Abstract
Cinnamaldehyde (CA) is a natural compound that has promising biological activity. The current study investigates the antitumor activity of CA in thioacetamide induced hepatocellular carcinoma (HCC) in rats through targeting the Wnt/β-catenin pathway and evaluates the capability of CA to relieve hepatocytes oxidative stress in the HCC-rat model. After 16 weeks of HCC induction by thioacetamide (TAA), rats were treated for 7 consecutive weeks with CA daily; i.p. injection, Alpha-fetoprotein (AFP) level, necroinflammatory score and fibrosis percentage were measured to assess HCC development. The Wnt/β-catenin pathway was evaluated by measuring the hepatic protein level of Wnt-3a, β-catenin, cyclin D, matrix metalloproteinase-9 (MMP-9), and vascular endothelial growth factor (VEGF). Furthermore, hepatocytes’ oxidative stress was assessed by measuring hepatic GSH and MDA contents. Results showed that CA was significantly inhibiting the Wnt/β-catenin pathway through the downregulation of hepatic Wnt-3a, β-catenin, cyclin D, MMP-9, and VEGF. Moreover, CA ameliorates hepatocytes’ oxidative stress via lowering hepatic MDA content and rising hepatic GSH content. Thus, in conclusion, CA is a promising treatment for HCC. It not only has an effective antitumor activity but also ameliorates hepatocytes’ oxidative stress.
1. Introduction
Hepatocellular carcinoma (HCC) exacts a heavy disease burden worldwide: it is the fourth most common cause of cancer related death, globally [1]. The increasing incidence of HCC has generated a high demand for the search for effective therapies with new molecular mechanisms in the hope of a reduction in HCC burden, globally.
The Wnt/β-catenin pathway is one of the signaling pathways that is most activated during HCC. It is activated in about 50% of HCC tissues [2]. The overexpression of β-catenin plays a critical role in HCC development via the regulation of many cellular processes, such as proliferation, differentiation, apoptosis, migration, invasion, epithelial–mesenchymal transition and cancer cell stemness [3]. This activation always occurs due to the deregulation of one of its components, either by mutation or secondary to the downregulation of Wnt signal inhibitors [4,5].
The hallmark of the canonical Wnt pathway is β-catenin accumulation in the cytoplasm and its subsequent translocation into the nucleus [6]. This occurs when the Wnt ligand binds to the frizzled receptor (Fz) and lipoprotein receptor related protein (LPR) 5/6 co-receptors, and this binding creates a signal that leads to the recruitment of cytoplasmic dishevelled (Dvl) protein. The Dvl protein interacts with the cytoplasmic part of FZ, subsequently leading to Dvl phosphorylation [7]. As a result, phosphorylated Dvl recruits Axin and glycogen synthase kinase 3 (GSK3) to the plasma membrane, away from the destruction complex that is responsible for β-catenin phosphorylation and its subsequent proteasomal degradation [8]. Thus, the destruction complex is deactivated and non-phosphorylated active β-catenin accumulates in the cytoplasm and enters the nucleus, where it binds to the lymphoid enhancer factor/T-cell factor (LEF/TCF). This binding activates the transcription of target genes such as vascular endothelial growth factor (VEGF), matrix metalloproteinase-9 (MMP-9), epithelial cell adhesion molecule, survivin, cyclin D1, glutamine synthase, inducible nitric oxide synthase, multidrug resistance (MDR), and c-Myc [5,9,10,11,12].
Nowadays, numerous therapeutic strategies are available for HCC treatment but they are often not sufficient due to their several undesirable side effects and tumor resistance increasing their toxicity. Therefore, there is a demand for finding novel therapies that evade those drawbacks. Natural products are obtaining a noticeable reputation in HCC treatment due to their active components, which have promising anticancer activity with lesser side effects [13].
Cinnamaldehyde (CA) is a major constituent isolated from the stem bark of Cinnamomum cassia. It is responsible for the flavor and the odor of cinnamon. CA is commonly used as a fragrance ingredient and as an antibacterial agent in the food industry [14,15,16,17]. Moreover, previous studies have shown that CA possesses many biological activities, such as anticancer, antioxidant, and anti-inflammatory [18,19,20].
Therefore, the current study aims to assess the antitumor activity of CA in HCC induced by thioacetamide (TAA) in rats through targeting the Wnt/β-catenin pathway. Moreover, it investigates the antioxidant activity of CA in HCC rats.
2. Materials and Methods
2.1. Materials
TAA with 99% purity and CA were purchased from Sigma Aldrich Chemicals Co. (St. Louise, MO, USA). All other chemicals are of high analytical grade.
2.2. Animals
In the current study, forty-five male Sprague Dawley rats weighing 200–250 g were used. All rats were kept under standard conditions of temperature (25 ± 2 °C), with a standard 12 h light/dark cycle, and permitted free entry to nourishment and water. Ethical committee in the Faculty of Pharmacy, Mansoura University, Mansoura, Egypt, approved the animal protocol (approval code: 2022-12) according to the “Principles of Laboratory Animal Care” (NIH publication No. 85-23, revised 1985).
2.3. Experimental Design
HCC was induced in rats by intraperitoneal (i.p.) injection of freshly prepared TAA (dissolved in normal saline), at a dose of 200 mg/kg, two times weekly for 16 consecutive weeks [21]. At the end of the 16 weeks, twelve rats had died.
To assess the efficacy of CA in the treatment of TAA induced HCC, rats were equally divided into three groups with ten animals in each group arranged as follows: Control group: received no treatment. HCC group: received freshly prepared TAA at the dose of 200 mg/kg i.p. twice weekly for 16 consecutive weeks. CA group: rats received CA (70 mg/kg/day); i.p. injection; for seven weeks following HCC induction [22].
2.4. Sample Collection
At the end of the experiment, the puncture of retroorbital venous plexus and centrifugation, blood samples were collected. The sera were collected and stored at −80 °C for further biochemical evaluation. HCC occurrence was proven by the assessment of serum level of α-fetoprotein (AFP) and histopathological examination for 3 rats.
Then, rats were sacrificed and the whole liver was isolated and divided into two parts. The first part was fixed in 10% phosphate buffered formalin (pH 7.2) for histopathological and immunohistochemical assays. The second part was homogenized in ice cold phosphate buffered saline (PBS) (pH 7.4), and were centrifuged and stored at −80 °C for further hepatic antioxidant state and hepatic Wnt/β-catenin signaling pathway elements.
2.5. Assessed Parameters
Serum samples were used for the evaluation of liver function tests (total protein, albumin (Spin react, Girona, Spain) and bilirubin levels (BioMed Company, Heliopolis, Egypt) and alanine aminotransferase (ALT), aspartate aminotransferase (AST) (Human, Wiesbaden, Germany), gamma-glutamyl transferase (GGT) (Spin react, Girona, Spain), and alkaline phosphatase (ALP) (Human, Wiesbaden, Germany) activities). Serum AFP was assessed by AFP ELISA Kit (Atlas Medical, Berlin, Germany) and expressed as ng/mL.
Liver homogenates were used for assay of hepatic oxidative stress status (glutathione (GSH) and malondialdehyde (MDA) contents), and ELISA assays for hepatic Wnt-3a, β-catenin, Cyclin D, and MMP-9 protein concentrations (Bioassay Technology Laboratory, Birmingham, England) expressed as ng/100 mg tissue.
2.6. Histopathological Examination of Liver Tissue
Liver tissues were fixed in 10% buffered formalin, were embedded into paraffin blocks, and then were divided into 5 μm-thickness sectors. Part of hepatic sectors was stained with hematoxylin and eosin (H&E) stain to assess necroinflammatory scores and others were stained with Masson’s trichome stain to quantify fibrosis percentage. Histopathological changes were detected using Ishak et al., modified histology activity index (HAI) system [23].
2.7. Immunohistochemistry (IHC)
The immunohistochemical staining procedures of VEGF and β-catenin were carried out according to protocols as described by Saber et al. [24]. Hepatic sectors were dewaxed and incubated with polyclonal rabbit antibodies against VEGF (Thermo Fisher Scientific, Cat. No. PA5-16754, dilution 1/100) and β-catenin (Servicebio, Cat. No. GB11015, dilution 1/1000). After rinsing with PBS, goat antirabbit secondary antibody (Cat. No. K4003, EnVision+™ System Horseradish Peroxidase Labelled Pomer; Dako) was added and incubated for 30 min at room temperature. Then, 3,3′-Diaminobenzidine (DAB) kit for visualizing the slides and image J 1.5.3 analysis software (NIH, USA) was used to assess the staining intensity that presented as a percentage of positive cells in about 8 high power fields.
