Development of Microemulsions Containing Glochidion wallichianum Leaf Extract and Potential for Transdermal and Topical Skin Delivery of Gallic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GW Leaf Extract
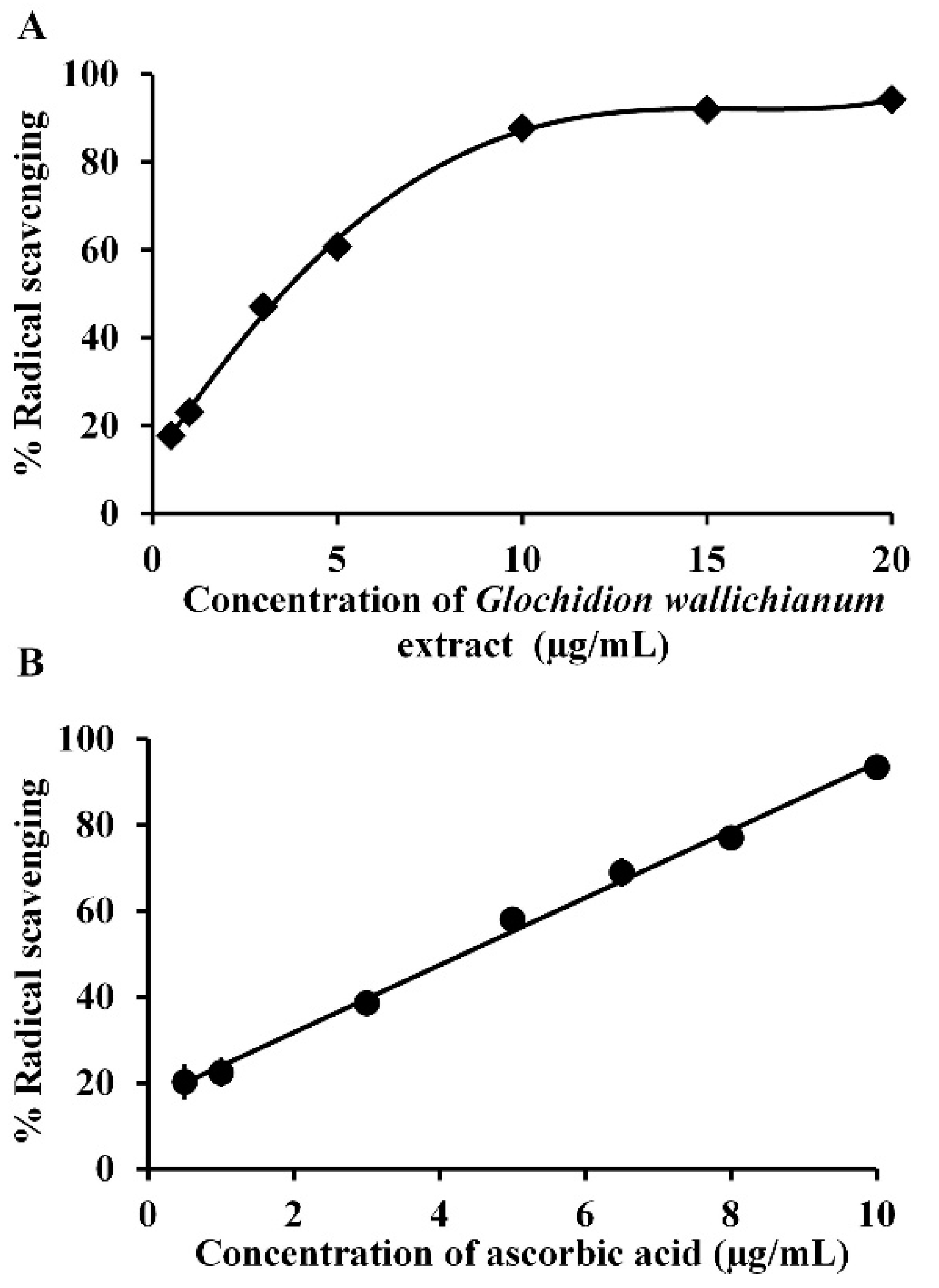
2.3. Determination of the Antioxidant Activity of GW Leaf Extract
2.4. Quantitative Analysis of Gallic Acid in GW Leaf Extract
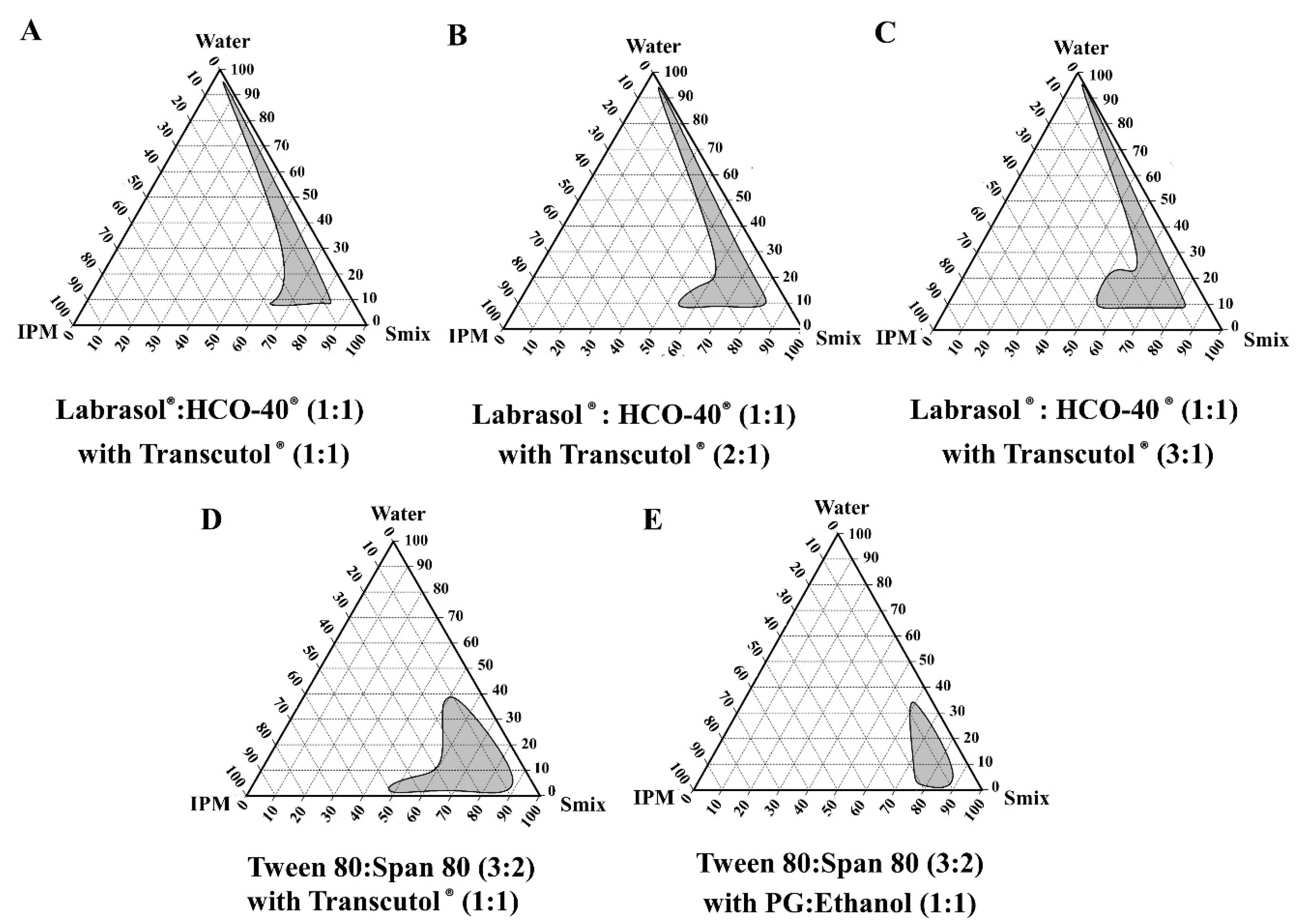
2.5. Construction of Pseudo-Ternary Phase Diagrams
2.6. Preparation of Blank Microemulsions and Microemulsions Containing the GW Extract
2.7. Characterization of Microemulsions
2.7.1. Droplet Size, Zeta Potential, and Conductivity Measurements
2.7.2. pH Measurement
2.7.3. Rheological Property
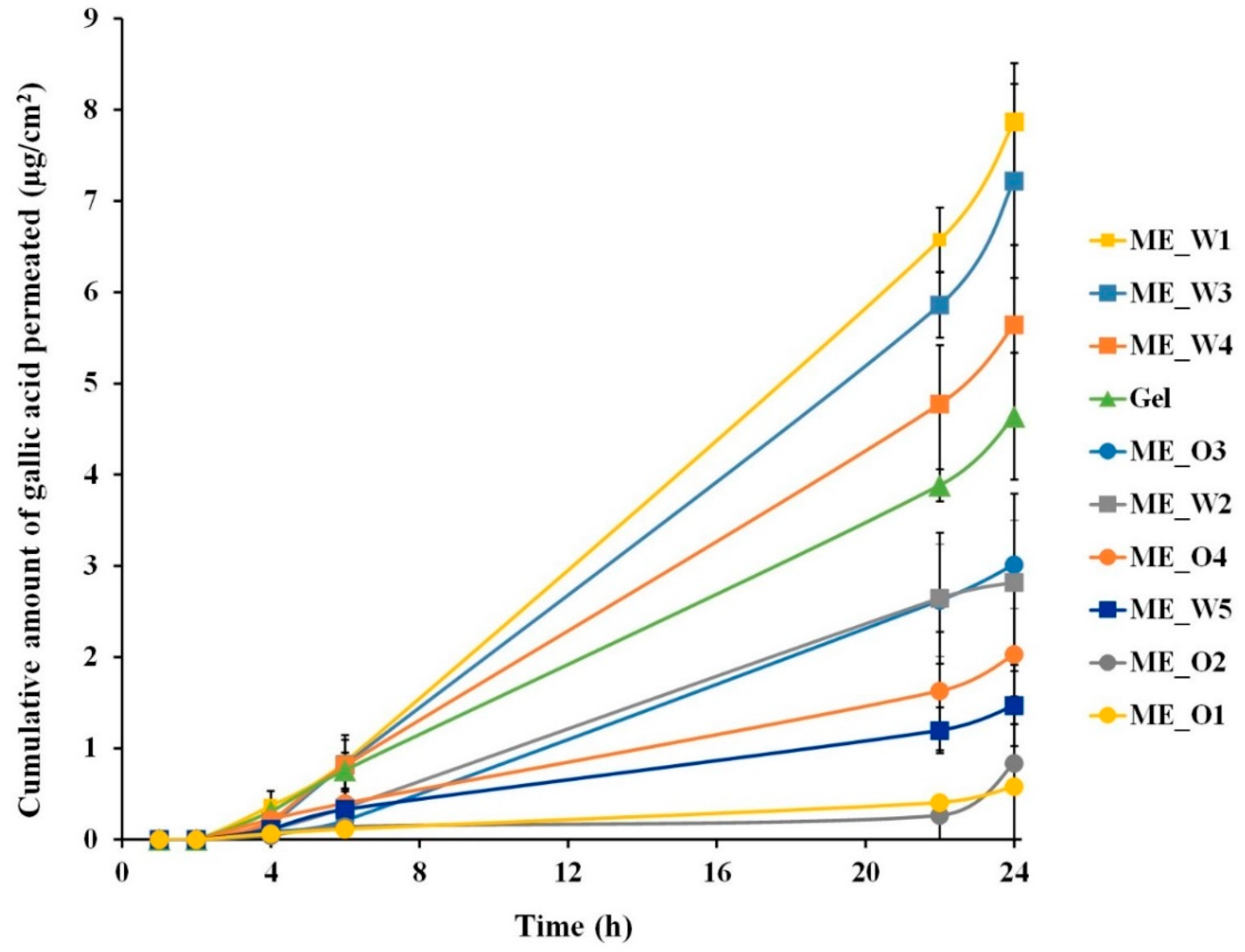
2.8. Skin Permeation Study of Microemulsions Containing GW Extract
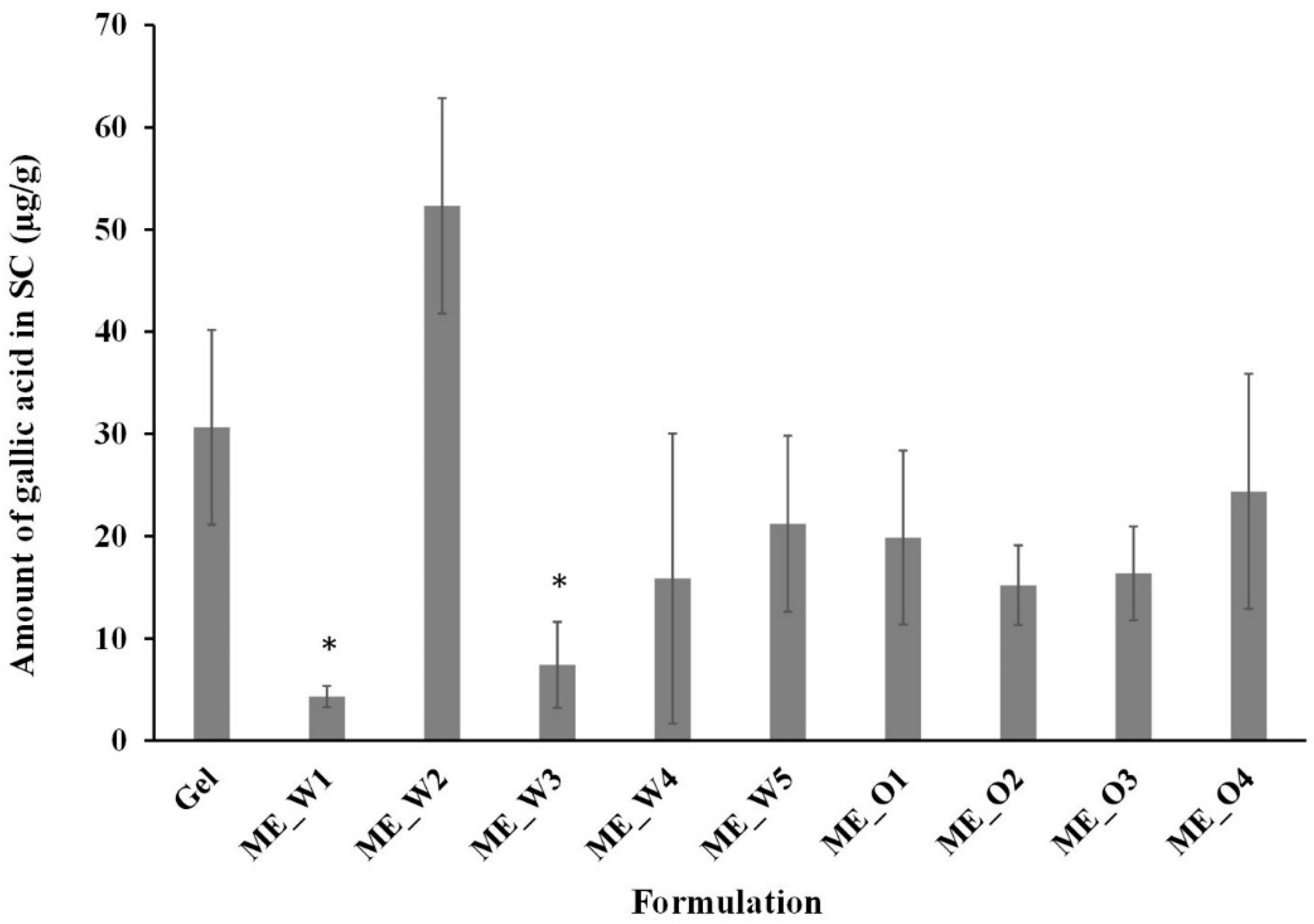
2.9. Skin Disposition Study of Microemulsions
2.10. Statistical Analysis
3. Results and Discussion
3.1. GW Leaf Extract, Antioxidant Activity, and Gallic Acid Content
3.2. Pseudo-Ternary Phase Diagrams of the Blank Microemulsions
3.3. Preparation and Characterization of Blank Microemulsions and Microemulsions Containing GW Extract
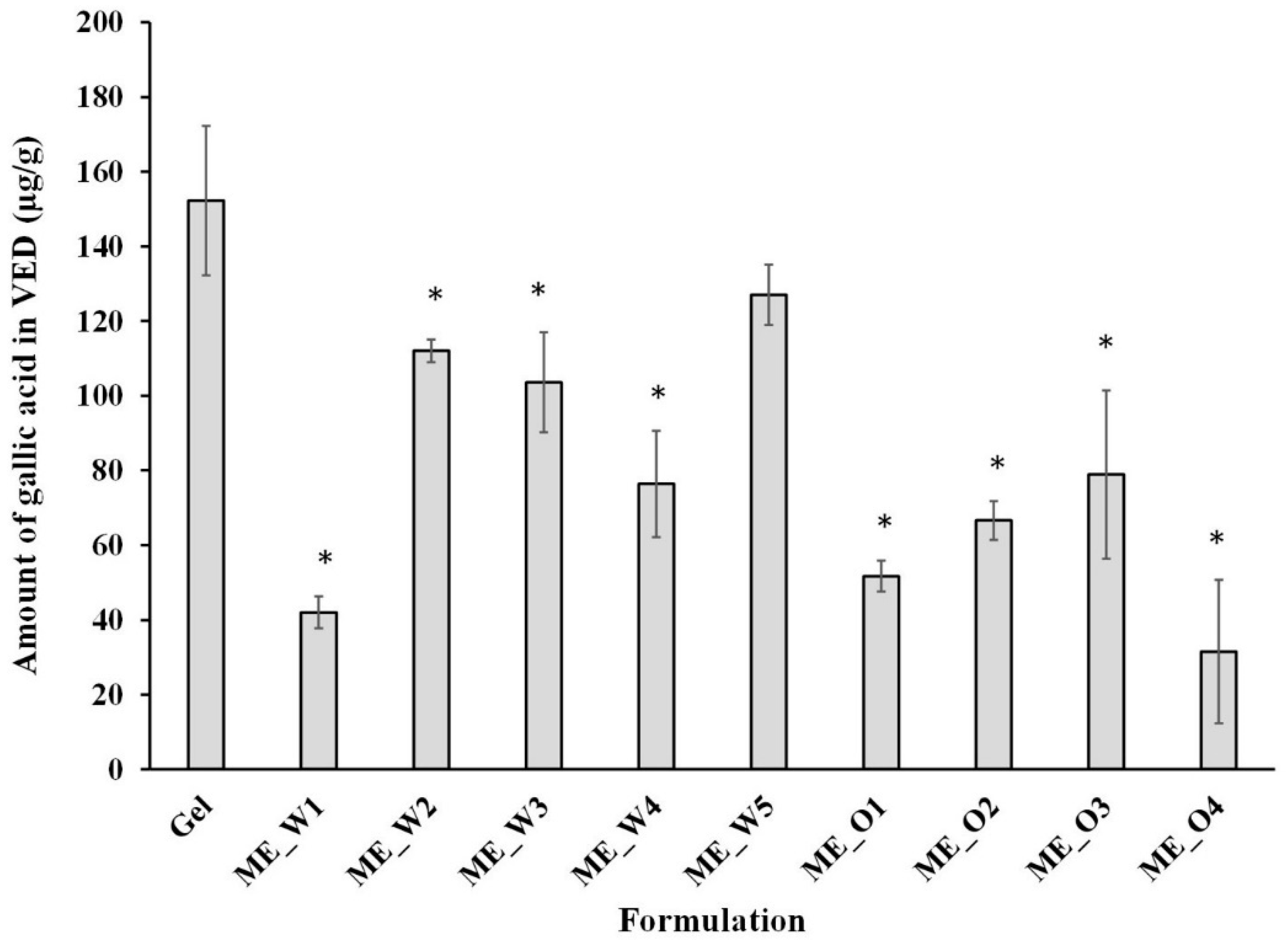
3.4. Skin Permeation and Disposition of Microemulsions Containing the GW Extract
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kongkachuichai, R.; Charoensiri, R.; Yakoh, K.; Kringkasemsee, A.; Insung, P. Nutrients value and antioxidant content of indigenous vegetables from Southern Thailand. Food Chem. 2015, 173, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Alzoreky, N.; Nakahara, K. Antioxidant activity of some edible Yemeni plants evaluated by ferrylmyoglobin/ABTS assay. Food Sci. Technol. Res. 2001, 7, 141–144. [Google Scholar] [CrossRef]
- Panpipat, W.; Suttirak, W.; Chaijan, M. Free radical scavenging activity and reducing capacity of five Southern Thai indigenous vegetable extracts. Walailak J. Sci. Technol. 2011, 7, 51–60. [Google Scholar]
