Nanocarrier Systems in Taste Masking
Abstract
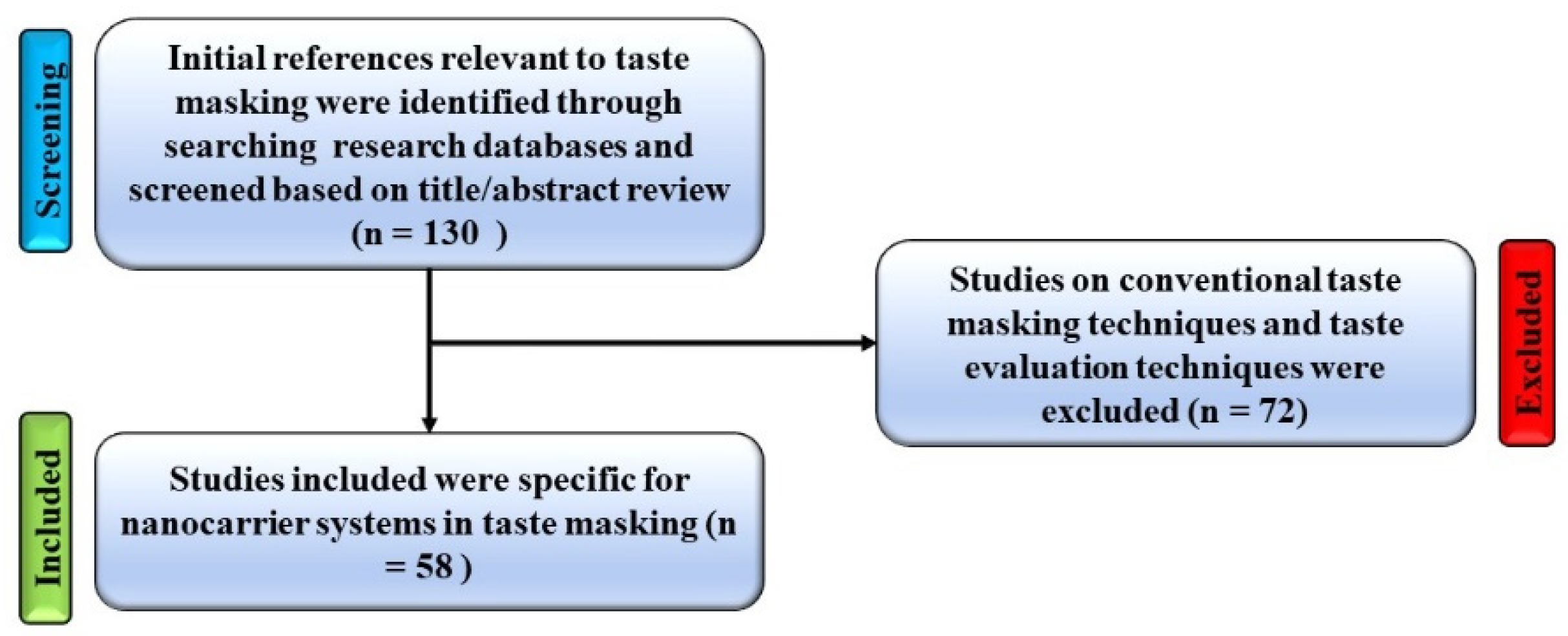
1. Introduction
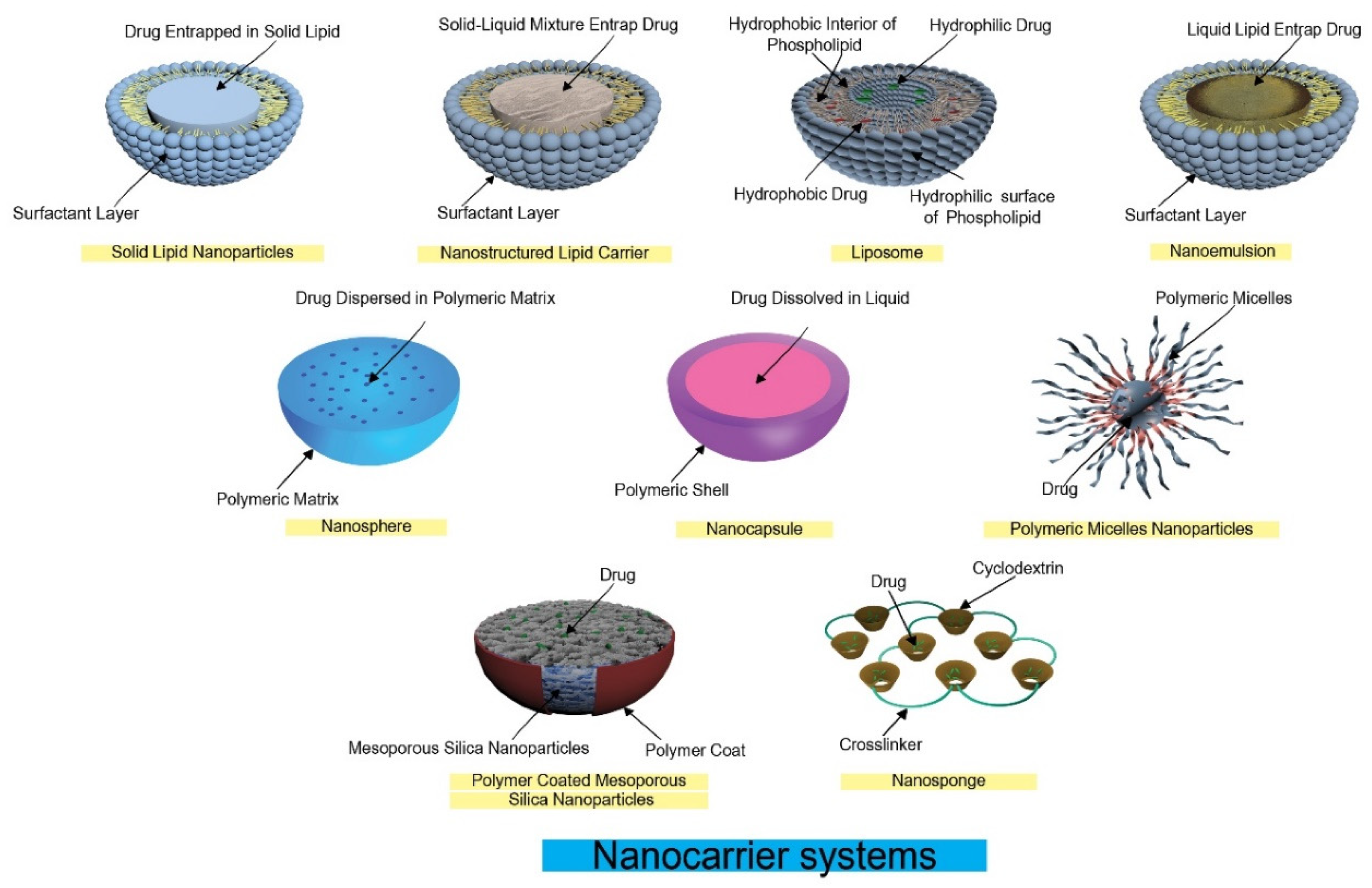
2. Nanocarrier Systems and Taste Masking
2.1. Liposomes
Liposomes and Taste Masking
2.2. Polymeric Nanoparticles
Polymeric Nanoparticles and Taste Masking
2.3. Solid Lipid Nanoparticles (SLNs)
Solid Lipid Nanoparticles and Taste Masking
2.4. Nanostructured Lipid Carriers (NLCs)
Nanostructured Lipid Carriers and Taste Masking
2.5. Polymeric Micelles
Polymeric Micelles and Taste Masking
2.6. Reverse Micelles Nanoparticles
Reverse Micelles Nanoparticles and Taste Masking
2.7. Submicron Lipid Emulsions
Submicron Lipid Emulsion and Taste Masking
2.8. Nanogels
Nanogels and Taste Masking
2.9. Nanosponges (NSs)
Nanosponges and Taste Masking
2.10. Inclusion Complex Formation
Inclusion Complex Formation and Taste Masking
2.11. PH-Responsive Co-Ordination Polymer Coated Mesoporous Silica Nanoparticles (MSNs)
PH-Responsive Co-Ordination Polymer Coated MSNs
2.12. Nanohybrid System
Nanohybrid System and Taste Masking
3. Conclusions
4. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| API | Active Pharmaceutical Ingredient |
| AEA | Polyvinylacetal diethylaminoacetate |
| β-CD | Beta-cyclodextrin |
| CP | Coordination polymer |
| Fe-bipy | Fe-4, 4′-bipyridine |
| HP-β-CD | Hydroxypropyl beta-cyclodextrin |
| MEQ | Mequindox |
| MMT | Montmorillonite |
| MSNs | Mesoporous silica nanoparticles |
| PCL | poly (ε-caprolactone) |
| PEG | Polyethylene Glycol |
| PLGA | Poly Lactic-co-Glycolic Acid |
| PLLA | poly (l-lactic acid) |
| PVP | Polyvinyl Pyrrolidone |
| SLNs | Solid lipid nanoparticles |
| NLC | Nanostructured lipid carriers |
| NS | Nanosponge |
| SMEDDS | Self microemulsifying drug delivery system |
References
- Bernkop-Schnurch, A. Nanocarrier systems for oral drug delivery: Do we really need them? Eur. J. Pharm. Sci. 2013, 49, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Sohi, H.; Sultana, Y.; Khar, R.K. Taste masking technologies in oral pharmaceuticals: Recent developments and approaches. Drug Dev. Ind. Pharm. 2004, 30, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Latha, R.S.; Lakshmi, P.K. Electronic tongue: An analytical gustatory tool. J. Adv. Pharm. Technol. Res. 2012, 3, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Cram, A.; Woertz, K.; Breitkreutz, J.; Winzenburg, G.; Turner, R.; Tuleu, C. Playing hide and seek with poorly tasting paediatric medicines: Do not forget the excipients. Adv. Drug Deliv. Rev. 2014, 73, 14–33. [Google Scholar] [CrossRef]