2.8. Statistical Analysis
Results were expressed as mean ± S.E.M. necroinflammatory scoring and they were expressed as median and range. All experimental results were investigated using one way analysis of variance (ANOVA) followed by Tukey’s posthoc test, with the exception of necroinflammatory scoring results, for which nonparametric Kruskal–Wallis test followed by Dunn’s post-hoc test was used. Statistical tests were performed by the SPSS Statistics version 20. Statistical significance was considered at p < 0.05.
3. Results
3.1. Effect of CA on Liver Function
CA showed a marked enhancement in liver function tests. The serum activities of ALT, AST, ALP, GGT, and bilirubin levels were significantly reduced, by 28.85%, 25.14%, 20.16%, 26.74% and 40.00%, respectively, compared to HCC. Furthermore, CA showed a significant elevation in albumin and total protein levels representing 23.14% and 43.90%, respectively, as compared to HCC group. Data are shown in Table 1.

Table 1.
Effect of CA on liver function parameters.
3.2. CA Decreases Fibrosis Percentage and Necroinflammatory Scores in Liver Homogenates
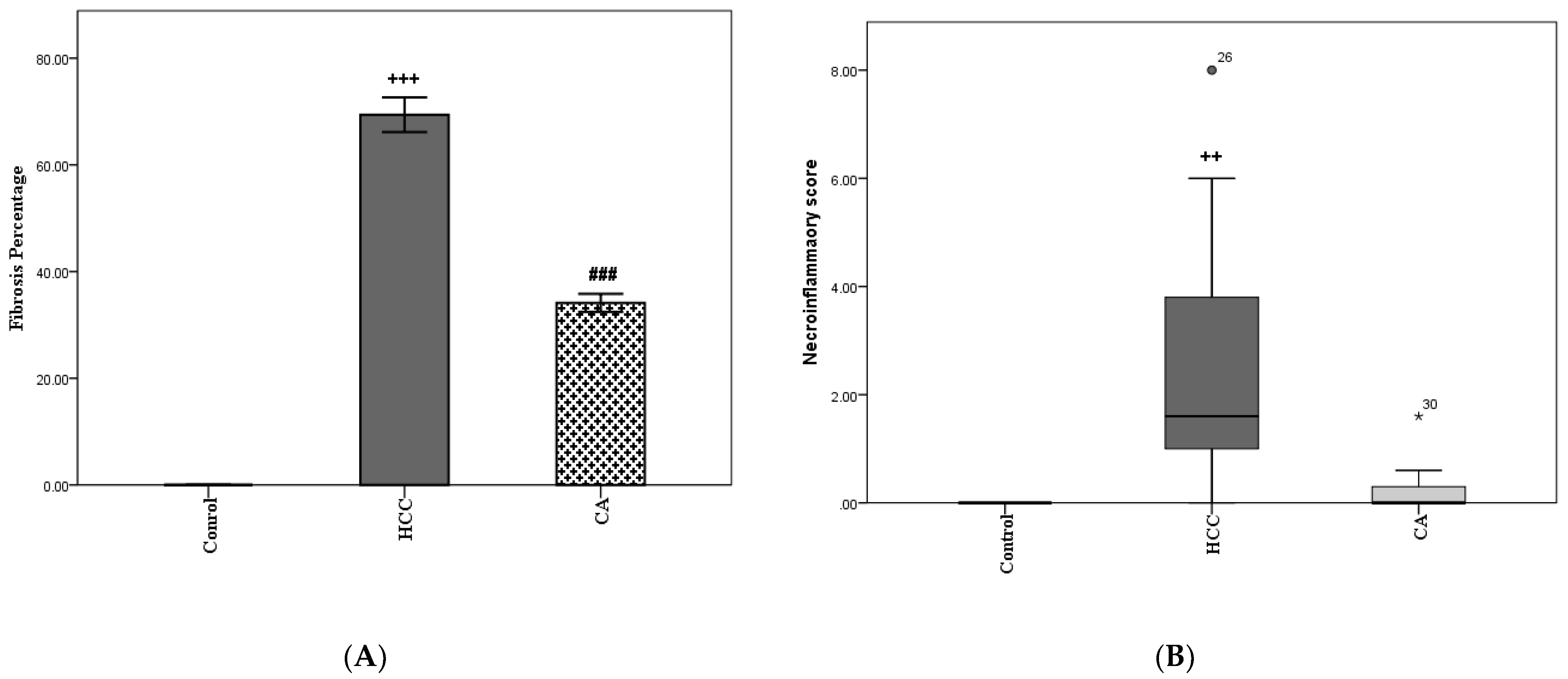
The HCC group showed a significant elevation in fibrosis percentage compared to the control group (p < 0.001). In addition, the CA group revealed a significant reduction in the percentage of fibrosis (p < 0.001), as compared to HCC group (Figure 1A). In parallel, the HCC group showed a significant increase in necroinflammatory scores compared to the control group (p < 0.01). While CA group showed a nonsignificant increase in necroinflammatory scores, as compared to control group (Figure 1B).
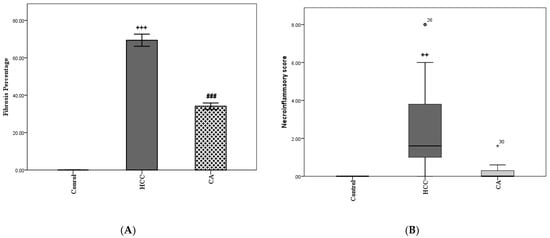
Figure 1.
CA decreases in fibrosis percentage (A) and necroinflammatory score (B) in liver homogenate. Data in A were expressed as mean ± S.E.M and data in B were expressed as median and range. Ten rats in each group. ++ p < 0.01, +++ p< 0.001 vs. control group, ### p < 0.001 vs. HCC group.
3.3. CA Attenuates TAA Induced HCC
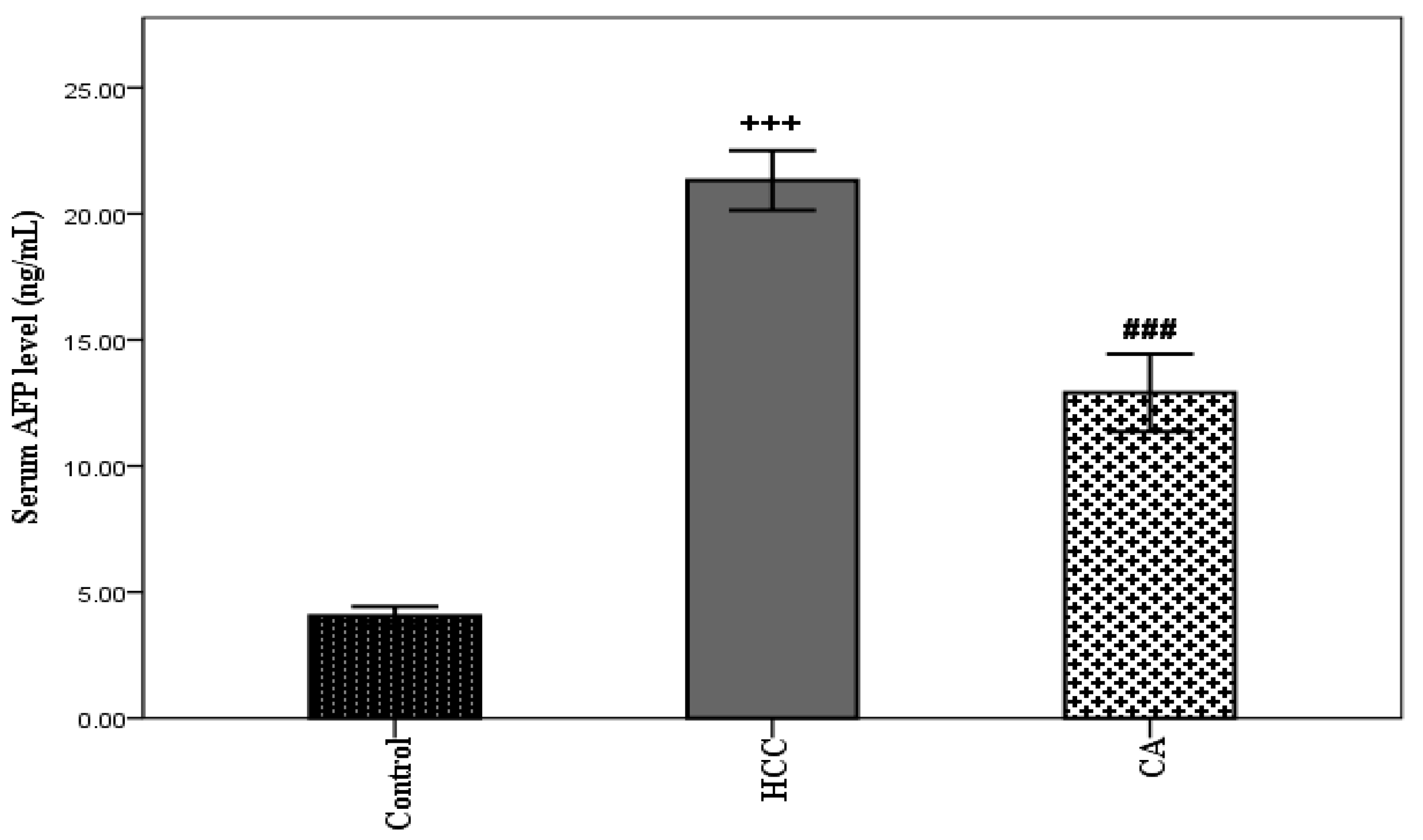
TAA induced HCC showed a significant elevation in serum AFP level (p < 0.001), compared with the control group. However, the CA group showed a significant decrease in serum AFP level, compared to the HCC group (p < 0.001) (Figure 2).
