Die Pharm. 2002, 57, 834–837. [Google Scholar]
- Fikru, A.; Makonnen, E.; Eguale, T.; Debella, A.; Mekonnen, G.A. Evaluation of in vivo wound healing activity of methanol extract of Achyranthes aspera L. J. Ethnopharmacol. 2012, 143, 469–474. [Google Scholar] [CrossRef]
- Cai, E.Z.; Ang, C.H.; Raju, A.; Tan, K.B.; Hing, E.C.; Loo, Y.; Wong, Y.C.; Lee, H.; Lim, J.; Moochhala, S.M.; et al. Creation of consistent burn wounds: A rat model. Arch. Plast. Surg. 2014, 41, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Nasr, M.; Teiama, M.; Ismail, A.; Ebada, A.; Saber, S. In vitro and in vivo evaluation of cubosomal nanoparticles as an ocular delivery system for fluconazole in treatment of keratomycosis. Drug Deliv. Transl. Res. 2020, 10, 1841–1852. [Google Scholar] [CrossRef]
- El-Baz, A.M.; Yahya, G.; Mansour, B.; El-Sokkary, M.M.A.; Alshaman, R.; Alattar, A.; El-Ganiny, A.M. The Link between Occurrence of Class I Integron and Acquired Aminoglycoside Resistance in Clinical MRSA Isolates. Antibiotics 2021, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Yahya, G.; Ebada, A.; Khalaf, E.M.; Mansour, B.; Nouh, N.A.; Mosbah, R.A.; Saber, S.; Moustafa, M.; Negm, S.; El-Sokkary, M.M.A.; et al. Soil-Associated Bacillus Species: A Reservoir of Bioactive Compounds with Potential Therapeutic Activity against Human Pathogens. Microorganisms 2021, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Patry, J.; Blanchette, V. Enzymatic debridement with collagenase in wounds and ulcers: A systematic review and meta-analysis. Int. Wound J. 2017, 14, 1055–1065. [Google Scholar] [CrossRef]
- Gilligan, A.M.; Waycaster, C.R.; Bizier, R.; Chu, B.C.; Carter, M.J.; Fife, C.E. Comparative Effectiveness of Clostridial Collagenase Ointment to Medicinal Honey for Treatment of Pressure Ulcers. Adv. Wound Care 2017, 6, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Zielins, E.R.; Brett, E.A.; Luan, A.; Hu, M.S.; Walmsley, G.G.; Paik, K.; Senarath-Yapa, K.; Atashroo, D.A.; Wearda, T.; Lorenz, H.P.; et al. Emerging drugs for the treatment of wound healing. Expert Opin. Emerg. Drugs 2015, 20, 235–246. [Google Scholar] [CrossRef]
- El-Fattah, E.E.A.; Saber, S.; Mourad, A.A.E.; El-Ahwany, E.; Amin, N.A.; Cavalu, S.; Yahya, G.; Saad, A.S.; Alsharidah, M.; Shata, A.; et al. The dynamic interplay between AMPK/NFκB signaling and NLRP3 is a new therapeutic target in inflammation: Emerging role of dapagliflozin in overcoming lipopolysaccharide-mediated lung injury. Biomed. Pharmacother. 2022, 147, 112628. [Google Scholar] [CrossRef]
- Saber, S.; Nasr, M.; Saad, A.S.; Mourad, A.A.E.; Gobba, N.A.; Shata, A.; Hafez, A.-M.; Elsergany, R.N.; Elagamy, H.I.; El-Ahwany, E.; et al. Albendazole-loaded cubosomes interrupt the ERK1/2-HIF-1α-p300/CREB axis in mice intoxicated with diethylnitrosamine: A new paradigm in drug repurposing for the inhibition of hepatocellular carcinoma progression. Biomed. Pharmacother. 2021, 142, 112029. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Gao, L.; Wang, X. In vitro anti-inflammatory mechanism of Folium Hibisci Mutabilis leaves ethanol extracts, African journal of traditional, complementary, and alternative medicines. AJTCAM 2014, 11, 127–130. [Google Scholar]
- Shah, A.; Amini-Nik, S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef] [Green Version]
- Finnerty, C.C.; Jeschke, M.G.; Branski, L.K.; Barret, J.P.; Dziewulski, P.; Herndon, D.N. Hypertrophic scarring: The greatest unmet challenge after burn injury. Lancet 2016, 388, 1427–1436. [Google Scholar] [CrossRef] [Green Version]
- Larsson, J.; Goumans, M.J.; Sjöstrand, L.J.; van Rooijen, M.A.; Ward, D.; Levéen, P.; Xu, X.; Dijke, P.t.; Mummery, C.L.; Karlsson, S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001, 20, 1663–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.I.; Kwak, J.H.; Na, H.J.; Kim, J.K.; Ding, Y.; Choi, M.E. Transforming growth factor-beta (TGF-beta1) activates TAK1 via TAB1-mediated autophosphorylation, independent of TGF-beta receptor kinase activity in mesangial cells. J. Biol. Chem. 2009, 284, 22285–22296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Süntar, I.; Akkol, E.K.; Nahar, L.; Sarker, S.D. Wound healing and antioxidant properties: Do they coexist in plants? Free. Radic. Antioxid. 2012, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Frank, T.; Netzel, G.; Kammerer, D.R.; Carle, R.; Kler, A.; Kriesl, E.; Bitsch, I.; Bitsch, R.; Netzel, M. Consumption of Hibiscus sabdariffa L. aqueous extract and its impact on systemic antioxidant potential in healthy subjects. J. Sci. Food Agric. 2012, 92, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Florina, M.; Luminița, F.; Simona, C.; Ioana, V.S. Formulation, Characterization, and Advantages of Using Liposomes in Multiple Therapies. Pharmacophore 2020, 11, 1–12. [Google Scholar]
- Peng, C.H.; Chyau, C.C.; Chan, K.C.; Chan, T.H.; Wang, C.J.; Huang, C.N. Hibiscus sabdariffa polyphenolic extract inhibits hyperglycemia, hyperlipidemia, and glycation-oxidative stress while improving insulin resistance. J. Agric. Food Chem. 2011, 59, 9901–9909. [Google Scholar] [CrossRef]
- Bakhtiari, E.; Hosseini, A.; Mousavi, S.H. Protective effect of Hibiscus sabdariffa against serum/glucose deprivation-induced PC12 cells injury. Avicenna J. Phytomedicine 2015, 5, 231–237. [Google Scholar]
- Guardiola, S.; Mach, N. (Therapeutic potential of Hibiscus sabdariffa: A review of the scientific evidence), Endocrinologia y nutricion. Organo Soc. Esp. Endocrinol. Nutr. 2014, 61, 274–295. [Google Scholar] [CrossRef]
- Bielecki, P.; Glik, J.; Kawecki, M.; Santos, V.A.M.d. Towards understanding Pseudomonas aeruginosa burn wound infections by profiling gene expression. Biotechnol. Lett. 2008, 30, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Rodríguez, A.S.; Nevárez-Baca, S.; Lerma-Hernández, J.C.; Hernández-Ochoa, L.R.; Nevárez-Moorillon, G.V.; Gutiérrez-Méndez, N.; Muñoz-Castellanos, L.N.; Salas, E. In Vitro Antibacterial Activity of Hibiscus sabdariffa L. Phenolic Extract and Its in Situ Application on Shelf-Life of Beef Meat. Foods 2020, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).