- Douroumis, D. Orally disintegrating dosage forms, and taste-masking technologies; 2010. Expert Opin. Drug Deliv. 2011, 8, 665–675. [Google Scholar] [CrossRef]
- Beck, T.K.; Jensen, S.; Bjoern, G.K.; Kidmose, U. The masking effect of sucrose on perception of bitter compounds in Brassica vegetables. J. Sens. Stud. 2014, 29, 190–200. [Google Scholar] [CrossRef]
- Lankpord, B.L.; Becker, C.H. The use of some imitation flavors for masking distasteful drugs. II. Quinine hydrochloride. J. Am. Pharm. Assoc. 1951, 40, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Douroumis, D. Practical approaches of taste masking technologies in oral solid forms. Expert Opin. Drug Deliv. 2007, 4, 417–426. [Google Scholar] [CrossRef]
- Bansal, A.; Kreig, B.; Sharma, N.; McGinnis, J.; Bhatia, I.; Paz, C. Taste masking of granulated acetaminophen by water insoluble ethylcellulose coating. Folia Med. 2021, 63, 97–104. [Google Scholar] [CrossRef]
- Borodkin, S.; Sundberg, D.P. Polycarboxylic acid ion-exchange resin adsorbates for taste coverage in chewable tablets. J. Pharm. Sci. 1971, 60, 1523–1527. [Google Scholar] [CrossRef]
- Al-Omran, M.F.; Al-Suwayeh, S.A.; El-Helw, A.M.; Saleh, S.I. Taste masking of diclofenac sodium using microencapsulation. J. Microencapsul. 2002, 19, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Karaman, R. Computationally designed prodrugs for masking the bitter taste of drugs. J. Drug Des. 2012, 1, 106. [Google Scholar] [CrossRef]
- Hussain, M.A.; Aungst, B.J.; Koval, C.A.; Shefter, E. Improved buccal delivery of opioid analgesics and antagonists with bitterless prodrugs. Pharm. Res. 1988, 5, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Kharb, V.; Saharan, V.A.; Kharb, V.; Jadhav, H.; Purohit, S. Formulation and characterization of taste masked ondansetron magnesium aluminum silicate adsorption systems. Drug Dev. Ind. Pharm. 2016, 42, 1291–1299. [Google Scholar] [CrossRef]
- Katsuragi, Y.; Kashiwayanagi, M.; Kurihara, K. Specific inhibitor for bitter taste: Inhibition of frog taste nerve responses and human taste sensation to bitter stimuli. Brain Res. Protoc. 1997, 1, 292–298. [Google Scholar] [CrossRef]
- Shah, P.P.; Mashru, R.C. Development and evaluation of artemether taste masked rapid disintegrating tablets with improved dissolution using solid dispersion technique. AAPS PharmSciTech 2008, 9, 494–500. [Google Scholar] [CrossRef][Green Version]
- Tang, W.L.; Tang, W.H.; Chen, W.C.; Diako, C.; Ross, C.F.; Li, S.D. Development of a Rapidly Dissolvable Oral Pediatric Formulation for Mefloquine Using Liposomes. Mol. Pharm. 2017, 14, 1969–1979. [Google Scholar] [CrossRef]