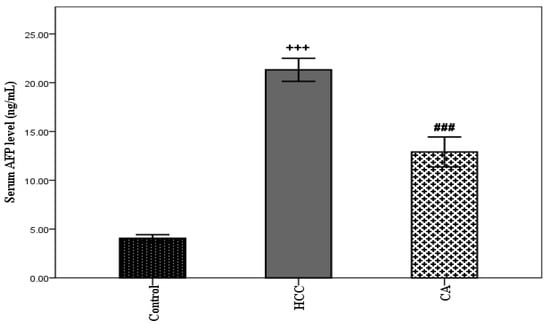
Figure 2.
Effect of CA on serum AFP level. Data were expressed as mean ± S.E.M. Ten rats in each group. +++ p < 0.001 vs. control group, ### p < 0.001 vs. HCC group.
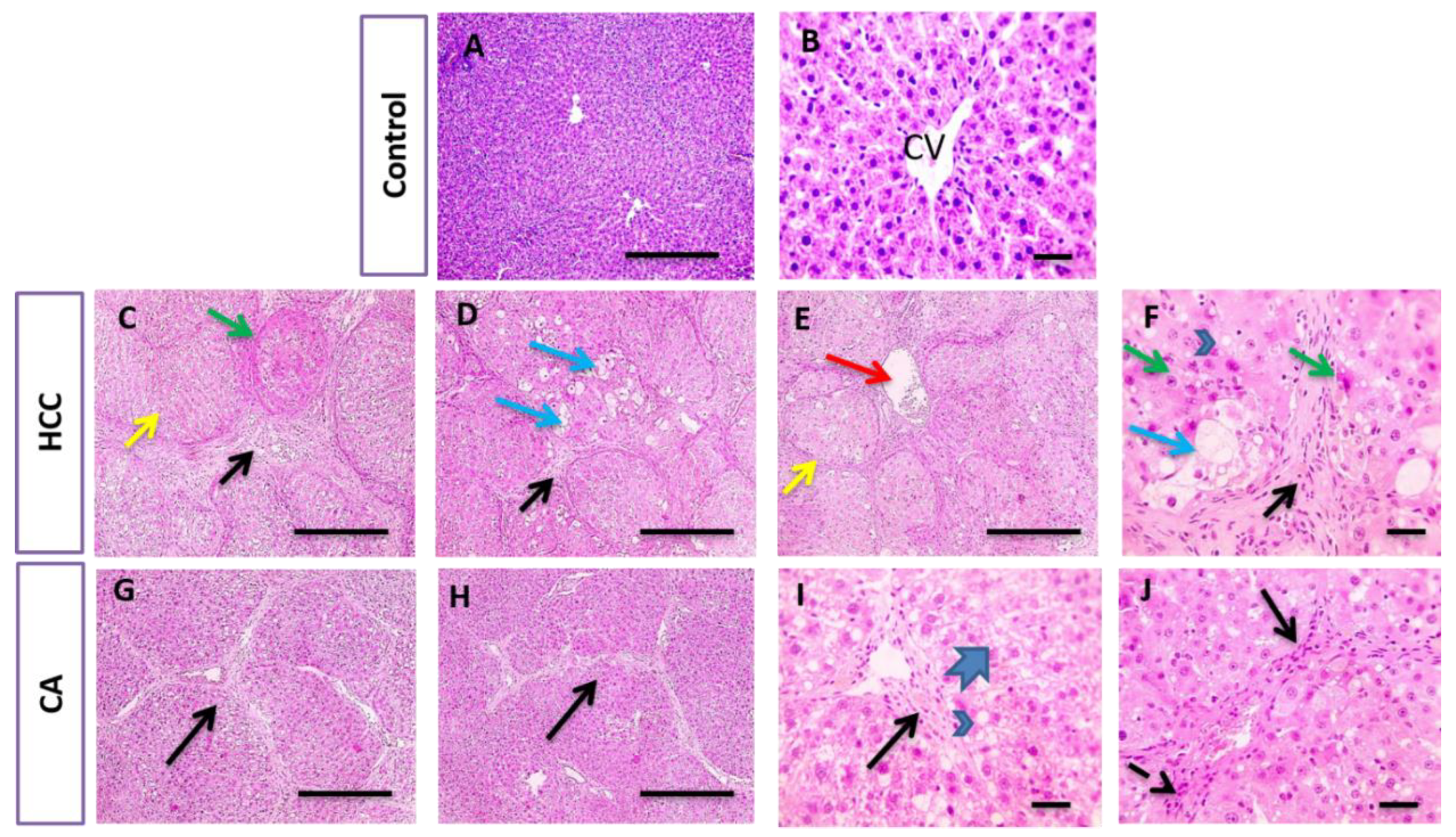
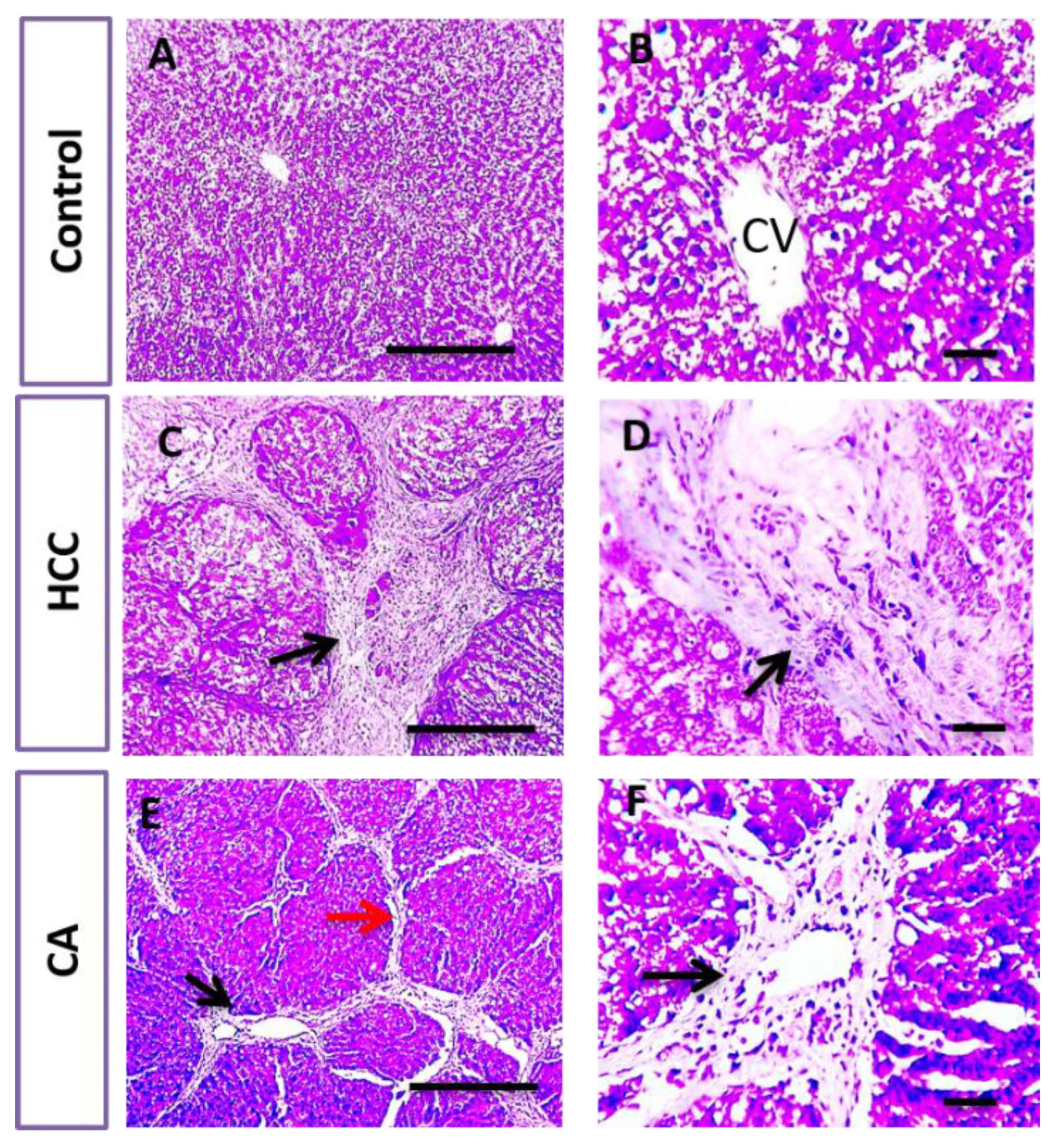
In parallel, liver sections stained with H&E and Masson trichrome in the control group showed the normal arrangement of hepatic cords around the central vein (CV), with normal portal areas and sinusoids and with no collagen deposition. The HCC group revealed disrupted hepatic architecture due to extensive fibrosis associated with the infiltration of inflammatory cells, with excessive collagen deposition. CA hepatic sections revealed a slight improvement in hepatic parenchymal structure with mild perivascular collagen deposition, when compared to HCC group (Figure 3 and Figure 4).
