Compatibility of Different Formulations in TrichoConceptTM Vehicles for Hair Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Active Pharmaceutical Ingredients Cream Samples
- F1. Clobetasol 0.05% in TrichoWashTM
- Weigh clobetasol propionate in a hood;
- Measure approximately 50% of the required TrichoWashTM and incorporate clobetasol propionate until a paste is obtained, using glass mortar and pestle;
- Transfer to a light-resistant polyethylene dispensing container, adding the required total amount of TrichoWashTM, rinsing the mortar;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F2. Ketoconazole 2% in TrichoWashTM
- Weigh ketoconazole in a hood;
- Dissolve ketoconazole with DMSO, using glass mortar and pestle;
- Slowly add approximately 80% of the required TrichoWashTM, using diametric dilution;
- Transfer to a light-resistant polyethylene dispensing container and bring to volume;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F3. Spironolactone 1% in TrichoWashTM
- Weigh spironolactone in a hood;
- Dissolve spironolactone with ethoxy diglycol, using a magnetic stirrer;
- In an Unguator jar, weigh approximately 50% of the required TrichoWashTM. Add the spironolactone/ethoxy diglycol solution, and then the rest of the required TrichoWashTM;
- Spin on Unguator (FagronLab, Scheßlitz, Germany) slowly, on direct speed 2/2/2/.
- Transfer to a light-resistant polyethylene dispensing container and bring to volume;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F4. Latanoprost 0.1% in TrichoCreamTM
- Measure required amount of latanoprost;
- Weigh 50% of required TrichoCreamTM;
- Incorporate latanoprost into TrichoCreamTM;
- Add the remaining amount of required TrichoCreamTM to Unguator jar and spin at normal speed;
- Transfer to a light-resistant polyethylene dispensing jar;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F5. Pyridoxine HCl 0.5%, Vitamin A acetate 1%, and Vitamin E succinate 12.1 IU in TrichoCondTM
- Weigh pyridoxine HCl, vitamin A acetate, and Vitamin E succinate in a hood;
- Place vitamin A in purified water and heat to 50 °C and mix until homogeneously suspended;
- Measure approximately 50% of the required TrichoCondTM (which is an emulsion) and incorporate Vitamin A, Vitamin E, and pyridoxine HCl into a paste for the solubilization of the APIs, using a glass mortar and pestle;
- Once mixed, transfer to a light-resistant polyethylene dispensing container and bring to volume with TrichoCondTM;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F6. Caffeine 2%, menthol 1%, and pyridoxine HCl 0.5% in TrichoWashTM
- Weigh caffeine, menthol, and pyridoxine HCl in a hood;
- Triturate and dissolve menthol in DMSO, using a separate glass mortar and pestle;
- Heat purified water to 150 °C and dissolve caffeine, mixing;
- Dissolve pyridoxine HCl in heated caffeine/water mixture, then let it achieve room temperature;
- Tare a speed mixer jar and add the pyridoxine HCl/caffeine/water mixture and the menthol/DMSO mixture;
- Bring to volume with the heated TrichoWashTM and lightly stir with a glass stir rod;
- Transfer to a light-resistant polyethylene dispensing container;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
- F7. Latanoprost 0.1%, minoxidil 5%, and finasteride 0.25% in TrichoSolTM
- Weigh minoxidil and finasteride in a hood;
- Triturate and dissolve finasteride in ethoxy diglycol, using a magnetic stirrer;
- Measure required amount of latanoprost;
- Measure approximately 80% of the required TrichoSolTM. Add finasteride/ethoxy diglycol, then dissolve minoxidil, with mixing;
- Add latanoprost to the previous solution and mix well;
- Transfer to a light-resistant polyethylene dispensing container and bring to volume with TrichoSolTM;
- Adjust pH to 4.0–5.0 with triethanolamine, if needed.
2.2. Compatibility Study
2.3. Chromatographic Conditions
3. Results
4. Conclusions
- F1 = clobetasol 0.05% in TrichoWashTM. BUD = 6 months.
- F2 = ketoconazole 2% in TrichoWashTM. BUD = 6 months.
- F3 = spironolactone 1% in TrichoWashTM. BUD = 6 months.
- F4 = latanoprost 0.1% in TrichoCreamTM. BUD = 3 months.
- F5 = pyridoxine HCl 0.5%, vitamin A acetate 1% and vitamin E succinate 12.1 IU in TrichoCondTM. BUD = 6 months.
- F6 = caffeine 2%, menthol 1%, and pyridoxine HCl 0.5% in TrichoWashTM. BUD = 6 months.