- Zhu, Y.; You, X.; Huang, K.; Raza, F.; Lu, X.; Chen, Y.; Dhinakar, A.; Zhang, Y.; Kang, Y.; Wu, J.; et al. Effect of taste masking technology on fast dissolving oral film: Dissolution rate and bioavailability. Nanotechnology 2018, 29, 304001. [Google Scholar] [CrossRef]
- Seyedabadi, M.M.; Rostami, H.; Jafari, S.M.; Fathi, M. Development and characterization of chitosan–coated nanoliposomes for encapsulation of caffeine. Food Biosci. 2021, 40, 100857. [Google Scholar] [CrossRef]
- Naik, J.; Rajput, R.; Singh, M.K. Development and Evaluation of Ibuprofen Loaded Hydrophilic Biocompatible Polymeric Nanoparticles for the Taste Masking and Solubility Enhancement. BioNanoScience 2021, 11, 21–31. [Google Scholar] [CrossRef]
- Krieser, K.; Emanuelli, J.; Daudt, R.M.; Bilatto, S.; Willig, J.B.; Guterres, S.S.; Pohlmann, A.R.; Buffon, A.; Correa, D.S.; Külkamp–Guerreiro, I.C. Taste-masked nanoparticles containing Saquinavir for pediatric oral administration. Mater. Sci. Eng. C 2020, 117, 111315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, L.; Wang, T.; Li, H.; Huang, R.; Zhang, Z.; Wang, Y.; Quan, D. Taste masking of water-soluble drug by solid lipid microspheres: A child-friendly system established by reversed lipid-based nanoparticle technique. J. Pharm. Pharmacol. 2020, 72, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Chen, D.; Xu, W.; Tao, Y.; Pan, Y.; Meng, K.; Abu Bakr Shabbir, M.; Liu, Q.; Huang, L.; et al. Solid lipid nanoparticles with enteric coating for improving stability, palatability, and oral bioavailability of enrofloxacin. Int. J. Nanomed. 2019, 14, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Dandagi, P.M.; Rath, S.P.; Gadad, A.P.; Mastiholimath, V.S. Taste masked quinine sulphate loaded solid lipid nanoparticles for flexible pediatric dosing. Indian J. Pharm. Educ. Res. 2014, 48, 93–99. [Google Scholar] [CrossRef]
- Akhoond Zardini, A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2018, 55, 287–298. [Google Scholar] [CrossRef]
- Li, P.; Tian, Y.; Ke, X.M.; Tan, Q.C.; Han, X.; Ma, H.Y.; Pei, J.; Lin, J.Z.; Xu, R.C.; Han, L.; et al. Amphiphilic Block Copolymers: A Novel Substance for Bitter–Masking in Aqueous Solutions. Mol. Pharm. 2020, 17, 1586–1595. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Y.; Wang, T.; Shen, L.; Zhang, Z.; Wang, Y.; Quan, D. Creation of an assessment system for measuring the bitterness of azithromycin–containing reverse micelles. Asian J. Pharm. Sci. 2018, 13, 343–352. [Google Scholar] [CrossRef]