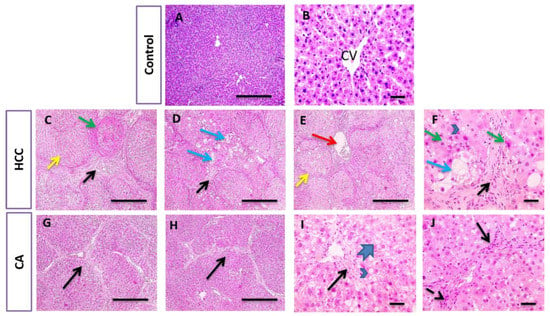
Figure 3.
Histopathological examination of liver tissue in control, HCC and CA groups. H&E-stained liver sections. Hepatic sections show the normal arrangement of hepatic cords around central vein (CV) with the normal portal areas and sinusoids in control group. In contrast, hepatic sections from HCC group showing disrupted parenchymal structure due to extensive fibrosis (black arrows), scored six, associated with inflammatory cells infiltration that includes hemosiderin laden macrophages, congested blood vessels (red arrows). Hepatocytes are present in solid nodules (yellow arrows), lacking normal structures of hepatic lobules, suffered from microvascular degeneration (arrowheads) to ballooning degeneration (blue arrows) and necrosis (green arrows). Hepatic sections from CA group showing slightly improved hepatic parenchymal structure characterized by portal fibrosis (black arrows), scored four, associated with inflammatory cells infiltration (dashed arrows). Hepatocytes suffered from macrovascular degeneration (arrowheads) to hydropic degeneration (thick arrows). Ten rats in each group. Scale bar = 100 µm (A,C–E,G,H), Scale bar = 50 µm (B,F,I,J).
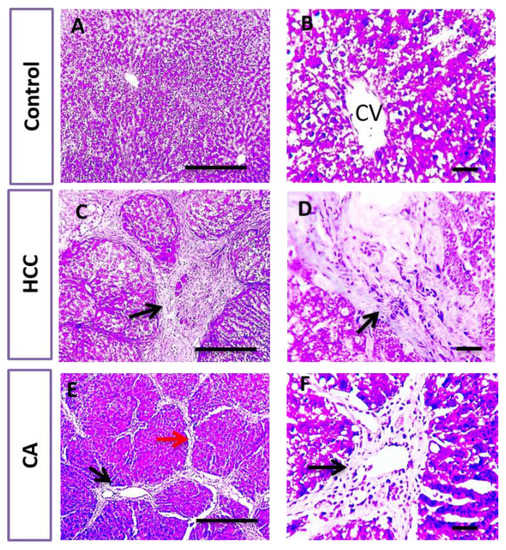
Figure 4.
Histopathological examination of liver tissue in control, HCC and CA groups. Masson’s trichome stained liver sections. Microscopic pictures of hepatic sections from control group showing no collagen deposition. Meanwhile, hepatic sections from HCC group showed excessive bluish collagen deposition (black arrows) dividing hepatic lobules into separate nodules. Hepatic sections from CA group showed mild perivascular bluish collagen deposition (black arrows) with thin collagen strands extending from portal areas (red arrow). Ten rats in each group. Scale bar = 100 µm (A,C,E), Scale bar = 50 µm (B,D,F).
3.4. CA Inhibits Wnt/β-Catenin Signaling Pathway
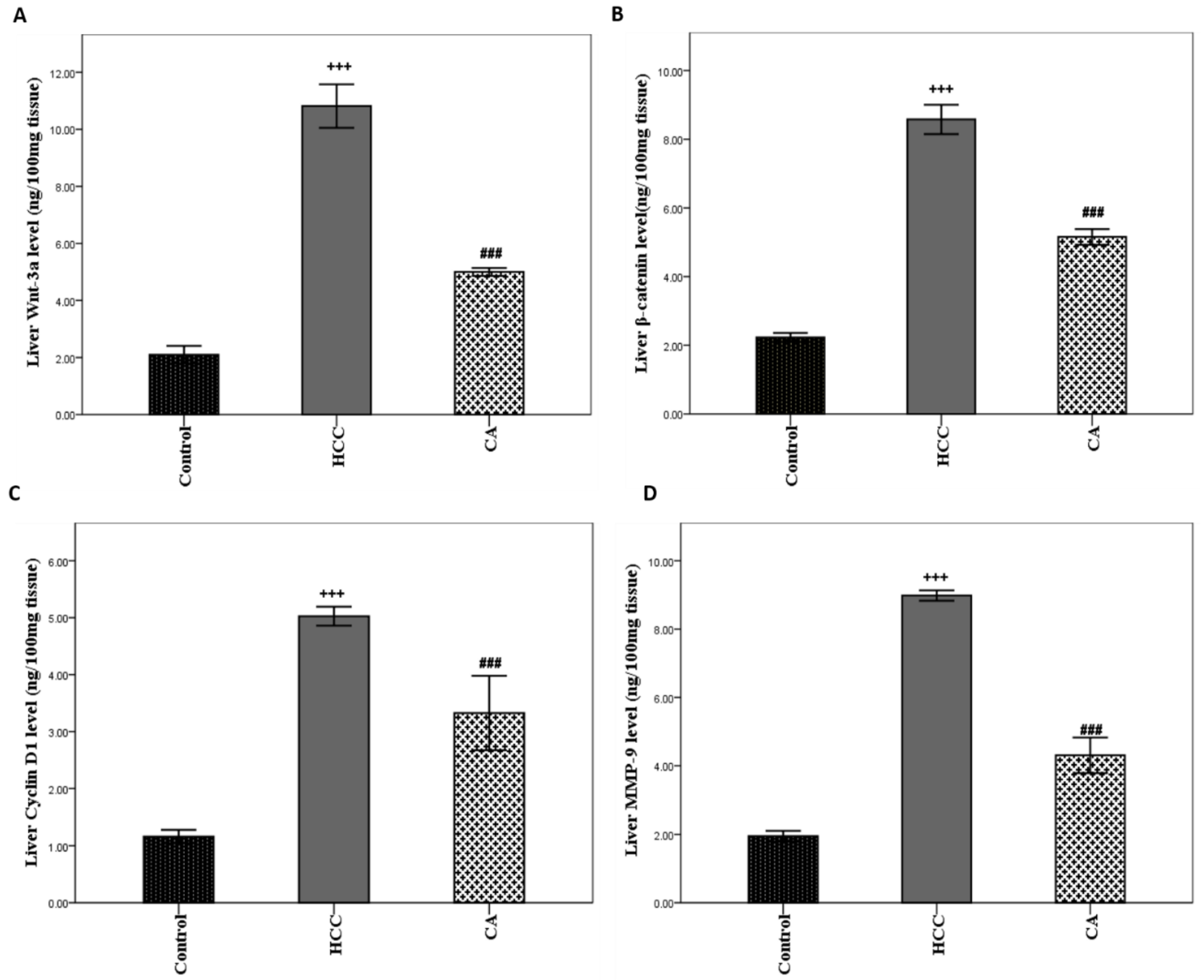
TAA induced HCC showed marked elevation in hepatic Wnt-3a, β-catenin, Cyclin D1, and MMP-9 protein levels (p < 0.001) compared to the control group. The CA group significantly decreased hepatic Wnt-3a, β-catenin, Cyclin D1, and MMP-9 protein levels by 53.84% (p < 0.001), 39.98% (p < 0.001), 33.80% (p < 0.001), and 52.00% (p < 0.001), respectively, as compared to the HCC group (Figure 5A–D).
