Abstract
The dry, powdered cladodes of Opuntia ficus-indica are often-used in over-the-counter (OTC) pharmaceutical formulations. Gentle drying techniques, such as lyophilization and vacuum drying are compared with convection drying for the cladodes and also compared with another species of economic importance, Opuntia joconostle. The heteropolysaccharide purified from the mucilage extracted from the dried powders were investigated in their monosaccharide composition (HPAEC-PAD, TLC), mineral and protein content, molecular dimensions (SEC) and fermentability by probiotic bacteria (Bioscreen technique) for evaluation of the prebiotic potential of the mucilage. The heteropolysaccharide is composed of galactose, arabinose, xylose, galacturonic acid and rhamnose. O. ficus-indica includes an additional 13% of glucose coming from an α-glucan. The content of Ca (0.3%) and Mg (0.4%) is relatively low in both species; the content of protein adds up to 1.5% in O. ficus-indica but is significantly lower in O. joconostle with 0.8%. The average molecular mass Mw of the extracted mucilage ranges from 3.7 to 4.7 × 105 g∙mol−1 for both species; only the mucilage from long-time convection drying (C2) delivers a lower average Mw of 2.6 × 105 g∙mol−1, due to partial breakdown of the mucilage matrix. All tested probiotic strains utilized the mucilage to some extent; C2 being the most active, and thus confirms the prebiotic potential of cladode’s powder and its derived products. In general, the molecular dimensions and prebiotic potential are not extremely sensitive to the drying treatment, yet temperature and drying time can modify the cladode’s powder to a profile with better prebiotic characteristics.
1. Introduction
The species belonging to the Cactaceae family are characterized for being extremely well adapted to hot, arid environments. The characteristics evolved to thrive in these ecosystems include: a Crassulacean Acid Metabolism (CAM); This type of metabolism involves CO2 uptake by night and its assimilation into carbohydrate during the following light period, with the stomata opening only at night, therefore minimizing water loss [1]; the transformation of the leaves into glochids, or small spines, and the trunk into flat, oval photosynthetic tissues called cladodes [2]. These adaptations help make members of this family hold among the highest biomass productivity in arid environments [3]. The Opuntia genus comprises 150 to 180 species [4], and its center of domestication was central Mexico [5], where the largest genotype diversity still remains [6]. Of these species, two are widely used in human nutrition: Opuntia ficus-indica and Opuntia joconostle. Several cultivars of O. ficus-indica, selected either for the production of fruit or the cladode are widely grown in Mexico, Brazil, Tunisia, Italy, and other arid regions of the world [7]. Their fruits, prickly pears (tuna), are consumed fresh or processed to obtain conserves and liquors among other products [8,9]; and in Mexico, the cladodes are widely consumed as a vegetable [10]. O. joconostle is mainly cultivated for its fruit, which in contrast to the one of O. ficus-indica, is quite acidic and is used mostly for cooking [11].
Both plants have been a part of the human diet since prehistoric times in arido- and Mesoamerica [12], and have had a role in traditional medicine, both in their original habitat (Mexico) [13] and elsewhere the plant has been transplanted [14]. In the case of the cladodes, several of these health-promoting effects can be ascribed to the high content of dietary fibre found in the form of mucilage. The mucilage from Opuntia spp. (e.g., Opuntia ficus-indica, Opunta microdasys, Opuntia spinulifera) has been reported to contain a highly branched polysaccharide, composed of xylose, rhamnose, galactose, arabinose and galacturonic acid [15], where the xylose and arabinose residues are located in the branches of the main backbone, composed of rhamnose, galactose and galacturonic acid. The composition is additionally affected by the species and development stage of the plant [16,17,18,19], affecting its properties. The use of cladodes, and the polysaccharides which are part of its mucilage, have shown several health-promoting effects: antidiabetic and hypoglycemic [20,21,22,23,24,25], antiulcer activity [14,26,27,28], a reduction of cholesterol, LDL and triglyceride plasma levels [29], immunomodulatory activity [30], increased bone mineral density [31] and a prebiotic impact in the gut microbiome.
The availability of oligo and/or polysaccharides as carbon source for the gut microbiome is highly dependent on their molecular composition, molecular dimensions and structure [32,33,34]. In the case of Opuntia this is an overlooked fact. In the case of O. ficus-indica, Yahia et al. and Reyes-Reyes et al. studied the effect on selected prebiotic strains of native and modified mucilages and report the molecular dimensions but not the monosaccharide composition [35,36]. Sánchez-Tapia et al., Guevara-Arauza et al. and Moran-Ramos et al. evaluated the positive changes on the microbiome and related health effects after mucilage supplementation but did not offer a characterization of the mucilage [37,38,39].
Moreover, the fresh cladodes have a limited shelf-life [40], so drying is a commonly used process to extend the shelf-life of Opuntia cladodes. However, the chosen drying conditions have an effect on the properties of the mucilage and other components of therapeutic interest [41,42]. As such, we have investigated the effect of different drying treatments on the physicochemical properties of the mucilage extracted from O. ficus-indica and O. joconostle, and their effect on their prebiotic potential.
2. Materials and Methods
2.1. Vegetable Material and Drying Methods
Cladodes from O. ficus-indica (L.) Mill (OFI) and O. joconostle F.A.C. Weber (OXO), both cultivated in a conventional commercial plantation in the same region (Cuquio, Jalisco, Mexico) and of the same age were collected and the thorns and glochids removed. The cladodes were then sliced transversally into 3–4 mm thin strips. Immediately after this, the strips from all cladodes from the same species were mixed into a homogeneous material and separated into five portions (ca. 500 g each) for drying. The following drying methods were applied: (1) liquid nitrogen freezing (N): the strips were frozen with liquid nitrogen and ground with a mortar to pass a 120 US standard sieve (125 µm opening) and lyophilized; (2) regular lyophilization (L): the strips were frozen at −20 °C and lyophilized; (3) vacuum-drying (V): the strips were placed in trays inside a vacuum oven (Binder GmbH, Tuttlingen, Germany) and dried for 48 h (40 °C, 10 mbar); (4) Convection drying: the strips were placed in a convection oven (Heraeus GmbH, Hanau, Germany) at 50 °C and were dried for 36 h (C1) or 96 h (C2). Once the material was dry, it was milled into a fine powder with a blender until it would pass a mesh 50 screen. Moisture was gravimetrically determined as loss-on-drying, i.e., weight after drying divided by fresh weight × 100.