- Tangkanakul, P.; Trakoontivakorn, G.; Jariyavattanavijit, C. Extracts of Thai indigenous vegetables as rancid inhibitor in a model system. Kasetsart J. (Nat. Sci.) 2005, 39, 274–283. [Google Scholar]
- Dzialo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Chhillar, R.; Dhingra, D. Antidepressant-like activity of gallic acid in mice subjected to unpredictable chronic mild stress. Fund. Clin. Pharmacol. 2013, 27, 409–418. [Google Scholar] [CrossRef]
- Mitsou, E.; Pletsa, V.; Sotiroudis, G.T.; Panine, P.; Zoumpanioti, M.; Xenakis, A. Development of a microemulsion for encapsulation and delivery of gallic acid. The role of chitosan. Colloids Surf. B Biointerfaces 2020, 190, 110974. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 370, Gallic Acid. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Gallic-acid (accessed on 27 August 2020).
- Benigni, M.; Pescina, S.; Grimaudo, M.A.; Padula, C.; Santi, P.; Nicoli, S. Development of microemulsions of suitable viscosity for cyclosporine skin delivery. Int. J. Pharm. 2018, 545, 197–205. [Google Scholar] [CrossRef]
- Boonme, P. Applications of microemulsions in cosmetics. J. Cosmet. Dermatol. 2007, 6, 223–228. [Google Scholar] [CrossRef]
- Pinsuwan, S.; Amnuaikit, T.; Ungphaiboon, S.; Itharat, A. Liposome-containing Hibiscus sabdariffa calyx extract formulations with increased antioxidant activity, improved dermal penetration and reduced dermal toxicity. J. Med. Assoc. Thail. 2010, 93, 216. [Google Scholar]
- Fernandes, F.H.A.; Batista, R.S.A.; Medeiros, F.D.; Santos, F.S.; Medeiros, A.C.D. Development of a rapid and simple HPLC-UV method for determination of gallic acid in Schinopsis brasiliensis. Rev. Bras. Farmacogn. 2015, 25, 208–211. [Google Scholar] [CrossRef]