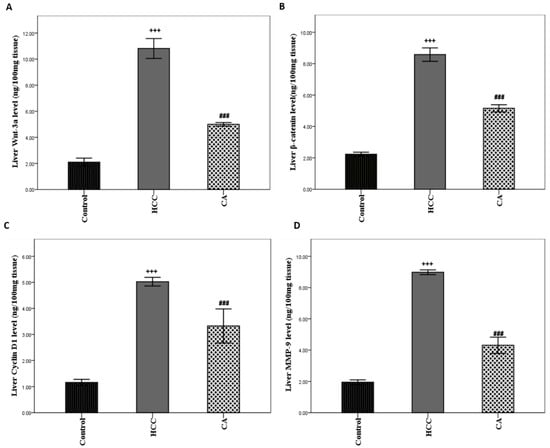
Figure 5.
Effect of CA on hepatic (A) Wnt-3a, (B) β-catenin, (C) Cyclin D, and (D) MMP-9 protein levels. Data were expressed as mean ± S.E.M. Ten rats in each group. +++ p < 0.001 vs. control group, ### p < 0.001 vs. HCC group.
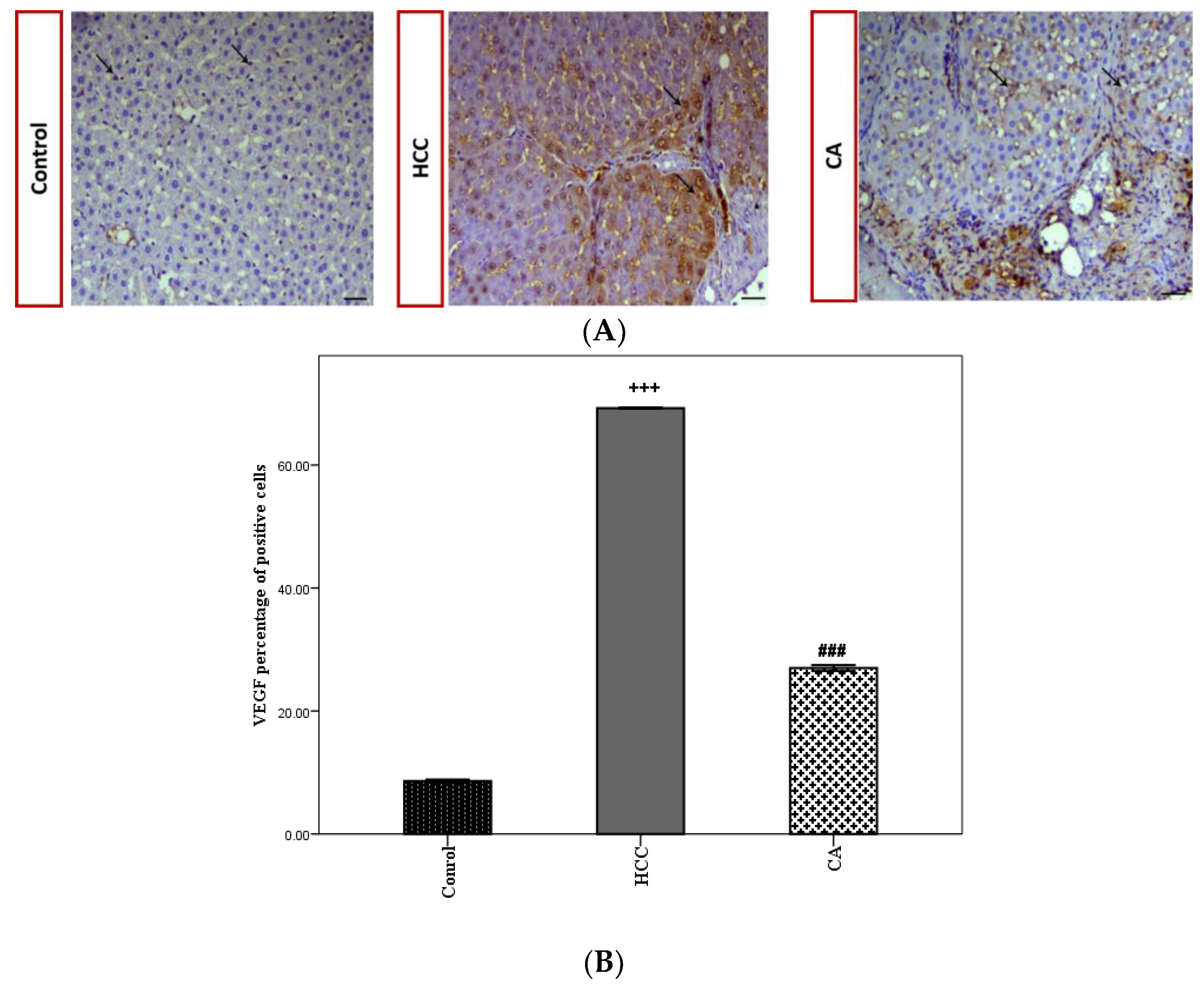
VEGF expression was evaluated by the immunohistochemical analysis of hepatic tissues in all studied groups (Figure 6A). The HCC group showed a significant increase in VEGF the percentage of positive cells (p < 0.001), compared to the control group. While the CA group significantly decreased the VEGF percentage of positive cells (p < 0.001), as compared to the HCC group (Figure 6B).
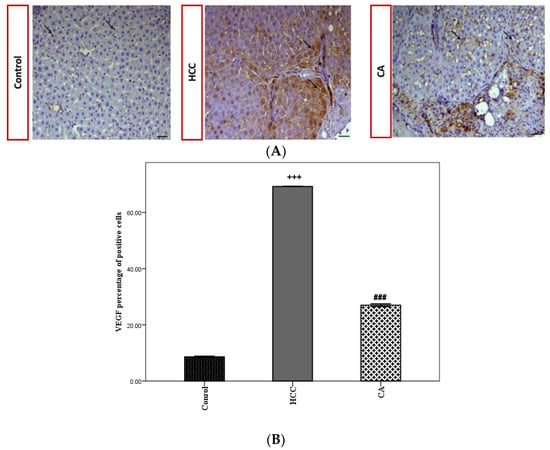
Figure 6.
Effect of CA on VEGF expression within the hepatic tissue. (A) Immunohistochemical stained liver sections of VEGF, black arrows indicate VEGF antibody positive regions. Scale bar = 50 µm. (B) VEGF percentage of positive cells in hepatic tissue. Data were expressed as mean ± S.E.M. Te rats in each group. +++ p < 0.001 vs. control group, ### p < 0.001 vs. HCC group.