- Monteagudo, E.; Langenheim, M.; Salerno, C.; Buontempo, F.; Bregni, C.; Carlucci, A. Pharmaceutical optimization of lipid-based dosage forms for the improvement of taste–masking, chemical stability and solubilizing capacity of phenobarbital. Drug Dev. Ind. Pharm. 2014, 40, 783–792. [Google Scholar] [CrossRef]
- Hasan, N.M.; Al–aram, M.S.; Al–wadie, M.S.; Althobaiti, F.A.; Al–Malki, M.J. Flavored self microemulsifying lipid formulations for masking the organoleptic taste of pharmaceutical actives. J. Appl. Pharm. Sci. 2015, 5, 127–134. [Google Scholar] [CrossRef][Green Version]
- Omar, S.M.; Ibrahim, F.; Ismail, A. Formulation and evaluation of cyclodextrin-based nanosponges of griseofulvin as pediatric oral liquid dosage form for enhancing bioavailability and masking bitter taste. Saudi Pharm. J. 2020, 28, 349–361. [Google Scholar] [CrossRef]
- Chay, S.K.; Keating, A.V.; James, C.; Aliev, A.E.; Haider, S.; Craig, D.Q. Evaluation of the taste-masking effects of (2–hydroxypropyl)–β–cyclodextrin on ranitidine hydrochloride; a combined biosensor, spectroscopic and molecular modelling assessment. RSC Adv. 2018, 8, 3564–3573. [Google Scholar] [CrossRef]
- Stojanov, M.; Wimmer, R.; Larsen, K.L. Study of the inclusion complexes formed between cetirizine and α-, β-, and γ-cyclodextrin and evaluation on their taste-masking properties. J. Pharm. Sci. 2011, 100, 3177–3185. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Mashru, R.C. Palatable reconstitutable dry suspension of artemether for flexible pediatric dosing using cyclodextrin inclusion complexation. Pharm. Dev. Technol. 2010, 15, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.M.; Wang, L.; Yuan, H.Q.; Wang, X.Y.; Mei, T.X.; Qu, M.R. Taste masking of a drug by pH–responsive coordination polymer-coated mesoporous silica nanoparticles. RSC Adv. 2016, 6, 109453–109459. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, G.; Oh, Y.J.; Park, J.W.; Choy, Y.B.; Park, M.C.; Yoon, Y.J.; Lee, H.J.; Chang, H.C.; Choy, J.H. A nanohybrid system for taste masking of sildenafil. Int. J. Nanomed. 2012, 7, 1635–1649. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, J.; Niu, M.; Gao, X.; Zhang, G.; Yu, H.; Yang, X.; Liu, L. Ten Years of Knowledge of Nano–Carrier Based Drug Delivery Systems in Ophthalmology: Current Evidence, Challenges, and Future Prospective. Int. J. Nanomed. 2021, 16, 6497–6530. [Google Scholar] [CrossRef]
- Alexander, T.; Florence, J.S. Modern Pharmaceutics, Volume 2: Applications and Advances, 5th ed.; CRC Press: Boca Raton, FL, USA, 2010; Volume 2, p. 560. [Google Scholar]