- Arce, F.; Asano, N.; See, G.L.; Oshizaka, T.; Itakura, S.; Todo, H.; Sugibayashi, K. Prediction of skin permeation and concentration of rhododendrol applied as finite dose from complex cosmetic vehicles. Int. J. Pharm. 2020, 578, 119186. [Google Scholar] [CrossRef]
- Dahlizar, S.; Futaki, M.; Okada, A.; Yatomi, C.; Todo, H.; Sugibayashi, K. Combined use of n-palmitoyl-glycine-histidine gel and several penetration enhancers on the skin permeation and concentration of metronidazole. Pharmaceutics 2018, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Panapisal, V.; Charoensri, S.; Tantituvanont, A. Formulation of microemulsion systems for dermal delivery of silymarin. AAPS PharmSciTech 2012, 13, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Sisak, M.A.A.; Daik, R.; Ramli, S. Study on the effect of oil phase and co-surfactant on microemulsion systems. Malays. J. Anal. Sci. 2017, 21, 1409–1416. [Google Scholar] [CrossRef]
- Moghimipour, E.; Salimi, A.; Eftekhari, S. Design and characterization of microemulsion systems for naproxen. Adv. Pharm. Bull. 2013, 3, 63–67. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Wu, S.; Ju, B.; Zhu, D.; Yan, Y.; Wang, M.; Hu, J. Preparation and evaluation of microemulsion based transdermal delivery of Cistanche tubulosa phenylethanoid glycosides. Mol. Med. Rep. 2017, 15, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Hu, Q.; Yang, J. Enhancement of transdermal delivery of ibuprofen using microemulsion vehicle. Iran J. Basic Med. Sci. 2014, 17, 760–766. [Google Scholar]
- Zhang, Y.; Zhao, J.; Zhang, S.; Zhong, Y.; Wang, Z.; Liu, Y.; Shi, F.; Feng, N. Enhanced transdermal delivery of evodiamine and rutaecarpine using microemulsion. Int. J. Nanomed. 2011, 6, 2469–2482. [Google Scholar] [CrossRef]
- Sintov, A.C. Transdermal delivery of curcumin via microemulsion. Int. J. Pharm. 2015, 481, 97–103. [Google Scholar] [CrossRef]
- Nazari, M.; Mehrnia, M.A.; Jooyandeh, H.; Barzegar, H. Preparation and characterization of water in sesame oil microemulsion by spontaneous method. J. Food Process Eng. 2019, 42, e13032. [Google Scholar] [CrossRef]