- F7 = latanoprost 0.1%, minoxidil 5%, and finasteride 0.25% in TrichoSolTM. BUD = 3 months.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rhodes, T.; Girman, C.J.; Savin, R.C.; Kaufman, K.D.; Guo, S.; Lilly, F.R.W.; Siervogel, R.M.; Chumlea, C.W. Prevalence of Male Pattern Hair Loss in 18–49 Year Old Men. Dermatol. Surg. 1998, 24, 1330–1332. [Google Scholar] [CrossRef]
- Emer, J.; Levy, L.L.L. Female pattern alopecia: Current perspectives. Int. J. Women’s Health 2013, 5, 541–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hunt, N.; McHale, S. The psychological impact of alopecia. BMJ 2005, 331, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.; Jardim, M.; Antunes, V.M.D.S.; Michelin, L.F.G.; dos Santos, B.A.R.; Barbosa, C.M.V.; Spindola, D.G.; Bincoletto, C.; Oliveira, C.R. In Vitro Effects of the Phytocomplex TrichoTechTM on Human Fibroblasts: Proliferative Potential and Effects on Gene Expression of FGF-7 and FGF-10. J. Cosmet. Dermatol. Sci. Appl. 2017, 7, 1–13. [Google Scholar] [CrossRef][Green Version]
- Dhurat, R.; Chitallia, J.; May, T.W.; Jayaraaman, A.M.; Madhukara, J.; Anandan, S.; Vaidya, P.; Klenk, A. An Open-Label Randomized Multicenter Study Assessing the Noninferiority of a Caffeine-Based Topical Liquid 0.2% versus Minoxidil 5% Solution in Male Androgenetic Alopecia. Ski. Pharmacol. Physiol. 2017, 30, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Lenane, P.; MacArthur, C.; Parkin, P.C.; Krafchik, B.; DeGroot, J.; Khambalia, A.; Pope, E. Clobetasol Propionate, 0.05%, vs Hydrocortisone, 1%, for Alopecia Areata in Children. JAMA Dermatol. 2014, 150, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Piraccini, B.M.; Pazzaglia, M.; Vincenzi, C. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J. Am. Acad. Dermatol. 2003, 49, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Loconsole, G.; Cammisa, G.; Mastrolonardo, G.; Vena, G. Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16-month therapy course. J. Dermatol. Treat. 1997, 8, 189–192. [Google Scholar] [CrossRef]
- Hajheydari, Z.; Akbari, J.; Saeedi, M.; Shokoohi, L. Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Srisuwanwattana, P.; Chalermroj, N.; Khunkhet, S. A randomized, double-blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride admixed with 3% minoxidil vs. 3% minoxidil solution in the treatment of male androgenetic alopecia. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2257–2263. [Google Scholar] [CrossRef] [PubMed]