2.2. Purification of the Powdered Cladodes
In order to remove low molecular weight components such as lipids, chlorophyll and its fragments, phenolic components, and mono- and disaccharides from the cladode’s powder, the following extraction steps were applied: (1) Acetone extraction: Approximately 50 g of powder were extracted with 250 mL of acetone (5 volumes) under vigorous stirring overnight; the acetone was removed by centrifugation and the procedure repeated three times. (2). Methanol extraction: The dry, acetone-washed powder was extracted with 95% methanol using the same procedure as before. The methanolic extracts were saved for further processing. The resulting mass was lyophilized to remove the traces of methanol and water left and stored in a desiccator.
2.3. Purification of Methanolic Extracts
The methanolic extracts from each treatment were pooled together and the methanol evaporated under reduced pressure until a thick, viscous consistency was reached. 25 mL of water were added, and the solution was extracted with hexane twice (50 and 25 mL) to remove the chlorophyll and other apolar compounds. The aqueous phase was then frozen and lyophilized until further use.
2.4. Mucilage Extraction
The mucilage was extracted from the purified cladode powder with deionized water at 80 °C for 1 h for three times. The water:solids ratio (w/w) was 35:1, 25:1 and 20:1 for each extraction step. After each extraction, the supernatant was separated by centrifugation (24,000× g, 30 min). The supernatants (mucilage) were pooled and centrifuged at 24,000× g for 90 min to discard fines. The clear supernatant was lyophilized, and the resulting mucilage samples were stored in a desiccator until further use. Yields were calculated gravimetrically as mass of dry mucilage, MeOH extract or insoluble material divided by cladode fresh weight.
2.5. Trifluoroacetic Acid (TFA) Hydrolysis
Mucilage samples were diluted in water to a final concentration of 10 mg/mL. To ensure complete dilution, the samples were placed in an ultrasonic bath at 70 °C for 4 h and then stirred overnight at 80 °C. To a 100 µL aliquot of each sample, 100 µL of 4 M TFA were added and the solution was allowed to react for three hours at 110 °C with vigorous stirring. TFA was removed by adding an equal volume of methanol and the solution evaporated to dryness using a warm stream of nitrogen. This procedure was repeated until there were no traces of TFA. 500 µL of water were added to the dry samples to reach a final concentration of 2 mg/mL.
2.6. Thin Layer Chromatography (TLC)
To evaluate the presence and type of monosaccharides in the methanolic extracts, 3 µL of sample, hydrolysate or standard were sprayed on HPTLC silica gel 60 plates (Merck KGaA, Darmstadt, Germany) using a Linomat 5 (Camag AG, Muttenz, Switzerland). The plates were developed thrice using acetonitrile: 0.3% ammonium hydroxide 17:3 as the mobile phase. Detection was achieved by spraying the plates with diphenyl-aniline reagent (DPA) [43] and the plates heated for 3 min at 95 °C.
2.7. High Performance Anion Exchange Chromatography–Pulsed Amperometric Detection (HPAEC-PAD)
The monosaccharides were quantified by HPAEC-PAD. An ICS-3000 system (Thermo Scientific, Sunnyvale, CA) was used with a Carbopac PA1 precolumn (4 × 50 mm) plus column (4 × 250 mm). Injection volume was 10 µL of the polysaccharide hydrolysate (see Section 2.5) after appropriate dilution to fall inside calibration curve, typically 1:50 or 1:100. The cell was fitted with a gold electrode, 2 mil gasket and operated with the Carbohydrates (standard quad) waveform. The flow rate was 1 mL/min and the column and detector temperature were set at 30 °C. The eluents were 200 mM NaOH, 200 mM NaOH + 200 mM NaOAc and H2O. The elution gradient was as follow: −20 to −2.5 min, 200 mM NaOH. −2.5 to 22 min, NaOH 10 mM + 2 mM NaOAc. 22 to 40 min, ramp up to 200 mM NaOH and 200 mM NaOAc. 40 to 50 min, 200 mM NaOH. With this gradient the pair xylose/mannose cannot be fully resolved; however, TLC indicates no presence of mannose in the hydrolysates, and was further confirmed by doing an exploratory HPAEC-PAD run using 4 mM NaOH as eluent; concentration which allows this pair to be almost baseline resolved. Samples and standards were evaluated in triplicates.
2.8. Size Exclusion Chromatography (SEC) Analysis
The molecular mass distribution was evaluated using a size exclusion chromatography column system composed of a precolumn (10 × 100 mm) filled with Toyopearl HW 40S (Tosoh Bioscience GmbH, Griesheim, Germany) followed by three columns (all 10 × 300 mm) filled with Superose 6 (GE Healthcare Life Sciences, Uppsala, Sweden), Superose 12 (GE Healthcare Life Sciences) and Toyopearl HW 40S, respectively. The injection volume was 300 µL of a 3.33 mg/mL mucilage solution. The eluent was 50 mM NaCl + 0.01% NaN3 at a flow rate of 0.6 mL/min. Differential refractive index detection was used (Shodex RI-101, Showa Denko Europe GmbH, Munich, Germany), and the whole system was calibrated using the dextran standard kit (Pharmacosmos A/S, Holbaek, Denmark). Data analysis was performed using CPCWin 32 (a.h. group, Graz, Austria)
2.9. Total Carbohydrate Content
A modified phenol-sulfuric method based on the work of Rao [44] was developed and used for the determination of carbohydrate content in the mucilage. A grid of 40 × 8 mm glass tubes (Witeg Labortechnik GmbH, Wertheim, Germany) with the same dimensions of a standard 96-well microplate was self-constructed. Ca. 2 mg of mucilage were weighed and adjusted to a 22.5 µg/mL solution in water (roughly equivalent to 0.15 mM). A stock standard solution, containing all the monosaccharides in the average composition as obtained by HPAEC-PAD, was prepared and used to make the corresponding dilutions to construct the calibration curve. 150 µL of sample or standard were added to the bottom of the tube. The grid was inserted in an ice water bath and the tubes left to cool. 600 µL of concentrated H2SO4 at 4 °C were added, the tube lattice was thoroughly mixed inside the water bath and capped with a Teflon-coated silicone microplate sealing mat. The grid was incubated at 80 °C for 30 min. and after the reaction was finalized, placed in a cold-water bath for 10 min. Once cool, 10 µL of 90% phenol were added, the contents completely mixed, and the reaction was left to proceed for 30 min. After this time, 200 µL aliquots of the reaction products were pipetted into a 96-well microplate and the absorbance read at 480 nm in an Infinite 200 Pro microplate reader (Tecan Trading AG, Männedorf, Switzerland).