- Üstündağ Okur, N.; Çağlar, E.Ş.; Arpa, M.D.; Karasulu, H.Y. Preparation and evaluation of novel microemulsion-based hydrogels for dermal delivery of benzocaine. Pharm. Dev. Technol. 2016, 22, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Subongkot, T.; Ngawhirunpat, T. Development of a novel microemulsion for oral absorption enhancement of all-trans retinoic acid. Int. J. Nanomed. 2017, 12, 5585–5599. [Google Scholar] [CrossRef]
- Subongkot, T.; Sirirak, T. Development and skin penetration pathway evaluation of microemulsions for enhancing the dermal delivery of celecoxib. Colloids Surf. B Biointerfaces 2020, 193, 111103. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaki, R.; Awadallah, A.; Tawfeek, H.M.; Nasr, M. Preparation, characterization and cytotoxic activity of new oleuropein microemulsion against HCT-116 colon cancer cells. Pharm. Chem. J. 2020, 53, 1118–1121. [Google Scholar] [CrossRef]
- Djordjevic, L.; Primorac, M.; Stupar, M.; Krajisnik, D. Characterization of caprylocaproyl macrogolglycerides based microemulsion drug delivery vehicles for an amphiphilic drug. Int. J. Pharm. 2004, 271, 11–19. [Google Scholar] [CrossRef]
- Poomanee, W.; Chaiyana, W.; Randall Wickett, R.; Leelapornpisid, P. Stability and solubility improvement of Sompoi (Acacia concinna Linn.) pod extract by topical microemulsion. Asian J. Pharm. Sci. 2017, 12, 386–393. [Google Scholar] [CrossRef]
- Chansiri, G.; Lyons, R.T.; Patel, M.V.; Hem, S.L. Effect of surface charge on the stability of oil/water emulsions during steam sterilization. J. Pharm. Sci. 1999, 88, 454–458. [Google Scholar] [CrossRef]
- Acharya, S.P.; Pundarikakshudu, K.; Panchal, A.; Lalwani, A. Preparation and evaluation of transnasal microemulsion of carbamazepine. Asian J. Pharm. Sci. 2013, 8, 64–70. [Google Scholar] [CrossRef]