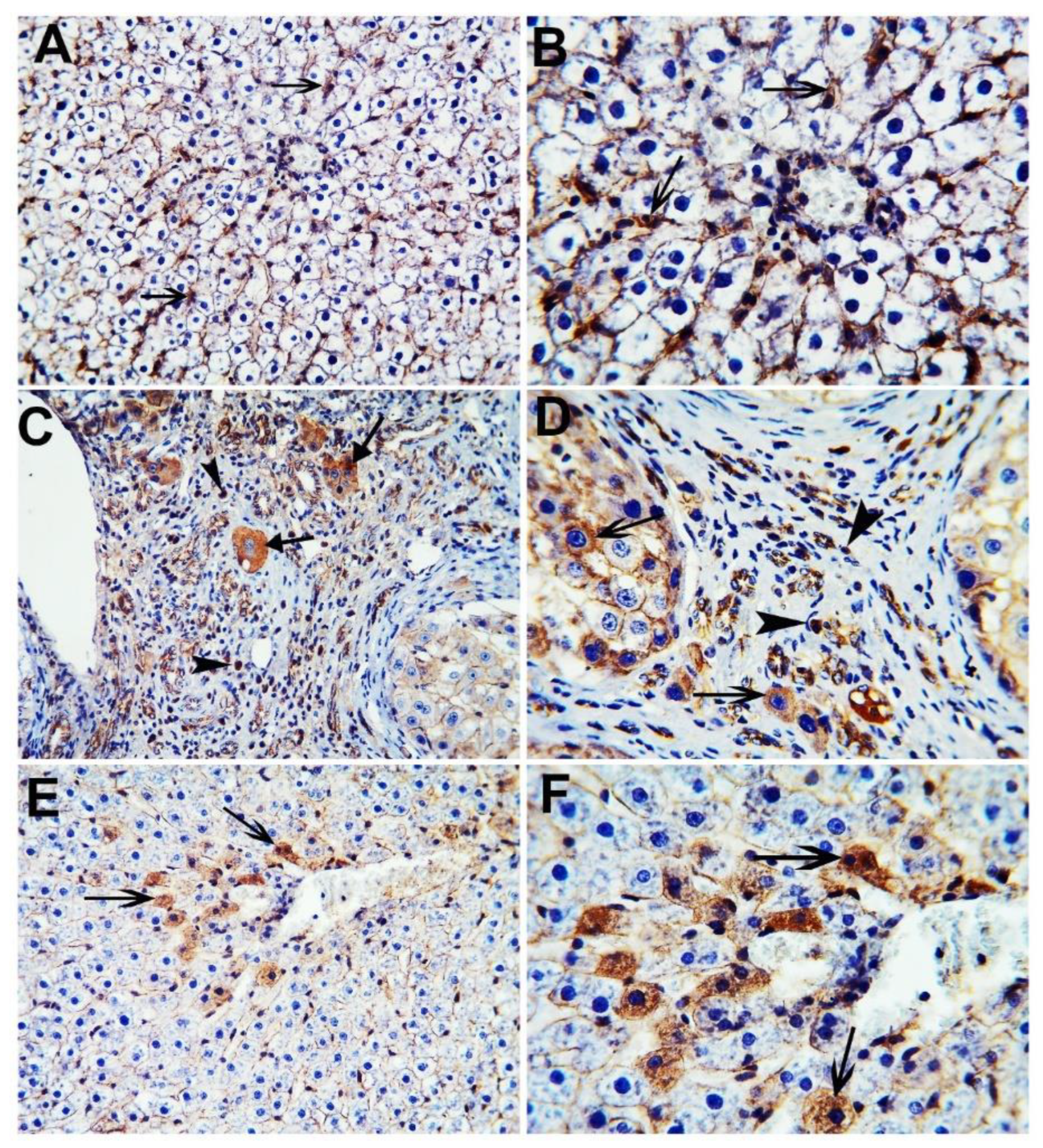
The nuclear and cytoplasmic localization of β-catenin was evaluated by the immunohistochemical analysis of hepatic tissues in all the studied groups (Figure 7). The control group showed minimal cytoplasmic staining of β-catenin positive cells, while the HCC group showed extensive nuclear and cytoplasmic staining of β-catenin positive cells. The CA group revealed only the cytoplasmic staining of scattered β-catenin positive cells.
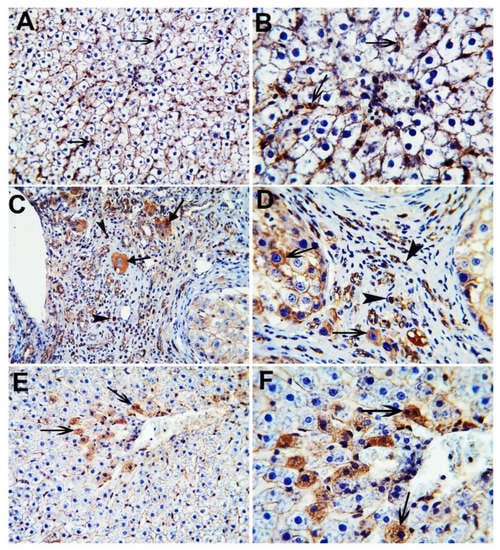
Figure 7.
Immunohistochemical stained liver sections of β-catenin. Control group (A,B); HCC group (C,D); CA group (E,F). Black arrowheads indicate nuclear localization of β-catenin positive cells and black arrows indicate cytoplasmic localization of β-catenin positive cells. X 200 (A,C,E), X 400 (B,D,F).
3.5. Antioxidant Effect of CA
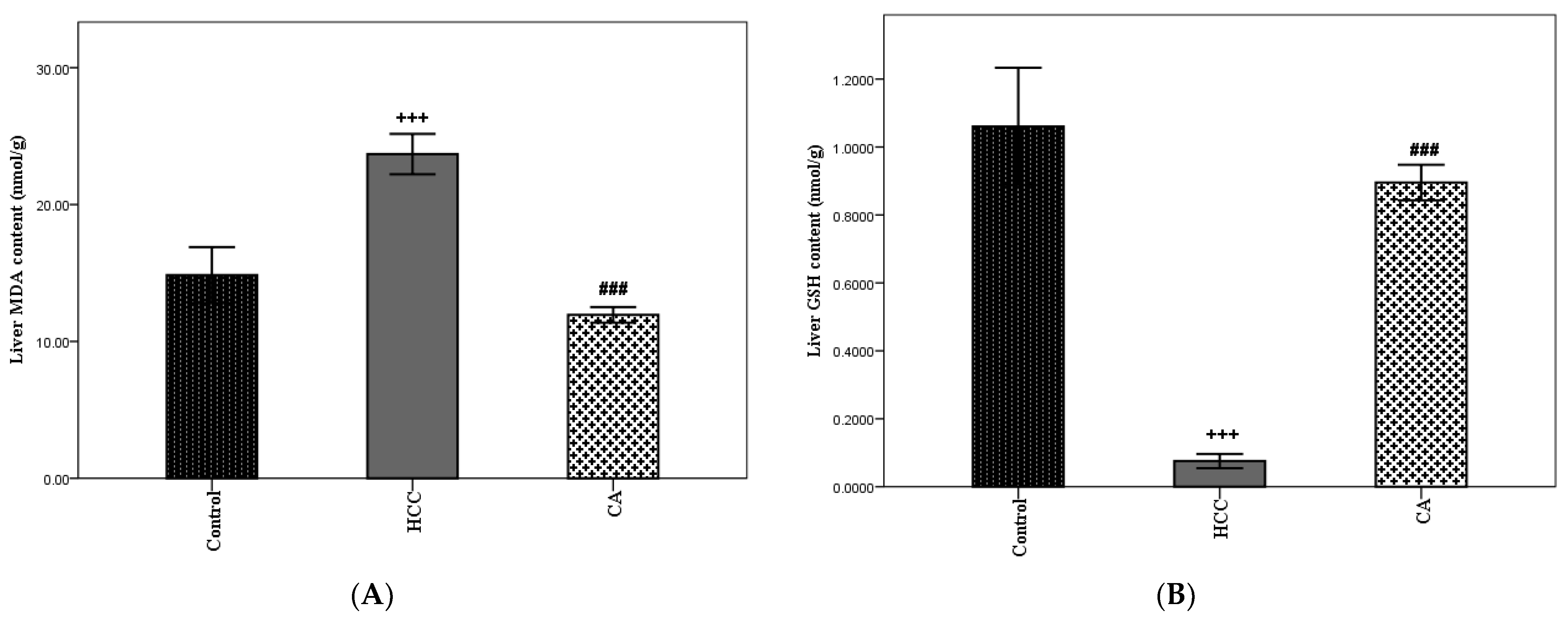
Oxidative stress is considered one of the important hallmarks of HCC. In the HCC group, hepatic MDA content was significantly elevated and hepatic GSH content was significantly lowered, as compared to the control group (p < 0.001). The CA group showed a marked decrease in hepatic MDA content (p < 0.001) and a marked increase in hepatic GSH content (p < 0.001), as compared to the HCC group. Moreover, CA revealed a nonsignificant difference in hepatic GSH content, when compared to the control group (Figure 8A,B).
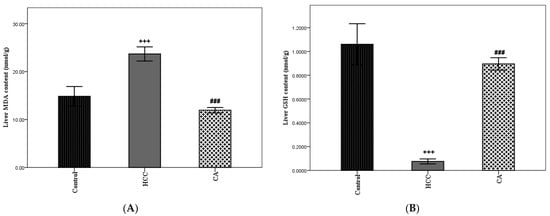
Figure 8.