- Dill-Müller, D.; Zaun, H. Topical Treatment of Androgenetic Alopecia with Spironolactone. J. Eur. Acad. Dermatol. Venereol. 1997, 9, S31. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Lönnfors, S.; Hillmann, K.; Bartels, N.G. A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J. Am. Acad. Dermatol. 2012, 66, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Aleid, N.M.; Fertig, R.; Maddy, A.; Tosti, A. Common Allergens Identified Based on Patch Test Results in Patients with Suspected Contact Dermatitis of the Scalp. Ski. Appendage Disord. 2016, 3, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2018, 9, 51–70. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia. <795> Pharmaceutical Compounding—Nonsterile Preparations; United States Pharmacopeia: Rockville, MD, USA, 2020. [Google Scholar]
| API | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
|---|---|---|---|---|---|---|---|
| Caffeine | - | - | - | - | - | 20 mg | - |
| Clobetasol propionate | 0.5 mg | - | - | - | - | - | - |
| DMSO | - | 0.02 mL | - | - | - | 0.01 mL | - |
| Ethoxy diglycol | - | - | 0.1 mL | - | - | - | 0.2 mL |
| Finasteride | - | - | - | - | - | - | 2.5 mg |
| Ketoconazole | - | 20 mg | - | - | - | - | - |
| Latanoprost 50 mg/mL in DMSO | - | - | - | 0.02 mL | - | - | 0.02 mL |
| Menthol crystal | - | - | - | - | - | 10 mg | - |
| Minoxidil | - | - | - | - | - | - | 50 mg |
| Purified water | - | - | - | - | 0.25 mL | 0.05 mL | - |
| Pyridoxine hydrochloride | - | - | - | - | 5 mg | 5 mg | - |
| Spironolactone | - | - | 10 mg | - | - | - | - |
| Vitamin A acetate | - | - | - | - | 10 mg | - | - |
| Vitamin E succinate | - | - | - | - | 10 mg | - | - |
| TrichoCondTM | - | - | - | - | q.s. 1 mL | - | - |
| TrichoCreamTM | - | - | - | q.s. 1 g | - | - | - |
| TrichoSolTM | - | - | - | - | - | - | q.s. 1 mL |
| TrichoWashTM | q.s. 1 mL | q.s. 1 mL | q.s. 1 mL | - | - | q.s. 1 mL | - |
| Formulation | Mobile Phase Composition | Work Concentration (μg/mL)/Injection Volume (µL) | Column | Flux (mL/min) | Ultraviolet Detection Wavelength (nm) |
|---|---|---|---|---|---|
| F1 | 190 mL acetonitrile + 40 methanol + 170 mL 0.05 M monobasic sodium phosphate | 40.0/10 | C18, 150 × 4.6 mm | 1.0 | 240 |
| F2 | 400 mL water + 650 mL acetonitrile + 0.5 mL triethylamine | 200.0/10 | C18, 100 × 4.6 mm | 2.0 | 240 |
| F3 | 220 mL water + 255 mL acetonitrile + 50 mL methanol + 1.35 mL phosphoric acid | 5.0/5 | C18, 150 × 4.6 mm | 1.0 | 230 |
| F4 | 180 mL water + 1.1 g potassium phosphate monobasic + 220 mL acetonitrile | 50.0/4 | C18, 300 × 3.9 mm | 2.0 | 200 |
| F5 | 10 mL glacial acetic acid + 0.6 g sodium 1-hexanesulfonate + 700 mL of water | 50.0 (pyridoxine)/40 | C18, 250 × 4.6 mm | 1.5 | 280 |