- Resende, K.X.; Correa, M.A.; Oliveira, A.G.; Scarpa, M.V. Effect of cosurfactant on the supramolecular structure and physicochemical properties of non-ionic biocompatible microemulsions. Rev. Bras. Cienc. Farm. 2008, 44, 35–42. [Google Scholar] [CrossRef]
- Sintov, A.C.; Shapiro, L. New microemulsion vehicle facilitates percutaneous penetration in vitro and cutaneous drug bioavailability in vivo. J. Control. Release 2004, 95, 173–183. [Google Scholar] [CrossRef]
- Araujo, L.M.; Thomazine, J.A.; Lopez, R.F. Development of microemulsions to topically deliver 5-aminolevulinic acid in photodynamic therapy. Eur. J. Pharm. Biopharm. 2010, 75, 48–55. [Google Scholar] [CrossRef]
- Zhang, J.; Michniak-Kohn, B. Investigation of microemulsion microstructures and their relationship to transdermal permeation of model drugs: Ketoprofen, lidocaine, and caffeine. Int. J. Pharm. 2011, 421, 34–44. [Google Scholar] [CrossRef]
- Lopes, L.B. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef]
- Yousef, S.; Mohammed, Y.; Namjoshi, S.; Grice, J.; Sakran, W.; Roberts, M. Mechanistic evaluation of hydration effects on the human epidermal permeation of salicylate esters. AAPS J. 2017, 19, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.W.; Musakhanian, J. Skin penetration and permeation properties of Transcutol®-neat or diluted mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical delivery of anthramycin I. Influence of neat solvents. Eur. J. Pharm. Sci. 2017, 104, 188–195. [Google Scholar] [CrossRef]
- Godwin, D.A.; Kim, N.H.; Felton, L.A. Influence of Transcutol CG on the skin accumulation and transdermal permeation of ultraviolet absorbers. Eur. J. Pharm. Biopharm. 2002, 53, 23–27. [Google Scholar] [CrossRef]