2.10. Protein Content
Protein content of the extracted mucilages was measured using the EZQ Protein Quantitation Kit (Molecular Probes Carlsbad, CA, USA), a fluorescence-based protein assay using an Infinite 200 Pro microplate reader (Tecan Trading AG), following the manufacturer instructions, with an excitation wavelength of 485 nm and emission wavelength of 590 nm. Samples and standards were analyzed by triplicate.
2.11. Mineral Content
Calcium, magnesium, potassium, and sodium were measured by flame atomic absorption spectrometry (FAAS) using the method EN-15505:2008. Shortly, samples were mineralized with 65% HNO3 for 40 min at 200 °C in a high-pressure PTFE container in a microwave oven (Mars Xpress, CEM Corporation, Matthews, NC, USA). Buffer solution of Schuhknecht and Schinkel was added for the potassium and sodium determination, while buffer solution of Schinkel was added for the calcium and magnesium determinations. The wavelengths for the determination of minerals were as follows: K, 766.5 nm; Ca, 222.7 nm; Mg, 285.2 nm, and Na, 589.0 and were measured in an AA240FS fast sequential atomic absorption spectrometer (Varian Inc., Palo Alto, CA, USA). The measurements were performed in duplicates.
2.12. Evaluation of Prebiotic Potential
The prebiotic potential was evaluated by measuring the growth of three probiotic bacterial strains [32]; Lactobacillus rhamnosus GG (ATCC 53103), Lactobacillus acidophilus (DSM 13241), and Bifidobacterium longum subsp. infantis (ATCC 15697). Shortly, the bacterial strains were incubated in glucose-free Man-Rogosa-Sharpe (MRS) liquid media supplemented with 2% of the polysaccharide to be tested. Glucose was used as the positive control, and sterility of the polysaccharide solutions was confirmed by concurrent incubation of uninoculated samples. The blank was the bacterial strain in MRS media without any carbohydrate added. Growth was monitored by measuring the optical density at 600 nm (OD600) for 72 h using a Bioscreen C (Oy Growth Curves Ab Ltd., Helsinki, Finland). The measurements were performed in duplicate, and the maximum OD600 was calculated using the GrowthRates software [45].
2.13. Statistical Analysis
Where performed, Welchs’ test and Games-Howell post-hoc test were used to evaluate the difference between treatments. Both were calculated using the Real Statistics Resource Pack software (release 7.2) for Excel (Charles Zaiontz) and confidence intervals were calculated manually using t-values for the calculation.
3. Results
3.1. Drying Methods and Composition of the Cladodes
Convection drying is the preferred method for commercial production of Opuntia powder, both for pharmaceutical and food uses, as it is the most economical method; this is especially true if it is done on a solar drier, which additionally is optimally suited to the dry, hot regions where Opuntia spp. grows [46]. The disadvantage of this technique is that it can bring thermal degradation and so change the quality profile of the cladodes, especially the quality of the mucilage. The polysaccharides in mucilage can be partly denatured, analogously to protein [47] and/or their supramolecular structure modified by thermal treatment. Additionally, the presence of reducing sugar and proteins in combination with temperature and time delivers possible chemical modifications, such as Maillard or caramelization reactions.
The chosen drying methods can be arranged in order of increasing harshness of the drying conditions: liquid nitrogen < lyophilized < vacuum dried < convection dried 36 h < convection dried 96 h (N2 < L < V < C1 < C2). The liquid nitrogen and lyophilization treatments represent the fastest and least damaging freezing but are the most expensive. Convection drying, while more economical, could generate a greater change in the quality profile of the cladode powder especially over a long period.
Table 1 shows no difference in water content of the cladode between treatments or species, at around 92%. Conversely, the dry matter is around 8% with a high proportion of insoluble material (4–5.3%w/w fresh material) for both species. Remarkable is the high level of insoluble material after long term convection drying C2. The low molecular weight, water-soluble substances (MeOH extract) were significantly higher (25%; p = 0.015, Welch’s test) in the cladodes of O. ficus-indica compared to O. joconostle. This was confirmed in the TLC-profile (Figure 1) of both species. The samples of O. ficus-indica show a clearly higher content of mono and disaccharides than those of O. joconostle. The identified sugars are fructose, glucose, and sucrose, documenting the high photosynthetic activity, particularly in the cladodes of O. ficus-indica. Furthermore, the sucrose content varied depending on the drying method. It is noticeable that the sucrose level in the N, L, V and C1 treatments was relative similar, and only in the C2 treatment, with the longer drying time, sucrose was partly destroyed. Moreover, the methanolic extract of C2 powder was the only one with deep brownish material, which could be originated from caramelization and Maillard reaction products by the long period of drying process.

Table 1.
Major component composition of O. ficus-indica (OFI) and O. joconostle (OXO) after different drying processes.
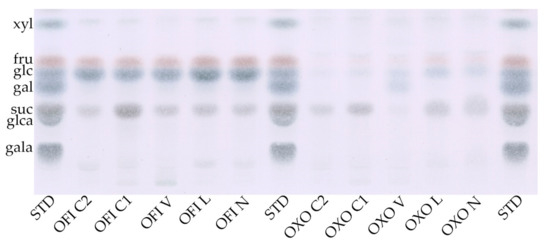
Figure 1.
TLC of methanolic extracts of O. ficus-indica (OFI) and O. joconostle (OXO); N, liquid nitrogen frozen; L, lyophilized; V, vacuum dried; C1, convection dried 36 h; C2, convection dried 96 h. STD are sugar standards; from top to bottom xylose, fructose, glucose, galactose, sucrose, glucoronic acid and galacturonic acid.