Effect of CA on TAA induced hepatic oxidative stress parameters. (A) Hepatic MDA content and (B) Hepatic GSH content. Data were expressed as mean ± S.E.M. Ten rats in each group. +++ p < 0.001 vs. control group, ### p < 0.001 vs. HCC group.
4. Discussion
Up to now, no successful therapeutic strategies have yet been found to be effective in the treatment of HCC. Current herbal drug therapy is a role player in the management of various hepatic disorders. The demand for natural drug therapies has been increasing day by day, due to the absence of side effects and their pivotal biological effect that reverses HCC development [13]. In context, the present study examined the efficacy of CA, the active constituent of cinnamon, in the treatment of TAA induced HCC in rats.
CA has been shown to have potent anticancer, antioxidant, and anti-inflammatory activities [18,19,20], and was found to induce apoptosis in cancer cells and prevent cancer cell invasion and metastasis in different tumor cell lines, including breast [25], prostate [26], colon [27], leukemia [28], HCC [19], nonsmall cell lung cancer [22], osteosarcoma [29], and oral cancer [28]. Therefore, we were motivated, in the current study, to evaluate the effect of CA in a HCC rat model.
The induction of HCC by TAA in rats dosed was proven by a significant increase in AFP level and disruption in parenchymal structure, as reflected by the histopathological examination of the liver. CA improved liver architecture, decreased both fibrosis percentage and necroinflammatory score, enhanced all liver functions and significantly decreased serum AFP level, which collectively proves its antitumor effect.
Wnt/β-catenin signaling is one of the most frequently activated pathways in HCC [30]. Wnt-3a is involved in the development of various cancer types via the Wnt/β-catenin pathway, including colorectal, prostate, HCC, and lung cancer [31,32]. In HCC, Wnt-3a is highly expressed in 92.5% of hepatic tissues and is correlated with tumor development and progression [33]. However, fewer studies consider Wnt-3a as a biomarker in diagnosis and prognosis and a novel target for HCC [34,35,36]. In context, the study revealed that hepatic Wnt-3a level was markedly elevated in HCC rats and CA succeeded in lowering hepatic Wnt-3a level significantly, when compared to the HCC group.
The binding of Wnt-3a to its surface coreceptors leads to downstream signaling events that end with stabilization and the accumulation of β-catenin in the cellular cytoplasm and nucleus translocation to induce the expression of the target gene. Thus, as the hepatic Wnt-3a legend increased in HCC, the substantial accumulation of the hepatic β-catenin protein level in the cytoplasm and nucleus will occur, leading to aberrant activation of Wnt/β-catenin signaling. In agreement with the results, hepatic β-catenin protein level was significantly elevated in the HCC group compared with the control group. It has been reported that β-catenin activation can be observed in 20–35% of HCC cases [12]. As cited before, CA markedly decreases hepatic Wnt-3a level, so, β-catenin may no longer be rescued from its fate by the destruction complex that leads to decreased total hepatic β-catenin protein levels and consequently inactivates the transcription of β-catenin target genes, which is important in HCC development and proliferation.
Cyclin D1, VEGF, and MMP-9 are important downstream targets of β-catenin. Cyclin D1 promotes liver cell growth and the overexpression of cyclin D1 initiates HCC development by promoting cell cycle progression [37,38,39]. Moreover, VEGF and MMP-9 are important contributors to the regulation of HCC angiogenesis, infiltration, and metastasis [10,40]. The overexpression of MMP-9 in HCC leads to a higher tumor stage through promoting metastasis, lymphocytic infiltration, and overall poor prognosis [41]. VEGF expression in HCC tissues was linked to the recurrence of HCC after hepatectomy and could be used to determine the risk of postoperative recurrence of HCC [42]. Moreover, VEGF was considered as a biochemical marker for the diagnosis of HCC, as serum VEGF level in HCC patients was significantly more elevated than the healthy controls [43].
In accordance with previously mentioned studies, this study revealed that HCC significantly elevated hepatic Cyclin D1 levels, MMP-9 levels, and VEGF expression. Interestingly, CA markedly suppressed Cyclin D1 levels, MMP-9 levels, and VEGF expression. Thus, CA could potentially act as a role player in opposing the activation of the Wnt⁄β-catenin signaling pathway during hepatocarcinogenesis provoked by TAA in rats.
A recent study revealed that oxidative stress and hypoxia promote HCC migration, invasion, and metastasis [44]. As seen in the results of this study, a marked elevation in hepatic MDA content and lowering in hepatic GSH content confirm that hepatocytes were exposed to oxidative stress induced by TAA. Treatment with CA lowered oxidative stress, as indicated by lowered MDA content and elevated GSH content. The established antioxidant capacity of CA was in agreement with a previous study [20].
5. Conclusions
CA has a hopeful anticancer activity for HCC treatment that is mainly related to pronounced inhibition of Wnt/β-catenin signaling pathway, which, in turn, downregulates the cell cycle and tumor angiogenesis. In addition, CA has notable antioxidant activity by lowering hepatocytes oxidative stress, which is a determinant factor for HCC initiation and progression.
Author Contributions
Data curation, A.S.G.A.E.S.; formal analysis, A.S.G.A.E.S.; funding acquisition, A.S.G.A.E.S.; investigation, A.S.G.A.E.S.; methodology, A.S.G.A.E.S., Y.A.S. and M.M.E.-S.; resources, A.S.G.A.E.S.; software, A.S.G.A.E.S.; writing—original draft, A.S.G.A.E.S.; conceptualization, Y.A.S. and M.M.E.-S.; supervision, Y.A.S. and M.M.E.-S.; writing—review and editing, Y.A.S. and M.M.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Faculty of Pharmacy, Mansoura University, Mansoura, Egypt (protocol code: 2022-12 and date of approval: 26 January 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated during this research are presented in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.M.; Fuentes, D.; Morshid, A.I.; Burke, M.R.; Kaseb, A.O.; Hassan, M.; Hazle, J.D.; Elsayes, K.M. Role of Wnt/β-catenin signaling in hepatocellular carcinoma, pathogenesis, and clinical significance. J. Hepatocell. Carcinoma 2018, 5, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wu, Y.; Awadasseid, A.; Tanaka, Y.; Zhang, W. New Advances in Canonical Wnt/β-Catenin Signaling in Cancer. Cancer Manag. Res. 2020, 12, 6987–6998. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.-S.; Ma, L.; Wei, W.; So, S. WNT/β-catenin pathway activation in hepatocellular carcinoma: A clinical perspective. Gastrointest. Cancer Targets Ther. 2014, 4, 49–63. [Google Scholar] [CrossRef]
- Mohammed, M.K.; Shao, C.; Wang, J.; Wei, Q.; Wang, X.; Collier, Z.; Tang, S.; Liu, H.; Zhang, F.; Huang, J.; et al. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 2016, 3, 11–40. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-C.; Bourdelas, A.; Krauss, A.; Lee, H.-J.; Shao, Y.; Wu, D.; Mlodzik, M.; Shi, D.-L.; Zheng, J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol. Cell 2003, 12, 1251–1260. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Zhang, H.; Wang, Z.; Nan, G.; Li, Y.; Zhang, F.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; et al. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: Implications in targeted cancer therapies. Lab Invest. 2016, 96, 116–136. [Google Scholar] [CrossRef] [PubMed]
- Nejak-Bowen, K.N.; Monga, S.P.S. Beta-catenin signaling, liver regeneration and hepatocellular cancer: Sorting the good from the bad. Semin. Cancer Biol. 2011, 21, 44–58. [Google Scholar] [CrossRef]