- Csizmazia, E.; Erős, G.; Berkesi, O.; Berkó, S.; Szabó-Révész, P.; Csányi, E. Penetration enhancer effect of sucrose laurate and Transcutol on ibuprofen. J. Drug Deliv. Sci. Technol. 2011, 21, 411–415. [Google Scholar] [CrossRef]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar] [CrossRef]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Touitou, E.; Levi-Schaffer, F.; Dayan, N.; Alhaique, F.; Riccieri, F. Modulation of caffeine skin delivery by carrier design: Liposomes versus permeation enhancers. Int. J. Pharm. 1994, 103, 131–136. [Google Scholar] [CrossRef]
- Manconi, M.; Caddeo, C.; Sinico, C.; Valenti, D.; Mostallino, M.C.; Biggio, G.; Fadda, A.M. Ex vivo skin delivery of diclofenac by transcutol containing liposomes and suggested mechanism of vesicle–skin interaction. Eur. J. Pharm. Biopharm. 2011, 78, 27–35. [Google Scholar] [CrossRef]
- Björklund, S.; Pham, Q.D.; Jensen, L.B.; Knudsen, N.O.; Nielsen, L.D.; Ekelund, K.; Ruzgas, T.; Engblom, J.; Sparr, E. The effects of polar excipients transcutol and dexpanthenol on molecular mobility, permeability, and electrical impedance of the skin barrier. J. Colloid Interface Sci. 2016, 479, 207–220. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Chen, H.; Chang, X.; Du, D.; Li, J.; Xu, H.; Yang, X. Microemulsion-based hydrogel formulation of ibuprofen for topical delivery. Int. J. Pharm. 2006, 315, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]

| Formulation | Water | Labrasol®:HCO40® (1:1) | Tween80:Span80 (3:2) | Transcutol® | PG:Ethanol | Oil (IPM) | Total |
|---|---|---|---|---|---|---|---|
| ME_W1 | 30 | 30 | - | 30 | - | 10 | 100 |
| ME_W2 | 30 | 40 | - | 20 | - | 10 | 100 |
| ME_W3 | 30 | 45 | - | 15 | - | 10 | 100 |
| ME_W4 | 30 | - | 30 | 30 | - | 10 | 100 |
| ME_W5 | 30 | - | 30 | - | 30 | 10 | 100 |
| ME_O1 | 10 | 30 | - | 30 | - | 30 | 100 |
| ME_O2 | 10 | 40 | - | 20 | - | 30 | 100 |
| ME_O3 | 10 | 45 | - | 15 | - | 30 | 100 |
| ME_O4 | 10 | - | 30 | 30 | - | 30 | 100 |
| Sample Name | Z-Ave (nm) | Polydispersity Index | Zeta Potential (mV) | Conductivity (mS/cm) | ME Type |
|---|---|---|---|---|---|
| ME_W1 | 27.11 ± 0.78 | 0.906 ± 0.059 | −0.0598 ± 0.0432 | 0.033 ± 0.004 | BP |