The water extraction yields of mucilage from the samples of all drying treatments (Table 1) are generally similar, but the content of mucilage between O. ficus-indica and O. joconostle was significantly different (p < 0.001). In O. joconostle cladodes the average level of mucilage was 2.5 g%w/w fresh material, compared to O. ficus-indica with only 1.5%w/w fresh material. Noticeably, the content of mucilage in both species after long time convection drying (C2) was relatively higher and in the range of gentle liquid nitrogen frozen drying.
3.2. Mucilage Composition
3.2.1. Main Components
The main components of the isolated purified mucilage are carbohydrates, particularly high molecular weight polysaccharides, with a small amount of associated protein and linked minerals such as calcium, magnesium, potassium, and sodium.
The carbohydrate content was in the range of 80% for all samples. Table 1 shows the influence of drying technique on the carbohydrate content; the gentler techniques (N, L, V) deliver a higher carbohydrate content compared to the harsher, convection techniques (C1, C2) in both species.
The mineral content was measured only for the mucilage extracted after the liquid nitrogen (N) and long-time convection (C2) drying treatments as an indicator of the purity of the mucilages after the washing steps. These two treatments were chosen as representative of the gentlest and harshest drying treatments. The amount of minerals was between 2.7 and 3.9%, with higher amount in the samples after the N treatment, compared to C2 (Table 1 and Table 2). Table 2 shows that potassium is by far the most abundant mineral at around 81 and 74% for O. ficus-indica and O. joconostle respectively. The content of calcium and magnesium in the mucilage was relatively low compared to the results of other studies [18,48]; however, as calcium in Opuntia spp. is mostly found in the form of insoluble oxalate, our results could be due to a more efficient salt removal from the cladode before extraction of the mucilage after the acetone and methanol wash steps. As divalent cations form aggregates with the polysaccharide chains in acidic polysaccharides such as pectin, it can be speculated then that only the calcium and magnesium crosslinked to the acidic residues of the polysaccharides were measured, as opposed to the sum of free plus bound calcium by other authors. Moreover, the lower level of divalent ions in the C2 treatment is further evidence of the degradation of the polysaccharide structure, as less cations are bound.

Table 2.
Mineral content of the mucilage after selected drying treatments.
The protein content was approx. 1.5%dm in O. ficus-indica, twice as much as in O. joconostle with 0.8%dm (Table 2). Moreover, C2 shows a significantly higher level of protein compared to all other drying treatments in both species (p < 0.05). The reason seems to originate in reaction products from the long-term convection drying, delivering artifactually high protein content results. The presented results (done with the EZQ Protein Quantitation Kit) are very close to the Kjeldahl results (not shown), with the advantage of being very quick and having a very reasonable cost per sample.
3.2.2. Monosaccharide Composition
Table 3 shows the monosaccharide composition of the mucilage after the different drying treatments. Since these treatments, especially N, L and V, are reasonably gentle drying processes, there is no significant difference due to degradation reactions in the composition of the polysaccharides between the treatments. The main monosaccharide is galactose, followed by arabinose, xylose and galacturonic acid. Other works [49,50,51] have found the same monosaccharides but in different proportions. Given the variability due to species, growing conditions and even extraction methods, this is not surprising. A stark difference between O. ficus-indica and O. joconostle is the relatively high presence of glucose in the former; however, this glucose is not part of the heteropolysaccharide forming the mucilage, as it belongs to an existing α-glucan population (identity confirmed by release of glucose after treatment with α-amylases, manuscript in preparation). Furthermore, the content of galacturonic acid was much higher in O. ficus-indica than in O. joconostle, which could be due to the epi- or genetic differences between both species.

Table 3.
Monosaccharide composition of the mucilage’s polysaccharide after different drying treatments.
3.2.3. Molecular Dimensions
Table 4 and Figure 2 show the molecular mass distribution and their related parameters for the polysaccharides present in the mucilage. Both species have a similar range of molecular mass distribution, from 104 to 106 g∙mol−1.

Table 4.
Molecular dimensions of Opuntia spp. mucilage after different drying treatments.
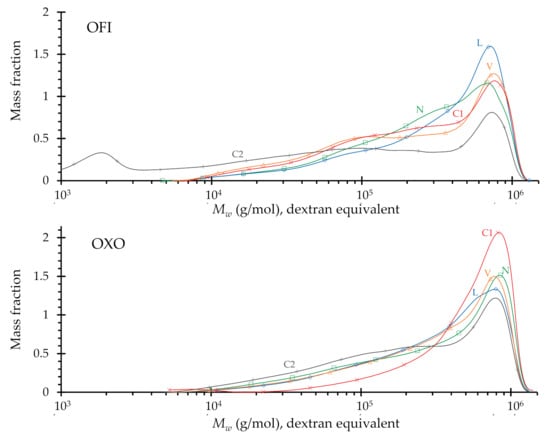
Figure 2.
Molar mass distribution (normalized to area = 1.0) of the purified mucilage of O. ficus-indica (OFI) and O. joconostle (OXO); N, liquid nitrogen frozen; L, lyophilized; V, vacuum dried; C1, convection dried 36 h; C2, convection dried 96 h.
In the case of O. ficus-indica, the mass average of molecular mass Mw is in the range of 3.7–4.3 × 105 for N, L, V and C1. Only the long-time convection technique (C2) results in a much lower molecular dimension, with an average Mw of 2.6 × 105 g∙mol−1, and as can be seen in Figure 2, a second polysaccharide population with a much lower molecular mass of around 2 × 103 g∙mol−1.
O. joconostle mucilage similarly has an average of molecular mass in the range of 4.3–4.7 × 105 g∙mol−1 for N, L, V and C1. The effect of decreasing average molecular mass after long-time convection drying (C2), is similar as in O. ficus-indica, with an average Mw of 3.6 × 105 g∙mol−1, yet no low molecular weight polysaccharides are seen in the molecular mass profile (Figure 2). These results would suggest that the heteropolysaccharides present in O. joconostle are more stable than those of O. ficus-indica.