- Chen, L.-J.; Yang, C.-X.; Yan, X.-P. Liposome–Coated Persistent Luminescence Nanoparticles as Luminescence Trackable Drug Carrier for Chemotherapy. Anal. Chem. 2017, 89, 6936–6939. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Lee, Y.W.; Scaletti, F.; Yu, R.; Rotello, V.M. Intracellular delivery of proteins by nanocarriers. Nanomedicine 2017, 12, 941–952. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei–Sadabady, R.; Davaran, S.; Woo Joo, S.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati–Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Khalil, H.; Wang, R.; Lu, T.; Zhao, W.; Zhang, Y.; Chen, Y.; Chen, T. Fusion between fluid liposomes and intact bacteria: Study of driving parameters and in vitro bactericidal efficacy. Int. J. Nanomed. 2016, 11, 4025–4036. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Betageri, G.V.; Singh, M. Factors affecting microencapsulation of drugs in liposomes. J. Microencapsul. 1995, 12, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [CrossRef]
- Mansha, M.; Khan, I.; Ullah, N.; Qurashi, A. Synthesis, characterization and visible-light-driven photoelectrochemical hydrogen evolution reaction of carbazole–containing conjugated polymers. Int. J. Hydrog. Energy 2017, 42, 10952–10961. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Alakhov, V.Y. Pluronic block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, R.S.; Hebbar, H.U. Reverse micelles for nanoparticle synthesis and biomolecule separation. In Nanoscience in Food and Agriculture 4; Springer: Cham, Switzerland, 2017; pp. 181–211. [Google Scholar]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Khan, Z.I.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Emerging role of microemulsions in cosmetics. Recent Pat. Drug Deliv. Formul. 2008, 2, 275–289. [Google Scholar] [CrossRef]
- Hippalgaonkar, K.; Majumdar, S.; Kansara, V. Injectable lipid emulsions–Advancements, opportunities and challenges. AAPS PharmSciTech 2010, 11, 1526–1540. [Google Scholar] [CrossRef]
- Hoar, T.P.; Schulman, J.H. Transparent Water-in-Oil Dispersions: The Oleopathic Hydro-Micelle. Nature 1943, 152, 102–103. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Tang, X. Chemical stability of teniposide in aqueous and parenteral lipid emulsions. Drug Dev. Ind. Pharm. 2009, 35, 508–513. [Google Scholar] [CrossRef]
- Simovic, S.; Hui, H.; Song, Y.; Davey, A.K.; Rades, T.; Prestidge, C.A. An oral delivery system for indomethicin engineered from cationic lipid emulsions and silica nanoparticles. J. Control. Release 2010, 143, 367–373. [Google Scholar] [CrossRef]