| ME_W2 | 43.25 ± 9.78 | 0.641 ± 0.037 | −0.2401 ± 0.2613 | 0.030 ± 0.000 | BP |
| ME_W3 | 30.35 ± 9.37 | 0.766 ± 0.086 | −0.1342 ± 0.0813 | 0.031 ± 0.000 | BP |
| ME_W4 | 13.78 ± 1.20 | 0.690 ± 0.178 | −0.0403 ± 0.0023 | 0.115 ± 0.004 | o/w |
| ME_W5 | 15.55 ± 4.50 | 1.000 ± 0.000 | −0.1041 ± 0.0234 | 0.152 ± 0.002 | o/w |
| ME_O1 | 19.74 ± 0.57 | 0.487 ± 0.104 | −0.1015 ± 0.1104 | 0.013 ± 0.009 | BP |
| ME_O2 | 9.02 ± 2.42 | 0.986 ± 0.024 | −0.0408 ± 0.0346 | 0.007 ± 0.004 | w/o |
| ME_O3 | 9.76 ± 0.77 | 0.939 ± 0.000 | −0.1232 ± 0.0000 | 0.004 ± 0.000 | w/o |
| ME_O4 | 6.55 ± 1.33 | 0.326 ± 0.031 | −0.0382 ± 0.0289 | 0.023 ± 0.001 | BP |
| Sample Name | Z-Ave (nm) | Polydispersity Index | Zeta Potential (mV) | Conductivity (mS/cm) | ME Type |
|---|---|---|---|---|---|
| ME_W1 | 338.40 ± 54.99 | 0.396 ± 0.125 | −0.036 ± 0.068 | 0.329 ± 0.006 | o/w |
| ME_W2 | 592.00 ± 78.71 | 0.617 ± 0.142 | −0.184 ± 0.107 | 0.288 ± 0.007 | o/w |
| ME_W3 | 551.13 ± 37.63 | 1.000 ± 0.000 | −0.038 ± 0.024 | 0.268 ± 0.006 | o/w |
| ME_W4 | 496.97 ± 84.85 | 0.419 ± 0.158 | −0.974 ± 0.518 | 0.324 ± 0.005 | o/w |
| ME_W5 | 392.40 ± 25.72 | 1.000 ± 0.000 | 0.247 ± 0.052 | 0.194 ± 0.004 | o/w |
| ME_O1 | 379.90 ± 81.33 | 0.813 ± 0.110 | −0.920 ± 0.708 | 0.024 ± 0.001 | BP |
| ME_O2 | 607.43 ± 41.24 | 0.448 ± 0.487 | −0.017 ± 0.043 | 0.008 ± 0.013 | w/o |
| ME_O3 | 764.47 ± 39.48 | 1.000 ± 0.000 | 0.428 ± 0.036 | 0.042 ± 0.002 | BP |
| ME_O4 | 338.60 ± 22.75 | 0.365 ± 0.186 | 0.010 ± 0.006 | 0.015 ± 0.001 | BP |
| Formulation | Viscosity (cP) | pH | ||
|---|---|---|---|---|
| Blank ME | ME Containing GW Extract | Blank ME | ME Containing GW Extract | |
| Gel (1% HPMC) | - | 228.7 ± 41.0 | - | 4.0 ± 0.3 |
| ME_W1 | 125.0 ± 27.9 | 487.7 ± 52.2 | 6.3 ± 0.3 | 4.2 ± 0.2 |
| ME_W2 | 311.7 ± 51.6 | 1248.7 ± 34.6 | 6.6 ± 0.1 | 4.1 ± 0.2 |
| ME_W3 | 383.1 ± 7.2 | 1577.7 ± 23.7 | 6.9 ± 0.2 | 4.4 ± 0.1 |
| ME_W4 | 155.2 ± 34.3 | 475.4 ± 126.6 | 6.9 ± 0.2 | 4.4 ± 0.4 |