3.3. Prebiotic Potential of Mucilage
Three strains of facultative anaerobic bacteria (Lactobacillus rhamnosus GG, Lactobacillus acidophilus and Bifidobacterium longum subsp. infantis), widely considered to be probiotic, were selected to evaluate their growth using the mucilage from Opuntia spp. as a carbon source. Previous work from our group has shown that these probiotics are able to ferment a variety of neutral and acidic oligo- and polysaccharides [32]. Given the monosaccharide composition of the mucilages from Opuntia spp. and the proposed structures mentioned in the literature [15,16,17,18,19], these strains seemed to be good candidates for testing. In general, all strains were able to utilize the mucilage as a substrate, albeit at lower maximum growth than glucose (Figure 3). None of these strains can ferment galacturonic acid, and the carbohydrate content is only around 80%, which help explain the difference. A big difference is the lag time, as growth with glucose started already within the first hour, while all the tested mucilages were delayed by 5–6 h. The highest growths in all cases were for the C2, the harshest drying condition. As described before, C2 mucilages have a lower molecular mass distribution, and coupled with the evidence of other degradation reactions (heavily colored solution, and a very noticeable reduction in viscosity), lower molecular mass polysaccharides were produced, and therefore could become more available for the bacteria, particularly B. longum subsp. infantis (RGR OFI 58% and OXO 50%) and L. rhamnosus GG (RGR OFI 56% and OXO 48%). As for the rest of samples (N, L, V and C1), the values are quite similar, showing relative growth rates in relation to glucose between 15 and 31% (Table 5).
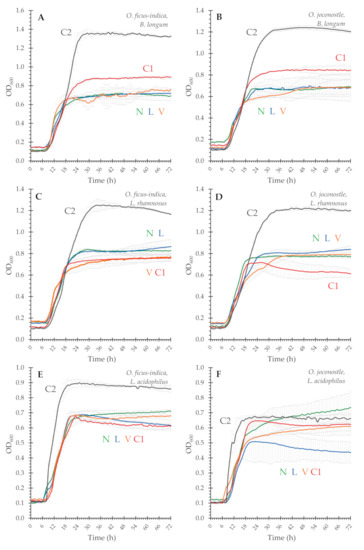
Figure 3.
Growth curves of three probiotic bacteria using mucilage of O. ficus-indica (OFI) and O. joconostle (OXO) as the carbon source; (A,B), B. longum subsp. infantis; (C,D), L. rhamnosus GG; (E,F) L. acidophilus. For all graphs, green correspond to liquid nitrogen frozen (N), blue to lyophilized (L), orange to vacuum dried (V), red to convection dried 36 h (C1) and grey to convection dried 96 h (C2). Error bars correspond to the standard deviation. Not all the stationary phase is plotted.

Table 5.
Prebiotic potential of Opuntia spp. mucilage after different drying treatments.
4. Discussion and Conclusions
The cladodes of Opuntia ficus-indica and Opuntia joconostle have a high commercial value due to their use in human nutrition and in traditional medicine. The fresh material is consumed directly as a vegetable or as an ingredient in different foodstuffs, particularly in Mexico [8,9,10]. Additionally, O. ficus-indica cladodes are dried, milled and sold as a dry powder for use in OTC pharmaceutical formulations. The most common commercial method for drying is drying with hot air (convection) heated with fossil fuels, although solar drying is gaining a foothold in new projects [7]. As the cladodes used as raw material are quite variable, and usually the only quality control parameter is the final moisture content, the drying method can have an important influence on the quality profile, and consequently on the properties of the produced powder, which includes the mucilage and other components of therapeutic interest [41,42]. Different drying techniques for cladodes were tested, evaluating their effect on the main constituents of the cladodes, including the mucilage composition and its prebiotic effectiveness.
The applied drying techniques include gentle methods such as liquid nitrogen freezing and lyophilizing (N), regular lyophilizing (L) and vacuum drying (V), and harsher methods such as convection drying at 50 °C, with short term (36 h, C1) and long term (96 h, C2) processing. While it was previously known that the C2 process produces an unattractive powder (heavily colored and with off-flavors), it was decided to be performed as an extreme form of convection drying, which could happen commercially if the drying time or conditions are not properly monitored and controlled. The gentler methods (N, L and V) represent the fastest and least damaging techniques, albeit the most expensive. Convection processing (C1, C2) is much more economical, but as we have demonstrated, generates changes in the quality profile of the cladode powder, especially with the long period of drying treatment.
After the different drying processes, the obtained cladode powder was purified in order to remove apolar components (such as lipids, chlorophyll and their fragments) as well as polar, low molecular weight components (such as mono- and disaccharides, salts) with acetone and 95% methanol extraction steps respectively. This purification was performed to avoid non-polysaccharide components influencing the growth of the selected probiotic strains. Only the C2 treatment resulted in a deep brownish material, extracted by methanol for both Opuntia species. This material could be originated from caramelization and Maillard reaction products, given the long period of drying process and documenting an over-drying of the cladodes, which also partly damages the plant matrix. This was confirmed in the TLC-analysis of both species. On one hand, the samples of O. ficus-indica showed a clearly higher content of mono- and disaccharides than O. joconostle, and on the other hand, a partial degradation of sucrose by long term convection drying (C2) is shown.
The cladodes have a very high water content of around 92% in both species and thus confirm the high-water binding capacity of succulent plants. A significant difference in the content of mucilage in both species is noticeable, as only 1.5% is present in O. ficus-indica while O. joconostle has a distinctly higher 2.5% content. In the two mucilage samples where the mineral contents were investigated, the content of minerals, particularly calcium and magnesium was relatively lower than the one reported in the literature [18,48]. After comparing with the mucilage extraction methods from the previous references, it appears that the acetone and methanol washes resulted in a better removal of the free Ca and Mg salts, and so the only remaining calcium and magnesium are the ones being linked to the acidic residues of the polysaccharide.
The protein levels are much lower than those reported in other publications [48,52], but this is likely due to artifactual effects of the methods chosen for protein measurement. A few reports utilized the widely used Bradford method [48,52]. In our experiments, we found inconsistently high results when using Bradford and Lowry’s methods compared to Kjeldahl’s method. We have found that the charged, acidic parts of the polysaccharide (in our case, the galacturonic acid residues) interact with the Coomassie blue, giving an artificially high false reading, and furthermore the reducing power of the polysaccharide is high enough to impact Lowry’s method.