- Ades, A.; Carvalho, J.P.; Graziani, S.R.; Amancio, R.F.; Souen, J.S.; Pinotti, J.A.; Maranhão, R.C. Uptake of a cholesterol-rich emulsion by neoplastic ovarian tissues. Gynecol. Oncol. 2001, 82, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers. In Multifunctional Pharmaceutical Nanocarriers; Torchilin, V., Ed.; Springer: New York, NY, USA, 2008; pp. 67–80. [Google Scholar]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano–Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.; Shende, P.; Trotta, F. Diversity of beta-cyclodextrin-based nanosponges for transformation of actives. Int. J. Pharm. 2019, 565, 333–350. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef]
- Sharma, N.; Baldi, A. Exploring versatile applications of cyclodextrins: An overview. Drug Deliv. 2016, 23, 739–757. [Google Scholar] [CrossRef]
| Study | Journal | Year | Taste Masking Technique | Ref No |
|---|---|---|---|---|
| Tang W.L. | Mol. Pharm. | 2017 | Liposomes | [17] |
| Zhu Y. | Nanotechnology. | 2018 | [18] | |
| Seyedabadi M.M. | Food Bioscience. | 2021 | [19] | |
| Naik J. | BioNanoScience. | 2021 | Polymeric Nanoparticles | [20] |
| Krieser K. | Materials Science and Engineering: C | 2020 | [21] | |
| Zhang Y. | J. Pharm. Pharmacol. | 2020 | Solid Lipid Nanoparticles (SLNs) | [22] |
| Li C. | Int. J. Nanomedicine. | 2018 | [23] | |
| Dandagi P.M. | Indian Journal of Pharmaceutical Education and Research. | 2014 | [24] | |
| Akhoond Zardini A. | J. Food Sci. Technol. | 2018 | Nanostructured Lipid Carriers (NLCs) | [25] |
| Li P. | Mol. Pharm. | 2020 | Polymeric Micelles | [26] |
| Huang R. | Asian J. Pharm. Sci. | 2018 | Reverse Micelles | [27] |
| Monteagudo E. | Drug Dev. Ind. Pharm. | 2014 | Submicron Lipid Emulsion | [28] |
| Hasan N.M. | Journal of Applied Pharmaceutical Science. | 2015 | [29] | |
| NA | NA | Nanogel | NA | |
| Omar S.M. | Saudi Pharm. J. | 2020 | Nanosponges (NSs) | [30] |
| Chay S.K. | RSC Advances. | 2018 | Inclusion complex | [31] |
| Stojanov M. | Journal of Pharmaceutical Sciences. | 2011 | [32] | |
| Shah P.P. | Pharm. Dev. Technol. | 2010 | [33] | |
| Bao G.M. | RSC Advances. | 2016 | PH-responsive co-ordination polymer coated mesoporous silica nanoparticles (MSNs) | [34] |
| Lee J.H. | Int. J. Nanomedicine. | 2012 | Nanohybrid system | [35] |
| Technique | Drug | Materials | Taste Evaluation Method | Ref | |
|---|---|---|---|---|---|
| 1 | Liposomes | Mefloquine | Phospholipids, cholesterol | Electronic tongue | [17] |
| Loratadine | Phospholipids, cholesterol | Sensory evaluation | [18] | ||
| Caffeine | Phospholipids, cholesterol, chitosan | NA | [19] | ||
| 2 | Polymeric Nanoparticles | Ibuprofen | Eudragit L100 PVP K30 β Cyclodextrin | In vitro dissolution | [20] |