| F6 | 25 mL acetonitrile + 20 mL tetrahydrofuran + 955 mL 0.82 g/L of anhydrous sodium acetate | 200.0 (caffeine), 50.0 (pyridoxine)/5 | C18, 150 × 4.6 mm | 1.0 | 275 |
| F7 | 280 mL water + 1.1 g potassium phosphate monobasic + 220 mL acetonitrile | 5.0 (latanoprost), 250.0 (minoxidil),12.5 (finasteride)/4.0 | C18, 300 × 3.9 mm | 2.0 | 200 |
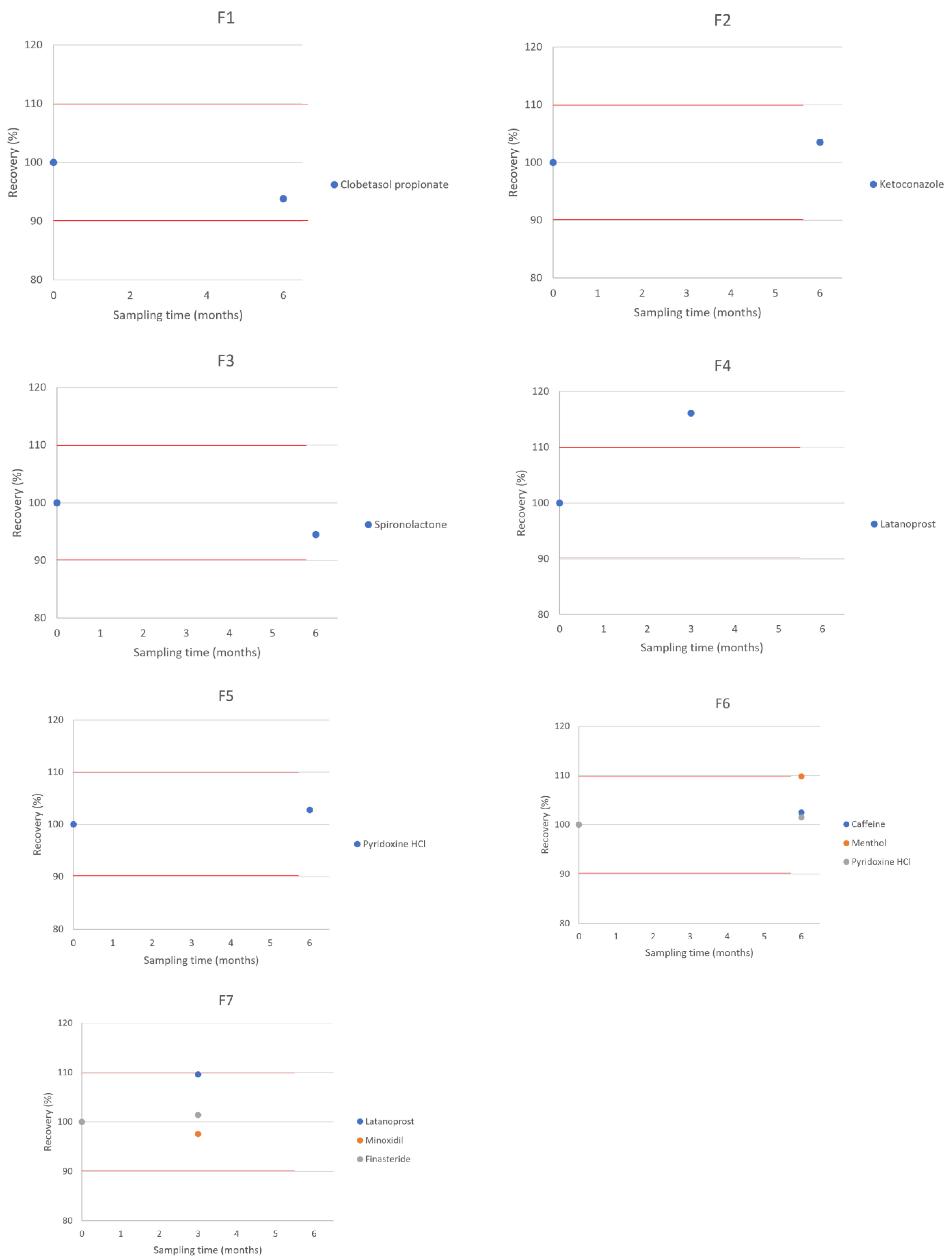
| Elapsed Time (Months) | % Recovery (Room Temperature, 20–25 °C) |
|---|---|
| Clobetasol propionate 0.05%—compounded in TrichoWashTM | |
| T = 0 | 100.0 |
| T = 6 | 93.8 |
| Ketoconazole 2%—compounded in TrichoWashTM | |
| T = 0 | 100.0 |
| T = 6 | 103.5 |
| Spironolactone 1%—compounded in TrichoWashTM | |
| T = 0 | 100.0 |
| T = 6 | 94.5 |
| Latanoprost 0.1%—compounded in TrichoCreamTM | |
| T = 0 | 100.0 |
| T = 3 | 106.6 |
| Pyridoxine HCl 0.5%—compounded with vitamin A acetate 1% + vitamin E succinate 12.1 IU in TrichoCondTM | |
| T = 0 | 100.0 |
| T = 6 | 102.78 |
| Caffeine 2%—compounded in combination with menthol 1% + pyridoxine HCl 0.5%, in TrichoWashTM | |
| T = 0 | 100.0 |
| T = 6 | 102.5 |
| Menthol 1%—compounded in combination with caffeine 2% + pyridoxine HCl 0.5%, in TrichoWashTM | |
| T = 0 | 100.0 |
| T = 6 | 109.8 |
| Pyridoxine HCl 0.5%—compounded in combination with caffeine 2% + menthol 1%, in TrichoWashTM | |
| T = 0 | 100.0 |
| T = 6 | 102.5 |
| Latanoprost 0.1%—compounded in combination with minoxidil 5% + finasteride 0.25%, in TrichoSolTM | |
| T = 0 | 100.0 |
| T = 3 | 109.6 |
| Minoxidil 5%—compounded in combination with latanoprost 0.1% + finasteride 0.25%, in TrichoSolTM | |
| T = 0 | 100.0 |
| T = 3 | 97.56 |
| Finasteride 0.25%—compounded in combination with latanoprost 0.1% + minoxidil 5%, in TrichoSolTM | |
| T = 0 | 100.0 |
| T = 3 | 101.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polonini, H.; Taylor, S.; Zander, C. Compatibility of Different Formulations in TrichoConceptTM Vehicles for Hair Treatments. Sci. Pharm. 2022, 90, 16. https://doi.org/10.3390/scipharm90010016
Polonini H, Taylor S, Zander C. Compatibility of Different Formulations in TrichoConceptTM Vehicles for Hair Treatments. Scientia Pharmaceutica. 2022; 90(1):16. https://doi.org/10.3390/scipharm90010016
Chicago/Turabian StylePolonini, Hudson, Sarah Taylor, and Clark Zander. 2022. "Compatibility of Different Formulations in TrichoConceptTM Vehicles for Hair Treatments" Scientia Pharmaceutica 90, no. 1: 16. https://doi.org/10.3390/scipharm90010016
APA StylePolonini, H., Taylor, S., & Zander, C. (2022). Compatibility of Different Formulations in TrichoConceptTM Vehicles for Hair Treatments. Scientia Pharmaceutica, 90(1), 16. https://doi.org/10.3390/scipharm90010016