After all the drying treatments, the purified polysaccharides from the mucilage deliver a similar and coherent presence of galactose, arabinose, xylose, galacturonic acid and rhamnose as the composing monosaccharides for both species. A comparison between both species shows that the mucilage of O. ficus-indica includes a relative high level of glucose (around 13%), but after further tests it was shown that this glucose is not part of the heteropolysaccharide molecules. Investigation suggests a small amount of α-glucan such as soluble starch in the O. ficus-indica mucilage sample. A higher content of galacturonic acid is also present in the polysaccharide of O. ficus-indica compared to O. joconostle. Contrariwise, O. joconostle shows a much higher level of arabinose than O. ficus-indica. These differences could be explained by epi- and/or genetic differences in these Opuntia species.
The molecular dimensions of the mucilage, such as mass distribution, average mass and dispersity show a high molecular weight for the polysaccharide for both species, with an average Mw in the range of 3.7 × 105 to 4.7 × 105 g∙mol−1. Only the mucilage of O. ficus-indica changes to a lower mass distribution after the long-time convection drying (C2) process. Its average Mw is 2.6 × 105 g∙mol−1, but also includes a second population with a lower molecular mass range with a maximum at around 2 × 103 g∙mol−1. This partial hydrolysis of the high Mw polysaccharide into lower polysaccharide populations confirms the over-drying of the cladodes by the C2 drying process and the breakdown of the polysaccharide matrix.
To evaluate the prebiotic potential of the mucilage, three probiotic strains, one bifidobacteria and two lactobacilli were tested. All strains were able to utilize the mucilage as a substrate, albeit at a lower level than glucose. The highest growths of these probiotics, however, was in the presence of C2 mucilage. This phenomenon can be explained by the higher availability of this mucilage, which includes the lower Mw α-glucan polysaccharide and the degraded heteropolysaccharide (Figure 3); it has been shown for other polysaccharides that as the molecular weight of the carbohydrate decreases, the availability becomes higher [33].
The present results show that the molecular dimension and the prebiotic efficiency are not overly sensitive to the drying treatment, yet by increasing the temperature and/or drying time some modification will happen. Therefore, depending on the objective desired, the final mucilage can be modified from the drying step of the cladodes. Furthermore, this is an important result, as it can be shown that convection drying, if done gently enough, yields results similar to the much more expensive lyophilization techniques. However, the viscosity of the solutions is quite affected by the drying treatment (not measured but readily apparent), and since there are health-promoting effects, such as the lowering of serum cholesterol, that appear to depend on the viscosity and not on the composition of the polysaccharide [53], further studies in this direction should be conducted.
In general, it can be said that bifidobacteria and lactobacilli can ferment the heteropolysaccharides of the mucilage from Opuntia spp., results which are in agreement with previous studies. A second article is in preparation where the composition and structure of the heteropolysaccharides of Opuntia will be described in greater detail, and how these parameters affect their availability as carbon source for other members of the gut microbiota. Meanwhile, it is possible to say that the consumption of dried Opuntia cladodes (Nopal flour), or their refined form as the dry, water soluble mucilage is an interesting source of dietary fiber, with several potential beneficial health effects.
Author Contributions
Conceptualization, J.M.C.-R., M.M. and W.P.; Funding acquisition, H.V. and M.M.; Investigation, J.M.C.-R.; Methodology, J.M.C.-R., M.M. and W.P.; Writing—original draft, J.M.C.-R. and W.P.; Writing—review & editing, R.L. and H.V.; Supervision H.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
J.M.C.-R. thanks CONACYT for scholarship 440539; Stadt Wien for Hochschuljubiläumstiftung grant H-275198/2016; Biotrendy SA de CV for the samples; Many thanks to Ewa Cieslik for her help in conducting the FAAS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nobel, P.S.; Bobich, E.G. Environmental Biology. In Cacti: Biology and Uses; Nobel, P.S., Ed.; University of California Press: Berkeley, CA, USA, 2002; p. 280. [Google Scholar]
- Durán, A.G.; López, M.E.R.; Nava, H.J.A. El Género Opuntia en Jalisco: Guía de Campo; Universidad de Guadalajara: Guadalajara, Mexico, 2001. [Google Scholar]
- Garcia de Cortázar, V.; Nobel, P.S. Biomass and Fruit Production for the Prickly Pear Cactus, Opuntia ficus-indica. J. Am. Soc. Hortic. Sci. 1992, 117, 558–562. [Google Scholar] [CrossRef]
- Majure, L.C.; Puente, R.; Griffith, M.P.; Judd, W.S.; Soltis, P.S.; Soltis, D.E. Phylogeny of Opuntia s.s. (Cactaceae): Clade delineation, geographic origins, and reticulate evolution. Am. J. Bot. 2012, 99, 847–864. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.P. The origins of an important cactus crop, Opuntia ficus-indica (Cactaceae): New molecular evidence. Am. J. Bot. 2004, 91, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Currò, S.; Las Casas, G.; La Malfa, S.; Gentile, A. Microsatellite markers help to assess genetic diversity among Opuntia ficus indica cultivated genotypes and their relation with related species. Plant Syst. Evol. 2010, 290, 85–97. [Google Scholar] [CrossRef]