| Saquinavir | Eudragit RS 100 Pullulan Triglyceride capric/caprylic | Electronic tongue | [21] | ||
| 3 | SLNs | Atomoxetine | Phospholipids, medium-chain triglycerides | In vitro human panel | [22] |
| Enrofloxacin | Octadecanoic acid, polyvinyl alcohol, polyacrylic resin | Pig feeding | [23] | ||
| Quinine Sulphate | Glyceryl monostearate, polysorbate 80, poloxamer 407, poloxamer 188 | Franz diffusion cell | [24] | ||
| 4 | NLCs | Lycopene | Glyceryl monostearate Glyceryl distearate Lecithin | Human panel | [25] |
| 5 | Polymeric Micelles | Berberine hydrochloride, Quinine sulfate, Gentiopicroside, Matrine | Amphiphilic block copolymers | Volunteer sensory test | [26] |
| 6 | Reverse Micelles | Azithromycin | Phospholipids, medium-chain triglycerides | In vitro human panel | [27] |
| 7 | Submicron Lipid Emulsion | Phenobarbital | Polyoxyl 40 hydrogenated castor oil, caprylocaproyl polyoxyl-8-glycerides, isopropyl myristate, glycerol, monocaprylocaprate, caprylic/capric triglyceride, propylene glycol caprylate, propylene glycol dicaprylate/dicaprate, diethylene glycol monoethyl ether, propylene glycol, glycerol, PEG 400 | Electronic tongue | [28] |
| Peppermint Oil | Medium-chain triglyceride, propylene glycol dicaprylate/caprate, PEG 6 caprylic/capric glycerides, PEG 40 hydrogenated castor oil, oleic Acid | NA | [29] | ||
| 8 | NSs | Griseofulvin | β Cyclodextrin, Diphenyl Carbonate | Human panel gustatory response palatability studies | [30] |
| 9 | Inclusion complex | Ranitidine | HP-β-CD | Electronic tongue | [31] |
| Cetirizine | β-CD | Volunteer sensory study | [32] | ||
| Artemether | HP-β-CD and PVP | Gustatory sensation test | [33] | ||
| 10 | PH-responsive co-ordination polymer coated MSNs | Mequindox (MEQ) | MSNs | In vitro drug release | [34] |
| 11 | Nanohybrid system | Sildenafil Citrate | MMT, PVA-EAA | In vitro drug release | [35] |
| Nanocarrier System | Advantages | Disadvantages | Characteristics |
|---|---|---|---|
| Liposomes | Entrap hydrophilic and hydrophobic drugs | Leakage of drug Low physical stability Inapplicable upscaling | Vesicles formed of bilayer amphiphilic lipids spherical in shape |
| Polymeric Nanoparticles | Drug encapsulated, dissolved, entrapped, or attached to the polymeric matrix | Process complexity and cost | Spherical particles composed of polymer; may be nanocapsule or nanosphere |
| SLNs | Less liable to coalescence and agglomeration | Low loading capacity Drug expulsion during stability | Particles are solid lipids at room temperature stabilized by surfactant |