| ME_W5 | 162.9 ± 2.8 | 2273.0 ± 131.5 | 7.0 ± 0.1 | 4.5 ± 0.3 |
| ME_O1 | 48.3 ± 26.4 | 372.6 ± 20.9 | 5.5 ± 0.1 | 4.7 ± 0.3 |
| ME_O2 | 109.1 ± 25.7 | 556.4 ± 23.6 | 6.6 ± 0.2 | 4.0 ± 0.2 |
| ME_O3 | 104.2 ± 16.5 | 765.5 ± 4.9 | 6.7 ± 0.3 | 4.2 ± 0.4 |
| ME_O4 | 82.4 ± 13.7 | 212.0 ± 29.2 | 7.1± 0.2 | 4.3± 0.2 |
| Formulation | Flux (Jss, µg/cm2/h) | Enhancement Ratio |
|---|---|---|
| Gel (1% HPMC) | 0.197 ± 0.004 | 1.00 ± 0.02 |
| ME_W1 | 0.350 ± 0.024 * | 1.78 ± 0.12 |
| ME_W2 | 0.142 ± 0.047 | 0.72 ± 0.24 |
| ME_W3 | 0.316 ± 0.014 * | 1.60 ± 0.07 |
| ME_W4 | 0.252 ± 0.034 | 1.28 ± 0.17 |
| ME_W5 | 0.058 ± 0.010 * | 0.29 ± 0.05 |
| ME_O1 | 0.019 ± 0.003 * | 0.10 ± 0.02 |
| ME_O2 | 0.010 ± 0.002 * | 0.05 ± 0.01 |
| ME_O3 | 0.145 ± 0.033 | 0.74 ± 0.17 |
| ME_O4 | 0.078 ± 0.029 * | 0.40 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sae Yoon, A.; Sakdiset, P. Development of Microemulsions Containing Glochidion wallichianum Leaf Extract and Potential for Transdermal and Topical Skin Delivery of Gallic Acid. Sci. Pharm. 2020, 88, 53. https://doi.org/10.3390/scipharm88040053
Sae Yoon A, Sakdiset P. Development of Microemulsions Containing Glochidion wallichianum Leaf Extract and Potential for Transdermal and Topical Skin Delivery of Gallic Acid. Scientia Pharmaceutica. 2020; 88(4):53. https://doi.org/10.3390/scipharm88040053
Chicago/Turabian StyleSae Yoon, Attawadee, and Pajaree Sakdiset. 2020. "Development of Microemulsions Containing Glochidion wallichianum Leaf Extract and Potential for Transdermal and Topical Skin Delivery of Gallic Acid" Scientia Pharmaceutica 88, no. 4: 53. https://doi.org/10.3390/scipharm88040053
APA StyleSae Yoon, A., & Sakdiset, P. (2020). Development of Microemulsions Containing Glochidion wallichianum Leaf Extract and Potential for Transdermal and Topical Skin Delivery of Gallic Acid. Scientia Pharmaceutica, 88(4), 53. https://doi.org/10.3390/scipharm88040053