- Ciriminna, R.; Chavarría-Hernández, N.; Rodríguez-Hernández, A.I.; Pagliaro, M. Toward unfolding the bioeconomy of nopal (Opuntia spp.). Biofuels Bioprod. Biorefining 2019, 13, 1417–1427. [Google Scholar] [CrossRef]
- Billiard, E. When tradition becomes trendy: Social distinction in Maltese food culture. Anthropol. Noteb. 2006, 12, 113–126. [Google Scholar]
- Sawaya, W.N.; Khatchadourian, H.A.; Safi, W.M.; Al-muhammad, H.M. Chemical characterization of prickly pear pulp, Opuntia ficus-indica, and the manufacturing of prickly pear jam. Int. J. Food Sci. Technol. 1983, 18, 183–193. [Google Scholar] [CrossRef]
- Ramírez-Moreno, E.; Córdoba-Díaz, D.; de Cortes Sánchez-Mata, M.; Díez-Marqués, C.; Goñi, I. Effect of boiling on nutritional, antioxidant and physicochemical characteristics in cladodes (Opuntia ficus indica). LWT Food Sci. Technol. 2013, 51, 296–302. [Google Scholar] [CrossRef]
- Morales, P.; Ramírez-Moreno, E.; Sanchez-Mata, M.d.C.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional and antioxidant properties of pulp and seeds of two xoconostle cultivars (Opuntia joconostle F.A.C. Weber ex Diguet and Opuntia matudae Scheinvar) of high consumption in Mexico. Food Res. Int. 2012, 46, 279–285. [Google Scholar] [CrossRef]
- Poinar, H.N.; Kuch, M.; Sobolik, K.D.; Barnes, I.; Stankiewicz, A.B.; Kuder, T.; Spaulding, W.G.; Bryant, V.M.; Cooper, A.; Paabo, S. A molecular analysis of dietary diversity for three archaic Native Americans. Proc. Natl. Acad. Sci. USA 2001, 98, 4317–4322. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Dominguez, F.; Zapata-Morales, J.R.; Carranza-Alvarez, C. Plants used in the traditional medicine of Mesoamerica (Mexico and Central America) and the Caribbean for the treatment of obesity. J. Ethnopharmacol. 2015, 175, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Monforte, M.T.; Tripodo, M.M.; d’Aquino, A.; Mondello, M.R. Antiulcer activity of Opuntia ficus indica (L.) Mill. (Cactaceae): Ultrastructural study. J. Ethnopharmacol. 2001, 76, 1–9. [Google Scholar] [CrossRef]
- McGarvie, D.; Parolis, H. The mucilage of Opuntia ficus-indica. Carbohydr. Res. 1979, 69, 171–179. [Google Scholar] [CrossRef]
- Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Díaz, M.d.S.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; de la Barba Rosa, A.P. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- Peña-Valdivia, C.B.; Trejo, C.; Arroyo-Pena, V.B.; Sanchez Urdaneta, A.B.; Balois Morales, R. Diversity of unavailable polysaccharides and dietary fiber in domesticated nopalito and cactus pear fruit (Opuntia spp.). Chem. Biodivers 2012, 9, 1599–1610. [Google Scholar] [CrossRef]
- du Toit, A.; de Wit, M.; Hugo, A. Cultivar and Harvest Month Influence the Nutrient Content of Opuntia spp. Cactus Pear Cladode Mucilage Extracts. Molecules 2018, 23, 916. [Google Scholar] [CrossRef]
- Hernandez-Urbiola, M.I.; Perez-Torrero, E.; Rodriguez-Garcia, M.E. Chemical analysis of nutritional content of prickly pads (Opuntia ficus indica) at varied ages in an organic harvest. Int. J. Environ. Res. Public Health 2011, 8, 1287–1295. [Google Scholar] [CrossRef]
- Butterweck, V.; Semlin, L.; Feistel, B.; Pischel, I.; Bauer, K.; Verspohl, E.J. Comparative evaluation of two different Opuntia ficus-indica extracts for blood sugar lowering effects in rats. Phytother. Res. 2011, 25, 370–375. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Lan, Q.J.; Huang, Z.C.; Ouyang, L.J.; Zeng, F.H. Antidiabetic effect of a newly identified component of Opuntia dillenii polysaccharides. Phytomedicine 2011, 18, 661–668. [Google Scholar] [CrossRef]
- Alarcon-Aguilar, F.J.; Valdes-Arzate, A.; Xolalpa-Molina, S.; Banderas-Dorantes, T.; Jimenez-Estrada, M.; Hernandez-Galicia, E.; Roman-Ramos, R. Hypoglycemic activity of two polysaccharides isolated from Opuntia ficus-indica and O. streptacantha. Proc. West. Pharm. Soc. 2003, 46, 139–142. [Google Scholar]
- Godard, M.P.; Ewing, B.A.; Pischel, I.; Ziegler, A.; Benedek, B.; Feistel, B. Acute blood glucose lowering effects and long-term safety of OpunDia supplementation in pre-diabetic males and females. J. Ethnopharmacol. 2010, 130, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Lopez, M.A.; Paredes-Lopez, O.; Reynoso-Camacho, R. Functional and hypoglycemic properties of nopal cladodes (O. ficus-indica) at different maturity stages using in vitro and in vivo tests. J. Agric. Food Chem. 2013, 61, 10981–10986. [Google Scholar] [CrossRef] [PubMed]
- Gouws, C.A.; Georgousopoulou, E.N.; Mellor, D.D.; McKune, A.; Naumovski, N. Effects of the Consumption of Prickly Pear Cacti (Opuntia spp.) and its Products on Blood Glucose Levels and Insulin: A Systematic Review. Medicina 2019, 55, 138. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Pergolizzi, S.; Miceli, N.; Monforte, M.T.; Tripodo, M.M. Study on the increment of the production of gastric mucus in rats treated with Opuntia ficus indica (L.) Mill. cladodes. J. Ethnopharmacol. 2002, 83, 229–233. [Google Scholar] [CrossRef]
- Lee, E.-B.; Hyun, J.-E.; Li, D.-W.; Moon, Y.-I. The Effect of Opuntia ficus-indica var. saboten Fruit on Gastric Lesion and Ulcer in Rats. Nat. Prod. Sci. 2001, 7, 90–93. [Google Scholar]
- Vazquez-Ramirez, R.; Olguin-Martinez, M.; Kubli-Garfias, C.; Hernandez-Munoz, R. Reversing gastric mucosal alterations during ethanol-induced chronic gastritis in rats by oral administration of Opuntia ficus-indica mucilage. World J. Gastroenterol. 2006, 12, 4318–4324. [Google Scholar] [CrossRef]
- Galati, E.M.; Tripodo, M.M.; Trovato, A.; d’Aquino, A.; Monforte, M.T. Biological Activity of Opuntia ficus indica Cladodes II: Effect on Experimental Hypercholesterolemia in Rats. Pharm. Biol. 2008, 41, 175–179. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Xie, G.; Kirpotina, L.N.; Klein, R.A.; Jutila, M.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. Int. Immunopharmacol. 2008, 8, 1455–1466. [Google Scholar] [CrossRef]