| NLCs | Higher loading capacity | Required specific machinery | Particles are a mix of solid lipids and liquid lipids stabilized by surfactants. |
| Polymeric Micelles | Easy dispersion in aqueous media Core entrap lipophilic drugs Suitable for poorly soluble drugs | Biodegradability and biocompatibility issues | Synthetic amphiphilic copolymers form micelles with lipophilic core dispersed in aqueous media |
| Reverse Micelles | Core entrap aqueous drugs | Process complexity | Micelles dispersed in organic medium with the help of surfactant |
| Submicron Lipid Emulsion | Higher stability than conventional emulsion | Higher cost than conventional emulsion | Dispersed droplets nanosized in the almost transparent liquid with the assistance of surfactant and co-surfactant |
| Nanogel | Could target specific site Encapsulating small biologically active agents and biomacromolecules. | Presence of harmful residual surfactants Premature drug leakage Drug loading capacity needs improvement | Swelling particles of polymer crosslinked physically or chemically in a suitable solvent |
| NSs | Convert physical from liquid to solid | Safety, toxicity, and biodegradability concerns | Colloidal lipophilic structure resulting from the interaction of the crosslinking agent with cyclodextrin |
| Inclusion complex | Improve stability and solubility | Safety, toxicity, and biodegradability concerns | Cyclodextrin complex aggregates in aqueous media |
| PH-responsive co-ordination polymer coated MSNs | Simple and efficient | Required safety and toxicity investigation | Mesoporous silica particles engulf the drug such particles coated with polymer control the drug release based on pH of the polymer coat |
| Nanohybrid system | Enhanced drug release | Long process Inapplicable upscaling | Drug intercalated with inorganic clay particles and intercalated particles coated with the polymer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, N.E.H.; ElMeshad, A.N.; Fares, A.R. Nanocarrier Systems in Taste Masking. Sci. Pharm. 2022, 90, 20. https://doi.org/10.3390/scipharm90010020
Nasr NEH, ElMeshad AN, Fares AR. Nanocarrier Systems in Taste Masking. Scientia Pharmaceutica. 2022; 90(1):20. https://doi.org/10.3390/scipharm90010020
Chicago/Turabian StyleNasr, Nasr Eldin Hussein, Aliaa Nabil ElMeshad, and Ahmed Roshdy Fares. 2022. "Nanocarrier Systems in Taste Masking" Scientia Pharmaceutica 90, no. 1: 20. https://doi.org/10.3390/scipharm90010020
APA StyleNasr, N. E. H., ElMeshad, A. N., & Fares, A. R. (2022). Nanocarrier Systems in Taste Masking. Scientia Pharmaceutica, 90(1), 20. https://doi.org/10.3390/scipharm90010020