- Vilchez, V.; Turcios, L.; Marti, F.; Gedaly, R. Targeting Wnt/β-catenin pathway in hepatocellular carcinoma treatment. World J. Gastroenterol. 2016, 22, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.O.; Monga, S.P. Wnt/β-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu. Rev. Pathol. 2018, 13, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, D.S.; Mandlik, S.K. Herbal and Natural Dietary Products: Upcoming Therapeutic Approach for Prevention and Treatment of Hepatocellular Carcinoma. Nutr. Cancer 2020, 73, 2130–2154. [Google Scholar] [CrossRef] [PubMed]
- Cocchiara, J.; Letizia, C.S.; Lalko, J.; Lapczynski, A.; Api, A.M. Fragrance material review on cinnamaldehyde. Food Chem. Toxicol. 2005, 43, 867–923. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Chen, J.; Wang, K.; Geng, Y.; Lai, W.; Huang, X.; Chen, D.; Guo, H.; Fang, J.; Chen, Z.; et al. Study the antibacterial mechanism of cinnamaldehyde against drug-resistant Aeromonas hydrophila in vitro. Microb. Pathog. 2020, 145, 104208. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Deng, J.S.; Chiu, C.S.; Hou, W.C.; Huang, S.S.; Shie, P.H.; Huang, G.J. Anti-Inflammatory Activities of Cinnamomum cassia Constituents In Vitro and In Vivo. Evid. Based Complement. Alternat. Med. 2012, 2012, 429320. [Google Scholar] [CrossRef]
- Lin, L.-T.; Wu, S.-J.; Lin, C.-C. The Anticancer Properties and Apoptosis-inducing Mechanisms of Cinnamaldehyde and the Herbal Prescription Huang-Lian-Jie-Du-Tang ( Huáng Lián Jiě Dú Tang) in Human Hepatoma Cells. J. Tradit. Complement. Med. 2013, 3, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Rehman, M.T.; Shahzad, S.; Naeem, S.S.; Faizy, A.F.; Khan, A.Q.; Khan, M.S.; Husain, F.M.; Moin, S. Anti-oxidant and anti-inflammatory effects of cinnamaldehyde and eugenol on mononuclear cells of rheumatoid arthritis patients. Eur. J. Pharm. 2019, 852, 14–24. [Google Scholar] [CrossRef]
- Elewa, M.A.; Al-Gayyar, M.M.; Schaalan, M.F.; Abd El Galil, K.H.; Ebrahim, M.A.; El-Shishtawy, M.M. Hepatoprotective and anti-tumor effects of targeting MMP-9 in hepatocellular carcinoma and its relation to vascular invasion markers. Clin. Exp. Metastasis 2015, 32, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhuang, Y.; Jiang, S.; Tian, F.; Teng, Y.; Chen, X.; Zheng, P.; Liu, S.; Zhou, J.; Wu, J.; et al. Cinnamaldehyde induces apoptosis and reverses epithelial-mesenchymal transition through inhibition of Wnt/β-catenin pathway in non-small cell lung cancer. Int. J. Biochem. Cell Biol. 2017, 84, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.M.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Saber, S.; Khalil, R.M.; Abdo, W.S.; Nassif, D.; El-Ahwany, E. Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicol. Appl. Pharmacol. 2019, 364, 120–132. [Google Scholar] [CrossRef]
- Liu, Y.; An, T.; Wan, D.; Yu, B.; Fan, Y.; Pei, X. Targets and Mechanism Used by Cinnamaldehyde, the Main Active Ingredient in Cinnamon, in the Treatment of Breast Cancer. Front. Pharmacol. 2020, 11, 582719. [Google Scholar] [CrossRef]
- Han, L.; Mei, J.; Ma, J.; Wang, F.; Gu, Z.; Li, J.; Zhang, Z.; Zeng, Y.; Lou, X.; Yao, X.; et al. Cinnamaldehyde induces endogenous apoptosis of the prostate cancer-associated fibroblasts via interfering the Glutathione-associated mitochondria function. Med. Oncol. 2020, 37, 91. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-e.; Zhuang, Y.-w.; Zhou, J.-y.; Liu, S.-l.; Wang, R.-p.; Shu, P. Cinnamaldehyde enhances apoptotic effect of oxaliplatin and reverses epithelial-mesenchymal transition and stemnness in hypoxic colorectal cancer cells. Exp. Cell Res. 2019, 383, 111500. [Google Scholar] [CrossRef] [PubMed]
- Ka, H.; Park, H.-J.; Jung, H.-J.; Choi, J.-W.; Cho, K.-S.; Ha, J.; Lee, K.-T. Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett. 2003, 196, 143–152. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, J.; Yang, S.; Tan, T.; Wang, N.; Wang, Y.; Zhang, L.; Yang, C.; Huang, H.; Luo, J.; et al. Cinnamaldehyde Inhibits the Function of Osteosarcoma by Suppressing the Wnt/β-Catenin and PI3K/Akt Signaling Pathways. Drug Des. Dev. Ther. 2020, 14, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-J.; Xie, S.-X.; Chen, Y.-T.; Xue, J.-L.; Zhang, C.-J.; Zhu, F. Aberrant regulation of Wnt signaling in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 7486–7499. [Google Scholar] [CrossRef]
- Lu, C.; He, Y.; Duan, J.; Yang, Y.; Zhong, C.; Zhang, J.; Liao, W.; Huang, X.; Zhu, R.; Li, M. Expression of Wnt3a in hepatocellular carcinoma and its effects on cell cycle and metastasis. Int. J. Oncol. 2017, 51, 1135–1145. [Google Scholar] [CrossRef]
- Pashirzad, M.; Fiuji, H.; Khazei, M.; Moradi-Binabaj, M.; Ryzhikov, M.; Shabani, M.; Avan, A.; Hassanian, S.M. Role of Wnt3a in the pathogenesis of cancer, current status and prospective. Mol. Biol. Rep. 2019, 46, 5609–5616. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yao, M.; Zheng, W.; Gu, J.; Yang, X.; Qiu, L.; Cai, Y.; Wu, W.; Yao, D. Abnormality of Wnt3a expression as novel specific biomarker for diagnosis and differentiation of hepatocellular carcinoma. Tumor Biol. 2016, 37, 5561–5568. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-H.; Yao, M.; Cai, Y.; Gu, J.-J.; Yang, X.-L.; Wang, L.; Yao, D.-F. Oncogenic Wnt3a expression as an estimable prognostic marker for hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 3829–3836. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yao, M.; Fang, M.; Pan, L.; Wang, L.; Yang, J.; Dong, Z.; Yao, D. Oncogenic Wnt3a: A Candidate Specific Marker and Novel Molecular Target for Hepatocellular Carcinoma. J. Cancer 2019, 10, 5862–5873. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Fang, M.; Zheng, W.-J.; Yao, D.-F. Oncogenic Wnt3a: A promising specific biomarker in hepatocellular carcinoma. Hepatoma Res. 2018, 4, 30. [Google Scholar] [CrossRef][Green Version]
- Deane, N.G.; Parker, M.A.; Aramandla, R.; Diehl, L.; Lee, W.J.; Washington, M.K.; Nanney, L.B.; Shyr, Y.; Beauchamp, R.D. Hepatocellular carcinoma results from chronic cyclin D1 overexpression in transgenic mice. Cancer Res. 2001, 61, 5389–5395. [Google Scholar] [PubMed]
- Joo, M.; Kang, Y.K.; Kim, M.R.; Lee, H.K.; Jang, J.J. Cyclin D1 overexpression in hepatocellular carcinoma. Liver 2001, 21, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Anna, C.H.; Iida, M.; Sills, R.C.; Devereux, T.R. Expression of potential beta-catenin targets, cyclin D1, c-Jun, c-Myc, E-cadherin, and EGFR in chemically induced hepatocellular neoplasms from B6C3F1 mice. Toxicol. Appl. Pharmacol. 2003, 190, 135–145. [Google Scholar] [CrossRef]
- Qu, B.; Liu, B.-R.; Du, Y.-J.; Chen, J.; Cheng, Y.-Q.; Xu, W.; Wang, X.-H. Wnt/β-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol. Lett. 2014, 7, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.J.; Wang, N.; Sun, Z.W.; Chen, J.; Cui, H.W. MiR-5692a promotes the invasion and metastasis of hepatocellular carcinoma via MMP9. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4869–4878. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Guo, R.P.; Shi, M.; Wei, W.; Yu, W.S.; Li, J.Q. Expression and clinical significance of VEGF and MMP-9 in hepatocellular carcinoma. Ai Zheng Aizheng Chin. J. Cancer 2006, 25, 599–603. [Google Scholar]
- Atta, M.M.; Atta, H.M.; Gad, M.A.; Rashed, L.A.; Said, E.M.; Hassanien Sel, S.; Kaseb, A.O. Clinical significance of vascular endothelial growth factor in hepatitis C related hepatocellular carcinoma in Egyptian patients. J. Hepatocell. Carcinoma 2016, 3, 19–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, M.Q.; You, A.B.; Cui, W.; Zhang, S.; Guo, Z.G.; Chen, L.; Zhu, X.D.; Zhang, W.; Zhu, X.L.; Guo, H.; et al. Cross talk between oxidative stress and hypoxia via thioredoxin and HIF-2α drives metastasis of hepatocellular carcinoma. FASEB J. 2020, 34, 5892–5905. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).