- Aguilera-Barreiro, M.d.L.; Rivera-Marquez, J.A.; Trujillo-Arriaga, H.M.; Tamayo, Y.O.J.A.; Barreira-Mercado, E.; Rodriguez-Garcia, M.E. Intake of dehydrated nopal (Opuntia ficus indica) improves bone mineral density and calciuria in adult Mexican women. Food Nutr. Res. 2013, 57. [Google Scholar] [CrossRef]
- Mueller, M.; Cavarkapa, A.; Unger, F.M.; Viernstein, H.; Praznik, W. Prebiotic potential of neutral oligo- and polysaccharides from seed mucilage of Hyptis suaveolens. Food Chem. 2017, 221, 508–514. [Google Scholar] [CrossRef]
- Koenen, M.E.; Cruz Rubio, J.M.; Mueller, M.; Venema, K. The effect of agave fructan products on the activity and composition of the microbiota determined in a dynamic in vitro model of the human proximal large intestine. J. Funct. Foods 2016, 22, 201–210. [Google Scholar] [CrossRef]
- Mueller, M.; Reiner, J.; Fleischhacker, L.; Viernstein, H.; Loeppert, R.; Praznik, W. Growth of selected probiotic strains with fructans from different sources relating to degree of polymerization and structure. J. Funct. Foods 2016, 24, 264–275. [Google Scholar] [CrossRef]
- Yahia, E.M.; Ornelas, J.D.J.; Anaya, A. Extraction and Chemical Characteristics of Mucilage from Mesquite, Aloe Vera, Maguey and Prickly Pear Cactus Cladodes (Nopal) and Evaluation of Its Prebiotic Effect on the Growth of 2 Probiotic Bacteria. Acta Hortic. 2009, 625–628. [Google Scholar] [CrossRef]
- Reyes-Reyes, M.; Salazar-Montoya, J.A.; Rodriguez-Paez, L.I.; Ramos-Ramirez, E.G. In vitro fermentation of oligosaccharides obtained from enzymatic hydrolysis of Opuntia streptacantha mucilage. J. Sci. Food Agric. 2019, 99, 2883–2891. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Tapia, M.; Aguilar-Lopez, M.; Perez-Cruz, C.; Pichardo-Ontiveros, E.; Wang, M.; Donovan, S.M.; Tovar, A.R.; Torres, N. Nopal (Opuntia ficus indica) protects from metabolic endotoxemia by modifying gut microbiota in obese rats fed high fat/sucrose diet. Sci. Rep. 2017, 7, 4716. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; He, X.; Chin, E.L.; Tovar, A.R.; Torres, N.; Slupsky, C.M.; Raybould, H.E. Nopal feeding reduces adiposity, intestinal inflammation and shifts the cecal microbiota and metabolism in high-fat fed rats. PLoS ONE 2017, 12, 171672. [Google Scholar] [CrossRef]
- Guevara-Arauza, J.C.; de Jesús Ornelas-Paz, J.; Pimentel-González, D.J.; Rosales Mendoza, S.; Soria Guerra, R.E.; Paz Maldonado, L.M.T. Prebiotic effect of mucilage and pectic-derived oligosaccharides from nopal (Opuntia ficus-indica). Food Sci. Biotechnol. 2012, 21, 997–1003. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Gutierrez-Cortez, E.; Valderrama-Bravo, M.d.C.; Rojas-Molina, I.; Espinosa-Arbelaez, D.G.; Suarez-Vargas, R.; Rodriguez-Garcia, M.E. Effects of drying process on the physicochemical properties of nopal cladodes at different maturity stages. Plant Foods Hum. Nutr. 2012, 67, 44–49. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Rodríguez-Ramírez, J.; Medina-Torres, L.L.; Méndez Lagunas, L.L.; Bernad-Bernad, M.J. Effects of drying conditions on the rheological properties of reconstituted mucilage solutions (Opuntia ficus-indica). Carbohydr. Polym. 2011, 84, 439–445. [Google Scholar] [CrossRef]
- Martínez-Soto, G.; Celis-Fabián, F.; Hernández-Pérez, T.; López, O.P. Effect of Drying Methods on the Nutraceutical Potential of Cactus Cladodes (Opuntia spp.). Int. J. Food Nutr. Sci. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Kocourek, J.; Tichá, M.; Koštíř, J. The use of diphenylamine-aniline-phosphoric acid reagent in the detection and differentiation of monosaccharides and their derivatives on paper chromatograms. J. Chromatogr. A 1966, 24, 117–124. [Google Scholar] [CrossRef]
- Rao, P.; Pattabiraman, T.N. Reevaluation of the phenol-sulfuric acid reaction for the estimation of hexoses and pentoses. Anal. Biochem. 1989, 181, 18–22. [Google Scholar] [CrossRef]
- Hall, B.G.; Acar, H.; Nandipati, A.; Barlow, M. Growth rates made easy. Mol. Biol. Evol. 2014, 31, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Morreale, V.; Pecoraino, M.; Pagliaro, M. Solar air drying for innovative Opuntia ficus-indica cladode dehydration. 4open 2019, 2. [Google Scholar] [CrossRef]
- Crispín-Isidro, G.; Hernández-Rodríguez, L.; Ramírez-Santiago, C.; Sandoval-Castilla, O.; Lobato-Calleros, C.; Vernon-Carter, E.J. Influence of purification on physicochemical and emulsifying properties of tamarind (Tamarindus indica L.) seed gum. Food Hydrocoll. 2019, 93, 402–412. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Trachtenberg, S.; Mayer, A.M. Composition and properties of Opuntia ficus-indica mucilage. Phytochemistry 1981, 20, 2665–2668. [Google Scholar] [CrossRef]
- Manhivi, V.E.; Venter, S.; Amonsou, E.O.; Kudanga, T. Composition, thermal and rheological properties of polysaccharides from amadumbe (Colocasia esculenta) and cactus (Opuntia spp.). Carbohydr. Polym. 2018, 195, 163–169. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Brito-De La Fuente, E.; Torrestiana-Sanchez, B.; Katthain, R. Rheological properties of the mucilage gum (Opuntia ficus indica). Food Hydrocoll. 2000, 14, 417–424. [Google Scholar] [CrossRef]
- Reyes-Ocampo, I.; Córdova-Aguilar, M.S.; Guzmán, G.; Blancas-Cabrera, A.; Ascanio, G. Solvent-free mechanical extraction of Opuntia ficus-indica mucilage. J. Food Process Eng. 2019, 42. [Google Scholar] [CrossRef]
- Gunness, P.; Gidley, M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010, 1, 149–155. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).